Introduction
Gastric cancer (GC) is one of the most commonly
diagnosed malignant cancers, with the third leading cause of
cancer-related death worldwide (1,2). Although
surgery, chemotherapy and radiation therapy are the main
conventional treatments for GC, its effect is limited in disease
control, and the median overall survival time is <1 year
(3). Due to the lack of effective
treatments currently available for patients with advanced GC, the
development of novel and effective anticancer agents with minimal
toxicity is crucial (4). In previous
years, traditional Chinese medicinal herbs have received increased
recognition and are now considered to be an important source in the
development of novel and effective anticancer agents, with
relatively few side effects (5,6).
Previously, a considerable number of promising anticancer agents
that have been isolated from Chinese herbs have been identified
(7,8),
shedding new light on potential therapeutic strategies for cancer
treatment.
Signaling transduction pathways regulate all major
cellular events in health and disease (9). The phosphatidylinositide 3-kinase
(PI3K)/protein kinase-B (Akt) pathway is a crucial signaling
transduction pathway involved in cellular proliferation and
apoptosis; and is activated in multiple types of cancer (10–13).
Phosphorylation of Akt is mediated by the activation of plasma
membrane-localized 3-phosphoinositide-dependent protein kinase 1
(PDK1), which when inhibited is associated with the inhibition of
cancer cell proliferation (14).
Alterations in Akt signaling are known to be associated with tumor
cell survival and proliferation; in addition to the inhibition of
apoptosis via the upregulation of apoptotic associated factors,
including B-cell lymphoma-2 (Bcl-2), B-cell lymphoma-extra large
(Bcl-xL) and Bcl-2-associated X protein (Bax) (15,16).
Therefore, inhibition of the PI3K/Akt pathway with proteasome
inhibitors may potentially be an alternative strategy for the
treatment of gastric cancer (17,18).
Serratulae chinensis S. Moore (SC; also known
as Guang Sheng Ma), belonging to the composite family, is a
medicinal herb widely distributed in southern China. In traditional
Chinese medicine (TCM), the rhizome of SC is used for
detoxification, promotion of blood circulation (19,20) and
has been commonly used as an anti-inflammatory agent, analgesic,
and antipyretic agent in TCM. Pharmacological studies have
demonstrated that it contains compounds with anti-inflammatory,
analgesic, antipyretic and anticancer activities, including
ceramides and flavones (21,22). However, previous research has mainly
focused on the isolation of components of SC rather than the
biological activity. Therefore, the aim of the present study was to
evaluate the anticancer effects of SC on the proliferation, colony
formation and apoptosis of gastric cancer cells.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA and
phosphate-buffered saline (PBS) were all purchased from HyClone; GE
Healthcare Life Sciences (Logan, UT, USA). TRIzol reagent and the
SuperScript II reverse transcriptase kit were purchased from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). PI3K
(cat. no. 4249), Akt (cat. no. 4691), phosphorylated (p-)Akt (cat.
no. 4060), Bcl-2 (cat. no. 3498), Bax (cat. no. 5023), Cyclin D1
(cat. no. 2978), CDK4 (cat. no. 12790) antibodies and horseradish
peroxidase (HRP)-conjugated secondary antibodies (anti-rabbit IgG;
cat. no. 7074) were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). All other chemicals used, unless otherwise
stated, were obtained from Sigma-Aldrich; Merck KGaG (Darmstadt,
Germany).
Preparation of CSC
The aerial parts of SC were purchased from Shanghang
county (Longyan, China) and identified by Professor Cheng-zi Yang
of the Department of Pharmacy, Fujian University of Traditional
Chinese Medicine (Fuzhou, China). To prepare the CSC, SC (500 g)
was extracted three times with 5,000 ml 95% ethanol using a reflux
method, followed by filtering with qualitative filter paper
(Hangzhou Xinhua Paper Industry Co. Ltd., Hangzhou, China). The
solvent was fractionated using a series of solvents, including
petroleum ether, chloroform, ethyl acetate and N-butanol. The
chloroform fraction of SC (CSC) was evaporated on a rotary
evaporator (Model RE-2000; Yarong Biochemistry Instrument Factory,
Shanghai, China). The CSC was obtained using the spraying
desiccation method with a spray dryer (Model B-290; BUCHI
Labortechnik AG, Flawil, Switzerland) (23). Subsequently, powders of the extracts
were dissolved in 100% DMSO to a stock concentration of 100 mg/ml
and stored at −20°C. The final concentration of DMSO in the DMEM
medium (HyClone; GE Healthcare, Logan, UT, USA) for all experiments
was <0.5%. The final concentrations of CSC were 0, 125, 250 and
500 µg/ml.
Cell culture
Human gastric cancer SGC-7901 cells were obtained
from the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China). Cells were cultured in DMEM
supplemented with 10% (v/v) FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified incubator supplemented with 5%
CO2 in air.
MTT assay
Cells were cultured in DMEM medium supplemented with
0, 125, 250 or 500 µg/ml CSC for 24, 48 or 72 h. Following
treatment, 20 µl MTT (Invitrogen; Thermo Fisher Scientific, Inc.)
was added to each well and incubated for 4 h at 37°C prior to
analysis. The formazan precipitate was dissolved in 100 µl DMSO.
The absorbance was measured at 570 nm using a microplate reader
(BioTek Instruments Inc., Winooski, VT, USA). Cell viability was
determined using the formula: Cell viability (%)=sample optical
density (OD)/control OD ×100.
Observation of morphologic
changes
SGC-7901 cells were seeded at a density of
2.0×105 cells/well into 6-well plates in 2 ml DMEM
medium, supplemented with the indicated concentrations of CSC (0,
125, 250 or 500 µg/ml) for 24 h at 37°C. Cell morphology was
observed using a phase-contrast microscope (Leica Microsystems,
GmbH, Wetzlar, Germany; magnification, ×200).
