Introduction
Lung cancer is one of the leading causes of
cancer-associated mortality worldwide (1). Non-small-cell lung cancer (NSCLC)
accounts for ~80% all cases of lung cancer and has a 5-year
survival rate of 15% (2). Resistance
can be developed to contemporary chemotherapy regimens for NSCLC
and various adverse side effects can be invoked (3). Therefore, the identification and
development of more effective, less toxic antitumor NSCLC therapies
is urgently required.
The cell cycle is a highly regulated mechanism of
controlling cell growth, proliferation and survival. Cancer cells
exhibit dysregulated cell cycles, with the overexpression of
positive regulators and inhibition of negative regulators,
resulting in unlimited replication potential (4). Therefore, the development of agents
targeting the dysregulated cell cycle has been considered to be a
suitable strategy for cancer therapy (3). Cell cycle progression depends on
cyclin-dependent kinase (CDK) activation status, which act
consecutively in G1 phase to initiate S phase and, in
G2 phase, to initiate mitosis. With mitogenic
stimulation, cyclin D is activated, in turn activating CDK4 and 6.
Cyclin D-dependent kinases phosphorylate retinoblastoma proteins
(Rb), which relieves transcription factor E2F1 from inhibition and
allows for the expression of specific E2F1-target genes (5). The cyclin E-CDK2 complex concludes Rb
phosphorylation and permits the activation of E2F1-responsive genes
(6). Cyclin-CDK complexes are
inhibited by two CDK inhibitor (CDKN) families: Inhibitors of CDKN4
(INK4) (p15INK4B, p16INK4A,
p18INK4C and p19INK4D) and CDKN1A
(p21CIP1/WAF1), 1B (p27KIP1) and 1C
(p57KIP2) (7). The CDKNs
also perform central cell cycle-regulating functions, coordinating
internal and external signals that modulate cell cycle progression
(7). Thus, the cell cycle is tightly
controlled in response to external and internal factors. In
abnormal conditions, including DNA damage, cell cycle progression
is inhibited to prevent harmful progression.
Reactive oxygen species (ROS) are chemically
reactive molecules, including the superoxide anion, hydrogen
peroxide and the hydroxyl radical. ROS are endogenously produced
during metabolic activities of the cell, including the oxidative
phosphorylation of mitochondria (8).
ROS may also arise from reactions with exogenous sources, including
xenobiotic compounds (9). Cellular
function requires moderate ROS levels for normal cell signaling.
However, ROS overproduction can be toxic to cells owing to their
peroxidative activity (10). One
proposed anticancer strategy is the use of exogenous ROS-stressing
agents or inhibition of endogenous antioxidants to elevate
intracellular ROS levels to toxic amounts, triggering cell cycle
arrest in the target cancer cell (11).
Ginsenosides are triterpene saponins and the major
pharmacologically active components extracted from the roots and
rhizomes of various Panax species. Ginsenosides are
responsible for the majority of functions of ginseng, including
angiogenesis, vasorelaxation and antioxidation (8). These molecules also possess
anti-inflammatory and anticancer properties (12–14). In a
previous study, four dammarane-type triterpene saponins isolated
from the Panax ginseng root exhibited effective hydroxyl
radical scavenging ability, as well as antibacterial and cytotoxic
activities (15). However, to the
best of our knowledge, there has been no research into the
anti-proliferative effects of these compounds or their underlying
molecular mechanisms. As part of an ongoing screening program for
the evaluation of the anti-proliferative potential of natural
compounds, the mechanisms underlying the cell cycle-arresting
activities of Rg18 in NSCLC A549 cells were investigated in the
present study.
