Introduction
Lung cancer is the leading cause of
cancer-associated mortalities in China (1). Non-small cell lung cancer (NSCLC)
accounts for 80% of all cases of lung cancer worldwide (2), with the majority of patients presenting
with progressive disease. Platinum-based chemotherapy remains the
standard first-line treatment for advanced NSCLC (3). The identification of activating driver
mutations in the epidermal growth factor receptor (EGFR) has
changed the course of therapy for patients with NSCLC harboring
these mutations (4,5). As second-line treatment, docetaxel,
pemetrexed and erlotinib exhibit low objective response rates (ORR)
and short progression-free survival (PFS) (6–8). The use
of immune checkpoint inhibitors such as nivolumab and pembrolizumab
may prolong overall survival (OS) and duration of response in
patients with NSCLC, particularly for those who express programmed
death ligand-1 (PD-L1) (9–11).
At present, there are no guidelines for the systemic
treatment for patients with NSCLC who have failed 2 therapy
regimens. In a previous retrospective study, although a number of
patients received third-line treatment, the results of salvage
treatment were not satisfactory (12). With additional first- and second-line
therapies being made available, the number of patients with NSCLC
who are candidates for third-line, or continuing, treatments has
increased during the previous decade. Therefore, an effective
treatment approach is urgently required, particularly for patients
with neither targetable molecular aberrations nor PD-L1
expressions.
Bevacizumab is a monoclonal antibody that targets
vascular endothelial growth factor (13), and has been demonstrated to be
effective when used in tandem with a number of chemotherapeutic
agents for the treatment of patients with NSCLC (14–16). A
combination of carboplatin, paclitaxel and bevacizumab has been
approved by the United States of America Food and Drug
Administration as a first-line treatment for advanced NSCLC due to
the results of the Eastern Cooperative Oncology Group (ECOG) 4599
study (14). A combination of
cisplatin, gemcitabine and bevacizumab or carboplatin, pemetrexed
and bevacizumab has also exhibited encouraging efficacy as a
first-line treatment (15,16).
Several studies have also evaluated using
bevacizumab as an adjunct to salvage treatments: One phase II study
evaluated pemetrexed plus bevacizumab as second-line treatment for
patients with NSCLC, and demonstrated a disease control rate (DCR)
of 50.0%, a median PFS of 4.0 months and a median OS of 8.6 months
(17). An additional retrospective
study identified the efficacy of weekly paclitaxel and bevacizumab
as a fourth-line, and continuing, treatment for patients with NSCLC
(18).
The combination of chemotherapy and bevacizumab as a
third-line, or continuing, treatments in NSCLC has not been
studied, and its efficacy remains unclear. For patients who have
not received bevacizumab previously, the combination of bevacizumab
and chemotherapy may be an effective salvage treatment based on
encouraging antitumor activity observed as a first-line treatment
(14–16). Therefore, the present study was
designed to compare the efficacy and safety of chemotherapy plus
bevacizumab (chemotherapy-bevacizumab group) with chemotherapy
alone (chemotherapy-alone group) as a third-line, or continuing,
treatment for patients with NSCLC using propensity score matching
(PSM).
Materials and methods
Patients
The present retrospective analysis of 165 patients
with stage IV NSCLC, diagnosed according to the 7th edition of
American Joint Committee on Cancer staging system (19), who had received single-agent
chemotherapy with or without bevacizumab following the failure of
≥2 prior standard systemic regimens was performed at the Department
of Thoracic Oncology, West China Hospital (Chengdu, China) between
January 2011 and June 2016. Inclusion criteria of patients were:
>18 years of age, ECOG performance status of 0–1 (20,21),
measurable lesions as defined by Response Evaluated Criteria in
Solid Tumors (RECIST; version 1.1) (22), and histological or cytological
confirmation of adenocarcinoma, or squamous cell and adenosquamous
carcinoma but without a central lesion or lesion abutting major
blood vessels, history of hemoptysis or presence of cavitation and
concomitant use of full-dose anticoagulants. Patients who had
previously received bevacizumab were not eligible. All patients
enrolled underwent computed tomography scanning. Baseline clinical
characteristics included age, sex, smoking history, histology, EGFR
mutation status, number of prior regimens and type of chemotherapy
regimen.
Treatment
All patients had received single-agent chemotherapy
with or without bevacizumab. Single-agent chemotherapy included
gemcitabine (days 1 and 8, 1,000 mg/m2), pemetrexed (day
1, 500 mg/m2), paclitaxel (day 1, 150 mg/m2)
and docetaxel (day 1, 75 mg/m2). Bevacizumab
(Avastin®; Roche Diagnostics GmbH, Mannheim, Germany)
was administered intravenously at a dose of 7.5 mg/kg on day 1.
Combined and monotherapy were repeated every 3 weeks and continued
until disease progression or development of unacceptable
toxicity.
Evaluation of efficacy, survival and
toxicity
Efficacy evaluation was performed every 6 weeks
following the administration of treatment. The treatment response
was assessed with RECIST 1.1 as follows: Complete response (CR);
partial response (PR); stable disease (SD); and progressive disease
(PD). The ORR included CR and PR. The DCR included CR, PR and SD.
OS was measured from the first day of chemotherapy-bevacizumab or
chemotherapy alone to the day of mortality or last follow-up. PFS
was defined as the time between the initiation of treatment and
disease progression or mortality from any cause. The adverse events
(AEs) of treatment were graded the by National Cancer Institute
Common Terminology Criteria for Adverse Events (version 4.0)
(23).