Colony formation
Cells were seeded at a density of 2.0×105
cells/well into 6-well plates in 2 ml DMEM. Following treatment
with the indicated concentrations of CSC (0, 125, 250 or 500 µg/ml)
for 24 h at 37°C, the cells were harvested and reseeded at a
density of 1.5×103 cells/well in fresh CSC-free medium.
The culture medium was renewed every three days. Following 7 days
of culture, cells were fixed with 10% formaldehyde for 15 min at
room temperature, stained with 0.01% crystal violet for 10 min at
room temperature and photographed with digital camera (D7000;
Nikon, Tokyo, Japan). The stained cell number was counted in 5
arbitrarily selected fields under a light microscope (DM1000; Leica
Microsystems GmbH; magnification, ×100). The cell survival rate was
calculated using the survival rate of the control cells as
100%.
Detection of apoptosis with
4′,6-diamidino-2-phenylindole (DAPI) staining
SGC-7901 cells were seeded at a density of
1.0×105 cells/well into 12-well plates in 1 ml DMEM.
Following CSC treatment with 0, 125, 250 or 500 µg/ml for 24 h at
37°C, the level of cell apoptosis was determined using DAPI
staining kit (Beyotime Institute of Biotechnology, Shanghai, China)
according to the manufacturer's protocol. Briefly, cells were fixed
with 4% polyoxymethylene for 10 min and then incubated in DAPI
solution for 10 min in the dark at room temperature. The stained
images were recorded using a phase-contrast fluorescence microscope
(Leica Microsystems, GmbH; magnification, ×400). Five visual fields
in each group were randomly selected.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from SGC-7901 cells using
TRIzol reagent according to the manufacturer's protocol. The
quantity of RNA were assessed on a NanoDrop spectrophotometer
(ND-2000C; Thermo Fisher Scientific, Inc.). Subsequently,
first-strand cDNA synthesis was performed with 2 µg total RNA using
SuperScript II reverse transcriptase kit (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. The cDNA
was used to determine the expression levels of Bax, Bcl-2, Cyclin
D1 and CDK4 mRNA using PCR with DreamTaq Green PCR Master Mix
(Fermentas; Thermo Fisher Scientific, Inc.) Samples were analyzed
by gel electrophoresis (1.5% agarose) and the DNA bands were
analyzed using a Gel Documentation system (Model Gel Doc 2000;
BioRad Laboratories, Hercules, CA, USA). The reference gene used
was glyceraldehyde 3-phosphate dehydrogenase (GADPH). The primer
sequences used were as follows: Bax forward,
5′-TGCTTCAGGGTTTCATCCAGG-3′ and reverse,
5′-TGGCAAAGTAGAAAAGGGCGA-3′; Bcl-2 forward,
5′-CAGCTGCACCTGACGCCCTT-3′ and reverse, 5′-GCCTCCGTTATCCTGGATCC-3′;
Cyclin D1 forward, 5′-CATCCCAATGTTGTCCG-3′ and reverse,
5′-GCAGCCCAATCAGGTCA-3′; CDK4 forward, 5′-CATGTAGACCAGGACCTAAGC-3′
and reverse, 5′-AACTGGCGCATCAGATCCTAG-3′, GAPDH, forward
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse
5′-AGGGGCCATCCACAGTCTTC-3′.
Western blot analysis
Cells, previously treated with CSC (0, 125, 250 or
500 µg/ml), were harvested for total protein extraction using RIPA
lysis buffer containing phosSTOP EASYpack and a protease inhibitor
cocktail (Roche Diagnostics GmbH, Mannheim, Germany) for 15 min at
4°C. Lysates were centrifuged for 15 min at 12,000 × g at 4°C and
supernatants were collected for further analysis. Protein
concentrations were measured using a BCA quantification assay
(Pierce; Thermo Fisher Scientific, Inc.). Proteins (50 µg) were
separated using 10% SDS-PAGE and transferred to PVDF membranes with
a 0.45-µm pore size (IPVH00010; Millipore, Billerica, MA, USA).
Following blocking with 5% non-fat milk powder at room temperature
for 2 h with the 5% non-fat milk powder dissolved using TBS with
Tween-20 (TBST, pH 8.0) containing 0.1% Tween. The membranes were
incubated with Akt, p-Akt, PI3K, Bcl-2, Bax, Cyclin D1, CDK4 and
β-actin (all 1:1,000) primary antibodies in immunoblot buffer (TBS
containing 0.05% Tween-20 and 5% non-fat dry milk) overnight at
4°C. The following day, membranes were washed three times with
Tris-buffered saline with Tween-20 (TBST), and then were incubated
with HRP-conjugated Goat anti-rabbit IgG secondary antibodies
(1:5,000) for 1 h at room temperature. Following washes, Clarity
Western enhanced chemiluminescence substrate (Bio-Rad Laboratories,
Inc., Hercules, CA, US) was used to visualize protein expression
using ChemiDoc XRS+ Imaging System (Bio-Rad Laboratories, Inc.).
Respective band intensities were quantified using Image Lab
software 4.0 (Bio-Rad Laboratories, Inc.). β-actin was used as the
internal control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
One-way analysis of variance was used to compare different
experimental groups. Fisher's least significant difference (for
equal variances) or Dunnett's correction (for unequal variances)
were used as post-hoc tests. Experiments were conducted in
triplicate, with the representative results presented. P<0.05
was considered to indicate a statistically significant
difference.