Materials and methods
Chemicals and reagents
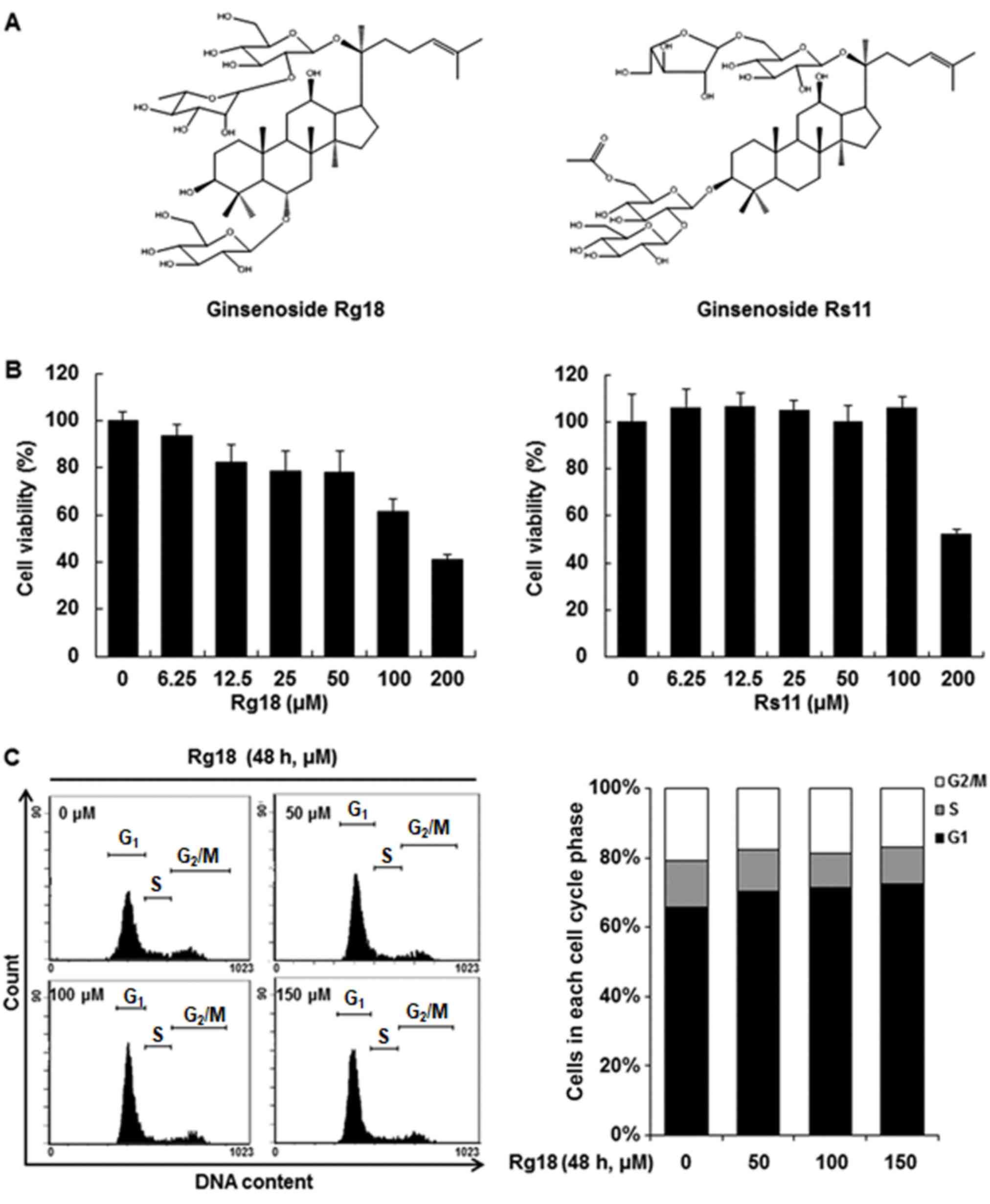
Rg18 and Rs11 (Fig.
1A) were kindly provided by Dr. Kyung-Tack Kim (Korea Food
Research Institute, Wanju-gun, South Korea), and its purity of
>96% was determined by high-performance liquid
chromatography-mass spectrometry analyses (15). RPMI-1640 medium, fetal bovine serum
(FBS), penicillin and streptomycin were all obtained from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). MTT,
phenylmethylsulfonyl fluoride (PMSF), N-acetylcysteine
(NAC), 2′,7′-dichlorofluorescin diacetate (DCFH-DA), bisacrylamide
and sodium dodecyl sulfate were purchased from Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany). CDK2 (sc-163), CDK4 (sc-260), CDK6
(sc-7961), cyclin D1 (sc-8396), cyclin D2 (sc-593), cyclin E
(sc-198), p21CIP1/WAF1 (sc-397), p27KIP1
(sc-1641), Rb (sc-102), p38 mitogen activated protein kinase (p38,
sc-535), c-Jun N-terminal kinase (JNK, sc-7345), extracellular
signal-regulated kinase (ERK, sc-94), p65 (sc-372), p-JNK
(sc-12882), p-ERK (sc-7383) and β-actin (sc-81178) primary
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Phosphorylated (p)-Rb (no. 9307), p-p38 (no.
9215), p-p65 (Ser536, no. 3031) primary antibodies were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Proteinase
K, ribonuclease A, and TEMED were purchased from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA).
Cell culture
The human lung adenocarcinoma A549 cell line was
obtained from the Korean Cell Line Bank (Seoul, Korea). A549 cells
were grown at 37°C in RPMI-1640, supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin
sulfate, in a humidified atmosphere of 5% CO2.
MTT assay
Cell viability was assessed by MTT assay, as
described previously (16). A total
of 5×104 cells/ml were seeded in wells containing 100 µl
complete medium in a 96-well plate. After 24 h, Rg18 or Rs11 was
added at concentrations of 0, 6.25, 12.5, 25, 50, 100 and 200 µM
and cells were incubated for 48 h. A total of 50 µl MTT (stock
solution, 5 mg/ml in PBS) was added, and the plates were incubated
for an additional 4 h. The medium was disposed and the formazan
blue was dissolved using 100 µl DMSO/well. The optical density was
measured at 540 nm.
