Introduction
Gastric cancer is a well-known malignant tumor,
which is harmful to human health. The incidence of gastric cancer
increases significantly with age, and the peak age of onset is
50–80 years, although it is showing a younger tendency every year
(1). The proportion of 19- to
35-year-old patients with gastric cancer has increased from 1.7 to
3.3% in the past 40 years (1).
Although surgical resection and adjuvant chemotherapy and
radiotherapy are primarily employed in the treatment of the
disease, most patients with gastric cancer succumb to tumor
recurrence and metastasis (2–4), and the five-year survival rate is
<24% (5). The occurrence of
gastric cancer is influenced by many factors (6,7). The
specific molecular mechanism of the occurrence of gastric cancer
remains to be determined. Current use of traditional cytotoxic
chemotherapy agents for gastric cancer is limited (8). At present, many studies involving
molecular pathways aim to identify new targets to treat gastric
cancer. The development of molecular diagnostic science has further
supported the finding of new molecular targets.
Transmembrane protein 119 (TMEM119) belongs to the
transmembrane proteins (TMEMs) family. Differential regulation of
TMEMs is observed in many types of cancer. TMEM176A and TMEM176B
were significantly elevated in lymphoma and associated with certain
cancer pathology (9). TMEM72 and
TMEM116 were downregulated in metastatic clear cell renal cell
carcinoma (ccRCC) tissue, and TMEM30B and TMEM45B were
downregulated in advanced-stage samples of ccRCC, suggesting that
TMEM could be utilized as a potential predictor for metastases and
cancer progression (10). TMEM45B was
overexpressed in lung cancer and the knockdown of TMEM45B
suppressed lung cancer cell invasion, migration and proliferation
and caused cell cycle arrest and cell apoptosis (11). TMEM106A is often methylated in human
gastric cancer and TMEM106A upregulation suppressed cell growth and
induced apoptosis in gastric cancer cell lines (12). TMEM119 is important in bone formation
and normal bone mineralization. Recently, TMEM119 induced the
transshipment and differentiation of muscle cells into osteoblasts
by enhancing the interaction of BMP2 and the bmp-runx2 pathway
(13). TMEM119 was increased in
osteosarcoma, connected with tumor size, clinical stage and overall
survival time, and associated with cell cycle, metastasis,
apoptosis as well as TGF-β signaling in osteosarcoma cell lines
(14). It was found that human
microglia, along with neuroblastoma cells and Alzheimer's disease,
expressed high levels of TMEM119 mRNA (15). However, the functions of TMEM119 in
gastric cancer remain to be investigated.
In the present study, the impact of TMEM119 on cell
viability and apoptosis of SGC-7901 and AGS cells, gastric
carcinoma MKN45 cells, as well as gastric epithelial cell lines
GES-1 was investigated. It was found that there was more TMEM119 in
gastric cancer tissues than in normal tissues and knockdown of
TMEM119 significantly curbed viability and induced cell apoptosis
of SGC-7901, AGS cells and MKN45 cells. In addition, TMEM119
downregulation decreased the expression of Bcl-2 but increased the
expression of Bax and caspase-3. Our results demonstrate a
significant role of TMEM119 in the regulation of SGC-7901 cell
viability and apoptosis.
Materials and methods
Analysis of bioinformatics
Data regarding gene expression were obtained from
The Cancer Genome Atlas website (TCGA; http://cancergenome.nih.gov/).
Patient specimens
Tumor and general gastric specimens were obtained
from 90 gastric cancer patients who experienced surgery at the
Zhejiang Hospital (Hangzhou, China) from June, 2010 to April, 2015.
The research program was approved by the Zhejiang Hospital Ethics
Committee. All the participants in this study provided informed
consent.
Cell culture
Gastric adenocarcinoma SGC-7901 and AGS cells,
gastric carcinoma MKN45 cells, as well as gastric epithelial cell
lines GES-1 (The Cell Bank of Type Culture Collection of Chinese
Academy of Sciences, Shanghai, China) were used in the present
study. The cell lines were cultured in DMEM medium followed by 10%
(v/v) fetal bovine serum (Life Technologies; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C and 5% of the
CO2 in an incubator.