Results
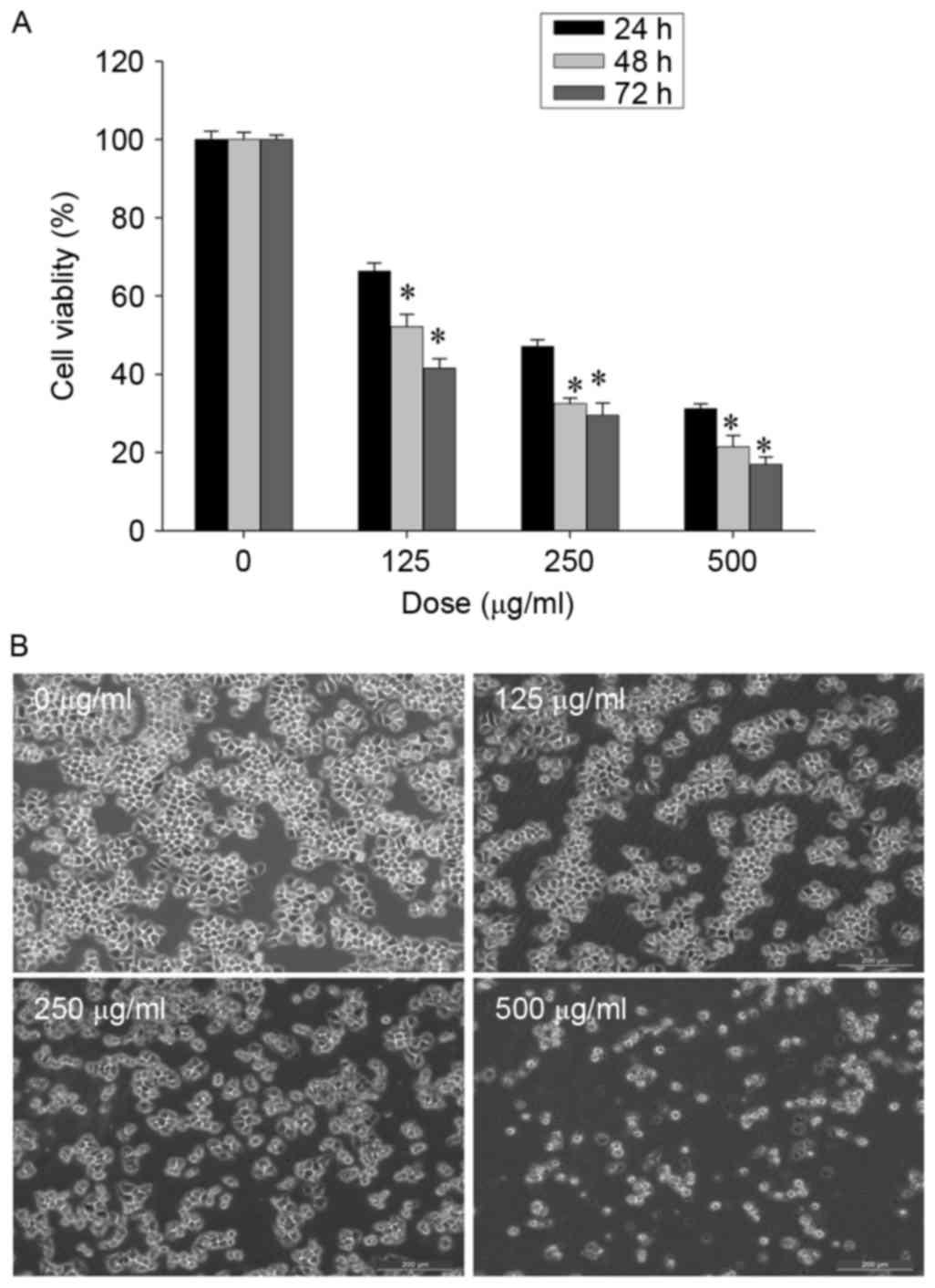
CSC inhibits the viability of SGC-7901
cells
Human SGC-7901 gastric cancer cells were treated
with CSC (0, 125, 250 or 500 µg/ml) for 24, 48 and 72 h, and cell
viability was measured using an MTT assay. CSC treatment inhibited
the viability of SGC-7901 cells in a dose-dependent and
time-dependent manner (Fig. 1A). To
further confirm the aforementioned results, the morphology of
SGC-7901 cells following CSC treatment was examined. Untreated
SGC-7901 cells appeared as densely packed, disorganized
multilayers. In contrast, cells which were treated with CSC for 24
h appeared predominantly round and shrunken, with visible cell
detachment (Fig. 1B). The results
from the present study demonstrated that CSC markedly inhibited the
viability of SGC-7901 cells.