Cell cycle analysis
The cell cycle distribution was analyzed using
propidium iodide staining as described previously (17). A total of 1×104 cells were
collected from each experimental group, washed twice with PBS
(4°C), fixed and permeabilized with 70% ethanol at 4°C for 1 h. The
cells were washed once more with PBS and resuspended in a solution
containing propidium iodide (50 µg/ml) and RNase A (250 µg/ml) for
30 min at room temperature. Fluorescence-activated cell sorting
(FACS) was then performed to determine the cell cycle stage of each
cell, using the Cytomics™ FC 500 flow cytometry and CXP cytometer
software (version 2.0; (Beckman Coulter, Inc, Indianapolis, IN,
USA).
Protein extraction and western blot
analysis
Cells were collected by centrifugation at 200 × g
for 10 min at 4°C, washed twice with PBS at 4°C, and centrifuged at
200 × g for 5 min. The resulting cell pellet was resuspended in 1X
protein lysis buffer (Intron Biotechnology, Inc., Seongnam, Korea).
The protein concentration was determined using the Bio-Rad protein
assay reagent (Bio-Rad Laboratories, Inc.) and bovine serum albumin
as a standard solution according to the manufacturer's instruction.
Equal amounts of cell lysates (5–15 µl) were separated by 8–12%
SDS-PAGE and transferred into nitrocellulose membranes. The
membranes were incubated with the aforementioned primary
antibodies. The membranes were then incubated with a 1:2,000
dilution of horseradish peroxidase-conjugated Goat anti-mouse IgG
(no. 31430), Goat anti-rabbit IgG (no. 31460), or Rabbit anti-Goat
IgG (no. 81-1620) (Thermo Fisher Scientific Inc.) and visualized
using an enhanced chemiluminescence detection system (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), according to the
manufacturer's protocol. β-actin was used as a loading control and
densitometric analysis was performed using Quantity One®
Software (version 4.6.3; Bio-Rad Laboratories, Inc.).
ROS detection (DCFH-DA assay)
Intracellular ROS levels were detected using a
DCFH-DA assay, as described previously (18). Cells (5×104 cells/ml) were
pretreated with and without 10 mM NAC for 1 h and then treated with
150 µM Rg18 for 24 h. The cells were collected and suspended in
pre-warmed PBS (37°C) prior to the addition of 20 µM DCFH-DA and
incubated for 30 min at 37°C. When transported into the cells,
DCFH-DA forms DCFH by deacetylation, and upon oxidation,
fluorescent 2′,7′-dichlorofluorescin is formed. Fluorescence
intensity was analyzed by Cytomics™ FC 500 flow cytometry and CXP
cytometer software (version 2.0; Beckman Coulter, Inc.,
Indianapolis, IN, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation of the results from three independent experiments.
One-way analysis of variance followed by Dunnett's post hoc test
was performed to identify differences between groups using GraphPad
Prism software (version 5.01; GraphPad Software, Inc., La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Rg18 inhibits cell proliferation and
induces cell cycle arrest in A549 cells
The anti-proliferative effects of Rg18 and Rs11 were
examined in A549 cells using an MTT assay (Fig. 1B) and half-maximal inhibitory
concentration (IC50) values. The A549 cells were treated
with different concentrations of Rg18 and Rs11 for 48 h, and it was
found that Rg18 significantly decreases the viability of A549
cells, and these decreases are concentration-dependent
(IC50=140.09 µM). However, Rs11 did not show obvious
cytotoxic effects up to 100 µM and it decreased cell viability at
200 µM (cell viability: 51.78 %). The following experiments in A549
cells were conducted using Rg18 owing to their greater sensitivity
to this ginsenoside. To investigate the mechanism of the
anti-proliferative effect of Rg18, flow cytometry was used to
identify changes in cell cycle distribution following treatment
with Rg18. A549 cells were treated with various concentrations of
Rg18 (0, 50, 100 and 150 µM) for 48 h, and the percentage of A549
cells in G1 phase increased from 63.3±1.5% (control
group) to 68.6±2.1, 69.8±1.4 and 71.1±2.5% upon treatment with 50,
100 and 150 µM Rg18, respectively (Fig.
1C).