RNA interference
TMEM119 mRNA (5′-ugggauaguggacuuc uuc-3′) and
non-specific interference siRNA sequences (control siRNA;
5′-uucuccgaacgucacgu-3′) siRNA were synthesized and transfected
with Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) as per the manufacturer's protocol. Assays were performed 48
h after transfection. The TMEM119 mRNA expression in 249 gastric
cancer tissues and 33 normal gastric tissues from the TCGA dataset
was first examined.
Cell Counting kit-8 (CCK-8) assay
Cell viability was determined using a Cell Counting
kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan),
where the substrate was
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,
4-disulfophenyl)-2H-tetrazolium monosodium salt (WST-8). After the
cells were incubated for 12, 24, 48 and 72 h, CCK-8 solution (10
µl) was placed into each well of the plate. The plates were
incubated at 37°C, 5% CO2, for 4 h.
Cell apoptosis assay
In accordance with the manufacturer's protocol,
SGC-7901 cell apoptosis was analyzed using the Annexin
V-FITC/iodide (PI) cell apoptosis kit (BD Biosciences, Franklin
Lakes, NJ, USA). The cells were washed with phosphate buffered
salline (PBS) three times, followed by trypsin digestion,
centrifugation at 400 × g at room temperature for 10 min, and were
adjusted to 5×104/ml, after which they were suspended in
binding buffer containing Annexin V-FITC and PI. The fluorescence
intensity was measured via flow cytometry (BD Biosciences) after 10
min of incubation in the dark.
Reverse transcription and quantitative
PCR
Using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) RNA was extracted from patient tissue samples and
cell lines, and were treated with DNase I (Roche Diagnostics,
Indianapolis, IN, USA) to eliminate residual DNA as per the
manufacturer's protocol. The Prime Script PTMP RT reagent kit
(Perfect Real Time; Takara Biotechnology Co., Ltd., Dalian, China)
was used to generate cDNA, and reverse transcribed with 1 µg total
RNA, followed by SYBR-Green qPCR Master (Thermo Fisher Scientific,
Inc.) to determine quantitative PCR in accordance with the
manufacturer's protocol. Primers used to amplify the coding region
were: 5′-CTGGCCTTTCTGCTGATGTTC-3′ (forward) and
5′-TCACTCTGGTCCACGTACTTC-3′ (reverse), for TMEM119, and
5′-CACCCACTCCTCCACCTTTG-3′ (forward) and 5′-CCACCACCCTGTTGCTGTAG-3′
(reverse) for GAPDH. GAPDH mRNA levels were employed for
normalization while the 2−ΔΔCq method was employed to
calculate the expression changes.
Western blot analysis
To determine the protein expression level, complete
cell extracts were prepared in the lysis buffer. After culture, the
medium was removed, SGC-7901 cells were obtained and washed with
PBS, and were immediately lysed in a buffer containing 20 mM
Tris-HCl. The total protein was quantitatively determined using BCA
protein. Loading buffer was added into the cytoplasm extract and
boiled for approximately 5 min. The gel was then separated from
each sample of the same amount by 10% SDS-PAGE, and transferred to
the nylon membrane. Following incubation for 1.5 h with a 5%
degreasing emulsion in the fresh block buffer, rabbit anti-human
Bax, Bcl-2, caspase-3 and GAPDH polyclonal antibodies (1:500; cat.
nos. PA5-11378, PA5-20068, PA5-16332, and PA1-987-HRP; Thermo
Fisher Scientific, Inc.) and rabbit anti-human TMEM119 polyclonal
antibody (1:500; cat. no. ab185333; Abcam, Cambridge, MA, USA) were
incubated overnight at 4°C in freshly prepared TBST containing 5%
skim milk. The membrane was cleaned 3 times with the TBST and then
incubated with the secondary antibody at room temperature for 2 h.
Specific proteins were tested with enhanced chemiluminescence after
goat anti-rabbit polyclonal secondary antibody (1:1,000; cat. no.
31460; Thermo Fisher Scientific, Inc.) binding.
Immunohistochemistry
Tissue sections (5 µm) were cut and mounted on
slides. After dewaxing and rehydration, the sections were
antigen-retrieved in 10 mm citrate buffer for 5 min at 100°C.
Endogenous peroxidase activity and non-specific antigens were
blocked with 3% hydrogen peroxide and serum, followed by incubation
with rabbit anti-human TMEM119 polyclonal antibody (1:500; cat. no.
HPA051870; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
over-night at 4°C. The slides were then incubated with goat
anti-rabbit secondary polyclonal antibody (1:2,000; cat. no. A0545;
Sigma-Aldrich; Merck KGaA), developed using 3,3′-diaminobenzidine
solution and counterstained with hematoxylin.