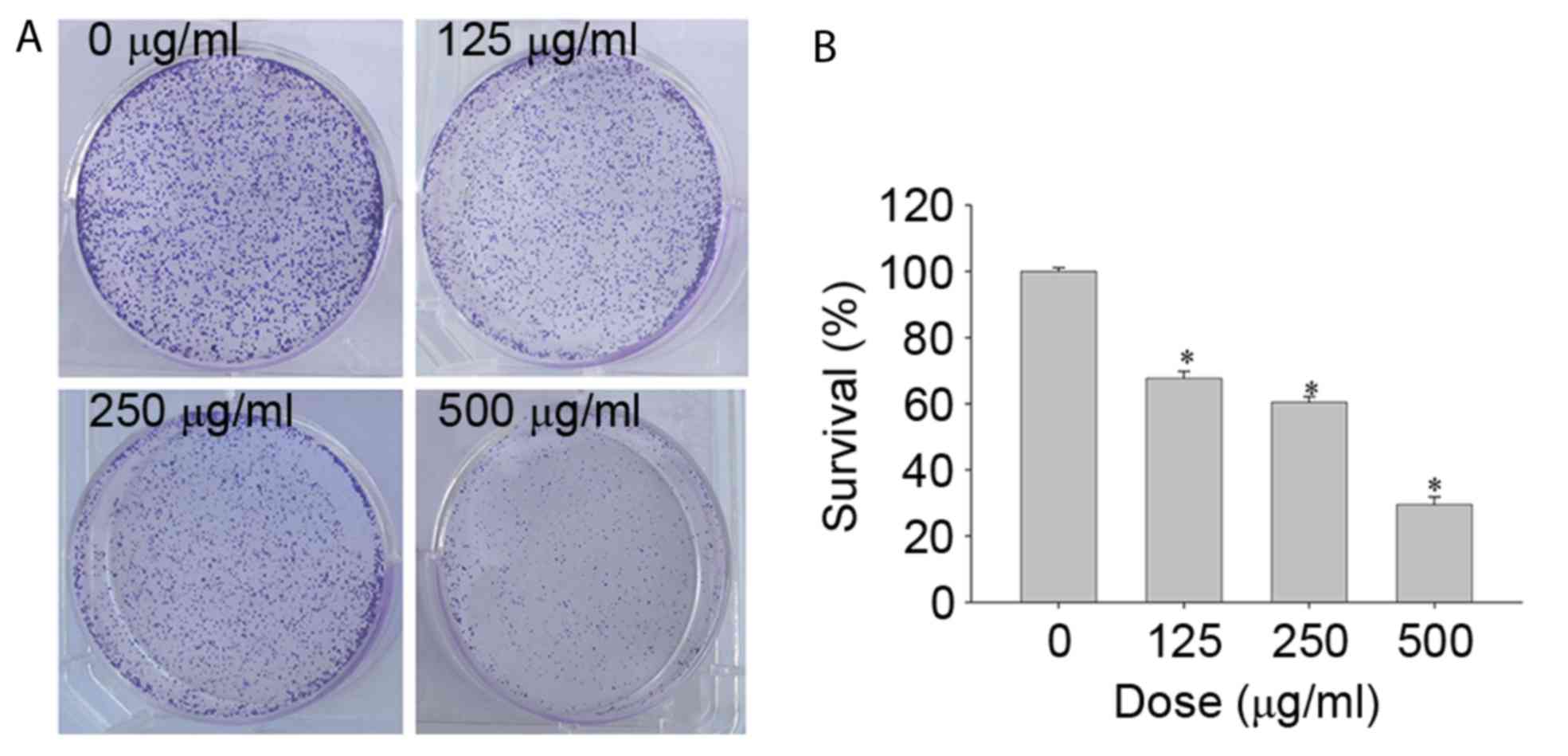
CSC inhibits colony formation of
SGC-7901 cells
To investigate the colony formation of SGC-7901
cells following CSC treatment at various concentrations, colonies
were counted manually after 1 week of culture. Increased
concentrations of CSC resulted in a significant decrease in colony
size and colony numbers of SGC-7901 cells (Fig. 2 and B). The results from the present
study indicated that CSC treatment significantly inhibited the
colony formation ability of SGC-7901 cells.
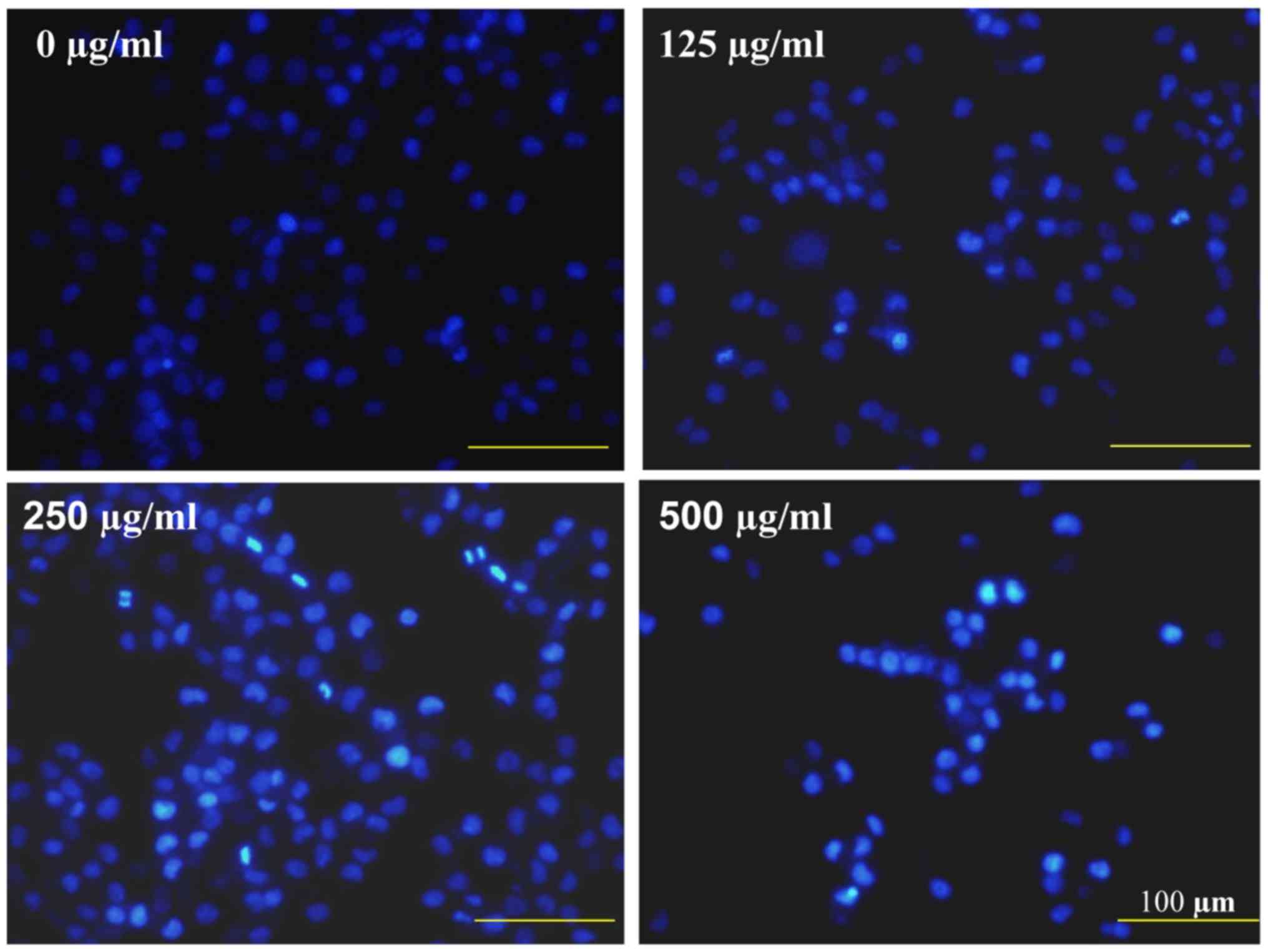
CSC induces apoptosis in SGC-7901
cells
To observe the pro-apoptotic effects of CSC, nuclear
morphological changes in SGC-7901 cells was investigated using the
DNA-binding dye DAPI. CSC-treated cells demonstrated typical
apoptotic morphological features including condensed chromatin and
fragmented nuclear morphology, whereas untreated cells exhibited a
homogeneously stained nucleus, yet at a lower intensity (Fig. 3).
