Introduction
Breast cancer is a common malignancy in women.
Angiogenesis serves an important role in the genesis and
development of various tumors, including breast, colon, pancreatic
and prostatic cancer (1,2). Out of all imaging techniques,
computerized tomography (CT) and magnetic resonance imaging (MRI)
are able to quantify the perfusion blood volume and permeability in
the level of capillaries so as to reveal the hemodynamics of tumors
with good spatial and temporal resolution; however, application of
these technique in breast tumor is restricted by limitations,
including high radioactivity of CT, and the complexity and
time-consumption of MRI. Conventional doppler ultrasonography is
not sensitive to low-velocity blood flow, which restricts its
application in the assessment of neovascularization in tumor.
Contrast enhanced ultrasonography (CEUS) make it possible to
reflect morphologic and functional changes of microvessel perfusion
in tumors. A previous study reported that CEUS may be useful for
distinguishing malignant breast lesions from benign ones (3). The possibility of using CEUS for the
evaluation of tumor perfusion following chemotherapy or other local
antiangiogenic treatments has been reported (4). A previous study revealed that CEUS
patterns and perfusion parameters are well correlated with the
microvascular density (MVD) values and distribution in lesion
sites, which have focused on the ultrasonographic characteristics
of high- and low-MVD lesions (5),
This study focused on the correlation between regional perfusion
patterns and microvascular distributions in breast cancer. In
addition, Wan et al (6)
reported that certain enhancement patterns and parameters correlate
with the prognostic factors of tumor invasiveness to some extent.
Hence, it is reasonable to assume that the different enhancement
characteristics in CEUS may not only indicate microvascular
distribution but also provide valuable information about tumor
biologic potential and prognosis. The present study aimed to
evaluate whether enhancement patterns and perfusion parameters
correlate with microvascular distribution in breast cancer. In
addition, the possible prognostic value of these parameters was
assessed.
Materials and methods
Ethics statement
All patients who accepted SonoVue CEUS and who
donated human breast tissues provided written informed consent
prior to participation in the study. The protocols for collection
and analysis of the samples were approved by the Institutional
Review Board of the Tianjin Medical University Cancer Institute and
Hospital (Tianjin, China), in accordance with the current revision
of The Declaration of Helsinki.
Patient selection
From September 2011 to April 2013 in Tianjin Medical
University Cancer Institute and Hospital, prospective analysis of
109 lesions in Breast Imaging Reporting and Data System (BI-RADS)
classes 4–5 (7) was conducted by
conventional breast ultrasound. Before core-needle biopsy, CEUS was
performed. Following CEUS, punctures were performed on different
enhancement regions in the same body position under the guidance of
ultrasound. With the exception of 8 benign lesions, and 2 lesions
in which biopsied tissue was too small to diagnose, the remaining
malignant lesions (n=99) confirmed by biopsy or surgical resection
were enrolled. All patients were females, ranging in age from 19 to
93 years (mean age, 53.0 years).
Instruments and methods
All ultrasonographic examinations were performed
using a Logiq E9 Ultrasound Machine (GE Healthcare Life Sciences,
Chicago, IL, USA) with probe frequency of 6–15 MHz. The patient was
required to lie down in a supine position with both breasts fully
exposed, then the patient was examined under routine
ultrasonographic mode for information including pathomorphology and
color Doppler flow image. The most suspected lesion site was
selected if the patient had multiple lesions. Once the routine mode
exhibited the section of largest size or most abundant blood
supply, it was switched to CEUS mode with probe frequency of 9 MHz
and SonoVue contrast agent was used (Bracco S.p.A., Milan, Italy;
prepared according to the manufacturer's instructions). Contrast
pulse sequencing was started and 2.5 ml contrast agent was quickly
administered using bolus injection via the cubital vein, followed
by a 5 ml normal saline flush. Dynamic perfusion of the lesion was
observed in real time for ≥3 min after the injection. The whole
process of dynamic CEUS was recorded and stored in the machine's
hard drive and ultrasound workstation for further analysis.
Two ultrasonographers independently and blindly
evaluated the CEUS images, and any disagreements were discussed
until a consensus was reached. Enhancement patterns were defined
based on the study objectives: 1) Homogeneous enhancement, all
areas in the lesion site were homogeneously and diffusely enhanced
with almost the same enhancement intensity; 2) peripheral
enhancement, the periphery of the lesion site was enhanced without
marked enhancement in the center of the lesion; or the periphery
and center of lesion site were enhanced but the range and/or
intensity of enhancement was more apparent in the periphery than
the center; or 3) regional enhancement: The enhancement areas were
distributed unevenly in the lesion site with different intensities;
or a specific area of a lesion site presented homogeneous and
diffuse enhancement.