Statistical analysis
Statistical analysis was performed using the ANOVA
and post-hoc test was SNK test. P<0.05 was considered to
indicate a statistically significant analysis. All statistical
analyses were carried out with the GraphPad Prism software
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
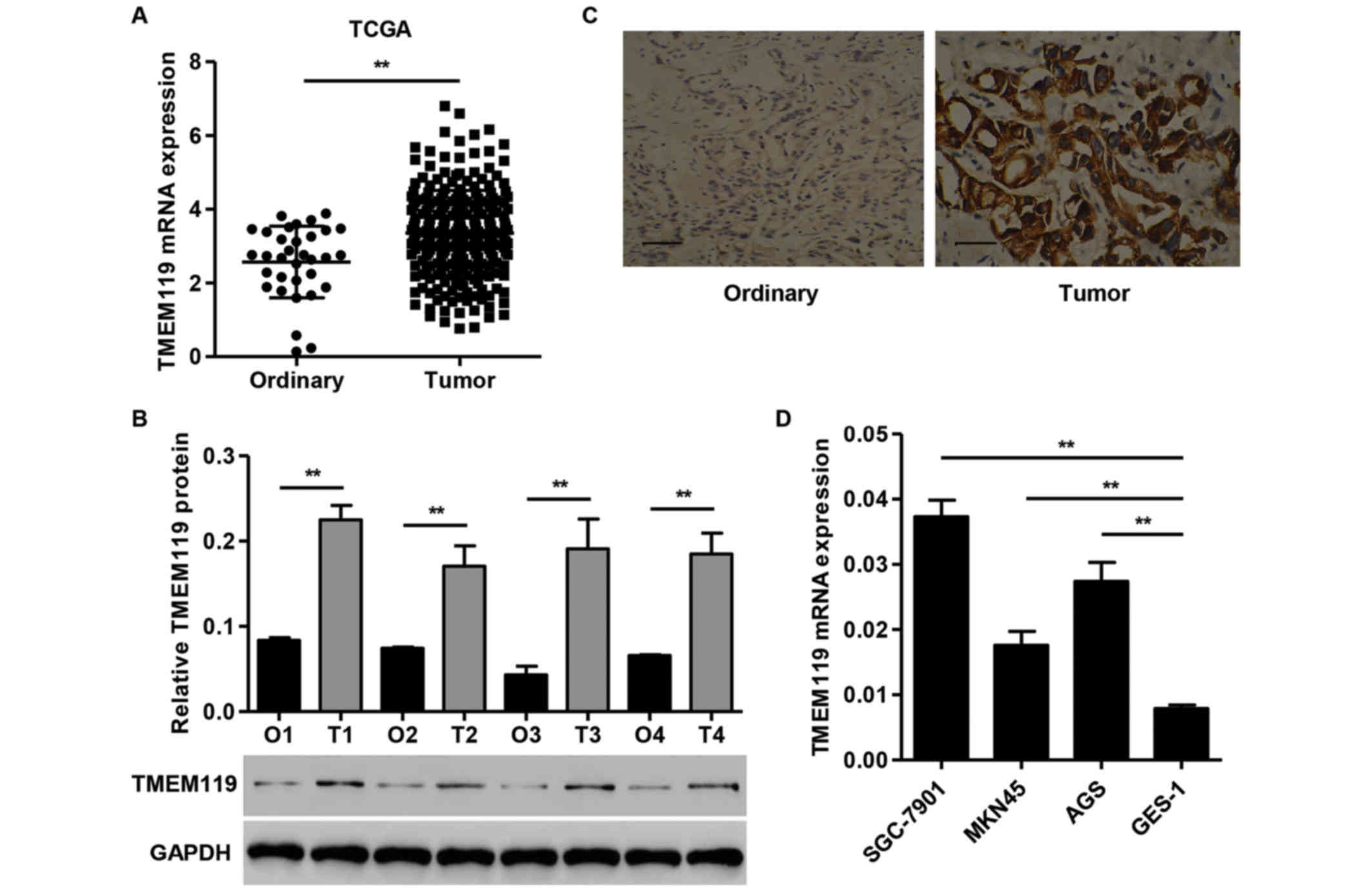
TMEM119 is upregulated in gastric
cancer tissues and cell lines
The TMEM119 mRNA expression in 249 gastric cancer
tissues and 33 normal gastric tissues from the TCGA dataset was
first examined (Fig. 1A). TMEM119
showed a significantly higher expression in gastric cancer tissues
than in ordinary tissues. TMEM119 protein levels in gastric cancer
tissues and normal gastric tissues obtained from the Zhejiang
Hospital were evaluated using western blot analysis. TMEM119
protein expression was increased in all four gastric cancer tissues
compared with the normal gastric tissues (Fig. 1B). Immunohistochemical analysis
revealed that the expression of TMEM119 was significantly
upregulated in gastric cancer tissues compared with the
corresponding normal gastric tissues (Fig. 1C). Quantitative PCR analysis showed a
higher expression of TMEM119 in all the gastric cancer cell lines
compared to GES-1 cells, with the highest expression detected in
SGC-7901 cells. The SGC-7901 cells were therefore used for
subsequent experiments (Fig. 1D).