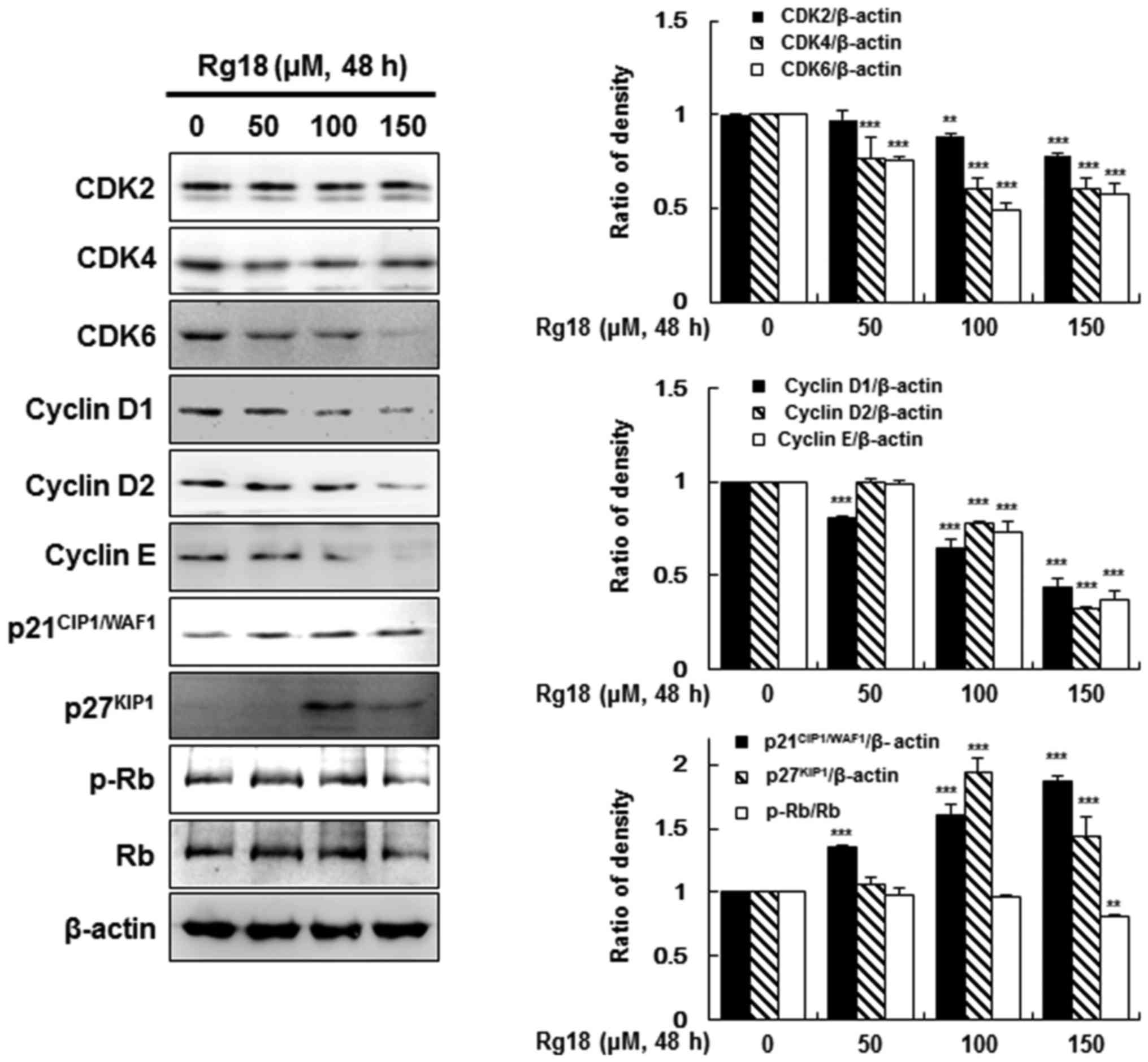
Effect of Rg18 on protein expression
levels of various G1 phase cell cycle regulators in A549
cells
Rg18-induced G1 arrest in A549 cells
(Fig. 1C), therefore, the protein
expression levels of cell cycle regulatory molecules were
investigated following 50, 100 or 150 µM Rg18 treatment. Rg18
treatment significantly reduced the protein expression level of
CDK2, CDK4, CDK6, cyclin D1, cyclin D2 and cyclin E in A549 cells.
Rg18 treatment also increased the protein expression levels of
p21CIP1/WAF1 and p27KIP1 after a 48-h
treatment. These proteins modulate CDK activity in various phases
of the cell cycle, including G1. As Rb phosphorylation
is influential in the transit from G1 to S phase
(19), the phosphorylation levels of
Rb were also examined. Following treatment with 150 µM Rg18 for 48
h, the degree of phosphorylation and expression levels of Rb
significantly decreased (Fig. 2).
Rg18 treatment results in upregulation
of intracellular ROS levels and downregulates p38, JNK and nuclear
factor-κB (NF-κB) activation in A549 cells
ROS generation is an early sign of cell cycle arrest
(20). To analyze ROS generation
induced by Rg18 treatment, a DCFH-DA assay was performed. Following
treatment with 150 µM Rg18 for 2 h, the generation of ROS increased
significantly, an effect that was blocked by pretreatment with the
antioxidant NAC (Fig. 3A). To
investigate the molecular mechanism of Rg18-induced G1
arrest, p38, ERK, JNK, and NF-κB (p65) phosphorylation were
analyzed in Rg18-treated A549 cells. These molecules serve notable
roles in cell cycle regulation (21,22). Rg18
treatment resulted in a concentration-dependent decrease in p38,
JNK and NF-κB/p65 phosphorylation, but there was no change to ERK
phosphorylation levels (Fig. 3B).