Statistical analysis
All statistical analyses were performed using SPSS
(version 20.0; IBM Corp., Armonk, NY, USA). To minimize the effects
of potential confounding factors between the
chemotherapy-bevacizumab and chemotherapy-alone groups, PSM was
performed. Associations between the treatment and baseline clinical
characteristics were analyzed by Pearson's χ2 tests.
Patients who had received chemotherapy plus bevacizumab were
matched 1:1 with patients who had received chemotherapy alone using
PSM based on the variables that were significantly different
between the two groups. Propensity scores were generated by using a
multivariate logistic regression (24). Patients were considered a match if the
absolute difference in their propensity scores was ≤0.02.
In the matched dataset, tumor responses and AEs were
compared using Pearson's χ2 and Fisher's exact tests.
Survival curves were compared using the Kaplan-Meier method using
the log-rank test. Univariate and multivariate Cox proportional
hazards regression were used to evaluate the prognostic factors and
to calculate hazard ratios (HRs) and 95% confidence intervals (CI)
for OS and PFS. Subgroup analyses of OS and PFS were also performed
by the Kaplan-Meier method using the log-rank test. All clinical
variables were included in the multivariate regressions, regardless
of their univariate significance level. Two-sided P<0.05 were
considered to indicate a statistically significant difference.
Results
Patient characteristics and
treatment
From January 2011 to June 2016, a total of 165
patients who had received chemotherapy plus bevacizumab (n=43) or
chemotherapy alone (n=122) as third-line, or continuing treatments
were initially enrolled in the present study. For PSM-matched
variables, the number of prior regimens (P=0.009) and type of
chemotherapy regimen (P<0.001) were significantly different
between the two groups. A total of 38 patients in the
chemotherapy-bevacizumab group were then matched to 38 patients in
the chemotherapy-alone group. The cut-off day of the present study
was December 2016, and the median follow-up of all patients was 7.9
months (range, 1.1–62.9 months).
The baseline clinical characteristics for patients
pre- and post-PSM are summarized in Table
I. The median age of patients was 52 years (range, 40–71 years)
in the chemotherapy-bevacizumab group and 49 years (range, 38–76
years) in the chemotherapy-alone group. In the
chemotherapy-bevacizumab group, 34 (34/38; 89.5%) comprised
patients with adenocarcinoma, 2 (2/38; 5.3%) with squamous cell
carcinoma and 2 (2/38; 5.3%) with adenosquamous carcinoma. In the
chemotherapy-alone group, 35 (35/38; 92.1%) comprised patients with
adenocarcinoma, 2 (2/38; 5.3%) with squamous cell carcinoma and 1
(1/38; 2.6%) with adenosquamous carcinoma. The median number of
prior regimens was 3 (range, 2–5) in the chemotherapy-bevacizumab
group and 3 (range, 2–4) in the chemotherapy-alone group. A total
of 10 (10/38; 26.3%) patients had failed >3 prior regimens in
the two groups.
 | Table I.Baseline clinical characteristics for
patients in the chemotherapy-bevacizumab group vs. chemotherapy
alone group prior and subsequent to propensity score matching. |
Table I.
Baseline clinical characteristics for
patients in the chemotherapy-bevacizumab group vs. chemotherapy
alone group prior and subsequent to propensity score matching.
| A, Unmatched
dataset |
|---|
|
|---|
|
Characteristics |
Chemotherapy-bevacizumab (n=43) | Chemotherapy alone
(n=122) | P-value |
|---|
| Age, years |
|
| 0.844 |
| Median
(range) | 52 (37–72) | 56 (27–76) |
|
|
≤60 | 32 | 88 |
|
|
>60 | 11 | 34 |
|
| Sex, n |
|
| 0.287 |
|
Male | 19 | 67 |
|
|
Female | 24 | 55 |
|
| Smoking history,
n |
|
| 0.583 |
|
Current/previous | 14 | 47 |
|
|
Never | 29 | 75 |
|
| Histology, n |
|
| 0.800 |
|
Adenocarcinoma | 38 | 105 |
|
|
Squamous cell/adenosquamous
carcinoma | 5 | 17 |
|
| EGFR mutation
status |
|
| 0.716 |
| Mutant
typea | 17 | 44 |
|
|
Wild-type/unknown | 26 | 78 |
|
| Number of prior
regimensb, n |
|
| 0.009 |
| Median
(range) | 3 (2–5) | 2 (2–5) |
|
| ≤3 | 28 | 103 |
|
|
>3 | 15 | 19 |
|
| Chemotherapy
regimen, n |
|
| <0.0001 |
|
Gemcitabine | 30 | 39 |
|
|
Pemetrexed | 5 | 31 |
|
|
Paclitaxel | 3 | 8 |
|
|
Docetaxel | 5 | 44 |
|
|
| B, Matched (1:1)
dataset |
|
|
Characteristics |
Chemotherapy-bevacizumab
(n=38) | Chemotherapy
alone (n=38) | P-value |
|
| Age, years |
|
| 1.000 |
| Median
(range) | 52 (40–71) | 49 (38–76) |
|
|
≤60 | 30 | 31 |
|
|
>60 | 8 |
|
|
| Sex |
|
| 0.492 |
|
Male | 17 | 21 |
|
|
Female | 21 | 17 |
|
| Smoking history,
n |
|
| 0.240 |
|
Current/previous | 26 | 20 |
|
|
Never | 12 | 18 |
|
| Histology, n |
|
| 1.000 |
|
Adenocarcinoma | 34 | 35 |
|
|
Squamous cell/ | 4 | 3 |
|
|
adenosquamous carcinoma |
| EGFR mutation
status |
|
| 0.641 |
| Mutant
typea | 17 | 14 |
|
|
Wild-type/unknown | 21 | 24 |
|
| Number of prior
regimensb, n |
|
| 1.000 |
| Median
(range) | 3 (2–5) | 3 (2–4) |
|
| ≤3 | 28 | 28 |
|
|
>3 | 10 | 10 |
|
| Chemotherapy
regimen, n |
|
| 1.000 |
|
Gemcitabine | 25 | 25 |
|
|
Pemetrexed | 5 | 5 |
|
|
Paclitaxel | 3 | 3 |
|
|
Docetaxel | 5 | 5 |
|
The majority (25/38; 65.8%) of patients received
gemcitabine as their monotherapy or combined therapy with
bevacizumab. All patients received ≥1 cycle of treatment. The
median number of cycles of treatment was 3 (range, 1–10) in the
chemotherapy-bevacizumab group and 2 (range, 1–6) in the
chemotherapy-alone group.
