Introduction
Cervical cancer is a common malignancy with an
estimated 485000 new cases and 236000 deaths annually worldwide
(1). Nearly all cases of cervical
cancer are caused by human papillomavirus infection (2). Advances in novel diagnostic and
therapeutic technologies have led to a considerable decline in
cervical cancer morbidity and mortality over the last decade
(3,4).
Current treatments for cervical cancer are surgery, radiotherapy
and chemotherapy (5); however,
additional therapies will be required to increase survival rates
and reduce the need for surgery.
TNF-related apoptosis-inducing ligand (TRAIL) is a
member of the tumor necrosis factor (TNF) superfamily and was
originally cloned from human myocardial cell. TRAIL can bind to
five receptors, of which two, DR4 (TRAILR1) and DR5 (TRAILR2),
induce apoptosis. Upon TRAIL binding, FAS-associated protein with
death domain (FADD) and caspase 8 sequentially recruited to the
DR4/DR5 intracellular domain, resulting in formation of a
death-inducing signaling complex (DISC) (6). Downstream effector molecules, including
caspase-3, are then activated and induce the biochemical and
morphological hallmarks of apoptosis via cleavage of numerous
cellular proteins (6–8). At present, several types of cancers have
been reported to be sensitive to TRAIL-induced apoptosis in
vitro and in vivo (6,9–11). In addition, radiotherapy and
chemotherapy are known to enhance the effects of TRAIL by inducing
the DR4 or DR5 expression (12–16).
Taxol, an important first-line drug in cervical
cancer therapy, was first isolated from Taxus brevifolia in
1962 (17). Taxol inhibits the
disassembly of microtubule polymers, which causes the arrest of
cancer cells in metaphase and leads to apoptosis (17). Taxol is a wide-spectrum anti-tumor
drug and is used to treat many cancers, including lung cancer,
ovarian cancer (18–20). However, Taxol has weak anti-tumor
effects and must be used in combination with cisplatin or other
chemotherapies (19).
Two major apoptosis signaling pathways exist in
mammalian cells: The intrinsic pathway, which is controlled by the
Bcl-2 family of proteins and is induced by chemotherapy and
radiotherapy, and the extrinsic pathway, which is mediated by the
TNF receptor superfamily (6,21). In cancer cells, intrinsic
pathway-induced death can be enhanced by concomitant activation of
the extrinsic pathway; thus, inhibition of both pathways could be a
powerful method of inducing apoptosis in cancer cells (6). To test this, we constructed a
recombinant plasmid to overexpress human TRAIL114–281
protein in the human cervical carcinoma cell line, HeLa. We
examined the effects of TRAIL overexpression in combination with
Taxol treatment on cell growth and apoptosis in vitro and
in vivo, and investigated their possible underlying
mechanisms of action.
Materials and methods
Plasmids construction, cell culture
and transfection
To generate a TRAIL overexpression plasmid, we
cloned the human TRAIL functional domain (amino acids 144–281;
GeneBank accession No. NM_003810), and the sequence was cloned into
a plasmid encoding GFP and ampicillin- and neosporin-resistance
genes. The TRAIL sequence was amplified by PCR with the following
primers: P1, 5′-CCGAGATCTGTGAGAGAAGAGGTCC-3′; P2,
5′-CTTGTCGACTTAGCCAACTAAAAAGGCCC-3′. A signal peptide sequence was
placed in front of TRAIL to ensure passage of the translated
protein through cell membranes into the extracellular medium for
interaction with receptors. The of signal peptide primers were: P1:
5′-CTAGCATGGCCCTGTGGATGCGCCTCCTGCCCCTGCTGGCGCTGCTGGCCCTCTGGGGACCTGACCCAGCCGCAGCC-3′;
P2,
5′-CTAGGGCTGCGGCTGGGTCAGGTCCCCAGAGGGCCAGCAGCGCCAGCAGGGGCAGGAGGCGCATCCACAGGGCCATG-3′.
The human cervical carcinoma cell line HeLa was obtained from the
Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in IMDM containing 10% fetal
bovine serum and were maintained at 37°C in a 5% CO2
atmosphere. The cells were transfected with plasmids pTRAIL or
pVector (empty vector control) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
G418 at 800 µg/µl was added to the medium to select for stable
HeLa-TRAIL and HeLa-vect cell lines.
Western blot analysis
Cell lysis protein quantification, and Western
blotting analysis were carried out as described previously
(22). Antibodies against β-actin,
Bcl-2, cleaved caspase-3, and TRAIL were purchased from Wuhan
Boster Biological Technology, Ltd. (Wuhan, China).
Cell proliferation, cell cycle and
apoptosis analysis
Cell proliferation was assayed using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
kit (Sigma, USA) according to the manufacturer's instructions.
Inhibition of the cell growth rate was calculated as %
inhibition=(1-absorbance of the experimental group/absorbance of
the control group) ×100%. Cell cycle phase and apoptosis ware
determined by flow cytometry. In brief, stably transfected HeLa
lines were resuspended in phosphate-buffered saline, and DNA was
stained by the addtion 50 µg/ml propidium iodide (PI) (Beckman
Coulter, USA). The percentage of apoptotic cells and cells in
different cell cycle phases were quantified using an Epics-XL-MCL
flow cytometer (Beckman Coulter). HeLa cell apoptosis was also
analyzed by staining with acridine orange/ethidium bromide (AO/EB)
followed by fluorescence microscopy. Cell death in excised tumor
xenografts was analyzed by hematoxylin and eosin (H&E) and
terminal deoxynucleotidyl transferase dUTP nick-end labeling
(TUNEL) staining as described previously (22,23). To
quantify cell death in tissue sections, we randomly selected five
views for each group and analyzed TUNEL-positive cells using an
HPIAS-100 image analysis system.