Following CEUS, punctures were performed on
differently enhanced regions in the same body position under
guidance of ultrasound. A slice of tissue was taken for biopsy from
the periphery and center of homogeneous and peripheral lesions, as
well as from the different enhancement regions of a regional
enhancement lesion. The selected cases consisted of >2 accurate
puncture cores, intratumoral and peritumoral of breast cancer, to
ensure that there were sufficient samples for a random selection of
5 different microscopic fields (5 within the tumor and 5
surrounding the tumor). The dynamic images stored on the machine's
hard drive were examined at the corresponding puncture sites for
time-intensity curves, in order to automatically obtain the
perfusion parameters, including peak intensity (PI), ascending
slope, time to peak, area under the time-intensity curve and
wash-in time.
Histological types were defined according to the
World Health Organization classification (8); histological grade was determined using
the modified Bloom and Richardson grading system (9). The tumors were staged using the American
Joint Committee on Cancer, 7th edition, TNM staging system
(10).
Immunohistochemistry (IHC) and
assessment of MVD
Tissues were fixed in 10% neutral-buffered formalin
for ~24 h at room temperature, then paraffin-embedded. Tissue
sections (5-µm) of intratumoral and peritumoral breast cancer from
each case were selected. IHC for CD34 was performed according to
standard procedures. In brief, 5-µm tissue sections were
sequentially dewaxed and rehydrated using xylene and graded alcohol
washes. Antigen retrieval was performed at 121°C for 2 min, using
citrate buffer, pH 6.0. After serial blocking with hydrogen
peroxide and normal goat serum (cat. no. ZLI-9056) for 10 min at
room temperature, the sections were incubated with primary
monoclonal antibody against CD34 (cat. no. ZA-0550; dilution 1:200)
(both from Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) for 16 h at 4°C. The sections then were
sequentially incubated with biotinylated goat anti-rabbit/mouse
immunoglobulin (cat. no. SP-9000, working solution, undiluted;
Zhongshan Golden Bridge Biotechnology Co., Ltd.) and
peroxidase-conjugated streptavidin (Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) for 20 min at 37°C. The enzyme
substrate was 3–3′-diaminobenzidine tetrahydrochloride. Incubation
of sections with phosphate-buffered saline served as the negative
control.
MVD, as visualized by anti-CD34, was evaluated light
microscopically. When CD34 IHC demonstrated cytoplasmic or membrane
staining of endothelial cells or endothelial cell clusters that was
clearly separated from adjacent clusters and background, with or
without a lumen, this was recorded as an individual vessel. The
fields containing the greatest numbers of microvessels (vascular
hot-spots) were identified at low magnification (×10 objective
lens). Then, microvessels within the above fields were counted at
higher magnification (×40 objective lens). The total number of
microvessel in each field was counted manually and confirmed by two
investigators who were blinded to the clinicopathological
characteristics and outcomes of patients. The average count from 5
fields within intratumoral and peritumoral breast cancer samples
were recorded separately and used for statistical analysis.
Additional IHC for estrogen (ER; cat. no. ZM-0104;
dilution 1:100), progesterone (PR; cat. no. ZM-0215; dilution
1:150) (both from Zymed; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), human epidermal growth factor receptor 2 (HER2, cat. no.
c-erbB-2, dilution 1:400; Dako; Agilent Technologies, Inc.) and
Ki-67 (cat. no. 081156; dilution 1:200; Zymed; Thermo Fisher
Scientific, Inc.) of the tumors was performed on serial tissue
sections of the core-needle biopsy using standard procedures. The
immunohistochemical procedure is the same as aforementioned; the
differences were that the sections were incubated with primary
monoclonal antibody against for ER, PR, HER2 and Ki-67 for 2 h at
37°C. The staining were analyzed using previously described
criteria (11). For ER, PR and HER2,
IHC was scored according to the American Society of Clinical
Oncology/College of American Pathologists guidelines (12). Cases with a HER2 score of ≥2 were
further evaluated by fluorescence in situ hybridization
using a Vysis kit (Abbott Laboratories, Abbott Park, IL, USA).
Interpretation and scoring of Ki-67 staining was performed as
described by Cheang et al (13).
Statistical analysis
SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses. MVD ratio and CEUS-enhanced
patterns were compared using the Mann-Whitney U test. Correlations
between MVD ratio and histological grade, TNM stage and CEUS
parameters were analyzed using Spearman's rank correlation
coefficient. The association between MVD ratio and histological
characteristics of prognosis (including lymph node status, ER
status, PR status, HER2 status and Ki67 proliferative index) was
analyzed using an independent-samples t-test. Two-sided P<0.05
was considered to indicate a statistically significant
difference.
Results
Characteristics of breast cancer
patients
Histological types were defined according to the
World Health Organization classification (8), including 83 cases (83.8%) of invasive
ductal carcinoma-not otherwise specified type (IDC-NOS), 3 cases
(3.0%) of ductal carcinoma in situ, 2 cases (2.0%) of
apocrine carcinoma, 2 cases (2.0%) of mucinous carcinoma, 1 case
(1.0%) of neuroendocrine carcinoma, and 8 cases (8.1%) of mixed
type carcinoma (Table I).
 | Table I.Histopathological types of breast
cancer among patients. |
Table I.
