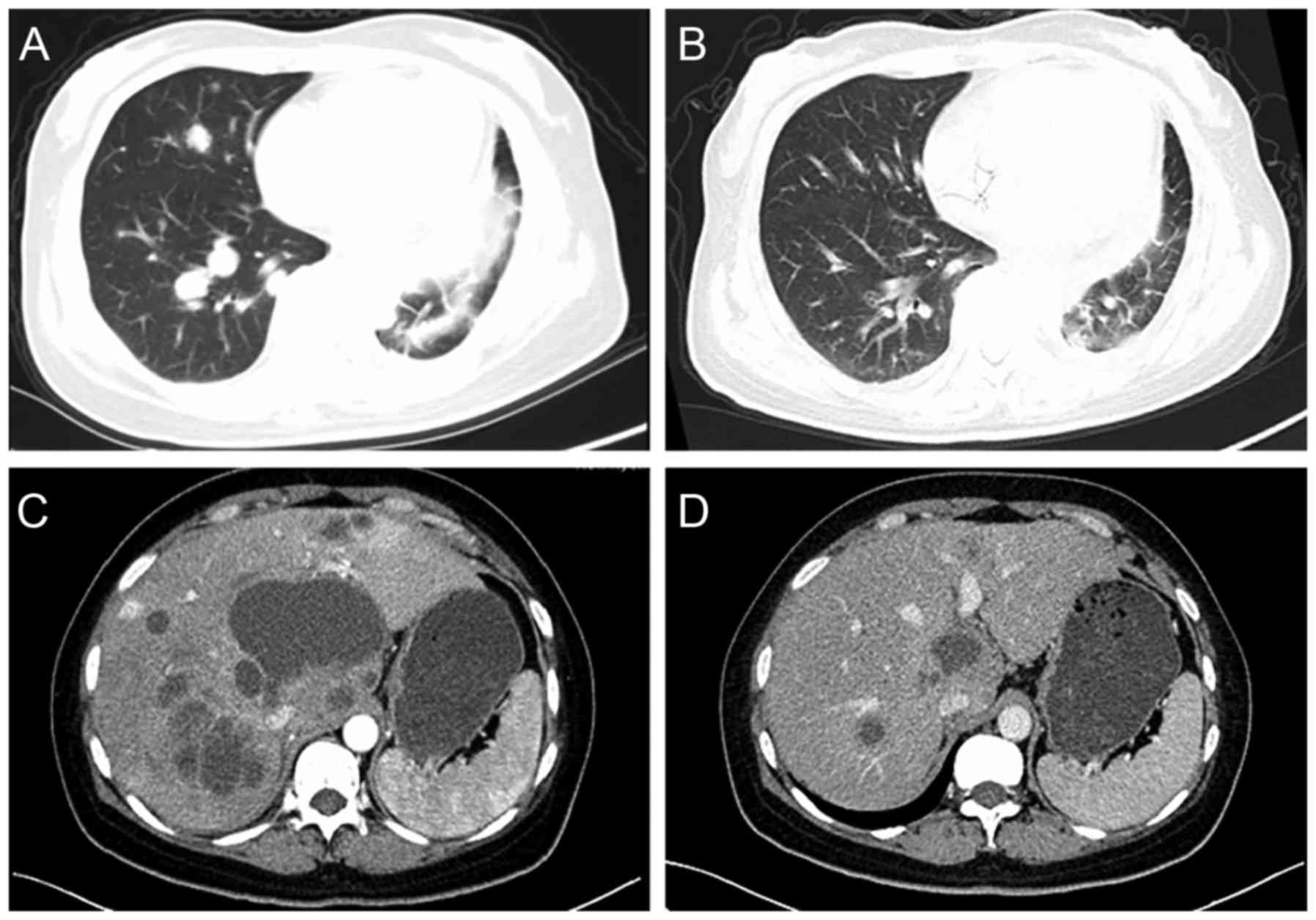
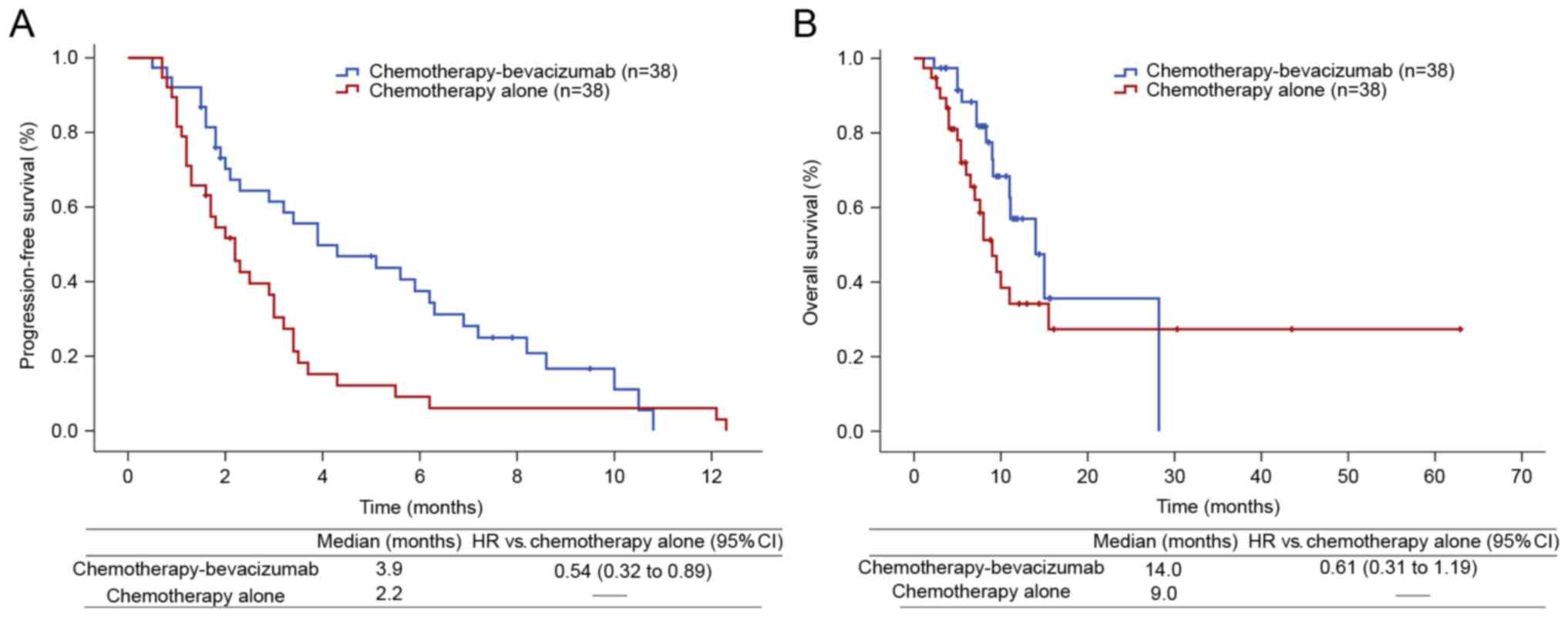
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, et al:
SEER cancer statistics review, 1975–2014, National cancer
institute. Bethesda, MD, based on November 2016 SEER data
submission, posted to the SEER web site. April;2017.
|
|
3
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shepherd FA, Dancey J, Ramlau R, Mattson
K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R,
et al: Prospective randomized trial of docetaxel versus best
supportive care in patients with non-small-cell lung cancer
previously treated with platinum-based chemotherapy. J Clin Oncol.
18:2095–2103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanna N, Shepherd FA, Fossella FV, Pereira
JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M,
Muller T, et al: Randomized phase III trial of pemetrexed versus
docetaxel in patients with non-small-cell lung cancer previously
treated with chemotherapy. J Clin Oncol. 22:1589–1597. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song Z, Yu Y, Chen Z and Lu S: Third-line
therapy for advanced non-small-cell lung cancer patients: Feasible
drugs for feasible patients. Med Oncol. 28 Suppl 1:S605–S612. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N and
Manegold C: Phase III trial of cisplatin plus gemcitabine with
either placebo or bevacizumab as first-line therapy for nonsquamous
non-small-cell lung cancer: AVAil. J Clin Oncol. 27:1227–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel JD, Socinski MA, Garon EB, Reynolds