Effects of CSC on cell cycle and
apoptosis-related proteins
To further investigate the mechanisms underlying the
pro-apoptotic and anti-proliferative effects of CSC, RT-PCR and
western blot analysis were performed to examine the respective mRNA
and protein expression levels of pro-proliferative Cyclin D1 and
CDK4, pro-apoptotic Bax and anti-apoptotic Bcl-2. CSC treatment
significantly reduced Bcl-2, Cyclin D1 and CDK4 mRNA
expression, but increased Bax expression in SGC-7901 cells
(Fig. 4A). In addition, western blot
analysis demonstrated that the pattern of protein expression was
similar to the respective mRNA expression levels (Fig. 4B).
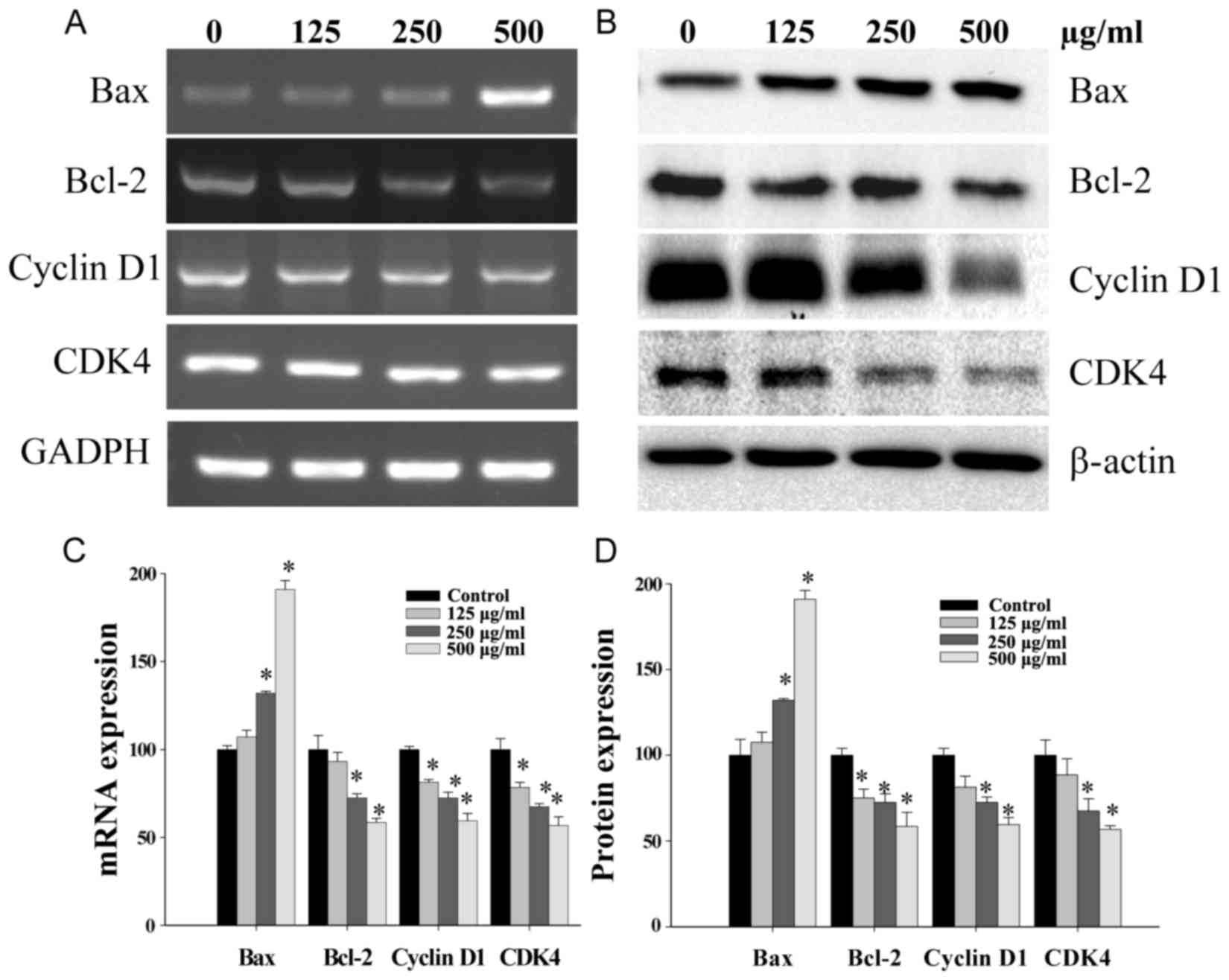 | Figure 4.Effects of CSC on the expression of
Bcl-2, Bax, Cyclin D1 and CDK4 in SGC-7901 cells. Cells were
treated with the indicated concentrations of CSC for 24 h. The mRNA
and protein expression of Bcl-2, Bax, Cyclin D1 and CDK4 were
evaluated by (A) RT-PCR and (B) Western blot analysis, (C) Analysis
of gene expression normalized to GAPDH and (D) respective
densitometric analysis of western blots. Data were normalized to
the mean mRNA or protein expression of untreated cells (100%).