Animal care
Female Nude mice were purchased from the Beijing
Institute for Experimental Animals. All animals were cared for in
compliance with institutional guidelines and ethical approval was
received from the Animal Experimental Ethics Committee of Jilin
University.
Tumor xenografts model
Four groups of female nude mice (n=5) were injected
subcutaneously (s.c.) in the right flank with 2×106 HeLa
cells, and the tumor were allowed to grow until they reached to 7
mm in diameter. After the point, the four groups of mice were
randomly assigned to receive injection of liposomes mixed with
pVector or pTRAIL plasmids (150 µg per mouse) and co-injections of
vehicle or Taxol (20 mg/kg) once a day for 3 weeks respectively.
The mice were then sacrificed and tumors were excised and evaluated
by Immunohistochemistry, H&E and TUNEL staining.
Immunohistochemical staining
Excised tumor samples were prepared for
immunohistochemical detection of cleaved caspase-3 and Bcl-2
protein using a streptavidin-biotin-peroxidase complex staining kit
(SABC; Wuhan Boster Biological Technology, Ltd.). Antibody to
cleaved caspase-3 and Bcl-2 were purchased from Wuhan Boster
Biological Technology, Ltd. To quantify staining, 5 fields of view
were randomly selected for each experimental group and cells were
analyzed using the HPIAS-100 image analysis system.
Semi-quantitation RT-PCR
Total RNA was isolated from cells using TRIzol
reagent (Ambion; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was generated by reverse
transcription of 2 µg samples of total RNA using a RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). The PCR
primers were: β-actin sense: 5′CTGGGACGACATGGAGAAAA-3′ and
antisense: 5′-AAGGAAGGCTGGAAGAGTGC-3′; DR4 sense
5′-CTGAGCAACGCAGACTCGCTGTCCAC-3′ and antisense
5′-TCCAAGGACACGGCAGAGCCTGTGCCAT-3′; and DR5 sense
5′-GCCTCATGGACAATGAGATAAAGGTGGCT-3′and antisense
5′-CCAAATCTCAAAGTACGCACAAACGG-3′. The PCR reaction conditions were
as follows: denaturation at 94°C for 3 min followed by 30
amplification cycles (94°C for 30 sec, 59°C for 30 sec, 72°C for 30
sec) and a final extension of 72°C for 5 min. The PCR products were
analyzed by 1% agarose gel electrophoresis containing 5 µg/ml EB,
