Introduction
Benzo(a)pyrene (BaP) is a polycyclic aromatic
hydrocarbon derived from incomplete combustion of organic materials
(including cigarette smoke). BaP is listed as a group I carcinogen
by the International Agency for Research on Cancer based on the
data from animal experiments and epidemiological studies (1). Numerous studies have documented the
associations between BaP exposure and the formation of different
types of cancer (2–7), including liver cancer (8–10). It has
been reported that gene mutations, chromosomal aberrations and
epigenetic alterations are involved in the process of BaP-induced
hepato-carcinogenesis (11–15). Recently, Ba et al (16) reported that BaP exposure had effects
on the metastasis of human liver cancer cell, but the underlying
mechanisms of this are not well understood.
Extracellular regulated protein kinase (ERK), a
pivotal regulator of the mitogen-activated protein kinase
(MAPK)/ERK pathway, has been implicated in the regulation of cell
proliferation, differentiation and survival (17,18). The
ERK cascade reaction may be activated by various stimuli, including
receptor tyrosine and G-protein-coupled receptors (19), and activated ERK may phosphorylate
various downstream molecules (20).
An increasing volume of evidence has demonstrated that the
activated ERK signaling pathway is associated with the development
and progression of liver cancer (21–25). For
example, Jiang et al (26)
reported that calcium binding protein 39 promoted the metastasis of
liver cancer by activating the ERK signaling pathway. Dang et
al (27) reported that loss of
protocadherin-17 promoted the metastasis and invasion of liver
cancer through hyperactivation of the epidermal growth factor
receptor (EGFR)/ERK signaling pathway.
Based on the observations of previous studies that
phosphorylated (p)-ERK served an important role in hepatocarcinoma
cell migration and invasion, and that BaP promoted hepatocarcinoma
cell migration and invasion, we hypothesized that ERK activation
may serve a pivotal role in BaP-induced migration and invasion of
hepatoma cells. In the present study, the most commonly studied
cell line of human hepatoblastoma, Hep-G2 (28) was used to investigate the potential
role of p-ERK in the BaP-induced migration and invasion of hepatoma
cells.
Materials and methods
Cell culture and BaP treatment
The human hepatoblastoma Hep-G2 cell line was
purchased from the Cell Resource Center, Peking Union Medical
College (National Infrastructure of Cell Line Resource, Beijing,
China). Hep-G2 cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA)
supplemented with 10% fetal bovine serum (FBS) (GE Healthcare
Bio-Sciences) and antibiotics (penicillin 100 U/ml and streptomycin
100 µg/ml) in an incubator with a humidified atmosphere of 5%
CO2 at 37°C. For BaP exposure, Hep-G2 cells were treated
with different concentrations (0, 2, 4, 8, 16, 32 or 64 µM) of BaP
(B1760; >96% HPLC; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at different time points (0, 24, 48 or 72 h), as described
previously (29,30). The final concentration of dimethyl
sulfoxide (DMSO) used as solvent control was 0.1% (v/v) or
less.
Cell proliferation assay
To observe the effects of BaP on Hep-G2 cell
proliferation, an MTT assay was performed as described previously
(30). In brief, 1×104
cells in 100 µl DMEM (GE Healthcare Bio-Sciences) were plated into
each well of 96-well plates (6 wells per group). A total of 24 h
after plating, cells were exposed to different concentrations of
BaP (0, 2, 4, 8, 16, 32 or 64 µM) for 0, 24, 48 or 72 h. A total of
10 µl of 5 mg/ml MTT was added to each well, and then the plates
were incubated at 37°C for 4 h. Finally, 150 µl DMSO was added to
each well to dissolve the purple formazan. The optical density was
read on a micro-plate reader (Multiskan Ascent Software, Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 492 nm. Relative cell
proliferation rates were determined by normalizing with that of the
solvent control at 0 h.