Histopathological types of breast
cancer among patients.
| Diagnosis | n | % |
|---|
| DCIS | 3 | 3.0 |
| Invasive
carcinoma | 96 | 97.0 |
| IDC-NOS | 83 | 83.8 |
| Mucinous
carcinoma | 2 | 2.0 |
| Apocrine
carcinoma | 2 | 2.0 |
| Neuroendocrine
carcinoma | 1 | 1.0 |
| Mixed type
carcinoma | 8 | 8.1 |
The characteristics of breast cancer patients are
summarized in Table II. The average
maximum tumor diameter was 23 mm (range, 6–75 mm). Histological
grade was determined using the modified Bloom and Richardson
grading system (9). Of the 83 IDC-NOS
cases, 8 cases (9.6%) were histological grade I, 57 cases (68.7%)
were histological grade II and 18 cases (21.7%) were histological
grade III. In all cases, the tumors were staged using the American
Joint Committee on Cancer, 7th edition, TNM staging system
(10) and included pTis (n=3, 3.0%),
pT1 (n=42, 42.4%), pT2 (n=51, 51.5%), pT3 (n=3, 3.0%). A total of
36 (36.4%) patients exhibited lymph node metastasis, 70 (70.7%) of
the tumors were positive for ER, 67 (67.7%) were positive for PR,
35 (35.4%) exhibited HER2 overexpression and 74 (74.7%) exhibited
high Ki-67 proliferative index.
 | Table II.Clinicopathological characteristics of
patients with breast cancer. |
Table II.
Clinicopathological characteristics of
patients with breast cancer.
| Characteristics | n |
|---|
| n | 99 |
| Age,
yearsb | 53.0±10.8 |
| Tumor size,
mmb | 22.7±9.4 |
| Histological grade, n
(%)a |
|
| I | 8 (9.6) |
| II | 57 (68.7) |
| III | 18 (21.7) |
| TNM stage, n (%) |
|
| Tis | 3 (3.0) |
| T1 | 42 (42.4) |
| T2 | 51 (51.5) |
| T3 | 3 (3.0) |