Tumor response
In the chemotherapy-bevacizumab group, 9 patients
achieved a PR, of which 3 were squamous cell or adenosquamous
carcinoma, and 16 exhibited SD, of which 1 was adenosquamous
carcinoma. In the chemotherapy-alone group, 2 patients achieved a
PR and 10 exhibited SD. There was a significant improvement in ORR
and DCR for the chemotherapy-bevacizumab group compared with the
chemotherapy-alone group (ORR, 23.7 vs. 5.3%, respectively,
P<0.001; DCR, 65.8 vs. 31.6%, respectively, P<0.001).
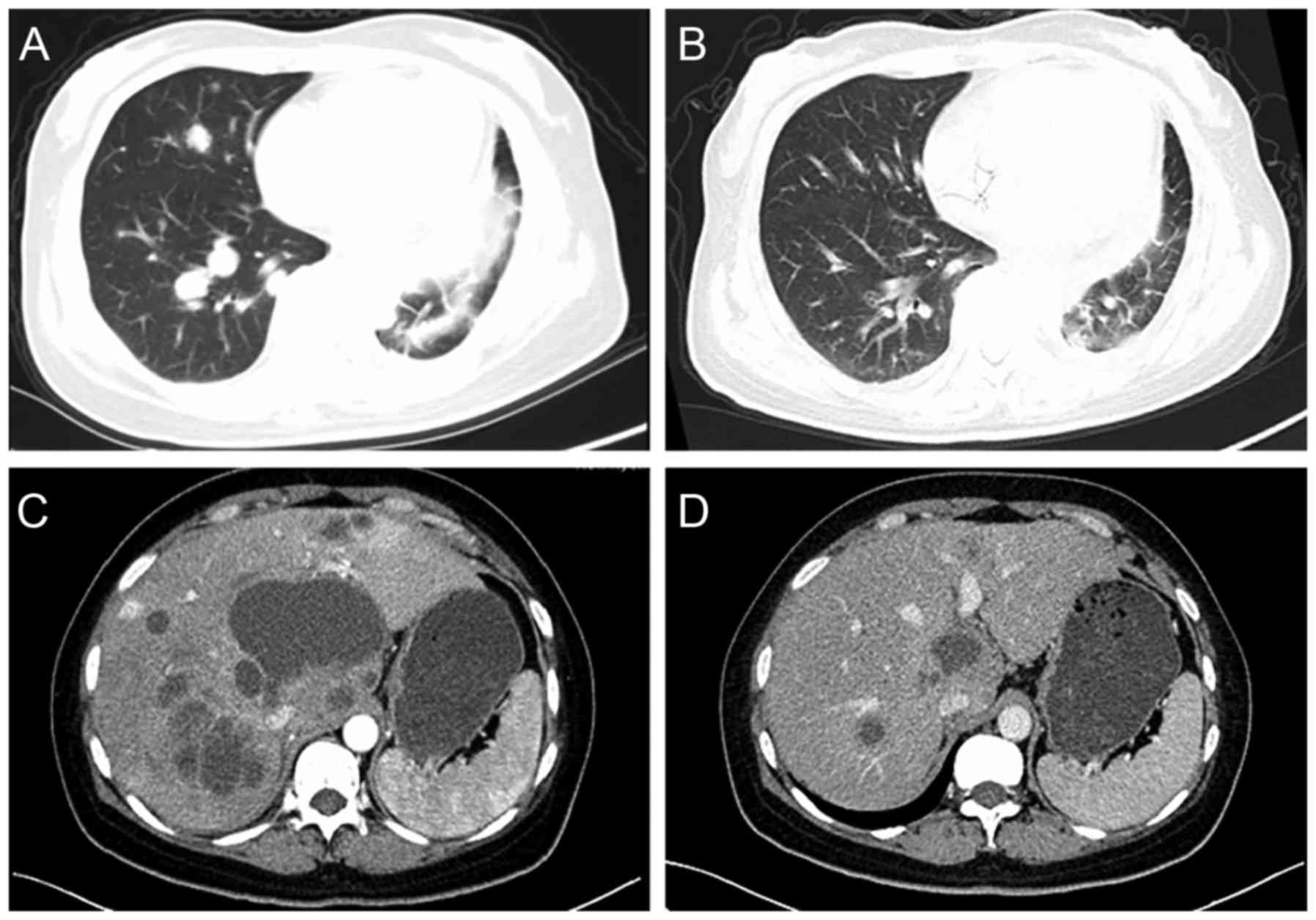
Computed tomography scans of 2 patients with PR are shown in
Fig. 1. Of these patients, 1 patient
was a 51-year-old female with stage IV adenocarcinoma, who received
gemcitabine plus bevacizumab as a fourth-line therapy. This patient
received a total of 6 cycles of treatment, and following 2 cycles
of treatment, there was a marked reduction in size of the pulmonary
lesions. The PFS of the patient was 3.9 months, but she succumbed
subsequent to follow-up for 9.1 months. The other patient was a
42-year-old female with stage IV squamous cell carcinoma, who
received gemcitabine plus bevacizumab as a fifth-line therapy, and
primarily targeted lesions in the liver. The hepatic lesions were
notably reduced in size following 2 cycles of treatment. This
patient exhibited a total of 10 months for PFS and survived until
the cut-off point.