*P<0.01 vs. control. CSC, chloroform extract of Serratulae
chinensis S. Moore; Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2-associated X protein; CDK4, cyclin-dependent kinase-4;
RT-PCR, reverse transcription-polymerase chain reaction; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
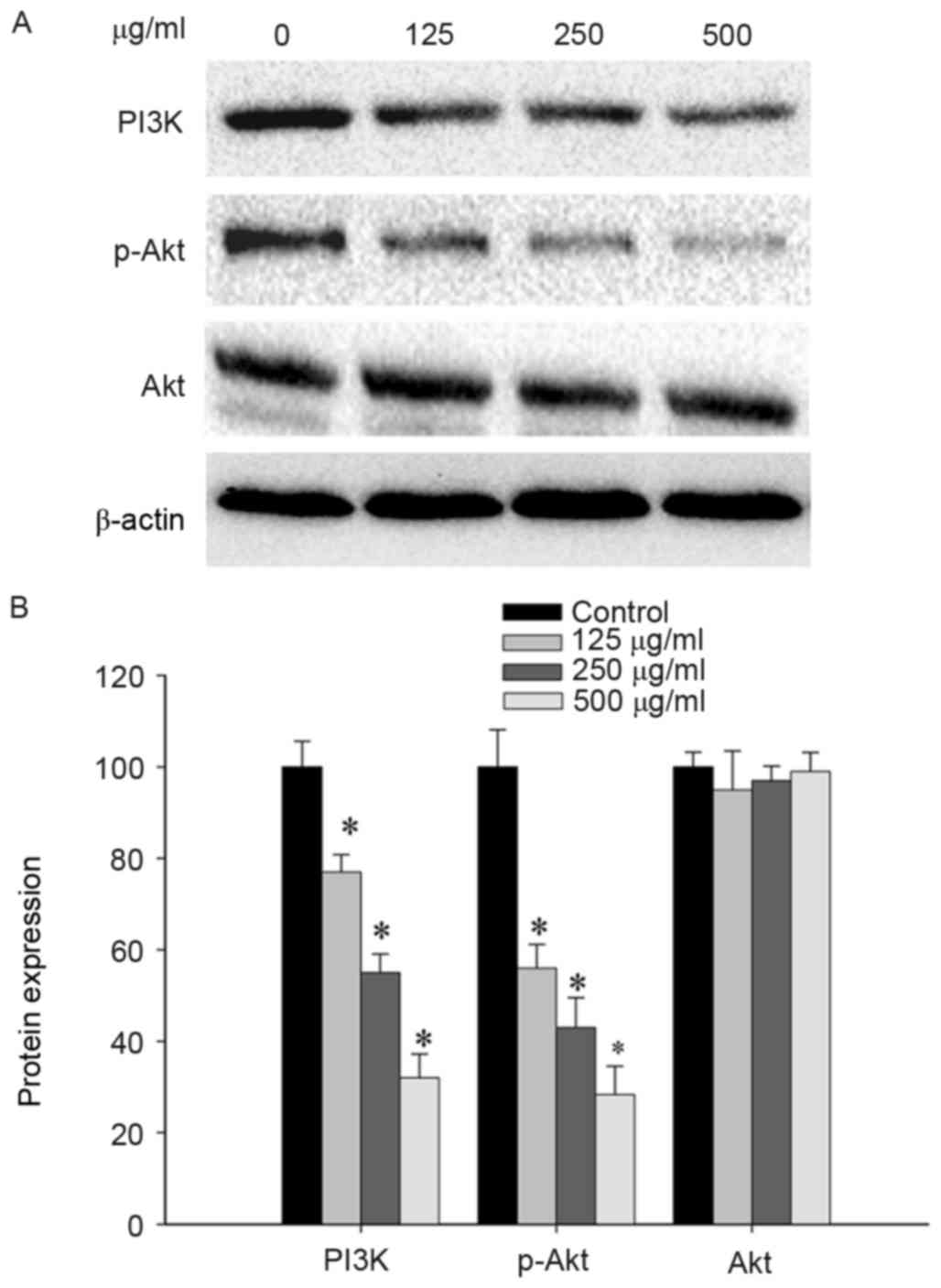
CSC modulated the PI3K/Akt pathway in
SGC-7901 cells
Considering the potential involvement of CSC in the
regulation of the PI3K/Akt pathway, the levels of p-Akt, Akt and
PI3K protein in SGC-7901 cells following CSC treatment were
examined. SGC-7901 cells, treated with CSC at different
concentrations (0, 125, 250, 500 µg/ml) for 30 min, demonstrated a
significant dose-dependent-reduction in expression levels of PI3K
and p-Akt. However, total Akt levels remained unchanged in response
to increasing concentrations of CSC (Fig.
5). Taken together, the results from the present study
indicated that CSC acts by suppressing the proliferation and
promoting the apoptosis of gastric cancer cells, which is mediated
by the suppression of the PI3K/Akt pathway.
Discussion
The underlying molecular mechanisms that accelerate
cancer development are complex and involve a multitude of cell
signaling transduction pathways. Therefore, a switch from the use
of single-drug to multiple-drug chemotherapy for the management and
treatment of various types of cancer has occurred (24). Multi-drug treatments aim to regulate
the complicated signaling processes responsible for the survival of
tumors via the activation or suppression of multiple targets
simultaneously (25). TCM has been
used extensively in China as a complementary or alternative
medicine for multiple diseases. TCM has received renewed interest
due to the discovery of its therapeutic anticancer effects, with
the advantage of reduced side effects compared with modern
chemotherapeutics (26–27). SC is a traditional Chinese herb with
multiple reported pharmacological applications (19–21).