and the bands were visualized using a GIS Gelatum imaging system
(Tanon Science and Technology Co., Ltd., Shanghai, China).
Statistical analysis
The data are expressed as mean values ± standard
deviation (SD). For both in vitro and in vivo
experiments, group differences were analyzed by Kruskal-Wallis test
followed by Dunn's multiple comparisons test. All experiments were
repeated at least three times. Statistical calculations were
performed using SigmaStat software (SPSS v20; SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Construction of a human TRAIL
overexpression plasmids
We choose the functional domain (amino acids
144–281) of human TRAIL by constructing a plasmid (pTRAIL) that
also encoded the GFP gene and a signal peptide preceding TRAIL to
ensure passage of the protein through membranes to the
extracellular medium. HeLa cells were transfected with the empty
vector (control, pVector) or pTRAIL, and stable transfectants were
selected by growth in G418. The transfection efficiency was
virtually identical for both cell types, as detected by the
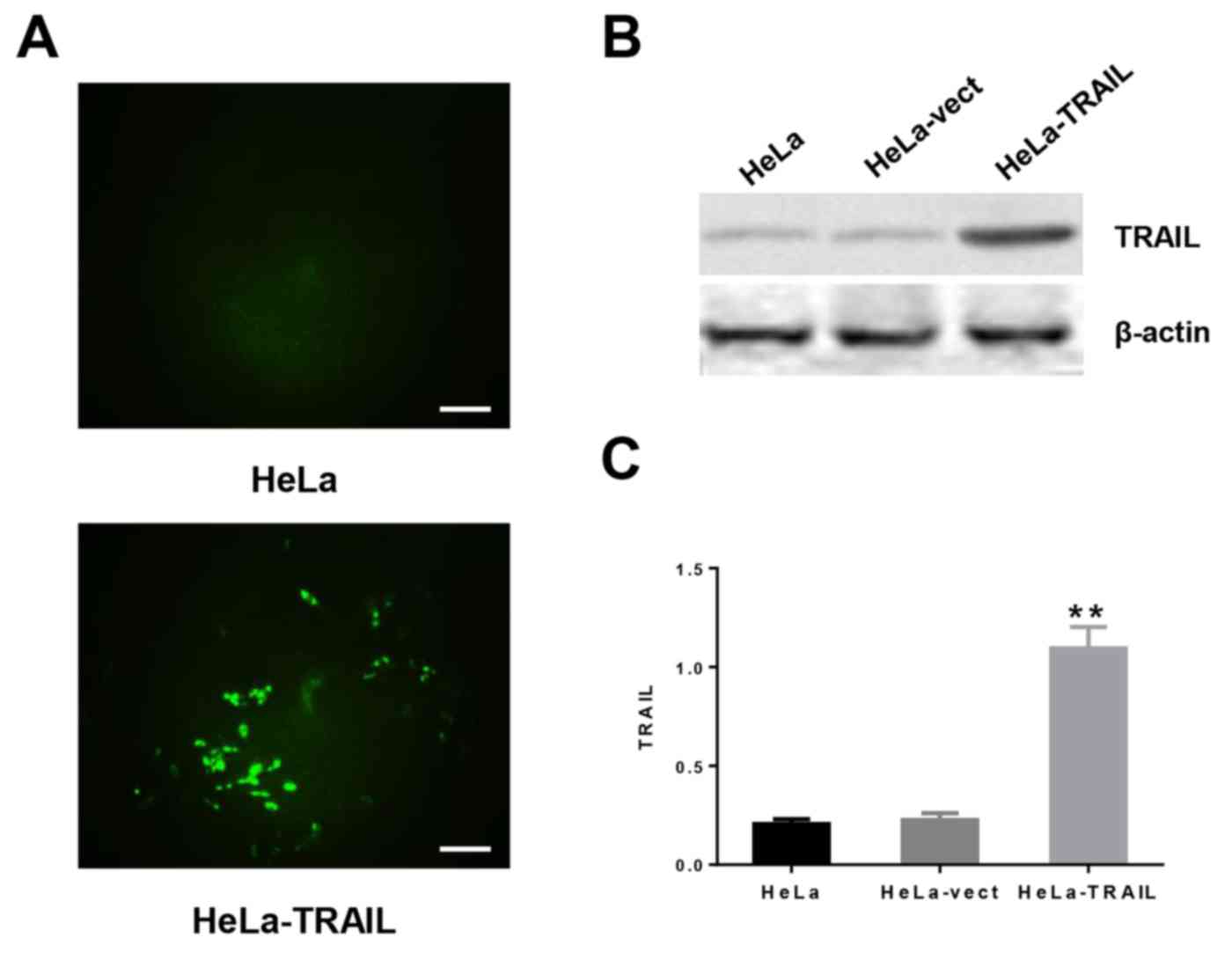
expression of GFP (Fig. 1A). As
expected, expression of TRAIL was significantly higher in HeLa
cells transfected with pTRAIL (HeLa-TRAIL) compared with cells
transfected with the empty vector (HeLa-vect) or untransfected HeLa
cells (Figs. 1B and C).
Effects of combination treatment with TRAIL and
Taxol
Suppression of HeLa cell
proliferation
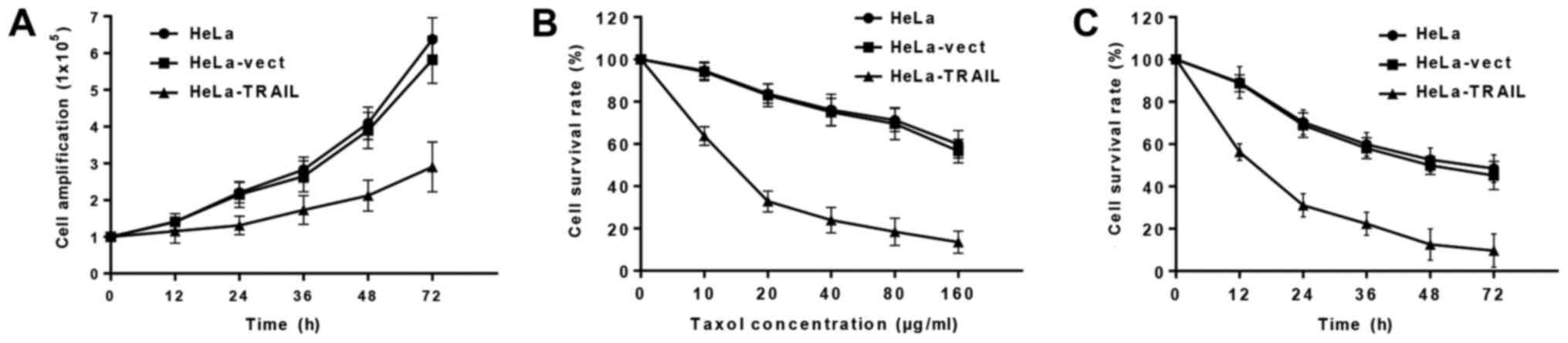
We first examined the growth of f HeLa, HeLa-vect,
and HeLa-TRAIL cells, and found that pTRAIL-transfected cells
showed reduced proliferation after 24 h compared to HeLa-vect and
uninfected Hela cells (Fig. 2A). We
next determined whether Taxol treatment could act in an additive
manner with TRAIL transfection to suppress HeLa cells survival. We
found that Taxol dose-dependently inhibited the survival of HeLa
cells, and fewer HeLa-TRAIL cells than HeLa-vect or HeLa cells were
alive after 24 h incubation in the presence of ≥10 µg/ml Taxol
(Fig. 2B). Moreover, Hela-TRAIL cells
treated with a constant Taxol concentration (40 ug/ml) showed a
decrease in survival over time (Fig.
2C).