Survival outcome
In the univariate analysis, bevacizumab treatment
was the only significant prognostic factor for PFS, but not for OS
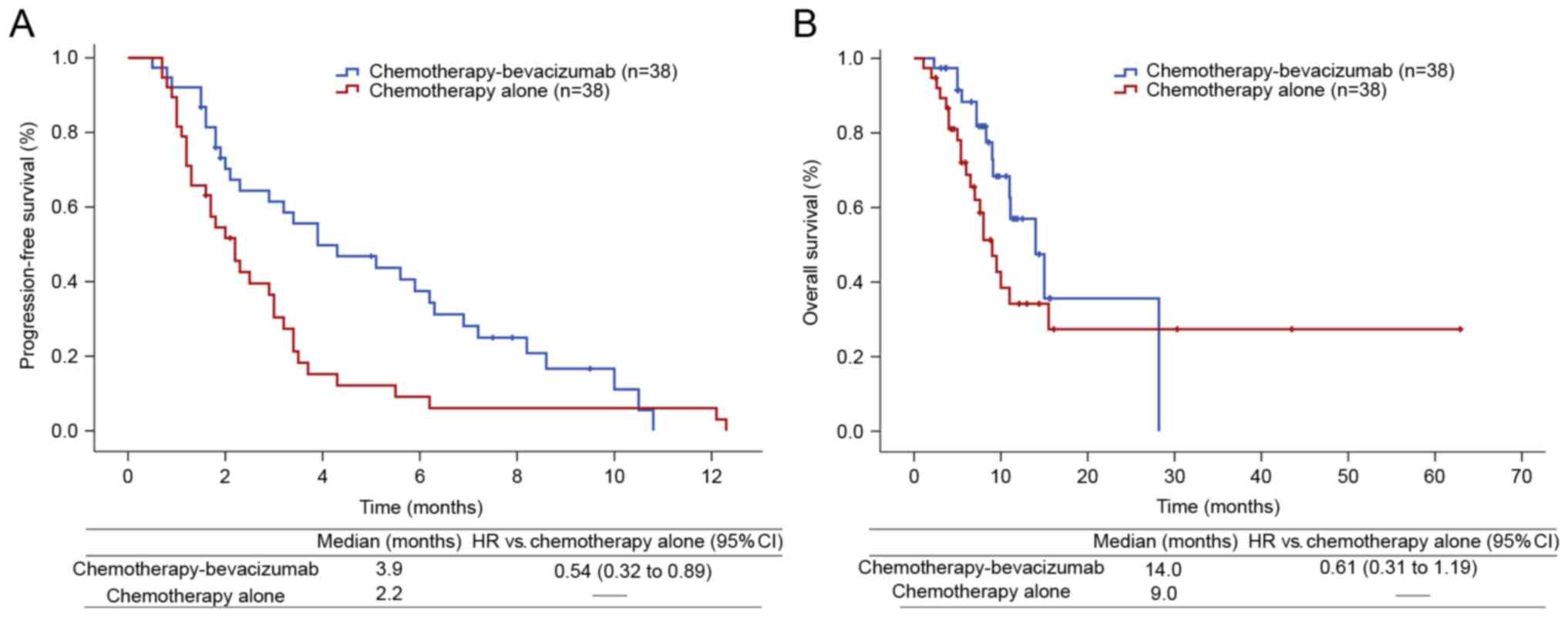
(data not shown). The duration of PFS was longer for the
chemotherapy-bevacizumab group compared with the chemotherapy-alone
group (median, 3.9 vs. 2.2 months, respectively; HR, 0.54; 95% CI,
0.32–0.89; P=0.014; Fig. 2A). The
duration of OS non-significantly increased with
chemotherapy-bevacizumab treatment compared with chemotherapy alone
(median, 14.0 vs. 9.0, respectively; HR, 0.61; 95% CI, 0.31–1.19;
P=0.141; Fig 2B). Smoking was
associated with poor OS (HR, 2.00; 95% CI, 1.04–3.86; P=0.033).
Multivariate analysis also demonstrated that bevacizumab treatment
was the only independent prognostic factor for PFS (HR, 0.48; 95%
CI, 0.27–0.85; P=0.011; Table II),
and age was the only independent risk factor for OS (HR, 4.19; 95%
CI, 1.17–14.97; P=0.027; Table
II).
 | Table II.Multivariate Cox regression analyses
for progression-free survival and overall survival in patients from
the matched dataset. |
Table II.
Multivariate Cox regression analyses
for progression-free survival and overall survival in patients from
the matched dataset.
| A, Progression-free
survival |
|---|
|
|---|
| Variables | HR (95% CI) | P-value |
|---|
| Groups |
|
|
|
Chemotherapy-bevacizumab vs.
chemotherapy alone | 0.48
(0.27–0.85) | 0.011 |
| Age, years |
|
|
| ≤60 vs.