| Lymph node, n
(%) |
|
|
Negative | 63 (63.6) |
|
Positive | 36 (36.4) |
| ER status, n (%) |
|
|
Negative | 29 (29.3) |
|
Positive | 70 (70.7) |
| PR status, n (%) |
|
|
Negative | 32 (32.3) |
|
Positive | 67 (67.7) |
| HER2 status, n
(%) |
|
|
Negative | 64 (64.6) |
|
Positive | 35 (35.4) |
| Ki67 proliferative
index, n (%) |
|
| Low | 25 (25.3) |
| High | 74 (74.7) |
Correlation of MVD ratio with
enhancement patterns and characteristics of breast cancer
In the present study, 44 (44.4%) cases of primary
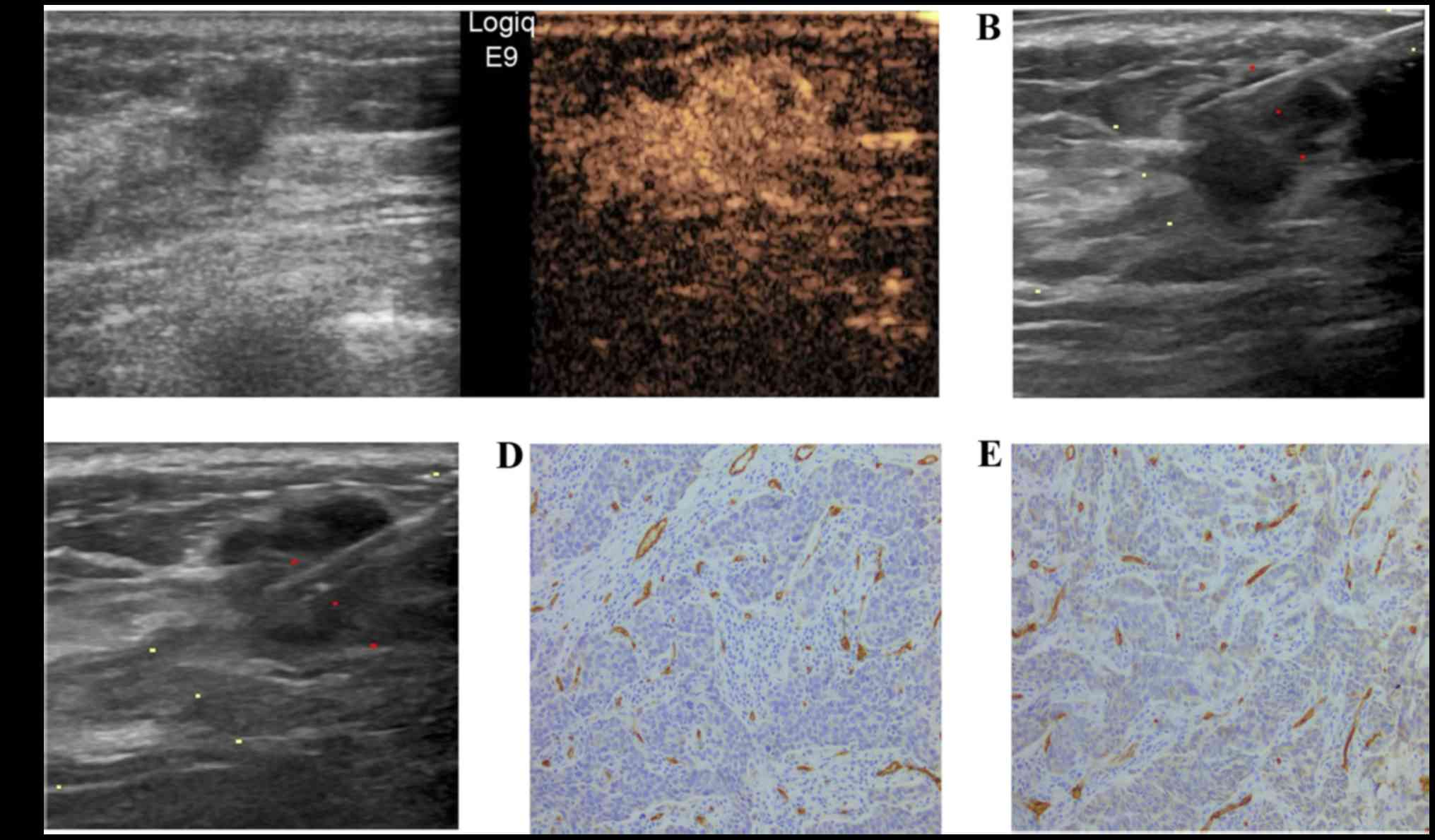
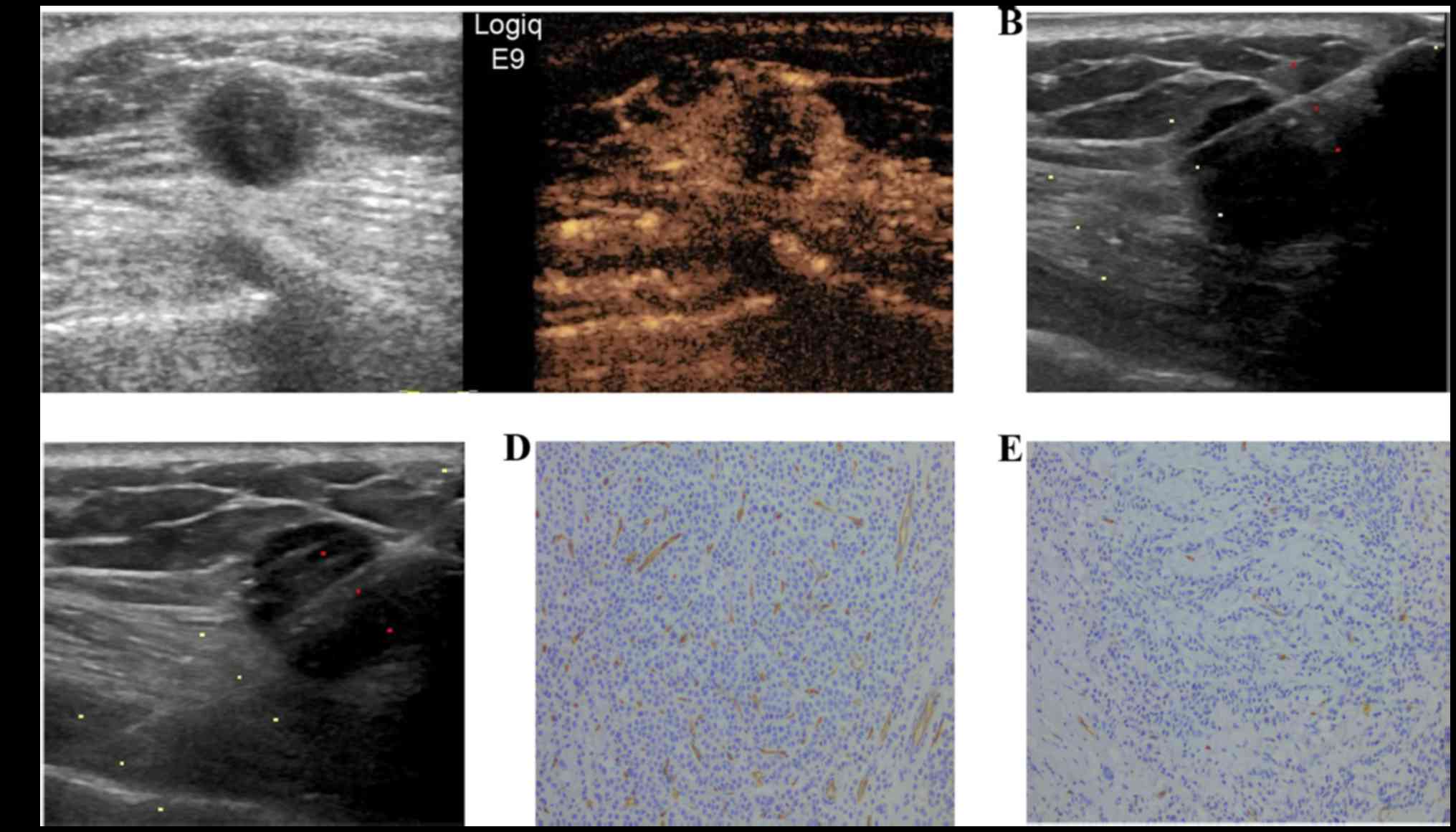
breast cancer exhibited homogenous enhancement (Fig. 1), 43 (43.4%) exhibited peripheral
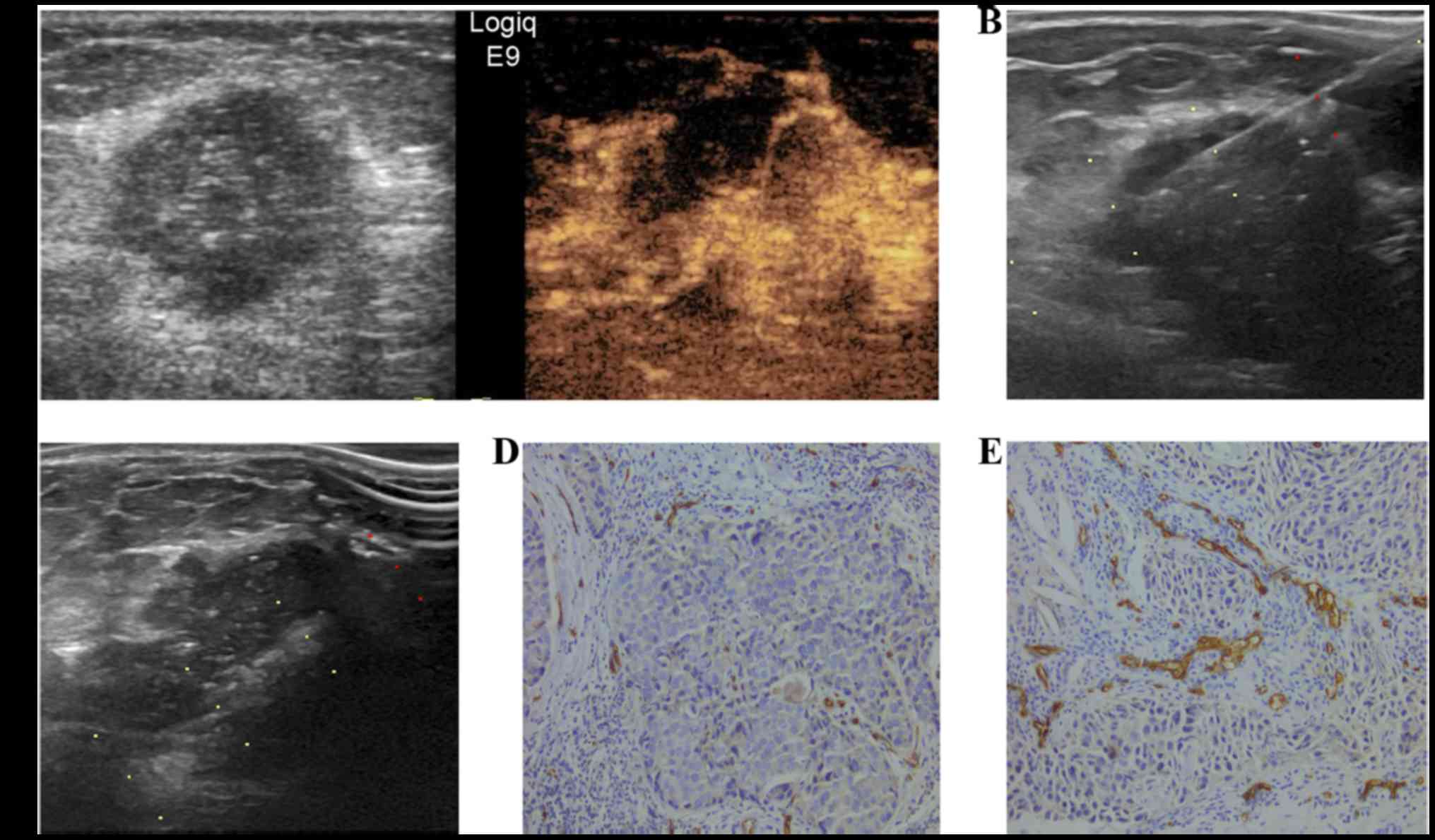
enhancement (Fig. 2) and 12 (12.1%)
exhibited regional enhancement (Fig.