However, the anticancer benefits of CSC and the mechanisms involved
are still largely unknown. Therefore, in order for CSC to be
further developed as an anticancer agent, there is an urgent
requirement to elucidate the underlying molecular mechanisms
involved.
A common feature of cancer cells is a reduction in
cell apoptosis and an uncontrolled increase in cell proliferation
(28). The Bcl-2 family of proteins
are key regulators of apoptosis, which either suppress apoptosis,
(for example, Bcl-2), or promote apoptosis (for example, Bax). The
ratio of Bax to Bcl-2 determines the fate of cells, and alterations
in the ratio caused by aberrant expression of these proteins
damages the normal apoptotic routine, thereby contributing to
various apoptosis-associated diseases including cancer. An
increased Bcl-2 to Bax ratio is associated with poor prognosis in
cancers (28,29). Cyclins and CDKs are two important
classes of cell cycle regulatory proteins that aid in determining
cellular fate during the cell cycle (26). Cyclin D binds to existing CDK4 to form
the active Cyclin D/CDK4 complex. Uncontrolled cell division and
malignancy are caused by hyperactivated or unchecked Cyclin D1/CDK4
complex (30,31). In the present study, in vitro
experiments using gastric cancer SGC-7901 cells revealed the marked
anti-proliferative activity of CSC treatment. In addition, CSC
treatment markedly increased the pro-apoptotic Bax/Bcl-2 ratio,
whilst downregulating pro-proliferative Cyclin D1 and CDK4
expression. Elucidation of the way CSC induces cell apoptosis and
inhibits cell cycle progression may provide a mechanistic basis for
an improved understanding of the anticancer effects of such herbal
medicines.
The PI3K/Akt signaling pathway is essential for the
regulation of cell proliferation and apoptosis. It was previously
reported that the activation of p-Akt signaling is increased in
gastric cancer tumor samples (32).
Following activation by extracellular stimuli, a shift of
phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol 3,4,5
trisphosphate (PIP3) is induced in the presence of PI3K. The
binding of Akt with PIP3 in the pleckstrin homology domain of Akt
causes phosphorylation at Thr308 on its activation loop by plasma
membrane localized 3-phosphoinositide-dependent protein kinase 1
(PDK1) (33). PDK-1 over-activation
leads to increased p-Akt, which in turn regulates the expression of
various key genes involved in cell proliferation and apoptosis,
including Bcl-2, Bax, Cyclin D1 and CDK4 (34,35).
Multiple PI3K or Akt inhibitors are currently in use in clinical or
preclinical studies (36). LY294002,
the first developed PI3K inhibitor, was demonstrated to inhibit
cell proliferation in several cancer cell lines and was able to to
potentiate the effects of chemotherapy (37–40).
Therefore, a re-balance in cell proliferation and apoptosis via
regulation of the PI3K/Akt pathway and its downstream target gene
expression is a promising route for the development of anticancer
therapies. In the present study, it was observed that CSC inhibited
cell viability and induced apoptosis in gastric cancer cells.
Furthermore, CSC significantly inhibited the expression of PI3K and
p-Akt in a dose-dependent manner, via upregulating Bax expression
and downregulating Cyclin D1, CDK4 and Bcl-2 expression, which are
major gene targets of the PI3K/Akt pathway.
In conclusion, the present study demonstrated for
the first time that CSC was able to inhibit the PI3K/Akt signaling
pathway, leading to the inhibition of cell viability, whilst
inducing apoptosis in gastric cancer cells. Thus, CSC may be a
potential novel therapeutic agent for the treatment of gastric
cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81403390) and the University
of Distinguished Young Research Talent Training Program of
Fujian.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
QC and JP conceived and designed the study. QC, JL,
LZ and SL performed the experiments. QC wrote the paper, and JP
reviewed and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu S, Soutto M, Chen Z, Peng D,
Romero-Gallo J, Krishna US, Belkhiri A, Washington MK, Peek R and
El-Rifai W: Helicobacter pylori-induced cell death is counteracted
by NF-κB-mediated transcription of DARPP-32. Gut. 66:761–762. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Power DG, Kelsen DP and Shah MA: Advanced
gastric cancer-slow but steady progress. Cancer Treat Rev.
36:384–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park HR, Tomida A, Sato S, Tsukumo Y, Yun
J, Yamori T, Hayakawa Y, Tsuruo T and Shin-ya K: Effect on tumor
cells of blocking survival response to glucose deprivation. J Natl
Cancer Inst. 96:1300–1310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li XJ and Zhang HY: Western healers in
traditional Chinese medicine. EMBO Rep. 9:112–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmidt BM, Ribnicky DM, Lipsky PE and
Raskin I: Revisiting the ancient concept of botanical therapeutics.
Nat Chem Biol. 3:360–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao J, Jiang P and Zhang W: Molecular
networks for the study of TCM pharmacology. Brief Bioinform.
11:417–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng Z, Garrett CR, Shen Y, Liu L, Yang P,
Huo Y, Zhao Q, Spelman AR, Ng CS, Chang DZ and Cohen L: Prospective
randomised evaluation of traditional Chinese medicine combined with
chemotherapy: A randomised phase II study of wild toad extract plus
gemcitabine in patients with advanced pancreatic adenocarcinomas.
Br J Cancer. 107:411–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Jin F, Higgins R and Mcknight K:
The current view for the silencing of the spindle assembly
checkpoint. Cell Cycle. 13:1694–1701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang XB, Song L, Wen HJ, Bai XX, Li ZJ
and Ma LJ: Upregulation of microRNA-31 targeting integrin α5
suppresses tumor cell invasion and metastasis by indirectly
regulating PI3K/AKT pathway in human gastric cancer SGC7901 cells.