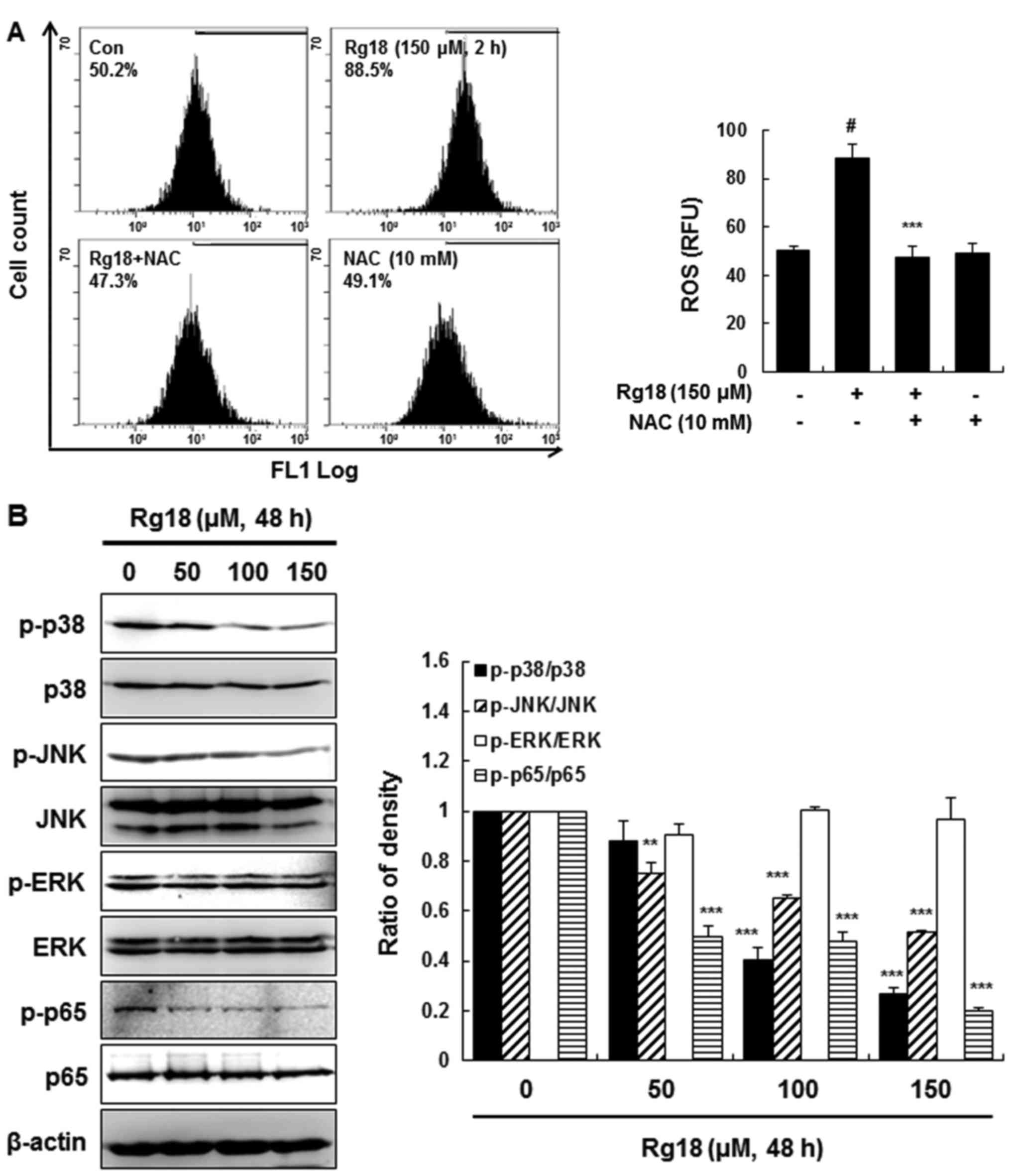 | Figure 3.Rg18 upregulates the level of
intracellular ROS and regulates the activation of p38, JNK and
NF-κB in A549 cells. (A) DCFH-DA was added to stain the A549 cells
following treatment with 150 µM Rg18 for 2 h. Flow cytometry was
used to analyze the level of ROS in the cells.