3). Further analysis revealed differences in MVD distribution
between homogeneous enhancement, peripheral enhancement and
regional enhancement. For homogeneous enhancement and peripheral
enhancement, the respective medians of peripheral MVD were 69
(range, 12–157) and 66 (range, 14–330) and those of central MVD
were 59 (range, 11–155) and 53 (range, 8–223). It was observed that
the MVD periphery/center ratio in peripheral enhancement (median,
1.23; range, 1.00–4.50) was significantly higher compared with
homogeneous enhancement of the lesions (median, 1.04; range,
0.90–2.13; P<0.001). Furthermore, the enhancement
characteristics and MVD values of corresponding biopsy sites were
relatively similar in 12 regionally enhanced cases. These cases
were not statistically analyzed or discussed further since there
was no apparent association between enhancement region and
microvascular distribution. The associations between CEUS
enhancement patterns and MVD ratios are indicated in Table III.
 | Table III.Association between MVD ratio and
contrast-enhanced ultrasound patterns in breast cancer. |
Table III.
Association between MVD ratio and
contrast-enhanced ultrasound patterns in breast cancer.
|
|
| MVD value, median
(range) |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Enhancement
pattern | n | Periphery | Center | MVD ratio of
periphery to center, median (range) | Z | P-value |
|---|
| Homogeneous
enhancement | 44 | 69 (12–157) | 59 (11–155) | 1.04 (0.90–2.13) | −3.604 | <0.001 |
| Peripheral
enhancement | 43 | 66 (14–330) | 53 (8–223) | 1.23 (1.00–4.50) |
|
|
| Regional
enhancementa | 12 |
|
|
|
|
|
Associations between MVD ratio and perfusion
parameters of CEUS in breast cancer are presented in Table IV. PI and ascending slope ratios
correlated with the corresponding MVD ratios (P=0.017 and P=0.016,
respectively). However, no significant correlation was observed
between MVD ratio and the other parameters, including wash-in time,
time to peak and area under the time-intensity curve.
 | Table IV.Correlation between microvessel
density ratio and contrast-enhanced ultrasound perfusion parameters
in breast cancer (n=87 cases). |
Table IV.
Correlation between microvessel
density ratio and contrast-enhanced ultrasound perfusion parameters
in breast cancer (n=87 cases).
| Statistic | Peak intensity
ratio | Time to peak
ratio | Area under the
time-intensity curve ratio | Ascending slope
ratio | Wash-in time
ratio |
|---|
| r-value | 0.267 | −0.061 | 0.159 | 0.271 | −0.050 |
| P-value | 0.017 | 0.591 | 0.163 | 0.016 | 0.660 |
Association between MVD ratio and
histological characteristics of breast cancer
Associations between MVD ratio and histological
characteristics of breast cancer are presented in Table V. MVD ratio was positively correlated
with histological grade (P=0.003). However, no significant
associations were observed between MVD ratio and other prognostic
factors, including TNM stage, lymph node status, expression of ER
and PR, Ki67 proliferative index and HER2 overexpression.
 | Table V.Association between MVD ratio and
histological characteristics of breast cancer. |
Table V.
Association between MVD ratio and
histological characteristics of breast cancer.
