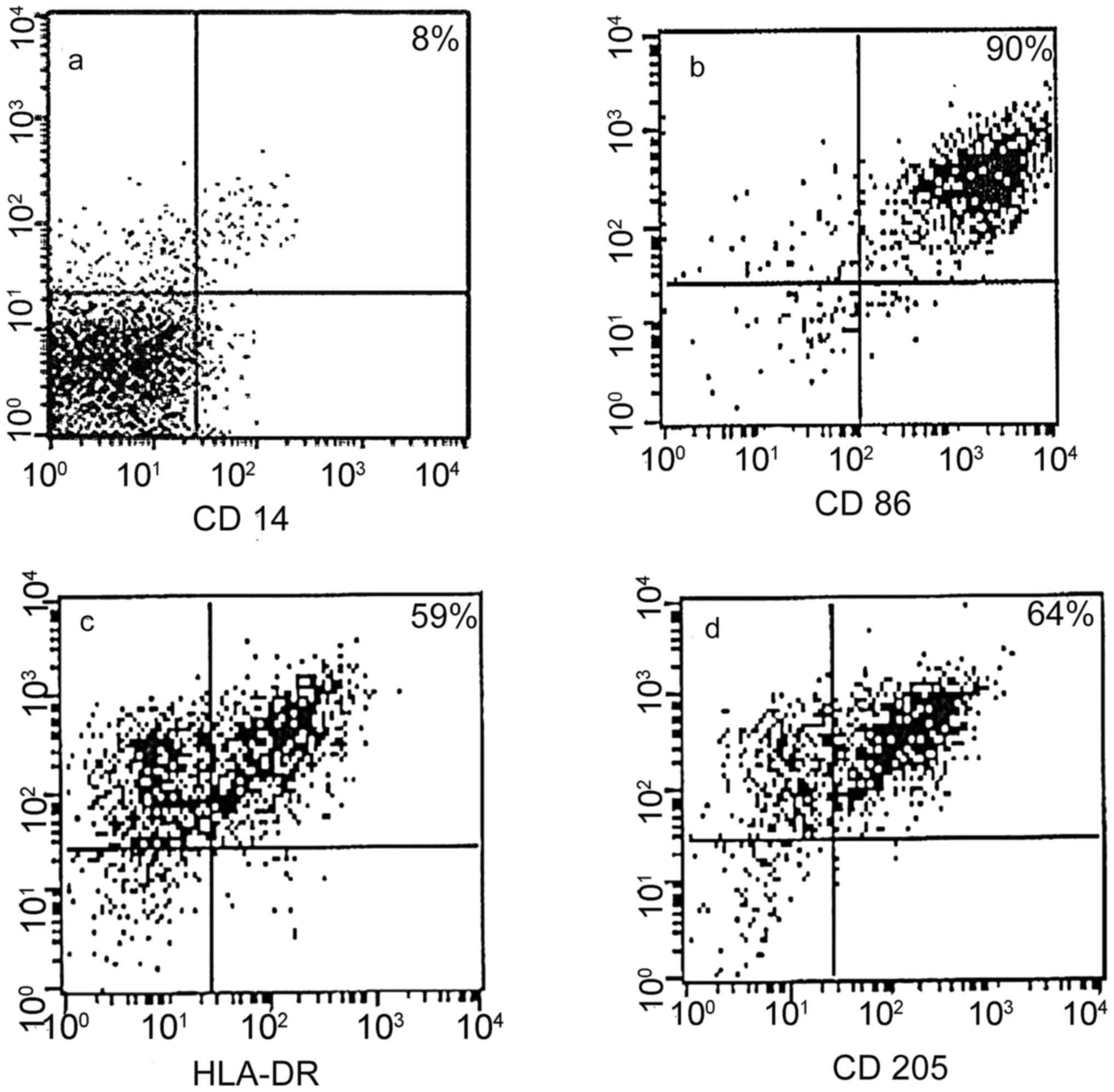
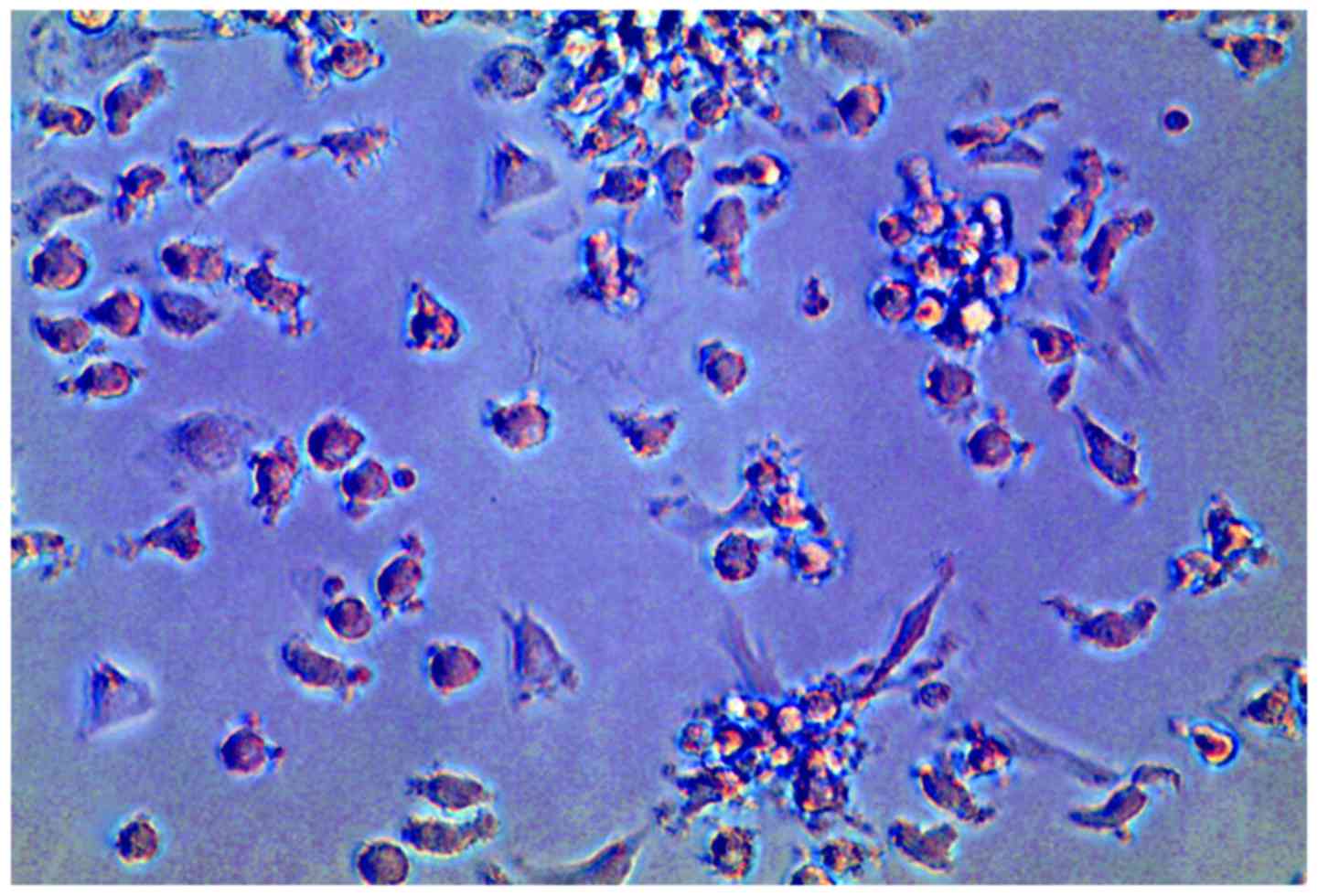
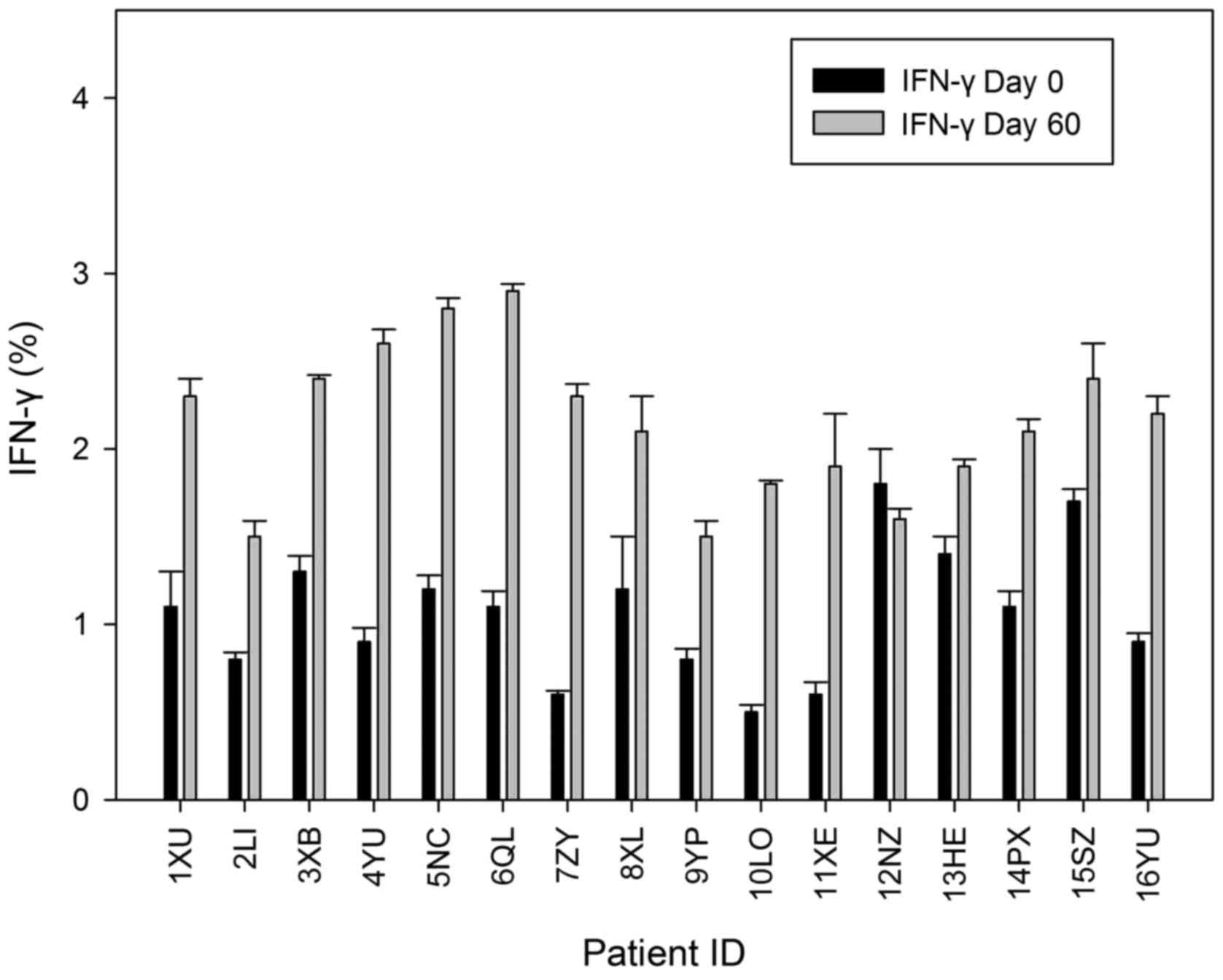
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clinic Proc. 83:584–594.
2008. View Article : Google Scholar
|
|
2
|
Reck M: What future opportunities may
immuno-oncology provide for improving the treatment of patients
with lung cancer? Ann Oncol. 23 suppl 8:viii28–viii34. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Romani N, Reider D, Heuer M, Ebner S,
Kämpgen E, Eibl B, Niederwieser D and Schuler G: Generation of
mature dendritic cells from human blood. An improved method with
special regard to clinical applicability. J Immunol Methods.
196:137–151. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sallusto F and Lanzavecchia A: Efficient
presentation of soluble antigen by cultured human dendritic cells
is maintained by granulocyte/macrophage colony-stimulating factor
plus interleukin 4 and downregulated by tumor necrosis factor
alpha. J Exp Med. 179:1109–1118. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He L, Feng H, Raymond A, Kreeger M, Zeng
Y, Graner M, Whitesell L and Katsanis E: Dendritic-cell-peptide
immunization provides immunoprotection against bcr-abl-positive
leukemia in mice. Cancer Immunol Immunother. 50:31–40. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mayordomo JI, Zorina T, Storkus WJ,
Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM,
Deleo AB, et al: Bone marrow-derived dendritic cells pulsed with
synthetic tumour peptides elicit protective and therapeutic
antitumour immunity. Nat Med. 1:1297–1302. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schuler G and Steinman RM: Dendritic cells
as adjuvants for immune-mediated resistance to tumors. J Exp Med.