#P<0.05 vs. the control group; ***P<0.001 vs. Rg18
treatment group. (B) Western blot analysis was performed with
antibodies against p-p38, p38, p-JNK, JNK, p-ERK, ERK, p-p65, p65
and β-actin. The histograms represent the mean fluorescence
intensity of samples analyzed **P<0.01 and ***P<0.001 vs. the
control group. Data presented are the mean ± standard deviation of
the results from three independent experiments. ROS, reactive
oxygen species; p-p38, phosphorylated p38 mitogen-activated protein
kinase; JNK, c-Jun N-terminal kinase; NF-κB, nuclear factor-κB;
DCFH-DA, 2′,7′-dichlorofluorescin diacetate; ERK, extracellular
signal-regulated kinase; NAC, N-acetylcysteine. |
Discussion
Previous studies have demonstrated that ginsenosides
isolated from the root of P. ginseng C. A. Meyer could
inhibit cancer cell growth in vitro and in vivo via
cell cycle arrest (14,23–25). In a
previous study, it was demonstrated that four novel ginsenosides
isolated from the P. ginseng root exhibited hydroxyl radical
scavenging, anti-bacterial and cytotoxic activities (15). The aim of the present study was to
determine whether Rg18 exerted an anti-proliferative effect on A549
cells and to characterize the molecular mechanism involved. The
results demonstrated that Rg18 inhibited the proliferation of A549
cells and flow cytometric assays indicated that treatment with Rg18
lead to G1 arrest in A549 cells.
Cell cycle progression is highly controlled by
interactions of various regulators, including the cyclins and their
catalytic partners, CDKs (6). CDK
complexes are formed and activated at specific cell cycle phases;
their activities are necessary for progression through distinct
cell cycle phases (7). Progressing
through the G1 phase requires either CDK4 or CDK6
activity, followed by the activation of CDK2. The cyclin-CDK
complex formed during G1 phase catalyzes the
phosphorylation of the dominant inhibitors of G1/S phase
cell cycle progression, the Rb family of tumor suppressor proteins,
thereby allowing progression to S phase (26,27).
Cyclin-CDK complexes can bind p21CIP1/WAF1 and
p27KIP1, which inhibit kinase activities and prevent
cell cycle progression (28). Western
blot analysis demonstrated that Rg18 decreased the expression
levels of cyclin D1, cyclin D2, cyclin E, CDK4, CDK6 and CDK2 in
A549 cells. Furthermore, decreased CDK expression has been
demonstrated to be associated with Rb under-phosphorylation, which
is known to result in the sequestering of E2F, and thereby
inhibition of the cell cycle progression (29).
The results indicate that Rg18 influences cell cycle
progression via the upregulation of p21CIP1/WAF1 and
p27KIP1 protein expression in A549 cells. It was
apparent that strong CKI upregulation mediated Rg18-induced
G1 phase arrest and the inhibition of cell growth.
Overall, the G1 phase blockade in A549 cells appeared to
be mediated by the downregulation of CDK activity associated with
CKI induction, such as by p21CIP1/WAF1 and
p27KIP1.
ROS are involved in multiple types of chemically
induced cell cycle arrest; evidence indicates that increased
oxidative stress is associated with cell cycle arrest induced by
certain anticancer agents (11,30). Among
the protopanaxadiols, ginsenoside-Rb2 has been
demonstrated to significantly increase the expression of genes
encoding antioxidant enzymes, including superoxide dismutase and
catalase in vitro (31). The
present study demonstrated that Rg18 treatment increased
intracellular ROS levels, which led to cell cycle arrest.
The mitogen-activated protein kinases (MAPKs) are
also involved in cell cycle regulation (21), and three pathways, ERK, JNK and p38,
are closely associated with the progression of a number of
malignant types of cancer, including breast and ovarian cancer, and
NSCLC (32,33). JNK and p38 function in stress
reactions and the induction of cell cycle arrest (34). The anticancer activity of
20(S)-protopanaxadiol in colon cancer cells is mediated by
downregulation of the ERK, JNK and NF-κB signaling pathways
(35). Additionally, compound K
significantly inhibited phorbol 12-myristate 13-acetate-induced
matrix metallopeptidase 9 protein expression and secretion via
suppression of DNA-binding and activator protein-1 transcriptional
activities, downstream of the p38, ERK and JNK pathways (36). However, it has been established that
selenite-induced ROS arrest the cell cycle of NB4 cells at the
G1 phase by inhibiting the JNK/activating transcription
factor 2 axis in vitro and in vivo (37). In the present study, it was
demonstrated that Rg18 treatment suppressed the phosphorylation of
JNK and p38 in A549 cells.
Data from previous studies indicated that blocking
the activation of NF-κB could be a critical target for the
regulation of cell proliferation and antioxidant behaviors
(38–40). Ginsenoside Rg3 has been reported to
inhibit NF-κB, induce G1 arrest and enhance
susceptibility to docetaxel and other chemicals in prostate cancer
cells (41). Furthermore, the
ginsenoside Rd has been demonstrated to elevate intracellular
glutathione levels by increasing γ-glutamyl cysteine ligase
activation in rat hepatocyte H4IIE cells through NF-κB-DNA binding
(42). This result indicates that
NF-κB serves as a cellular marker for cell cycle arrest in HL-60
cells. In the present study, Rg18 treatment inhibited the
phosphorylation of NF-κB/p65 in A549 cells. However, the exact
mechanism of this effect, and whether it took place at the
transcriptional and/or translational levels, requires further
investigation.