|
| MVD value, median
(range) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Prognostic
factors | Peripheral | Central | MVD ratio periphery
to center, median (range) | R- or T-value | P-value |
|---|
| Histologic
grade |
|
|
| 0.364a | 0.003c |
| I | 57 (25–136) | 47 (24–63) | 1.05
(1.04–1.61) |
|
|
| II | 67 (45–88) | 59 (33–74) | 1.09
(1.02–1.29) |
|
|
|
III | 67(37–114) | 38 (15–96) | 1.72
(1.29–2.08) |
|
|
| TNM stage |
|
|
| 0.041a | 0.751c |
| T1 | 59 (37–79) | 55 (30–66) | 1.09
(1.03–1.32) |
|
|
| T2 | 70 (64–92) | 60 (36–75) | 1.07
(1.02–1.41) |
|
|
| T3 | 51 (41–61) | 35 (23–49) | 1.50
(1.24–1.75) |
|
|
| Lymph node
status |
|
|
| −0.339b | 0.735d |
|
Negative | 67 (53–88) | 58 (34–68) | 1.05
(1.02–1.35) |
|
|
|
Positive | 70 (42–95) | 56 (31–78) | 1.17
(1.03–1.48) |
|
|
| ER status |
|
|
| 0.052b | 0.958d |
|
Negative | 79 (59–117) | 61 (41–104) | 1.18
(1.03–1.70) |
|
|
|
Positive | 67 (42–82) | 53 (31–69) | 1.10
(1.02–1.33) |
|
|
| PR status |
|
|
| −0.119b | 0.906d |
|
Negative | 76 (57–118) | 60 (40–106) | 1.17
(1.03–1.68) |
|
|
|
Positive | 66 (42–82) | 51 (29–68) | 1.10
(1.02–1.33) |
|
|
| HER2 status |
|
|
| 1.725b | 0.088d |
|
Negative | 67 (48–94) | 53 (32–67) | 1.16
(1.03–1.62) |
|
|
|
Positive | 73 (43–92) | 65 (34–90) | 1.06
(1.02–1.31) |
|
|
| Ki67 proliferative
index |
|
|
| 1.308b | 0.195d |
|
Low | 67 (34–77) | 29 (21–57) | 1.13
(1.01–1.61) |
|
|
|
High | 67 (52–81) | 58 (38–68) | 1.10
(1.02–1.44) |
|
|
Discussion
Multiple factors correlate with different
enhancement presentations in images of breast cancer, among which
MVD and microvascular distribution play the most important roles in
the enhancement patterns of tumors (14). CEUS of breast is a non-invasive method
for evaluating the degree of tumor vascularization, which uses
blood pool contrast agents that cannot easily pass into
intercellular spaces. Thus, it is appropriate for the evaluation of
microcirculation perfusion (15). A
previous study demonstrated that CEUS patterns and parameters
correlate well with MVD and microvascular distribution in lesions,
which have focused on the CEUS characteristics of high- and low-MVD
lesions (5). In the present study,
different CEUS-enhanced regions of breast cancer were used for
biopsy specimens, aiming at a more accurate evaluation of the
distribution differences of microvascular regions in breast cancer
and the possible prognoses indicated by these differences.
Different CEUS enhancement patterns at different
regions of breast cancer are primarily attributed to blood
perfusion disparities. Pathological examinations reveal that blood
vessel-rich regions are located at the peritumoral areas of breast
cancer (14). The majority of
peripheries have tortuous and dilated large vessels, while
intratumoral vessels of breast cancer are often naive, narrow and
obstructed (16,17). Previous studies have demonstrated that
peripheral enhancement of magnetic resonance imaging in breast
cancer is associated with a higher MVD of the peripheral region as
compared with the central region (14,18).
However, to the best of our knowledge, no reports have focused on
the correlation between different CEUS enhancement patterns of
breast cancer and microvascular distribution. In the present study,
it was observed that MVD of the periphery was higher compared with
MVD of the center in breast cancer. A higher peripheral/central MVD
ratio was observed in peripherally enhanced lesions compared with
homogeneously enhanced lesions, indicating a higher extent of
vascularization in peripherally enhanced lesions. Furthermore, the
CEUS enhancement characteristics and MVD values of corresponding
puncturing sites were relatively similar in 12 regionally enhanced
cases. These cases were not statistically analyzed or discussed
further since there was no apparent association between enhancement
region and microvascular distribution.
Quantitative analysis with CEUS provides an
objective and reproducible method for the evaluation of the degree
of vascularization. The results of the present study reveal that
peripheral/central MVD ratio is associated with PI ratio and
ascending slope ratio. PI reflects the quantity of contrast agent
microbubbles in the vascular bed of the lesion; while ascending
slope reflects the early flow quantity and velocity during contrast
agent perfusion. The two parameters are associated with the degree
of vascularization.
Biological prognostic factors of tumors (including
histological grade, TNM stage, MVD, vascular endothelial growth
factor, Ki-67, HER2, ER and PR) reflect the biological behaviors
and prognosis of breast cancer to some extent (19). Furthermore, MVD is regarded as an
independent prognostic factor of breast cancer and is correlated
with histological grade and proliferative activity to a certain
degree (18,20,21). The
current results indicated a significant correlation between the
peripheral/central MVD ratio and histological grade of breast
cancer (P=0.003), consistent with the findings of Fridman et
al (21). A higher
peripheral/central MVD ratio was observed in peripherally enhanced
lesions compared with homogeneously enhanced lesions, which may be
associated with poor prognosis.
While advantages of CEUS application in breast
cancer have been demonstrated, limitations also exist in the
present study. First, only a specific section from a lesion site
can be selected by CEUS for the evaluation of enhancement patterns
and parameters, and it is impossible to wholly observe the lesion.
Thus, the histopathological characteristics obtained from
core-needle biopsy cannot represent the status of the whole tumor.
Second, certain subjective and procedure errors are unavoidable. In
conclusion, the enhancement patterns and parameters of CEUS may not
only reflect the microvessel distribution but also indirectly
indicate the histological grade of breast cancer. As a non-invasive
examination technique, CEUS of breast cancer can objectively
reflect the basic pathological characteristics of blood supply to
the breast tumor, and is helpful in evaluating the biological
behaviors and prognosis of breast tumor.