These data further indicate that TMEM119 was significantly
upregulated in gastric cancer, and that TMEM119 may facilitate
gastric cancer carcinogenesis.
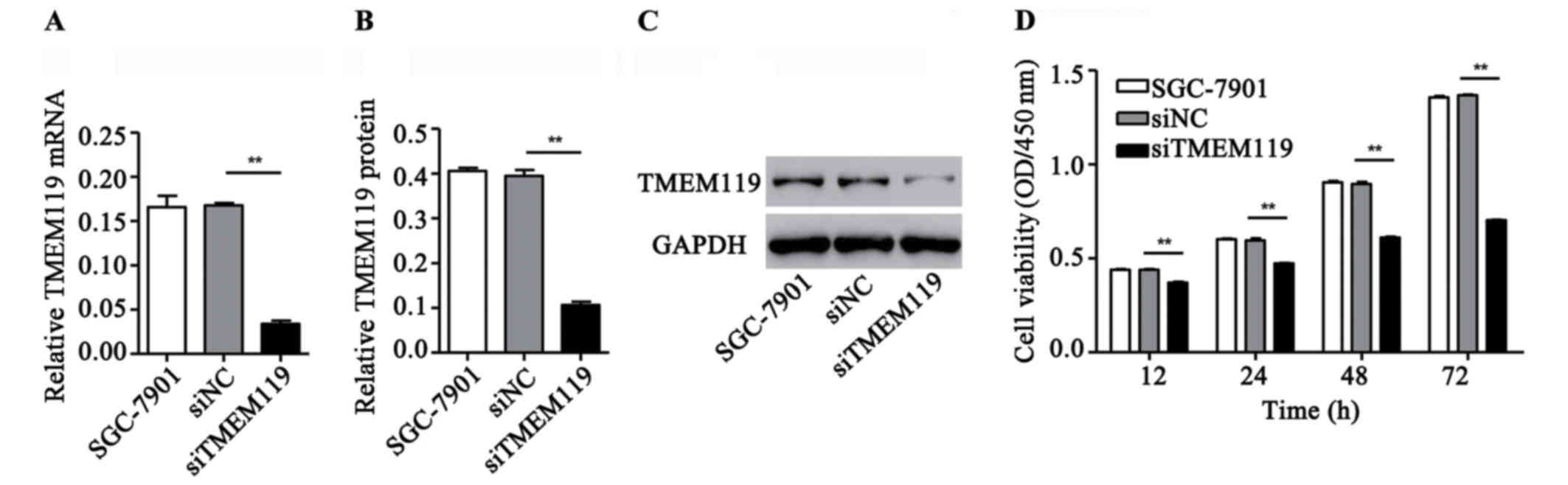
TMEM119 silencing inhibits SGC-7901
cell viability
To validate the role of TMEM119 in gastric cancer
in vitro, a special siRNA targeting TMEM119 and a scramble
siRNA (control siRNA) were transfected into the gastric cancer cell
lines, SGC-7901. According to Fig.
2A-C, siTMEM119 transfection significantly decreased the mRNA
and protein expression of TMEM119 by 79.8 and 73.1% in SGC-7901,
respectively. Moreover, the cell viability after siTMEM119
transfection was also measured by CCK-8 assay. Our results showed
that siTMEM119 transfection significantly inhibited SGC-7901 cell
viability by 15.8, 20.6, 31.9 and 48.6% at 12, 24, 48 and 72 h,
respectively (Fig. 2D).