>60 | 0.95
(0.49–1.88) | 0.892 |
| Sex |
|
|
| Female
vs. male | 0.82
(0.34–1.97) | 0.656 |
| Smoking
history |
|
|
|
Current/previous vs.
never | 1.15
(0.48–2.77) | 0.753 |
| Histology |
|
|
|
Adenocarcinoma vs. squamous
cell/adenosquamous carcinoma | 1.25
(0.47–3.36) | 0.655 |
| EGFR mutation
status |
|
|
|
Wild-type/unknown vs. mutant
type | 0.74
(0.41–1.36) | 0.333 |
| Number of prior
regimens |
|
|
| ≤3 vs.
>3 | 1.06
(0.57–1.98) | 0.858 |
| Chemotherapy
regimen |
| 0.326 |
|
Gemcitabine | Ref. |
|
|
Pemetrexed | 0.50
(0.21–1.24) | 0.137 |
|
Paclitaxel | 1.42
(0.54–3.77) | 0.481 |
|
Docetaxel | 1.31
(0.58–2.97) | 0.511 |
|
| B, Overall
survival |
|
|
Variables | HR (95%
CI) | P-value |
|
| Group |
|
|
|
Chemotherapy-bevacizumab vs.
chemotherapy alone | 0.53
(0.26–1.07) | 0.076 |
| Age, years |
|
|
| ≤60 vs.
>60 | 4.19
(1.17–14.97) | 0.027 |
| Sex |
|
|
| Female
vs. male | 1.30
(0.36–4.74) | 0.694 |
| Smoking
history |
|
|
|
Current/previous vs.
never | 3.32
(0.86–12.76) | 0.081 |
| Histology |
|
|
|
Adenocarcinoma vs. squamous
cell/adenosquamous carcinoma | 1.33
(0.29–6.11) | 0.711 |
| EGFR mutation
status |
|
|
|
Wild-type/unknown vs. mutant
type | 1.10
(0.51–2.37) | 0.812 |
| Number of prior
regimens |
|
|
| ≤3 vs.
>3 | 1.05
(0.49–2.26) | 0.908 |
| Chemotherapy
regimen |
| 0.064 |
|
Gemcitabine | Ref. |
|
|
Pemetrexed | 0.19
(0.05–0.84) | 0.029 |
|
Paclitaxel | 0.25
(0.05–1.24) | 0.090 |
|
Docetaxel | 0.57
(0.21–1.57) | 0.275 |
In the subgroup analyses, PFS was significantly
prolonged following chemotherapy-bevacizumab treatment compared
with chemotherapy alone in the following subgroups: Male sex;
current or former smokers; adenocarcinoma subtypes; EGFR wild-type
or unknown; and patients with ≤3 prior regimens (Table III). PFS was notably prolonged in
the subgroup of patients treated with gemcitabine (Table III). OS was significantly prolonged
for chemotherapy-bevacizumab treatment compared with chemotherapy
alone in the subgroup of patients with >3 prior regimens
(Table III).
 | Table III.Subgroup analyses in patients with
chemotherapy-bevacizumab vs. chemotherapy alone from the matched
dataset. |
Table III.
Subgroup analyses in patients with
chemotherapy-bevacizumab vs. chemotherapy alone from the matched
dataset.
| Subgroups | Median PFS
(months) | Median OS
(months) | HR (95% CI) | P-value |
|---|
| Male sex | 5.6 vs. 2.0 | – | 0.24
(0.10–0.54) | <0.0001 |
| Current/former
smokers | 4.3 vs. 2.2 | – | 0.32
(0.13–0.78) | 0.008 |
| Adenocarcinoma | 3.9 vs. 2.2 | – | 0.59
(0.35–0.99) | 0.037 |
| EGFR
wild-type/unknown | 6.3 vs. 2.9 | – | 0.26
(0.12–0.58) | <0.0001 |
| Prior regimens
≤3 | 3.9 vs. 2.2 | – | 0.55
(0.30–0.99) | 0.038 |
| Gemcitabine | 4.3 vs. 2.5 | – | 0.55
(0.30–1.01) |
|
| Prior regimens
>3 | – | 14.0 vs. 5.4 | 0.29
(0.09–0.92) | 0.025 |
| Docetaxel | – | 14.0 vs. 8.0 | 0.01
(0.00–37.70) |
|
Toxicity
Hematological and non-hematological toxicities of
patients in the two groups are summarized in Table IV. The incidence of severe (≥ grade
3) AEs was low and comparable in the two groups
(chemotherapy-bevacizumab vs. chemotherapy alone, 28.9 vs. 26.3%).
In the chemotherapy-bevacizumab group, the most common AEs of all
grades were leukopenia (19/38; 50.0%), increased alanine
transaminase (ALT) or aspartate transaminase (AST; 17/38; 44.8%)
and fatigue (16/38; 42.1%). In the chemotherapy-alone group, the
most common AEs of all grades were nausea (16/38; 42.1%),
leukopenia (15/38; 39.4%) and increased ALT or AST (14/38; 36.8%).
The rates of bleeding (P=0.001) and hypertension (P=0.025) were
significantly increased in the chemotherapy-bevacizumab group
compared with the chemotherapy-alone group. Although 15 (15/38;
39.5%) patients in the chemotherapy-bevacizumab group experienced
grade 1/2 bleeding, of which 3 were squamous cell carcinoma, there
were no reports of ≥grade 3 bleeding events.
 | Table IV.Toxicity grades of patients in the
two groups from the matched dataset. |
Table IV.