Acknowledgements
This study was supported by the Program of Science
and Technology Project of Anticancer, Tianjin, China (grant no.
12ZCDZSY16000) and the Medical University of Tianjin Cancer
Hospital Doctor Startup Foundation (grant no. 1314) awarded to
Yaqing Li.
References
|
1
|
Fan F, Schimming A, Jaeger D and Podar K:
Targeting the tumor microenvironment: Focus on angiogenesis. J
Oncol. 2012:2812612012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hida K, Kawamoto T, Ohga N, Akiyama K,
Hida Y and Shindoh M: Altered angiogenesis in the tumor
microenvironment. Pathol Int. 61:630–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao H, Xu R, Ouyang Q, Chen L, Dong B and
Huihua Y: Contrast-enhanced ultrasound is helpful in the
differentiation of malignant and benign breast lesions. Eur J
Radiol. 73:288–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vallone P, D'Angelo R, Filice S, Petrosino
T, Rinaldo M, De Chiara A and Gallipoli A: Color-doppler using
contrast medium in evaluating the response to neoadjuvant treatment
in patients with locally advanced breast carcinoma. Anticancer Res.
25:595–599. 2005.PubMed/NCBI
|
|
5
|
Du J, Li FH, Fang H, Xia JG and Zhu CX:
Correlation of real-time gray scale contrast-enhanced
ultrasonography with microvessel density and vascular endothelial
growth factor expression for assessment of angiogenesis in breast
lesions. J Ultrasound Med. 27:821–831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wan CF, Du J, Fang H, Li FH, Zhu JS and
Liu Q: Enhancement patterns and parameters of breast cancers at
contrast-enhanced US: Correlation with prognostic factors.
Radiology. 262:450–459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
American College of Radiology (ACR), .
Breast Imaging Reporting and Data System Atlas (BI-RADS Athas).
4th. ACR; Reston, VA: 2013
|
|
8
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO Classification of Tumours of the Breast.
4. 4th. IARC Press; 2012
|
|
9
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 41:154–151. 2002.PubMed/NCBI
|
|
10
|
AJCC Cancer Staging Handbook: From the
AJCC Cancer Staging Manual. Edge S, Byrd DR, Compton CC, Fritz AG,
Greene F and Trotti A: 7th. Springer-Verlag; NY: 2010
|
|
11
|
Liu F, Lang R, Zhao J, Zhang X, Pringle
GA, Fan Y, Yin D, Gu F, Yao Z and Fu L: CD8+ cytotoxic T
cell and FOXP3+ regulatory T cell infiltration in relation to
breast cancer survival and molecular subtypes. Breast Cancer Res
Treat. 130:645–655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer (unabridged version). Arch Pathol Lab Med.
134:e48–e72. 2010.PubMed/NCBI
|
|
13
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buadu LD, Murakami J, Murayama S,
Hashiguchi N, Sakai S, Masuda K, Toyoshima S, Kuroki S and Ohno S:
Breast lesions: Correlation of contrast medium enhancement patterns
on MR images with histopathologic findings and tumor angiogenesis.
Radiology. 200:639–649. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Jiang YX, Liu JB, Zhu QL and Sun Q:
Evaluation of breast lesions with contrast-enhanced ultrasound
using the microvascular imaging technique: Initial observations.
Breast. 17:532–539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagy JA, Chang SH, Shih SC, Dvorak AM and
Dvorak HF: Heterogeneity of the tumor vasculature. Semin Thromb
Hemost. 36:321–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jackson A, O'Connor JP, Parker GJ and
Jayson GC: Imaging tumor vascular heterogeneity and angiogenesis
using dynamic contrast-enhanced magnetic resonance imaging. Clin
Cancer Res. 13:3449–3459. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teifke A, Behr O, Schmidt M, Victor A,
Vomweg TW, Thelen M and Lehr HA: Dynamic MR imaging of breast
lesions: Correlation with microvessel distribution pattern and
histologic characteristics of prognosis. Radiology. 239:351–360.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kushlinskii NE, Orinovskii MB, Gurevich
LE, Kazantseva IA, Talaeva ShZh, Shirokii VP, Ermilova VD, Dvorova
EK and Ozherelev AS: Expression of biomolecular markers (Ki-67,
PCNA, Bcl-2, BAX, BclX, VEGF) in breast tumors. Bull Exp Biol Med.
137:182–185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weidner N, Folkman J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
A new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fridman V, Humblet C, Bonjean K and
Boniver J: Assessment of tumor angiogenesis in invasive breast
carcinomas: Absence of correlation with prognosis and pathological
factors. Virchows Arch. 437:611–617. 2000. View Article : Google Scholar : PubMed/NCBI
|