In summary, Rg18 was found to inhibit the
proliferation of A549 cells by arresting the cell cycle at the
G1 phase by downregulating CDK2, CDK4 and CDK6
expression, in association with the induction of
p21CIP1/WAF1 and p27KIP1. The results
indicated that this was, at least in part, due to intracellular ROS
production and the downregulation of multiple signaling pathways,
including JNK, p38 and NF-κB/p65. Further research is required to
dissect the underlying mechanisms of these pathway changes.
However, these results illustrate the potential use of Rg18 in
cancer treatment, either alone or in combination with other
anticancer agents.
Acknowledgements
Not applicable.
Funding
This study was supported by a research grant from
the Korea Food Research Institute (E0145202).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
DGL and JSS designed experiments, analyzed the data
and statistics, and drafted the manuscript. KTK, SYC, and MHL. did
isolation, structural analysis, and a purity analysis of Rg18 and
Rs11. KTL designed the study, coordinated the project and gave
final approval of publication. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:pp.
584–594. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang CY, Ju DT, Chang CF, Muralidhar
Reddy P and Velmurugan BK: A review on the effects of current
chemotherapy drugs and natural agents in treating non-small cell
lung cancer. Biomedicine (Taipei). 7:232017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen HS, Liu Y, Lin LQ, Zhao JL, Zhang CP,
Jin JC, Wang L, Bai MH, Wang YC, Liu M and Shen BZ:
Anti-proliferative effect of an extract of the root of Polygonum
multiflorum Thunb. On MCF-7 human breast cancer cells and the
possible mechanisms. Mol Med Rep. 4:1313–1319. 2011.PubMed/NCBI
|
|
5
|
Dickson MA and Schwartz GK: Development of
cell-cycle inhibitors for cancer therapy. Curr Oncol. 16:36–43.
2009.PubMed/NCBI
|
|
6
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foster DA, Yellen P, Xu L and Saqcena M:
Regulation of G1 cell cycle progression: Distinguishing the
restriction point from a nutrient-sensing cell growth
checkpoint(s). Genes Cancer. 1:1124–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nita M and Grzybowski A: The role of the
reactive oxygen species and oxidative stress in the pathomechanism
of the age-related ocular diseases and other pathologies of the
anterior and posterior eye segments in adults. Oxid Med Cell
Longev. 2016:31647342016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Zhao X and Gong X: Costunolide
induces lung adenocarcinoma cell line A549 cells apoptosis through
ROS (reactive oxygen species)-mediated endoplasmic reticulum
stress. Cell Biol Int. 40:289–297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao W, Jiang Y, Men Q, Yuan L, Huang Z,
Liu T, Li W and Liu X: Tetrandrine induces G1/S cell cycle arrest
through the ROS/Akt pathway in EOMA cells and inhibits angiogenesis
in vivo. Int J Oncol. 46:360–368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi B, Zhang L, Zhang Z, Ouyang J and Huang
H: Effects of ginsenosides-Rb1 on exercise-induced oxidative stress
in forced swimming mice. Pharmacogn Mag. 10:458–463. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Zhong W, Wang W, Hu S, Yuan J, Zhang
B, Hu T and Song G: Ginsenoside metabolite compound K promotes
recovery of dextran sulfate sodium-induced colitis and inhibits
inflammatory responses by suppressing NF-κB activation. PLoS One.
9:e878102014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung KS, Cho SH, Shin JS, Kim DH, Choi
JH, Choi SY, Rhee YK, Hong HD and Lee KT: Ginsenoside Rh2 induces
cell cycle arrest and differentiation in human leukemia cells by
upregulating TGF-β expression. Carcinogenesis. 34:331–340. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee DG, Lee AY, Kim KT, Cho EJ and Lee S:
Novel dammarane-type triterpene saponins from Panax ginseng root.
Chem Pharm Bull (Tokyo). 63:927–934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Plumb JA, Milroy R and Kaye SB: Effects of
the pH dependence of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium
bromide-formazan absorption on chemosensitivity determined by a
novel tetrazolium-based assay. Cancer Res. 49:4435–4440.