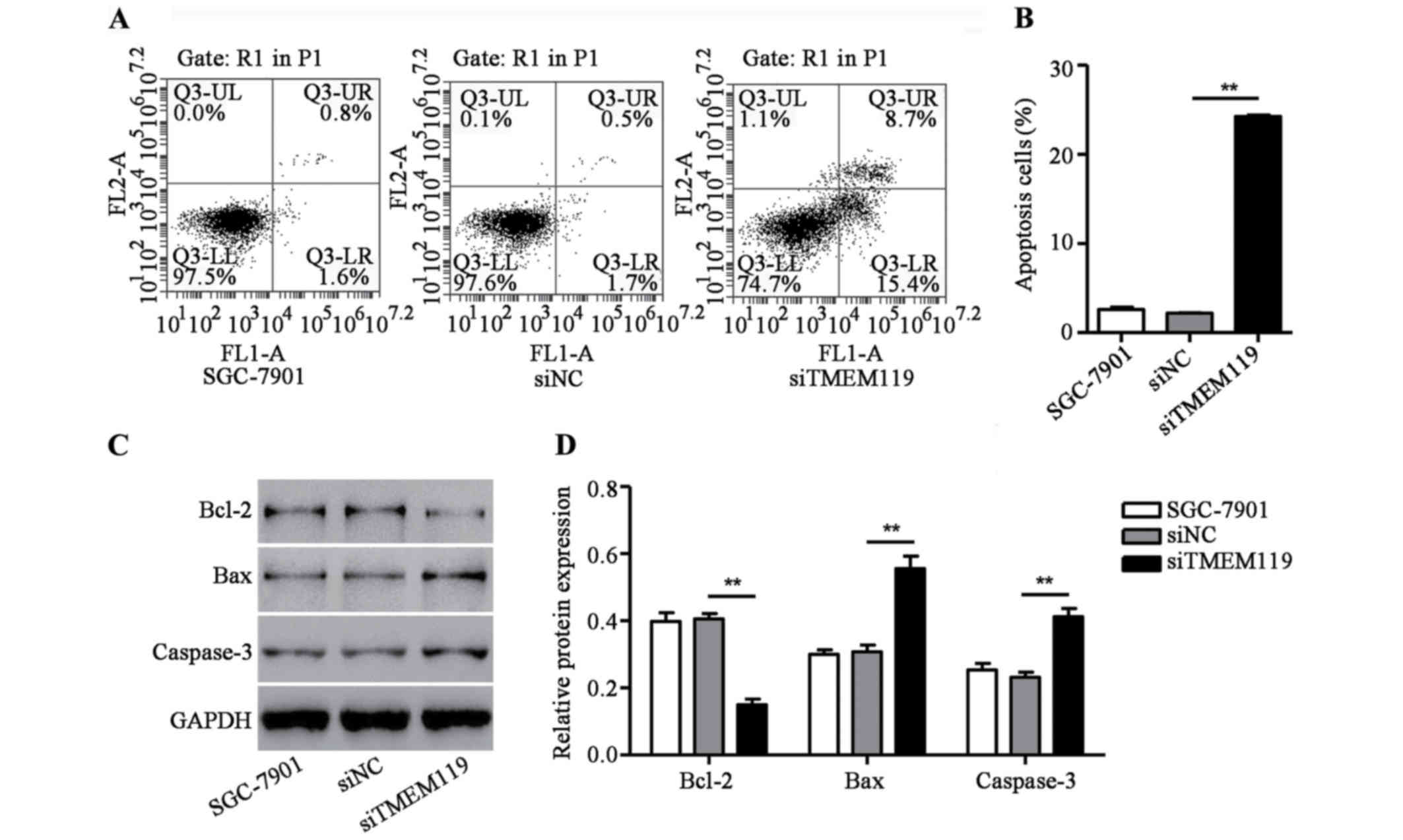
TMEM119 silencing induces SGC-7901
cell apoptosis
To determine the function of TMEM119 in the cell
apoptosis of SGC-7901, a flow cytometry assay was performed. As
shown in Fig. 3A and B, siTMEM119
transfection significantly increased apoptotic cells of SGC-7901 by
10.2-fold. Additionally, some proteins associated with apoptosis,
including Bax, Bcl-2 and caspase-3, were also measured by western
blot analysis. We found that siTMEM119 transfection greatly
decreased Bcl-2 expression but increased the expression of Bax and
caspase-3 in SGC-7901 cells (Fig. 3C and
D). According to the results, TMEM119 silencing induces
SGC-7901 cell apoptosis through an increase of Bax/Bcl-2 ratio and
caspase-3 expression.