Toxicity grades of patients in the
two groups from the matched dataset.
|
| No. of patients
(%) |
|---|
|
|
|
|---|
|
|
Chemotherapy-bevacizumab (n=38) | Chemotherapy alone
(n=38) |
|---|
|
|
|
|
|---|
| Toxicity | Grade 1/2 | Grade ≥3 | Grade 1/2 | Grade ≥3 |
|---|
| Hematological
toxicity |
|
|
|
|
|
Leukopenia | 14 (36.8) | 5 (13.2) | 11 (28.9) | 4 (10.5) |
|
Neutropenia | 11 (28.9) | 3 (7.9) | 9 (23.7) | 4 (10.5) |
|
Anemia | 15 (39.5) | 0 (0.0) | 12 (31.6) | 1 (2.6) |
|
Thrombocytopenia | 6 (15.8) | 1 (2.6) | 7 (18.4) | 2 (5.3) |
| Non-hematological
toxicity |
|
|
|
|
|
Increased ALT or AST | 15 (39.5) | 2 (5.3) | 13 (34.2) | 1 (2.6) |
|
Fatigue | 14 (36.8) | 2 (5.3) | 12 (31.6) | 0 (0.0) |
|
Nausea | 12 (31.6) | 0 (0.0) | 15 (39.5) | 1 (2.6) |
|
Vomiting | 7 (18.4) | 0 (0.0) | 7 (18.4) | 1 (2.6) |
|
Rash | 4 (10.5) | 0 (0.0) | 3 (7.9) | 0 (0.0) |
| Joint
or muscle pain | 4 (10.5) | 0 (0.0) | 1 (2.6) | 0 (0.0) |
|
Hypertension | 6 (15.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Bleeding | 15 (39.5) | 0 (0.0) | 2 (5.3) | 0 (0.0) |
|
Proteinuria | 4 (10.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Continuous bevacizumab treatment
following disease progression
In the chemotherapy-bevacizumab group, 22 patients
received post-progression treatment, of which 14 continued to
receive bevacizumab. This treatment regimen included docetaxel plus
bevacizumab in 4 patients (4/14; 28.6%), pemetrexed plus
bevacizumab in 4 patients (4/14; 28.6%), paclitaxel plus
bevacizumab in 3 patients (3/14; 21.4%), gemcitabine plus
bevacizumab in 2 patients (2/14; 14.3%) and vinorelbine plus
bevacizumab in 1 patient (1/14; 7.1%). The median number of cycles
of treatment was 2 (range, 1–4). Among the 14 patients, one patient
had not received an initial evaluation of efficacy as they had not
completed two cycles of treatment. Of the remaining 13 patients
whose response was assessed, PR was observed in 2 patients (2/13;
15.4%) and SD was observed in 6 patients (6/13; 46.1%). PFS was 3.4
months (95% CI, 2.8–4.0 months). No incident ≥grade 3 AEs were
observed.
Discussion
There is currently no standard systemic therapy for
third-line, or continuing, treatments of NSCLC. With an increasing
number of patients who meet the criteria for receiving third-line
treatment or beyond, it is imperative to optimize therapy regimens
for eligible candidates. When combined with chemotherapy,
bevacizumab has demonstrated efficacy as a first-line treatment for
NSCLC (14–16), and it may also be effective as a
salvage treatment for bevacizumab treatment-naïve patients with
NSCLC. The present retrospective study aimed to additionally
evaluate the efficacy and safety of bevacizumab in combination with
chemotherapy as a third-line, or continuing, treatment for NSCLC
patients. The results of the present study indicated favorable
clinical outcomes with chemotherapy-bevacizumab therapy.
The values for ORR (23.7%) and DCR (65.8%) for the
chemotherapy-bevacizumab treatment observed in the present study
were higher compared with the values obtained previously, where
6.45% was reported for ORR and 54.84% was reported for DCR by Ding
et al (25). Improvements in
ORR and DCR in NSCLC were also noted with the
chemotherapy-bevacizumab treatment in other studies (17,26).
Previously, ULTIMATE, a randomized, phase III study evaluated the
efficacy and safety of bevacizumab as a salvage treatment for
patients with NSCLC, who had previously failed first-, second- and
third-line treatments and were randomized to receive weekly
paclitaxel and bevacizumab or docetaxel alone. It was reported that
significant improvement was observed in ORR for bevacizumab
compared with docetaxel alone (22.5 vs. 5.5%, respectively).
Additionally, PFS was prolonged compared with docetaxel alone (5.4
vs. 3.9 months, respectively), but not OS (27). Similarly, in the present study,
chemotherapy-bevacizumab prolonged PFS compared with chemotherapy
alone (median, 3.9 vs. 2.2 months). However, improvements in PFS
did not translate into significant OS benefits. This may be
attributed to the enrollment of patients who had failed several
regimens prior to receiving bevacizumab, which may have affected
the OS. An additional possibility may be the small sample size in
the present study, which may have reduced statistical significance
between groups.
In the subgroup analyses, PFS was notably prolonged
following chemotherapy-bevacizumab treatment compared with
chemotherapy alone in the subgroup of patients treated with
gemcitabine (median, 4.3 vs. 2.5 months; HR, 0.55; 95% CI,
0.30–1.01). Gemcitabine may be a prognostic factor for PFS when the
sample size of a study is enlarged. Additionally, gemcitabine is
rarely employed as a first- or second-line treatment for patients
with advanced non-squamous NSCLC (28), so it may be considered an alternative
choice for salvage treatment in patients eligible to receive
third-line, or continuing, treatments. The majority of patients
(25/38; 65.8%) in the present study received gemcitabine as
monotherapy or combined therapy with bevacizumab, potentially
informing whether gemcitabine-bevacizumab is an effective
third-line, or continuing, treatment for patients with NSCLC.