1989.PubMed/NCBI
|
|
17
|
Choi JH and Lee KT: Costunolide-induced
apoptosis in human leukemia cells: Involvement of c-jun N-terminal
kinase activation. Biol Pharm Bull. 32:1803–1808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park EY, Kim JI, Leem DG, Shin JS, Kim KT,
Choi SY, Lee MH, Choi JH, Lee YS and Lee KT: Resveratrol analogue
(E)-8-acetoxy-2-[2-(3,4-diacetoxyphenyl)ethenyl]-quinazoline
induces apoptosis via Fas-mediated pathway in HL-60 human leukemia
cells. Oncol Rep. 36:3577–3587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taya Y: RB kinases and RB-binding
proteins: New points of view. Trends Biochem Sci. 22:14–17. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verbon EH, Post JA and Boonstra J: The
influence of reactive oxygen species on cell cycle progression in
mammalian cells. Gene. 511:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Joyce D, Albanese C, Steer J, Fu M,
Bouzahzah B and Pestell RG: NF-kappaB and cell-cycle regulation:
The cyclin connection. Cytokine Growth Factor Rev. 12:73–90. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abe H, Arichi S, Hayashi T and Odashima S:
Ultrastructural studies of Morris hepatoma cells reversely
transformed by ginsenosides. Experientia. 35:1647–1649. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HS, Lee EH, Ko SR, Choi KJ, Park JH
and Im DS: Effects of ginsenosides Rg3 and Rh2 on the proliferation
of prostate cancer cells. Arch Pharm Res. 27:429–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng CC, Yang SM, Huang CY, Chen JC,
Chang WM and Hsu SL: Molecular mechanisms of ginsenoside
Rh2-mediated G1 growth arrest and apoptosis in human lung
adenocarcinoma A549 cells. Cancer Chemother Pharmacol. 55:531–540.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang HC and Clurman BE: Cyclin E in
normal and neoplastic cell cycles. Oncogene. 24:2776–2786. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Connell-Crowley L, Harper JW and Goodrich
DW: Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell
cycle arrest by site-specific phosphorylation. Mol Biol Cell.
8:287–301. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmidt M, Lu Y, Parant JM, Lozano G,
Bacher G, Beckers T and Fan Z: Differential roles of p21(Waf1) and
p27(Kip1) in modulating chemosensitivity and their possible
application in drug discovery studies. Mol Pharmacol. 60:900–906.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee KW, Kim HJ, Lee YS, Park HJ, Choi JW,
Ha J and Lee KT: Acteoside inhibits human promyelocytic HL-60
leukemia cell proliferation via inducing cell cycle arrest at G0/G1
phase and differentiation into monocyte. Carcinogenesis.
28:1928–1936. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang R, Zhang Q, Peng X, Zhou C, Zhong Y,
Chen X, Qiu Y, Jin M, Gong M and Kong D: Stellettin B induces G1
arrest, apoptosis and autophagy in human non-small cell lung cancer
A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep.
6:270712016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang MS, Lee SG and Rho HM:
Transcriptional activation of Cu/Zn superoxide dismutase and
catalase genes by panaxadiol ginsenosides extracted from Panax
ginseng. Phytother Res. 13:641–644. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao JL, Lv GY, He BC, Zhang BQ, Zhang H,
Wang N, Wang CZ, Du W, Yuan CS and He TC: Ginseng saponin
metabolite 20(S)-protopanaxadiol inhibits tumor growth by targeting
multiple cancer signaling pathways. Oncol Rep. 30:292–298. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung SH, Woo MS, Kim SY, Kim WK, Hyun JW,
Kim EJ, Kim DH and Kim HS: Ginseng saponin metabolite suppresses
phorbol ester-induced matrix metalloproteinase-9 expression through
inhibition of activator protein-1 and mitogen-activated protein
kinase signaling pathways in human astroglioma cells. Int J Cancer.
118:490–497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
An JJ, Shi KJ, Wei W, Hua FY, Ci YL, Jiang
Q, Li F, Wu P, Hui KY, Yang Y and Xu CM: The ROS/JNK/ATF2 pathway
mediates selenite-induced leukemia NB4 cell cycle arrest and
apoptosis in vitro and in vivo. Cell Death Dis. 4:e9732013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaltschmidt B, Kaltschmidt C, Hehner SP,
Dröge W and Schmitz ML: Repression of NF-kappaB impairs HeLa cell
proliferation by functional interference with cell cycle checkpoint
regulators. Oncogene. 18:3213–3225. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rajitha B, Belalcazar A, Nagaraju GP,
Shaib WL, Snyder JP, Shoji M, Pattnaik S, Alam A and El-Rayes BF:
Inhibition of NF-κB translocation by curcumin analogs induces G0/G1
arrest and downregulates thymidylate synthase in colorectal cancer.
Cancer Lett. 373:227–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tran KQ, Tin AS and Firestone GL:
Artemisinin triggers a G1 cell cycle arrest of human Ishikawa
endometrial cancer cells and inhibits cyclin-dependent kinase-4
promoter activity and expression by disrupting nuclear factor-κB
transcriptional signaling. Anticancer Drugs. 25:270–281. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim SM, Lee SY, Cho JS, Son SM, Choi SS,
Yun YP, Yoo HS, Yoon DY, Oh KW, Han SB and Hong JT: Combination of
ginsenoside Rg3 with docetaxel enhances the susceptibility of
prostate cancer cells via inhibition of NF-kappaB. Eur J Pharmacol.
631:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim ND, Pokharel YR and Kang KW:
Ginsenoside Rd enhances glutathione levels in H4IIE cells via
NF-kappaB-dependent gamma-glutamylcysteine ligase induction.
Pharmazie. 62:933–936. 2007.PubMed/NCBI
|