Discussion
TMEM proteins have been recently extensively studied
in different types of malignant tumors (9–11) and
other members of TMEM proteins have been found to be overexpressed
in gastric cancer. TMEM106A is often methylated in human gastric
cancer and TMEM106A upregulation suppressed cell growth and induced
apoptosis in gastric cancer cell lines (12). TMEM16A overexpression contributes to
tumor invasion and poor prognosis of human gastric cancer (16). However, knowledge concerning the
aberrant expression and potential role of TMEM119 in gastric cancer
is lacking. In the present study, we demonstrated that TMEM119 was
overexpressed in gastric cancer tissues by analyzing independent
dataset downloaded from the TCGA website, and our western blot
analysis results on 90 pairs of gastric cancer as well as normal
tissues. TMEM119 silencing inhibited cell viability and caused cell
apoptosis of gastric cancer cells.
Previous findings revealed that TMEM119 was
overexpressed in osteosarcoma tissues and the knockdown of TMEM119
suppressed the migratory and invasive abilities of osteosarcoma
cells and osteosarcoma cell growth in vitro and in
vivo, causing G0/G1-phase arrest and apoptosis (14). In line with the previous study, it was
identified that TMEM119 was significantly upregulated in gastric
cancer tissues compared to ordinary tissues from the TCGA database
and our hospital samples. TMEM119 silencing significantly prevented
SGC-7901 cell viability as well as induced cell apoptosis. There
are several pathways to induce cancer cell apoptosis, such as
regulating apoptosis-related genes, and changing intracellular
Ca2+ concentration in endoplasmic reticulum and
mitochondrial pathway (17,18). Apoptosis is a complex signaling
pathway in which there are a number of genes responsible for pro-
and anti-apoptosis, including the Bax, Fas, p38, p53 and caspase
family and the Bcl-2 family (19,20).
Numerous studies have shown that Bcl-2 plays a key role in the
complex cell death signaling pathways (21,22). Bcl-2
family proteins contain anti- as well as pro-apoptotic genes.
Bcl-2 belongs to anti-apoptotic genes, while Bax
belongs to pro-apoptotic genes, and a ratio between them determines
cell apoptosis or not. The anti-apoptotic role of Bcl-2 is very
significant in cell life and cell response to adverse stimulation
(23). In the present study, our
results revealed that TMEM119 silencing significantly caused
apoptosis of SGC-7901 cells and an increase of the Bax/Bcl-2 ratio
as well as caspase-3 expression.
In conclusion, we have demonstrated that TMEM119 was
increased in gastric cancer tissues and is essential in regulating
cell viability and apoptosis in vitro. In addition, the
molecular mechanism involved may be the increase in the Bax/Bcl-2
ratio as well as caspase-3 expression, indicating novel targets for
therapeutic interventions to prevent gastric cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science
Foundation of Zhejiang Province (Y17H030031) and the Medical and
Health Science and Technology Plan of Zhejiang Province
(2016ZDA001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ and WW wrote the the manuscript and made
contributions to cell culture. MJ and QZ performed and analysed
quantitative PCR. YF helped with western blot analysis. FZ
contributed in immunohistochemistry. QH conducted cell apoptosis
assay. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The research program was approved by the Zhejiang
Hospital Ethics Committee (Hangzhou, China). All the participants
in this study provided informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei J, Wu ND and Liu BR: Regional but
fatal: Intraperitoneal metastasis in gastric cancer. World J
Gastroenterol. 22:7478–7485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeon MY, Park JC, Hahn KY, Shin SK, Lee SK
and Lee YC: Long-term outcomes after noncurative endoscopic
resection of early gastric cancer: The optimal time for additional
endoscopic treatment. Gastrointest Endosc. Oct 12–2017.(Epub ahead
of print).
|
|
4
|
Peixoto A, Santos-Antunes J, Silva M and
Macedo G: Duodenal stump recurrence of gastric adenocarcinoma after
subtotal gastrectomy. Rev Esp Enferm Dig. 108:739–740.