Tumour Biol. 37:8317–8325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Franke TF, Kaplan DR and Cantley LC: PI3K:
Downstream AKTion blocks apoptosis. Cell. 88:435–437. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franke TF, Yang SI, Chan TO, Datta K,
Kazlauskas A, Morrison DK, Kaplan DR and Tsichlis PN: The protein
kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okuzumi T, Fiedler D, Zhang C, Gray DC,
Aizenstein B, Hoffman R and Shokat KM: Inhibitor hijacking of Akt
activation. Nat Chem Biol. 5:484–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pugazhenthi S, Nesterova A, Sable C,
Heidenreich KA, Boxer LM, Heasley LE and Reusch JE: Akt/protein
kinase B up-regulates Bcl-2 expression through cAMP-response
element-binding protein. J Biol Chem. 275:10761–10766. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qian J, Zou Y, Rahman JS, Lu B and Massion
PP: Synergy between phosphatidylinositol 3-kinase/Akt pathway and
Bcl-xL in the control of apoptosis in adenocarcinoma cells of the
lung. Mol Cancer Ther. 8:101–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu CY, Shiau CW, Kuo HY, Huang HP, Chen
MH, Tzeng CH and Chen KF: Cancerous inhibitor of protein
phosphatase 2A determines bortezomib-induced apoptosis in leukemia
cells. Haematologica. 98:729–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen KF, Yeh PY, Yeh KH, Lu YS, Huang SY
and Cheng AL: Down-regulation of phospho-Akt is a major molecular
determinant of bortezomib-induced apoptosis in hepatocellular
carcinoma cells. Cancer Res. 68:6698–6707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ling TJ, Xia T, Wan XC, Li DX and Wei XY:
Cerebrosides from the roots of Serratula chinensis.
Molecules. 11:677–683. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ling TJ, Ping WU, Liu MF and Wei XY:
Ceramides from the roots of Serratula chinensis. J Trop
Subtrop Botany. 13:403–407. 2005.
|
|
21
|
Abdel Hadi L, Di Vito C, Marfia G,
Ferraretto A, Tringali C, Viani P and Riboni L: Sphingosine kinase
2 and ceramide transport as key targets of the natural flavonoid
luteolin to induce apoptosis in colon cancer cells. PloS One.
10:e01433842015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Zhu Z, Fei SJ, Liu L, Sun M and
Zhang Q: Ceramide promoting apoptosis of SGC7901 cell. Cancer Res
Prev Treat. 991–994. 2011.
|
|
23
|
Zhang L, Cai Q, Lin J, Fang Y, Zhan Y,
Shen A, Wei L, Wang L and Peng J: Chloroform fraction of
Scutellaria barbata D. Don promotes apoptosis and suppresses
proliferation in human colon cancer cells. Mol Med Rep. 9:701–706.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Petrelli A and Giordano S: From single- to
multi-target drugs in cancer therapy: When aspecificity becomes an
advantage. Curr Med Chem. 15:422–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gagliostro V, Casas J, Caretti A, Abad JL,
Tagliavacca L, Ghidoni R, Fabrias G and Signorelli P:
Dihydroceramide delays cell cycle G1/S transition via activation of
ER stress and induction of autophagy. Int J Biochem Cell Biol.
44:2135–2143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Feng Y, Wang N, Cheung F, Tan HY,
Zhong S, Li C and Kobayashi S: Chinese medicines induce cell death:
The molecular and cellular mechanisms for cancer therapy. Biomed
Res Int. 2014:5303422014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li-Weber M: Targeting apoptosis pathways
in cancer by Chinese medicine. Cancer Lett. 332:304–312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muntean AG, Pang L, Poncz M, Dowdy SF,
Blobel GA and Crispino JD: Cyclin D-Cdk4 is regulated by GATA-1 and
required for megakaryocyte growth and polyploidization. Blood.
109:5199–5207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nigg EA: Cyclin-dependent protein kinases:
Key regulators of the eukaryotic cell cycle. Bioessays. 17:471–480.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orlando DA, Lin CY, Bernard A, Wang JY,
Socolar JE, Iversen ES, Hartemink AJ and Haase SB: Global control
of cell-cycle transcription by coupled CDK and network oscillators.
Nature. 453:944–947. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson SM, Gulhati P, Rampy BA, Han Y,
Rychahou PG, Doan HQ, Weiss HL and Evers BM: Novel expression
patterns of PI3K/AKT/mTOR signaling pathway components in
colorectal cancer. J Am Coll Surg. 210:767–778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Umemura S, Yoshida S, Ohta Y, Naito K,
Osamura RY and Tokuda Y: Increased phosphorylation of Akt in
triple-negative breast cancers. Cancer Sci. 98:1889–1892. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu G, Song Y, Cui L, Wen Z and Lu X:
Inositol hexaphosphate suppresses growth and induces apoptosis in
HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a
potential target. Int J Clin Exp Pathol. 8:1402–1410.
2015.PubMed/NCBI
|
|
37
|
Badinloo M and Esmaeili-Mahani S:
Phosphatidylinositol 3-kinases inhibitor LY294002 potentiates the
cytotoxic effects of doxorubicin, vincristine, and etoposide in a
panel of cancer cell lines. Fundam Clin Pharmacol. 28:414–422.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang ZP, Zhao Y, Huang F, Chen J, Yao YH,
Li J and Wu XN: Equol inhibits proliferation of human gastric
carcinoma cells via modulating Akt pathway. World J Gastroenterol.
21:10385–10399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu D, Tao J, Xu B, Qing W, Li P, Lu Q and
Zhang W: Phosphatidylinositol 3-kinase inhibitor LY294002
suppresses proliferation and sensitizes doxorubicin chemotherapy in
bladder cancer cells. Urol Int. 86:346–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xia LJ, Wu YL and Zhang FC: Combination of
cecropinXJ and LY294002 induces synergistic cytotoxicity, and
apoptosis in human gastric cancer cells via inhibition of the
PI3K/Akt signaling pathway. Oncol Lett. 14:7522–7528.
2017.PubMed/NCBI
|