Effects of TRAIL and Taxol on
apoptosis
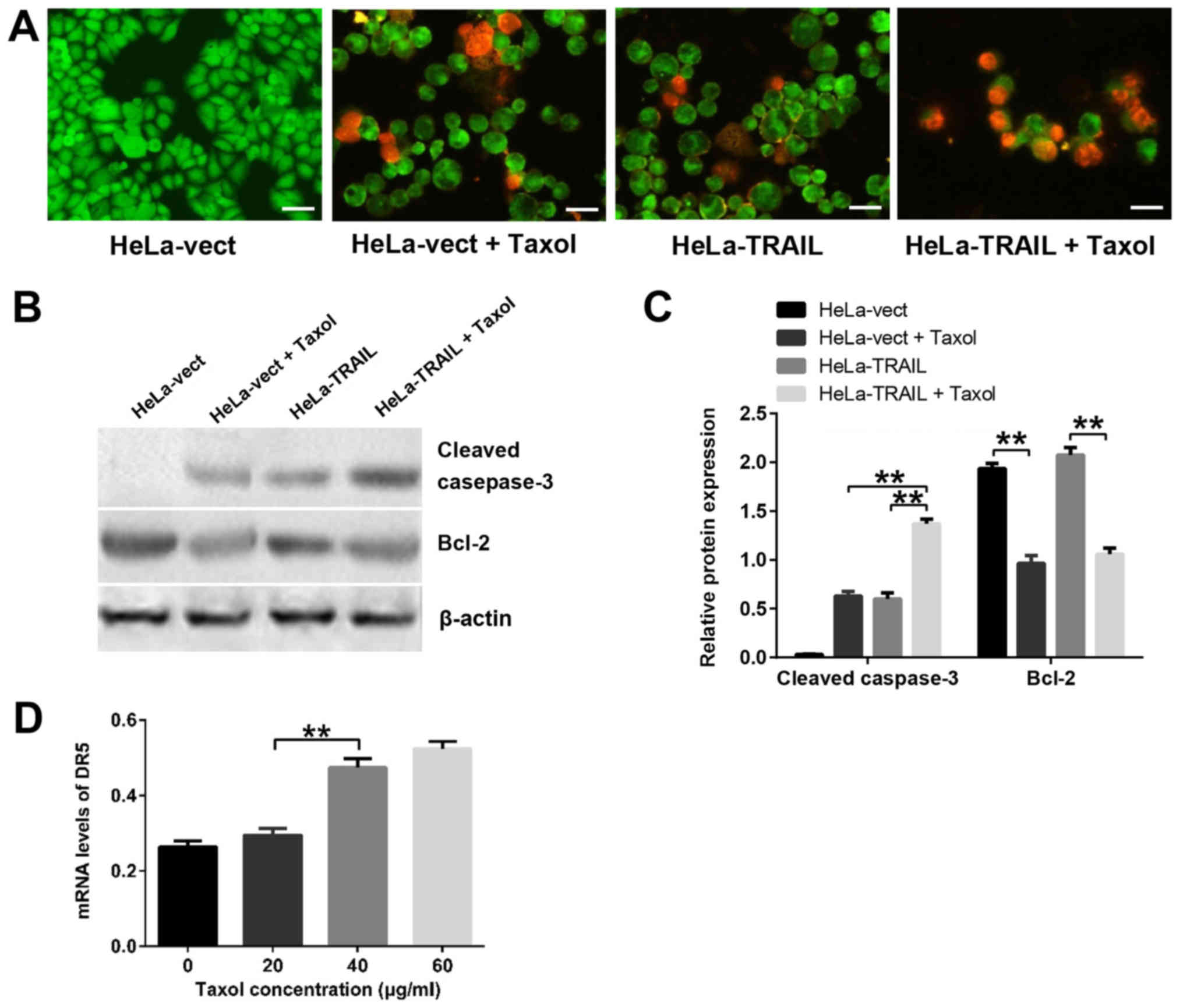
To determine whether TRAIL and Taxol suppress HeLa
cell growth and survival by inducing apoptosis, we used two assays:
Flow cytometry of PI-stained cells and fluorescence microscopy of
AO/EB-stained cells. Flow cytometry analysis showed that treatment
of HeLa-vect cells with Taxol or transfection with pTRAIL increased
the percentage of apoptotic cells to a similar extent (Table I). However, apoptosis was further
increased by treatment of HeLa-TRAIL cells with Taxol (Table I), indicating an additive effect. In
the second approach, cells were stained with AO/EB and analyzed by
fluorescence microscopy. This dual staining method canc identify
early (AO+) and late (AO+EB+) apoptotic cells. HeLa-TRAIL,
Hela-vect + Taxol and HeLa-TRAIL + Taxol cultures all showed
evidence of apoptosis, whereas HeLa-vect cells did not (Fig. 3A). More apoptotic cells were detected
in the HeLa-TRAIL + Taxol group compared with either the HeLa-TRAIL
or HeLa-vect + Taxol groups, indicating that TRAIL and Taxol induce
both early and late apoptosis in HeLa cells. These data suggest
that TRAIL overexpression and Taxol treatment in combination
induced HeLa cell apoptosis more efficiently than either one
alone.
 | Table I.Effect of TRAIL overexpression and
Taxol treatment on HeLa cell cycle phase and apoptosis. |
Table I.
Effect of TRAIL overexpression and
Taxol treatment on HeLa cell cycle phase and apoptosis.
| Group (n=5) | Apoptotic cells (%,
mean ± SD) | G2-M (%, mean ±
SD) | S (%, mean ± SD) |
|---|
| Hela-vect | 6.8±0.65 | 2.8±1.83 | 20.6±2.17 |
| Hela-vect+Taxol |
17.5±2.32a |
35.7±2.96a | 4.4±0.94a |
| Hela-TRAIL |
18.6±3.66a |
13.5±2.46a |
38.3±3.87a |
| Hela-TRAIL +
Taxol |
42.3±6.21a–c |
32.1±2.53a,c |
17.2±4.38b,c |
Expression of apoptosis-related
proteins in HeLa cells
To determine the mechanism by which TRAIL and Taxol
induce Hela cell apoptosis, we examined the expression of two
apoptosis-associated proteins; pro-apoptotic cleaved caspase-3 and
anti-apoptotic Bcl-2 (Fig. 3B and C).