2016.PubMed/NCBI
|
|
5
|
Yasumoto K, Koizumi K, Kawashima A, Saitoh
Y, Arita Y, Shinohara K, Minami T, Nakayama T, Sakurai H, Takahashi
Y, et al: Role of the CXCL12/CXCR4 axis in peritoneal
carcinomatosis of gastric cancer. Cancer Res. 66:2181–2187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10:49–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Charalampakis N, Elimova E, Shimodaira Y,
Shiozaki H, Wadhwa R and Ajani JA: Biologics in combination with
chemotherapy for gastric cancer: Is this the answer? Expert Opin
Pharmacother. 16:955–960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cuajungco MP, Podevin W, Valluri VK, Bui
Q, Nguyen VH and Taylor K: Abnormal accumulation of human
transmembrane (TMEM)-176A and 176B proteins is associated with
cancer pathology. Acta Histochem. 114:705–712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wrzesiński T, Szelag M, Cieślikowski WA,
Ida A, Giles R, Zodro E, Szumska J, Poźniak J, Kwias Z, Bluyssen
HA, et al: Expression of pre-selected TMEMs with predicted ER
localization as potential classifiers of ccRCC tumors. BMC Cancer.
15:5182015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu R, Hu F, Xie X, Wang L, Li G, Qiao T,
Wang M and Xiao H: TMEM45B, up-regulated in human lung cancer,
enhances tumorigenicity of lung cancer cells. Tumour Biol.
37:12181–12191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu D, Qu L, Hu J, Li G, Lv P, Ma D, Guo M
and Chen Y: Transmembrane protein 106A is silenced by promoter
region hypermethylation and suppresses gastric cancer growth by
inducing apoptosis. J Cell Mol Med. 18:1655–1666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hisa I, Inoue Y, Hendy GN, Canaff L,
Kitazawa R, Kitazawa S, Komori T, Sugimoto T, Seino S and Kaji H:
Parathyroid hormone-responsive Smad3-related factor, Tmem119,
promotes osteoblast differentiation and interacts with the bone
morphogenetic protein-Runx2 pathway. J Biol Chem. 286:9787–9796.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang ZH, Peng J, Yang HL, Fu XL, Wang JZ,
Liu L, Jiang JN, Tan YF and Ge ZJ: Upregulation and biological
function of transmembrane protein 119 in osteosarcoma. Exp Mol Med.
49:3292017. View Article : Google Scholar
|
|
15
|
Satoh J, Kino Y, Asahina N, Takitani M,
Miyoshi J, Ishida T and Saito Y: TMEM119 marks a subset of
microglia in the human brain. Neuropathology. 36:39–49. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu F, Cao QH, Lu DJ, Luo B, Lu XF, Luo RC
and Wang XG: TMEM16A overexpression contributes to tumor invasion
and poor prognosis of human gastric cancer through TGF-β signaling.
Oncotarget. 6:11585–11599. 2015.PubMed/NCBI
|
|
17
|
Kim KY, Cho HJ, Yu SN, Kim SH, Yu HS, Park
YM, Mirkheshti N, Kim SY, Song CS, Chatterjee B, et al: Interplay
of reactive oxygen species, intracellular Ca2+ and mitochondrial
homeostasis in the apoptosis of prostate cancer cells by
deoxypodophyllotoxin. J Cell Biochem. 114:1124–1134. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharifi S, Barar J, Hejazi MS and Samadi
N: Doxorubicin Changes Bax /Bcl-xL Ratio, Caspase-8 and 9 in breast
cancer cells. Adv Pharm Bull. 5:351–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song Y, Li X, Li Y, Li N, Shi X, Ding H,
Zhang Y, Li X, Liu G and Wang Z: Non-esterified fatty acids
activate the ROS-p38-p53/Nrf2 signaling pathway to induce bovine
hepatocyte apoptosis in vitro. Apoptosis. 19:984–997. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Gao J, Qian L, Wang X, Wu M, Zhang
Y, Ye H, Zhu S, Yu Y and Han W: Activation of p38-MAPK by
CXCL4/CXCR3 axis contributes to p53-dependent intestinal apoptosis
initiated by 5-fluorouracil. Cancer Biol Ther. 15:982–991. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akl H, Vervloessem T, Kiviluoto S,
Bittremieux M, Parys JB, De Smedt H and Bultynck G: A dual role for
the anti-apoptotic Bcl-2 protein in cancer: Mitochondria versus
endoplasmic reticulum. Biochim Biophys Acta. 1843:2240–2252. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|