CH, Spigel DR, Olsen MR, Hermann RC, Jotte RM, Beck T, Richards DA,
et al: PointBreak: A randomized phase III study of pemetrexed plus
carboplatin and bevacizumab followed by maintenance pemetrexed and
bevacizumab versus paclitaxel plus carboplatin and bevacizumab
followed by maintenance bevacizumab in patients with stage IIIB or
IV nonsquamous non-small-cell lung cancer. J Clin Oncol.
31:4349–4357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adjei AA, Mandrekar SJ, Dy GK, Molina JR,
Adjei AA, Gandara DR, Ziegler KL, Stella PJ, Rowland KM Jr, Schild
SE and Zinner RG: Phase II trial of pemetrexed plus bevacizumab for
second-line therapy of patients with advanced non-small-cell lung
cancer: NCCTG and SWOG study N0426. J Clin Oncol. 28:614–619. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Habib S, Delourme J, Dhalluin X, Petyt G,
Tacelli N, Scherpereel A, Lafitte JJ and Cortot AB: Bevacizumab and
weekly paclitaxel for non-squamous non small cell lung cancer
patients: A retrospective study. Lung Cancer. 80:197–202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. Springer
International Publishing; 2017, View Article : Google Scholar
|
|
20
|
Charles GZ, Marvin Schneiderman MA, Emil
FI, Clyde B, Lennard GG, Bruce S, Raul O, John G, Ralph J Jr, Ulfar
J, et al: Appraisal of methods for the study of chemotherapy of
cancer in man: Comparative therapeutic trial of nitrogen mustard
and triethylene thiophosphoramide. J Chronic Dis. 11:7–33. 1960.