The highest expression of cleaved caspase-3 was observed in
Taxol-treated HeLa-TRAIL cells, whereas only the groups treated
with Taxol showed a decrease in Bcl-2 levels. These finding suggest
that Taxol, but not TRAIL, modulates Bcl-2 expression during HeLa
cell apoptosis. In contrast, cleaved caspase-3 appears to
contribute to apoptosis induced by both TRAIL and Taxol. We next
examined the mRNA expression level of DR4 and DR5, two
apoptosis-related TRAIL receptors, in HeLa cells treated with
different concentrations of Taxol. As shown in Fig. 3D, Taxol caused a dose-dependent
increase in DR5 mRNA levels but had no effect on DR4 mRNA (data not
shown). Thus, Taxol may enhance the pro-apoptosis function of TRAIL
by increasing the expression of DR5, but not DR4 in HeLa cells.
Antitumor activity of TRAIL and Taxol
in vivo
To evaluate the effects of the combinative treatment
of TRAIL and Taxol on cervical cancer growth in vivo, we
used a nude mouse tumor xenograft model. Hela cells were inoculated
s.c. into female nude mice, and tumors allowed to grow to a
diameter of 7 mm. The groups were then randomly assigned to receive
injections of pVector or pTRAIL plasmid and either vehicle or Taxol
for 3 weeks. Animals were then sacrificed and the tumors were
excised for analysis.
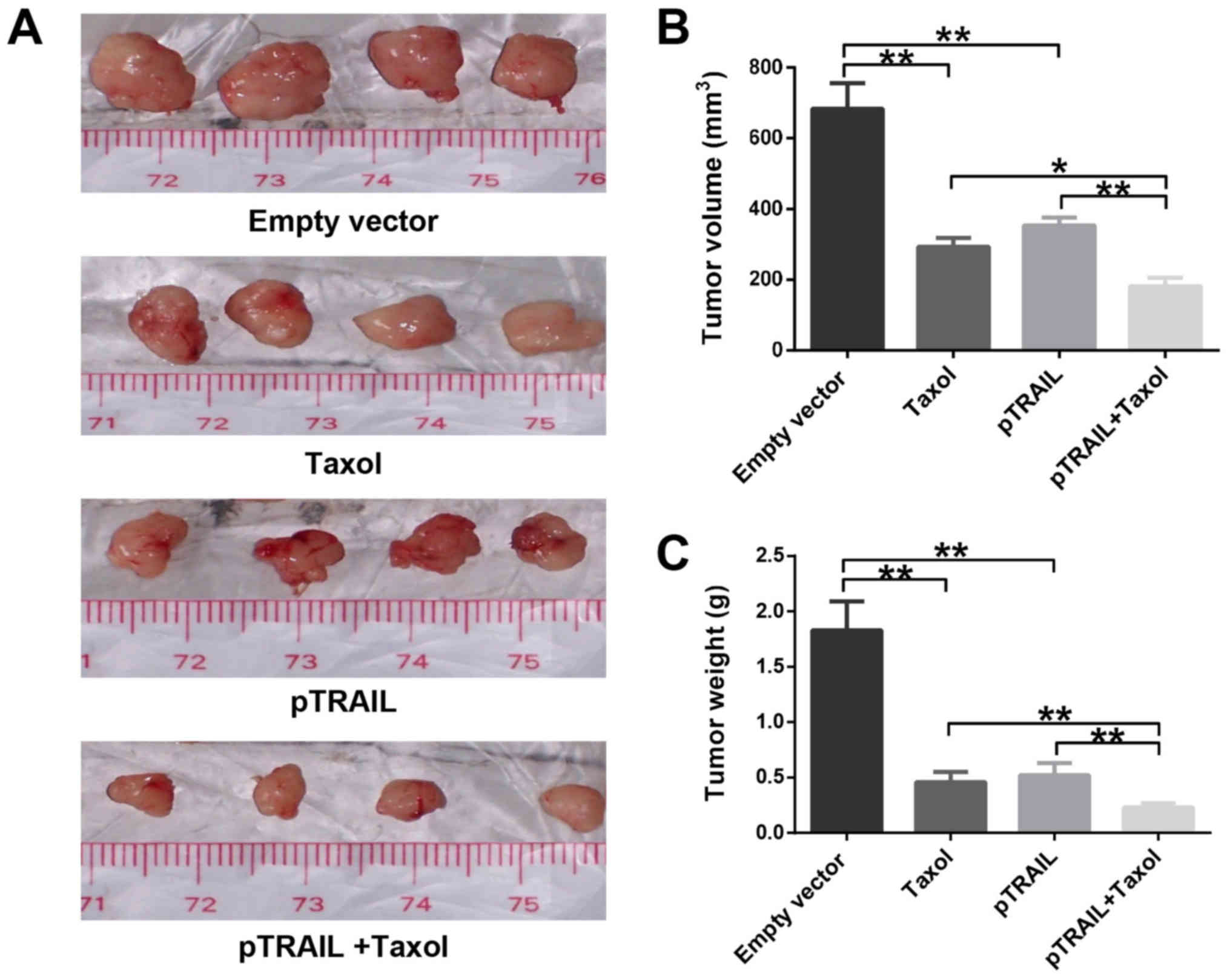
The tumor volumes and weights and are shown in
Fig. 4A-C. Tumors from mice treated
with pTRAIL and/or Taxol were significantly diminished compared
with tumors from mice receiving vehicle (saline) or pVector
treatments. As shown in Table II,
there were no significant difference in average mouse weights
between groups (P>0.05). However, tumors from mice treated with
pTRAIL + Taxol had markedly reduced weight and volume compared with
tumors from the other three mouse groups.
 | Table II.Analysis of tumors and mice bearing
HeLa tumor xenografts. |
Table II.