The toxicity of chemotherapy-bevacizumab in the
present study was well tolerated. The incidence of severe (≥ grade
3) AEs was low and comparable in the two groups. For AEs of all
grades, hematological toxicities were commonly observed in the
chemotherapy-bevacizumab group, which was also observed previously
with bevacizumab (14–16,26). The
high incidence of increased ALT or AST observed in the
chemotherapy-bevacizumab group may be due to a high proportion of
patients treated with gemcitabine. No severe bleeding events
occurred, indicating that hemorrhage was managed.
Patients with squamous NSCLC have generally been
excluded from studies investigating bevacizumab treatment, as
squamous histology was identified as a possible risk factor for
severe (grade ≥3) pulmonary hemorrhage in a phase II study
(29). However, patients with
squamous histology have been enrolled in a randomized phase IIIb
trial, ATLAS (30). A phase II study,
BRIDGE, evaluated the safety of carboplatin, paclitaxel and
bevacizumab as a first-line treatment for patients with advanced
squamous NSCLC and identified that 1 patient (1/31; 3.23%)
experienced grade ≥3 pulmonary hemorrhage, but no other AEs. The
authors of the BRIDGE study suggested that treatment of squamous
NSCLC with bevacizumab should be considered experimental (31). Based on these data, patients with
squamous histology were cautiously enrolled in the present study. A
total of 4 patients with squamous cell or adenosquamous histology,
who received bevacizumab, did not experience grade ≥3 pulmonary
hemorrhage. Additionally, all of these patients responded to
treatment, where 3 patients exhibited PR and 1 exhibited SD.
Although the sample size was small in the present study, efficacy
of bevacizumab was demonstrated in patients with squamous
NSCLC.
Assessment of the efficacy and safety of patients
who were treated with bevacizumab following previous failure of the
drug in the present study demonstrated that the majority of
patients continued to benefit from the therapy, with an ORR of
14.3%, DCR of 56.2% and PFS of 3.4 months. In patients treated with
chemotherapy-bevacizumab, progressive disease may be considered a
failure of chemotherapy, but not necessarily of bevacizumab.
Patients whose colorectal cancer had progressed with first-line
treatment with bevacizumab, but who continued to receive
chemotherapy-bevacizumab treatment beyond first PD, exhibited a
statistically significant improvement in survival (32). In a phase II trial which compared
docetaxel and docetaxel plus bevacizumab in patients with NSCLC
whose disease had progressed following first-line treatment with
bevacizumab plus a platinum-based doublet, significant increases in
the median PFS was observed, and a longer median OS was reported in
the docetaxel plus bevacizumab group (33). To validate these data, the AvaALL
trial (ClinicalTrials.gov identifier,
NCT01351415) evaluated the efficacy of standard of care (SOC) with
or without continuous bevacizumab treatment beyond progression in
patients with NSCLC progression following first-line
chemotherapy-bevacizumab treatment. Results reported in 2017 ASCO
demonstrated that though there was not a significantly longer OS
observed in SOC+ bevacizumab compared with SOC (median, 11.86 vs.
10.22 months, P=0.1044), there were significant increases in the
PFS was observed in the SOC +bevacizumab groups when patients were
administered third-line SOC (median, 4.0 vs. 2.6 months, P=0.045)
(34).
There are several limitations in the present study.
Firstly, the review was retrospective and monocentric, and the
number of patients included was small. Secondly, prior regimens
received by patients prior to the administration of bevacizumab in
the two groups were different, which may have affected the
efficacy.
In conclusion, to the best of our knowledge, this is
the first retrospective study to compare the outcomes of
chemotherapy-bevacizumab vs. chemotherapy alone as third-line or
continuing, treatment for patients with NSCLC who were
bevacizumab-treatment naïve. Treatment with
chemotherapy-bevacizumab was able to improve DCR and ORR, and
prolong PFS in patients with NSCLC. As the number of studies on the
efficacy of chemotherapy-bevacizumab as salvage treatment is
limited at present, results from the present study may provide
guidance for designing treatment regimens for patients with NSCLC.
However, the efficacy of chemotherapy-bevacizumab in patients with
NSCLC requires additional investigation in prospective trials.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81672982) and the
Sichuan Provincial Research Foundation for Basic Research (grant
no. 2016JY0050).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, et al:
SEER cancer statistics review, 1975–2014, National cancer
institute. Bethesda, MD, based on November 2016 SEER data
submission, posted to the SEER web site. April;2017.
|
|
3
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shepherd FA, Dancey J, Ramlau R, Mattson
K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R,
et al: Prospective randomized trial of docetaxel versus best
supportive care in patients with non-small-cell lung cancer
previously treated with platinum-based chemotherapy. J Clin Oncol.
18:2095–2103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanna N, Shepherd FA, Fossella FV, Pereira
JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M,
Muller T, et al: Randomized phase III trial of pemetrexed versus
docetaxel in patients with non-small-cell lung cancer previously
treated with chemotherapy. J Clin Oncol. 22:1589–1597. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song Z, Yu Y, Chen Z and Lu S: Third-line
therapy for advanced non-small-cell lung cancer patients: Feasible
drugs for feasible patients. Med Oncol. 28 Suppl 1:S605–S612. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N and
Manegold C: Phase III trial of cisplatin plus gemcitabine with
either placebo or bevacizumab as first-line therapy for nonsquamous
non-small-cell lung cancer: AVAil. J Clin Oncol. 27:1227–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel JD, Socinski MA, Garon EB, Reynolds
CH, Spigel DR, Olsen MR, Hermann RC, Jotte RM, Beck T, Richards DA,
et al: PointBreak: A randomized phase III study of pemetrexed plus
carboplatin and bevacizumab followed by maintenance pemetrexed and
bevacizumab versus paclitaxel plus carboplatin and bevacizumab
followed by maintenance bevacizumab in patients with stage IIIB or
IV nonsquamous non-small-cell lung cancer. J Clin Oncol.