View Article : Google Scholar
|
|
21
|
Oken M, Creech R, Tormey D, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe H, Okada M, Kaji Y, Satouchi M,
Sato Y, Yamabe Y, Onaya H, Endo M, Sone M and Arai Y: New response
evaluation criteria in solid tumours-revised RECIST guideline
(version 1.1). Gan To Kagaku Ryoho. 36:2495–2501. 2009.(In
Japanese). PubMed/NCBI
|
|
23
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events v4.0 (CTCAE). 2009,
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.InternetAugust
6–2010
|
|
24
|
D'Agostino RB Jr: Propensity score methods
for bias reduction in the comparison of a treatment to a
non-randomized control group. Stat Med. 17:2265–2281. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding L, Liu K, Jiang Z, Chen Q, Zhou N,
Liang Y, Gao H, Hong X and Wu H: The efficacy and safety of
pemetrexed plus bevacizumab in previously treated patients with
advanced non-squamous non-small cell lung cancer (ns-NSCLC). Tumour
Biol. 36:2491–2499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S,
Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized,
double-blind, placebo-controlled, multicenter, Phase III study of
first-line carboplatin/paclitaxel plus bevacizumab or placebo in
chinese patients with advanced or recurrent nonsquamous
non-small-cell lung cancer. J Clin Oncol. 33:2197–2204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cortot AB, Audigier-Valette C, Molinier O,
Le Moulec S, Barlesi F and Zalcman G: Weekly paclitaxel plus
bevacizumab versus docetaxel as second or third-line treatment in
advanced non-squamous non-small cell lung cancer (NSCLC): Results
from the phase III study IFCT-1103 ULTIMATE. J Clin Oncol. 34
Suppl-15:90052016.
|
|
28
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnson DH, Fehrenbacher L, Novotny WF,
Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF III,
Gaudreault J, Damico LA, et al: Randomized phase II trial comparing
bevacizumab plus carboplatin and paclitaxel with carboplatin and
paclitaxel alone in previously untreated locally advanced or
metastatic non-small-cell lung cancer. J Clin Oncol. 22:2184–2191.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnson BE, Kabbinavar F, Fehrenbacher L,
Hainsworth J, Kasubhai S, Kressel B, Lin CY, Marsland T, Patel T,
Polikoff J, et al: ATLAS: Randomized, double-blind,
placebo-controlled, phase IIIB trial comparing bevacizumab therapy
with or without erlotinib, after completion of chemotherapy, with
bevacizumab for first-line treatment of advanced non-small-cell
lung cancer. J Clin Oncol. 31:3926–3934. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hainsworth JD, Fang L, Huang JE, Karlin D,
Russell K, Faoro L and Azzoli C: BRIDGE: An open-label phase II
trial evaluating the safety of bevacizumab + carboplatin/paclitaxel
as first-line treatment for patients with advanced, previously
untreated, squamous non-small cell lung cancer. J Thorac Oncol.
6:109–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grothey A, Sugrue MM, Purdie DM, Dong W,
Sargent D, Hedrick E and Kozloff M: Bevacizumab beyond first
progression is associated with prolonged overall survival in
metastatic colorectal cancer: Results from a large observational
cohort study (BRiTE). J Clin Oncol. 26:5326–5334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takeda M, Yamanaka T, Seto T, Hayashi H,
Azuma K, Okada M, Sugawara S, Daga H, Hirashima T, Yonesaka K, et
al: Bevacizumab beyond disease progression after first-line
treatment with bevacizumab plus chemotherapy in advanced
nonsquamous non-small cell lung cancer (West Japan Oncology Group
5910L): An open-label, randomized, phase 2 trial. Cancer.
122:1050–1059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bennouna J, de Castro J, Dingemans AC,
Griesinger F and Grossi Flanger CJ: Efficacy and safety results
from AvaALL: An open-label, randomized phase III trial of standard
of care (SOC) with or without continuous bevacizumab (Bev)
treatment beyond progression (PD) in patients (pts) with advanced
non-small cell lung cancer (NSCLC) progressing after first-line Bev
and chemotherapy (chemo). J Clin Oncol. 35 Suppl 15:90042017.
|