Analysis of tumors and mice bearing
HeLa tumor xenografts.
| Group (n=4) | Mean weight of the
nude mice (g) | Mean weight of
tumor (g) | Mean volume of
tumor (mm3) |
|---|
| Empty vector | 25.21±2.94 | 1.98±0.35 | 702.75±117.63 |
| Taxol | 24.18±2.23 |
0.46±0.09a |
292.68±25.30a |
| pTRAIL | 24.34±2.76 |
0.52±0.11a |
353.14±22.39a |
| pTRAIL+Taxol | 25.23±2.71 |
0.23±0.04a–c |
181.31±24.57a,c |
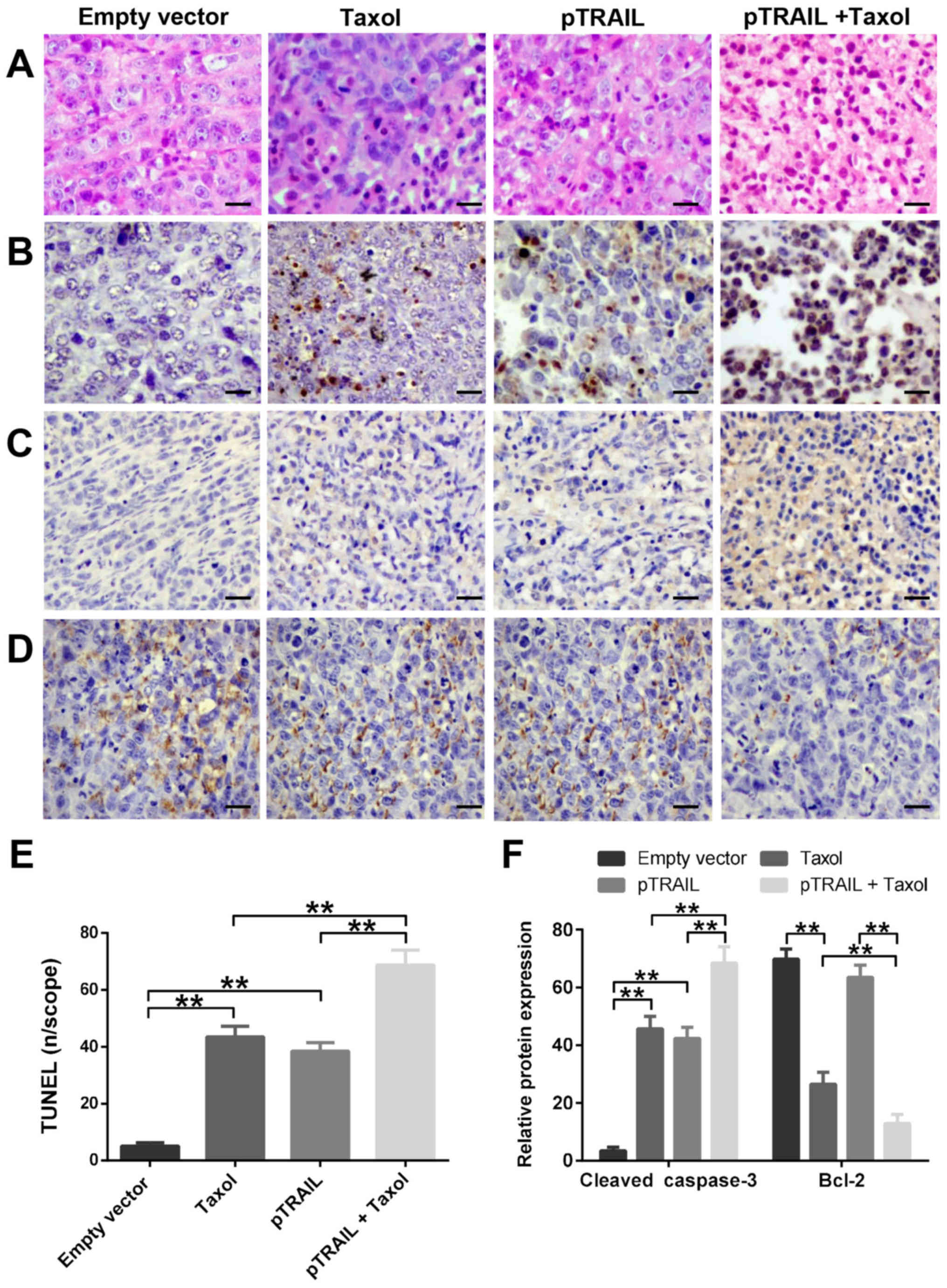
The excised tumors were sectioned and examined by
H&E staining and TUNEL staining to detect fragmented DNA
(Fig. 5A, B and E). Tumors from the
mouse groups treated with pTRAIL, Taxol, or pTRAIL + Taxol showed
elevated levels of apoptotic cells compared with the pVector group,
with the pTRAIL + Taxol group showing the highest levels. These
tumor sections were characterized by increased nuclear condensation
and DNA fragmentation (indicated by TUNEL-positive cells), whereas
tumor sections from the pVector group had granular cytoplasm and no
TUNEL-positive cells. These data demonstrated that the combination
of pTRAIL and Taxol exerts a stronger anti-tumor pro-apoptotic
effect in vivo compared with either treatment alone.