31:4349–4357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adjei AA, Mandrekar SJ, Dy GK, Molina JR,
Adjei AA, Gandara DR, Ziegler KL, Stella PJ, Rowland KM Jr, Schild
SE and Zinner RG: Phase II trial of pemetrexed plus bevacizumab for
second-line therapy of patients with advanced non-small-cell lung
cancer: NCCTG and SWOG study N0426. J Clin Oncol. 28:614–619. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Habib S, Delourme J, Dhalluin X, Petyt G,
Tacelli N, Scherpereel A, Lafitte JJ and Cortot AB: Bevacizumab and
weekly paclitaxel for non-squamous non small cell lung cancer
patients: A retrospective study. Lung Cancer. 80:197–202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. Springer
International Publishing; 2017, View Article : Google Scholar
|
|
20
|
Charles GZ, Marvin Schneiderman MA, Emil
FI, Clyde B, Lennard GG, Bruce S, Raul O, John G, Ralph J Jr, Ulfar
J, et al: Appraisal of methods for the study of chemotherapy of
cancer in man: Comparative therapeutic trial of nitrogen mustard
and triethylene thiophosphoramide. J Chronic Dis. 11:7–33. 1960.
View Article : Google Scholar
|
|
21
|
Oken M, Creech R, Tormey D, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe H, Okada M, Kaji Y, Satouchi M,
Sato Y, Yamabe Y, Onaya H, Endo M, Sone M and Arai Y: New response
evaluation criteria in solid tumours-revised RECIST guideline
(version 1.1). Gan To Kagaku Ryoho. 36:2495–2501. 2009.(In
Japanese). PubMed/NCBI
|
|
23
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events v4.0 (CTCAE). 2009,
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.InternetAugust
6–2010
|
|
24
|
D'Agostino RB Jr: Propensity score methods
for bias reduction in the comparison of a treatment to a
non-randomized control group. Stat Med. 17:2265–2281. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding L, Liu K, Jiang Z, Chen Q, Zhou N,
Liang Y, Gao H, Hong X and Wu H: The efficacy and safety of
pemetrexed plus bevacizumab in previously treated patients with
advanced non-squamous non-small cell lung cancer (ns-NSCLC). Tumour
Biol. 36:2491–2499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S,
Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized,
double-blind, placebo-controlled, multicenter, Phase III study of
first-line carboplatin/paclitaxel plus bevacizumab or placebo in
chinese patients with advanced or recurrent nonsquamous
non-small-cell lung cancer. J Clin Oncol. 33:2197–2204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cortot AB, Audigier-Valette C, Molinier O,
Le Moulec S, Barlesi F and Zalcman G: Weekly paclitaxel plus
bevacizumab versus docetaxel as second or third-line treatment in
advanced non-squamous non-small cell lung cancer (NSCLC): Results
from the phase III study IFCT-1103 ULTIMATE. J Clin Oncol. 34
Suppl-15:90052016.
|
|
28
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnson DH, Fehrenbacher L, Novotny WF,
Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF III,
Gaudreault J, Damico LA, et al: Randomized phase II trial comparing
bevacizumab plus carboplatin and paclitaxel with carboplatin and
paclitaxel alone in previously untreated locally advanced or
metastatic non-small-cell lung cancer. J Clin Oncol. 22:2184–2191.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnson BE, Kabbinavar F, Fehrenbacher L,
Hainsworth J, Kasubhai S, Kressel B, Lin CY, Marsland T, Patel T,
Polikoff J, et al: ATLAS: Randomized, double-blind,
placebo-controlled, phase IIIB trial comparing bevacizumab therapy
with or without erlotinib, after completion of chemotherapy, with
bevacizumab for first-line treatment of advanced non-small-cell
lung cancer. J Clin Oncol. 31:3926–3934. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hainsworth JD, Fang L, Huang JE, Karlin D,
Russell K, Faoro L and Azzoli C: BRIDGE: An open-label phase II
trial evaluating the safety of bevacizumab + carboplatin/paclitaxel
as first-line treatment for patients with advanced, previously
untreated, squamous non-small cell lung cancer. J Thorac Oncol.
6:109–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grothey A, Sugrue MM, Purdie DM, Dong W,
Sargent D, Hedrick E and Kozloff M: Bevacizumab beyond first
progression is associated with prolonged overall survival in
metastatic colorectal cancer: Results from a large observational
cohort study (BRiTE). J Clin Oncol. 26:5326–5334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takeda M, Yamanaka T, Seto T, Hayashi H,
Azuma K, Okada M, Sugawara S, Daga H, Hirashima T, Yonesaka K, et
al: Bevacizumab beyond disease progression after first-line
treatment with bevacizumab plus chemotherapy in advanced
nonsquamous non-small cell lung cancer (West Japan Oncology Group
5910L): An open-label, randomized, phase 2 trial. Cancer.
122:1050–1059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bennouna J, de Castro J, Dingemans AC,
Griesinger F and Grossi Flanger CJ: Efficacy and safety results
from AvaALL: An open-label, randomized phase III trial of standard
of care (SOC) with or without continuous bevacizumab (Bev)
treatment beyond progression (PD) in patients (pts) with advanced
non-small cell lung cancer (NSCLC) progressing after first-line Bev
and chemotherapy (chemo). J Clin Oncol. 35 Suppl 15:90042017.
|