186:1183–1187. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fong L, Hou Y, Rivas A, Benike C, Yuen A,
Fisher GA, Davis MM and Engleman EG: Altered peptide ligand
vaccination with Flt3 ligand expanded dendritic cells for tumor
immunotherapy. Proc Natl Acad Sci USA. 98:8809–8814. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nair SK, Hull S, Coleman D, Gilboa E,
Lyerly HK and Morse MA: Induction of carcinoembryonic antigen
(CEA)-specific cytotoxic T-lymphocyte responses in vitro using
autologous dendritic cells loaded with CEA peptide or CEA RNA in
patients with metastatic malignancies expressing CEA. Int J Cancer.
82:121–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falleni M, Pellegrini C, Marchetti A,
Oprandi B, Buttitta F, Barassi F, Santambrogio L, Coggi G and
Bosari S: Survivin gene expression in early-stage non-small cell
lung cancer. J Pathol. 200:620–626. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weiser TS, Ohnmacht GA, Guo ZS, Fischette
MR, Chen GA, Hong JA, Nguyen DM and Schrump DS: Induction of MAGE-3
expression in lung and esophageal cancer cells. Ann Thorac Surg.
71:295–302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
US Department of Health and Human
Services, National Institutes of Health, National Cancer Institute:
Common terminology criteria for adverse events. version, 4.0.
https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfOctober.
2014
|
|
14
|
Hirschowitz EA, Foody T, Kryscio R,
Dickson L, Sturgill J and Yannelli J: Autologous dendritic cell
vaccines for non-small-cell lung cancer. J Clin Oncol.
22:2808–2815. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim D, Jeon C, Kim JH, Kim MS, Yoon CH,
Choi IS, Kim SH and Bae YS: Cytoplasmic transduction peptide (CTP):
New approach for the delivery of biomolecules into cytoplasm in
vitro and in vivo. Exp Cell Res. 312:1277–1288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kern F, Surel IP, Brock C, Freistedt B,
Radtke H, Scheffold A, Blasczyk R, Reinke P, Schneider-Mergener J,
Radbruch A, et al: T-cell epitope mapping by flow cytometry. Nat
Med. 4:975–978. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bapsy PP, Sharan B, Kumar C, Das RP,
Rangarajan B, Jain M, Attili Suresh VS, Subramanian S, Aggarwal S,
Srivastava M and Vaid A: Open-label, multi-center, non-randomized,
single-arm study to evaluate the safety and efficacy of dendritic
cell immunotherapy in patients with refractory solid malignancies,
on supportive care. Cytotherapy. 16:234–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sabado RL, Miller E, Spadaccia M, Vengco
I, Hasan F and Bhardwaj N: Preparation of tumor antigen-loaded
mature dendritic cells for immunotherapy. J Vis Exp. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arriagada R, Bergman B, Dunant A, Le
Chevalier T, Pignon JP and Vansteenkiste J: International Adjuvant
Lung Cancer Trial Collaborative Group: Cisplatin-based adjuvant
chemotherapy in patients with completely resected non-small-cell
lung cancer. N Engl J Med. 350:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dy GK and Adjei AA: Novel targets for lung
cancer therapy: Part II. J Clin Oncol. 20:3016–3028. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dy GK and Adjei AA: Novel targets for lung
cancer therapy: Part I. J Clin Oncol. 20:2881–2894. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Sun N, Dong Y, Li J, Liu Y, Ren Y,
Yang C, Zhang L, Zhou Y, Tong Z, et al: Anticancer effects of
adenovirus-mediated calreticulin and melanoma-associated antigen 3
expression on non-small cell lung cancer cells. Int
Immunopharmacol. 25:416–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hobo W, Strobbe L, Maas F, Fredrix H,
Greupink-Draaisma A, Esendam B, de Witte T, Preijers F, Levenga H,
van Rees B, et al: Immunogenicity of dendritic cells pulsed with
MAGE3, Survivin and B-cell maturation antigen mRNA for vaccination
of multiple myeloma patients. Cancer Immunol Immunother.
62:1381–1392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheever MA and Higano CS: PROVENGE
(Sipuleucel-T) in prostate cancer: The first FDA-approved
therapeutic cancer vaccine. Clin Cancer Res. 17:3520–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tuyaerts S, Aerts JL, Corthals J, Neyns B,
Heirman C, Breckpot K, Thielemans K and Bonehill A: Current
approaches in dendritic cell generation and future implications for
cancer immunotherapy. Cancer Immunol Immunother. 56:1513–1537.
2007. View Article : Google Scholar : PubMed/NCBI
|