Finally, we examined the expression of cleaved
caspase-3 and Bcle-2 protein in xenograft tumors by
immunohistochemical staining (Fig. 5C and
D). Consistent with the TUNEL staining results, tumors from
mice treated with pTRAIL and/or Taxol showed increased cleaved
caspase-3 and decreased Bcl-2 expression compared with tumors from
HeLa-vect and/or saline-treated mice. Quantification of protein
staining showed that expression was highest in the tumors from
pTRAIL + Taxol-treated mice (Fig.
5F).
Discussion
Deaths from uterine cancer declined by more than 80%
between 1930 and 2012, largely due to the introduction of the
Papanicolaou test for early cervical cancer, which is predominantly
caused by human papillomavirus infection (4). However, cervical cancer is still the
third most common carcinoma among women worldwide and results in
236000 deaths each year (1); thus
there is a pressing need for new and/or improved treatments.
TRAIL, a member of the TNF protein superfamily,
induces apoptosis by binding to the cell surface death receptors
DR4 and DR5 (6). TRAIL-induced
apoptosis has been shown to be enhanced by combination treatment
with radiotherapy or chemotherapy (12,13).
Taxol, a classical chemotherapeutic agent, exerts its anti-tumor
effect by arresting cells in metaphase, which induces apoptosis.
Cisplatin and Taxol both show synergistic effects with TRAIL in
non-small cell lung cancer (24).
There are also reports that the combination of TRAIL and Taxol
enhances apoptosis in gastric cancer and hepatocellular carcinoma
cells (25,26). Here, we examined the efficacy of TRAIL
and Taxol combination therapy in cervical cancer cells in
vitro and in vivo.
To study the anti-tumor effect of TRAIL, we
transfected HeLa cells with a plasmid encoding a signal sequence
and the functional domain of TRAIL, resulting in constitutive
protein production and secretion. As expected, Hela cells
overexpressing TRAIL showed poorer proliferation than normal Hela
cells or the pVector control cells. We also showed that Taxol could
enhance the damaging effect of high TRAIL expression and
significantly decreased the survival of HeLa-TRAIL cells in a dose-
and time dependent manner. Since the combination of TRAIL and Taxol
had previously been shown to enhance apoptosis in non-small cell
lung cancer cells (24), we also
investigated their effects on apoptosis in HeLa cells. Indeed,
microscopic and flow cytometric analysis identified a significantly
higher degree of apoptosis in the pTRAIL + Taxol group compared
with the pTRAIL or Taxol groups. To determine the mechanism of
enhanced apoptosis, we measured cellular levels of cleaved
caspase-3 and Bcl-2 as markers of extrinsic and intrinsic
apoptosis, respectively. As expected, the expression of cleaved
caspase-3 was consistent with the degree of apoptosis induced by
pTRAIL and/or Taxol. However, Bcl-2, a hallmark anti-apoptotic
protein, was only downregulated after treatment with Taxol. TRAIL
has been reported to synergize with luteolin, a naturally occurring
flavonoid that upregulates DR5, to induce apoptosis in HeLa cell
(26). In the present study, we found
that DR5 expression in HeLa cells increased in parallel with Taxol
concentration, indicating that Taxol may enhance the pro-apoptotic
function of TRAIL by increasing DR5 expression.
Using a mouse tumor xenograft model, we showed that
the volumes and weights of HeLa-derived tumors were decreased
significantly by combined overexpression of TRAIL and treatment
with Taxol compared with either treatment alone. We evaluated the
tumor sections by H&E and TUNEL staining and found that the
cell morphology and degree of DNA fragmentation were in accordance
with the in vitro findings. Cleaved caspase-3 protein
expression was elevated in TRAIL-transfected and Taxol-treated
tumors compared with either treatment alone. Similarly, tumors from
the pTRAIL + Taxol group expressed lower Bcl-2 levels than tumors
from mice treated with Taxol alone, confirming that TRAIL enhances
the Taxol-induced intrinsic apoptotic pathway in vivo.
However, the mechanism by which this occurs remains unclear.
In this study, we found that the combination of
TRAIL overexpression and Taxol treatment significantly increased
apoptosis of a human cervical carcinoma cell line compared with
either TRAIL overexpression or Taxol treatment alone. Moreover,
this additive effect may derive from Taxol-induced upregulation of
DR5, a TRAIL receptor that connects to the downstream extrinsic
apoptosis pathway.
A number of different vectors are currently being
used for gene therapy of malignant tumors. Whereas early vectors
were non-specific, novel vectors, especially viruses, have been
developed that target cancer cells but leave normal cells unharmed
(27). We have reported that
attenuated Salmonella typhimurium has potential utility for
transgene delivery, since it shows >1,000-fold preferential
accumulation in tumors compared with normal tissues (28). Our results suggest that this delivery
system might be useful for combination therapy with TRAIL plasmids
and Taxol for the treatment of cancer.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81472344 and 81773217) and
Jilin Provincial Education Department (grant no. Jijiaokehezi
[2016]455) and the International Cooperation Project of Jilin
Provincial Science and Technology Department (grant no.
20150414031GH) and Jilin University Bethune Plan B Projects (No.
2015220). The authors would like to thank Dr Anne M. O'Rourke for
editing the English text of a draft of this manuscript.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crosbie EJ, Einstein MH, Franceschi S and
Kitchener HC: Human papillomavirus and cervical cancer. Lancet.
382:889–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yee GP, de Souza P and Khachigian LM:
Current and potential treatments for cervical cancer. Curr Cancer
Drug Targets. 13:205–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnstone RW, Frew AJ and Smyth MJ: The
TRAIL apoptotic pathway in cancer onset, progression and therapy.
Nat Rev Cancer. 8:782–798. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ashkenazi A and Dixit VM: Apoptosis
control by death and decoy receptors. Curr Opin Cell Biol.
11:255–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Falschlehner C, Emmerich CH, Gerlach B and
Walczak H: TRAIL signalling: Decisions between life and death. Int
J Biochem Cell Biol. 39:1462–1475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horak P, Pils D, Kaider A, Pinter A,
Elandt K, Sax C, Zielinski CC, Horvat R, Zeillinger R, Reinthaller
A and Krainer M: Perturbation of the tumor necrosis factor-related
apoptosis-inducing ligand cascade in ovarian cancer: Overexpression
of FLIPL and deregulation of the functional receptors DR4 and DR5.
Clin Cancer Res. 11:8585–8591. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinez-Ferrandis JI, Rodríguez-López R,
Milne RL, González E, Cebolla E, Chirivella I, Zamora P, Arias JI,
Palacios S, Cervantes A, et al: Polymorphisms in TRAIL receptor
genes and risk of breast cancer in Spanish women. Cancer Biomark.
3:89–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanlioglu AD, Korcum AF, Pestereli E,
Erdogan G, Karaveli S, Savas B, Griffith TS and Sanlioglu S: TRAIL
death receptor-4 expression positively correlates with the tumor
grade in breast cancer patients with invasive ductal carcinoma. Int
J Radiat Oncol Biol Phys. 69:716–723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gliniak B and Le T: Tumor necrosis
factor-related apoptosis-inducing ligand's antitumor activity in
vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res.
59:6153–6158. 1999.PubMed/NCBI
|
|
13
|
Hori T, Kondo T, Kanamori M, Tabuchi Y,
Ogawa R, Zhao QL, Ahmed K, Yasuda T, Seki S, Suzuki K and Kimura T:
Ionizing radiation enhances tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis through
up-regulations of death receptor 4 (DR4) and death receptor 5 (DR5)
in human osteosarcoma cells. J Orthop Res. 28:739–745.
2010.PubMed/NCBI
|
|
14
|
Sussman RT, Ricci MS, Hart LS, Sun SY and
El-Deiry WS: Chemotherapy-resistant side-population of colon cancer
cells has a higher sensitivity to TRAIL than the non-SP, a higher
expression of c-Myc and TRAIL-receptor DR4. Cancer Biol Ther.
6:1490–1495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Finnberg NK, Gokare P, Navaraj A, Lang
Kuhs KA, Cerniglia G, Yagita H, Takeda K, Motoyama N and El-Deiry
WS: Agonists of the TRAIL death receptor DR5 sensitize intestinal
stem cells to chemotherapy-induced cell death and trigger
gastrointestinal toxicity. Cancer Res. 76:700–712. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oliver PG, LoBuglio AF, Zhou T, Forero A,
Kim H, Zinn KR, Zhai G, Li Y, Lee CH and Buchsbaum DJ: Effect of
anti-DR5 and chemotherapy on basal-like breast cancer. Breast
Cancer Res Treat. 133:417–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ao X, Nie P, Wu B, Xu W, Zhang T, Wang S,
Chang H and Zou Z: Decreased expression of microRNA-17 and
microRNA-20b promotes breast cancer resistance to taxol therapy by
upregulation of NCOA3. Cell Death Dis. 7:e24632016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Wan L, Zhai LY and Wang J: Effects
of SC-560 in combination with cisplatin or taxol on angiogenesis in
human ovarian cancer xenografts. Int J Mol Sci. 15:19265–19280.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng C, Hao Y, Zhao Y, Sun Q, Zhao X and
Cong B: Effect of Smac and Taxol on non-small-cell lung cancer.
Acta Biochim Biophys Sin (Shanghai). 46:387–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao L, Zhang L, Hu J, Li F, Shao Y, Zhao
D, Kalvakolanu DV, Kopecko DJ, Zhao X and Xu DQ: Down-regulation of
signal transducer and activator of transcription 3 expression using
vector-based small interfering RNAs suppresses growth of human
prostate tumor in vivo. Clin Cancer Res. 11:6333–6341. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Zhang L, Shao Y, Liang Z, Shao C,
Wang B, Guo B, Li N, Zhao X, Li Y and Xu D: Effects of a human
plasma membrane-associated sialidase siRNA on prostate cancer
invasion. Biochem Biophys Res Commun. 416:1–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan QL, Zou WY, Song LH and Wei W:
Synergistic antitumor activity of TRAIL combined with
chemotherapeutic agents in A549 cell lines in vitro and in vivo.
Cancer Chemother Pharmacol. 55:189–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen L, Chen D, Gong M, Na M, Li L, Wu H,
Jiang L, Qian Y, Fang G and Xue X: Concomitant use of Ad5/35
chimeric oncolytic adenovirus with TRAIL gene and taxol produces
synergistic cytotoxicity in gastric cancer cells. Cancer Lett.
284:141–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Horinaka M, Yoshida T, Shiraishi T, Nakata
S, Wakada M, Nakanishi R, Nishino H and Sakai T: The combination of
TRAIL and luteolin enhances apoptosis in human cervical cancer HeLa
cells. Biochem Biophys Res Commun. 333:833–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pazarentzos E and Mazarakis ND: Anticancer
gene transfer for cancer gene therapy. Adv Exp Med Biol.
818:255–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Gao L, Zhao L, Guo B, Ji K, Tian
Y, Wang J, Yu H, Hu J, Kalvakolanu DV, et al: Intratumoral delivery
and suppression of prostate tumor growth by attenuated Salmonella
enterica serovar typhimurium carrying plasmid-based small
interfering RNAs. Cancer Res. 67:5859–5864. 2007. View Article : Google Scholar : PubMed/NCBI
|