Wound healing assay
A Wound-healing assay was conducted to evaluate the
migratory ability of Hep-G2 cells in accordance with our previous
study (30). In brief, cells were
seeded onto 6-well plates and were wounded by scratching the
surface of plates with a sterile 200-µl-pipette tip. Floating cells
were removed by washing with phosphate-buffered saline (PBS) three
times. A total of 2 ml DMEM (GE Healthcare Bio-Sciences) with 4 µM
BaP or 20 µM ERK inhibitor, U0126 (U120-1MG; ≥98% HPLC;
Sigma-Aldrich; Merck KGaA), was added to each well. Images of the
process of cell migration into the wound were captured using an
inverted microscope (magnification, ×10 and ×20) equipped with a
camera (Leica Microsystems GmbH, Wetzlar, Germany). The healing
width was calculated as the wound width at 0 h minus wound width at
48 h, and was normalized to the control.
Invasion assay
A Matrigel invasion assay was performed using a
24-well Transwell chamber (Corning Incorporated, Corning, NY, USA).
A total of 5×104 cells in DMEM (GE Healthcare
Bio-Sciences) without FBS were seeded into the upper chamber with
Matrigel. A total of 4 µM BaP or 20 µM U0126 were added to the
upper chamber. A total of 600 µl DMEM supplemented with 10% FBS
(both GE Healthcare Bio-Sciences) were added to the lower chamber.
After 24 h of incubation at 37°C, non-migrated cells on the upper
side of the membrane were removed with cotton swabs. Cells on the
lower surface of the membrane were fixed with pure methanol at room
temperature for 10 min and stained with crystal violet at room
temperature for 30 min. The number of invaded cells was counted in
six randomly selected fields. Relative ability of invasion was
calculated by normalizing with control.
Western blot analysis
Western blot analysis was performed as previously
described (30). In brief, cells were
harvested and washed with ice-cold PBS three times. Whole-cell
lysates were prepared in radioimmunoprecipitation assay buffer with
protease inhibitors and phosphatase inhibitors (Pierce; Thermo
Fisher Scientific, Inc.) at 4°C for 15 min, followed by 15 min of
centrifugation (14,000 × g) at 4°C. Total protein was determined by
a bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.).
Equal amounts of protein (50 µg) were electrophoresed on 10% sodium
dodecyl sulfate polyacrylamide gel. Following electrophoresis,
proteins were transferred onto nitrocellulose membranes. The
membranes were washed and blocked with 5% bovine serum albumin in
Tris-buffered saline and 0.1% Tween-20 at room temperature for 1 h.
The membranes were incubated with primary antibodies against the
following: p-ERK1/2 (1:2,000; cat. no. 4370S), ERK1/2 (1:2,000;
cat. no. 4695S), p-STAT3 (1:1,000; cat. no. 9145S), vimentin
(1:1,000; cat. no. 5741S; all Cell Signaling Technology, Inc.,
Danvers, MA, USA), tubulin (1:1,000; cat. no. sc-365791), β-catenin
(1:1,000; cat. no. sc-7199), p-protein kinase B (Akt; 1:1,000; cat.
no. sc-33437), MMP2 (1:1,000; cat. no. sc-10736), N-cadherin
(1:1,000; cat. no. sc-7939), TGIF (1:1,000; cat. no. sc-9084),
STIM1 (1:1,000; cat. no. sc-68897), Twist (1:500; cat. no.
sc-81417) and E-cadherin (1:1,000; cat. no. sc-7870; all Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. The members
were washed and incubated with Peroxidase-Conjugated Goat
anti-Rabbit IgG (1:5,000; cat. no. ZB-2301) or
Peroxidase-Conjugated Goat anti-Mouse IgG (1:5,000; cat. no.
ZB-2305; all ZSGB-BIO; OriGene Technologies, Inc., Beijing, China)
for 1 h at room temperature. The signals were captured in the
ChemiDoc™ XRS+ imaging system (Bio-Rad
Laboratories, Inc., Hercules, MA, USA) by a Bio-Rad
Clarity™ western enhanced chemiluminescence substrate
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Data were analyzed by one-way analysis, followed by
the least significant difference test, using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). All values are expressed as the
mean ± standard deviation (SD). P<0.05 was considered to
indicate a statistically significant difference.
Results
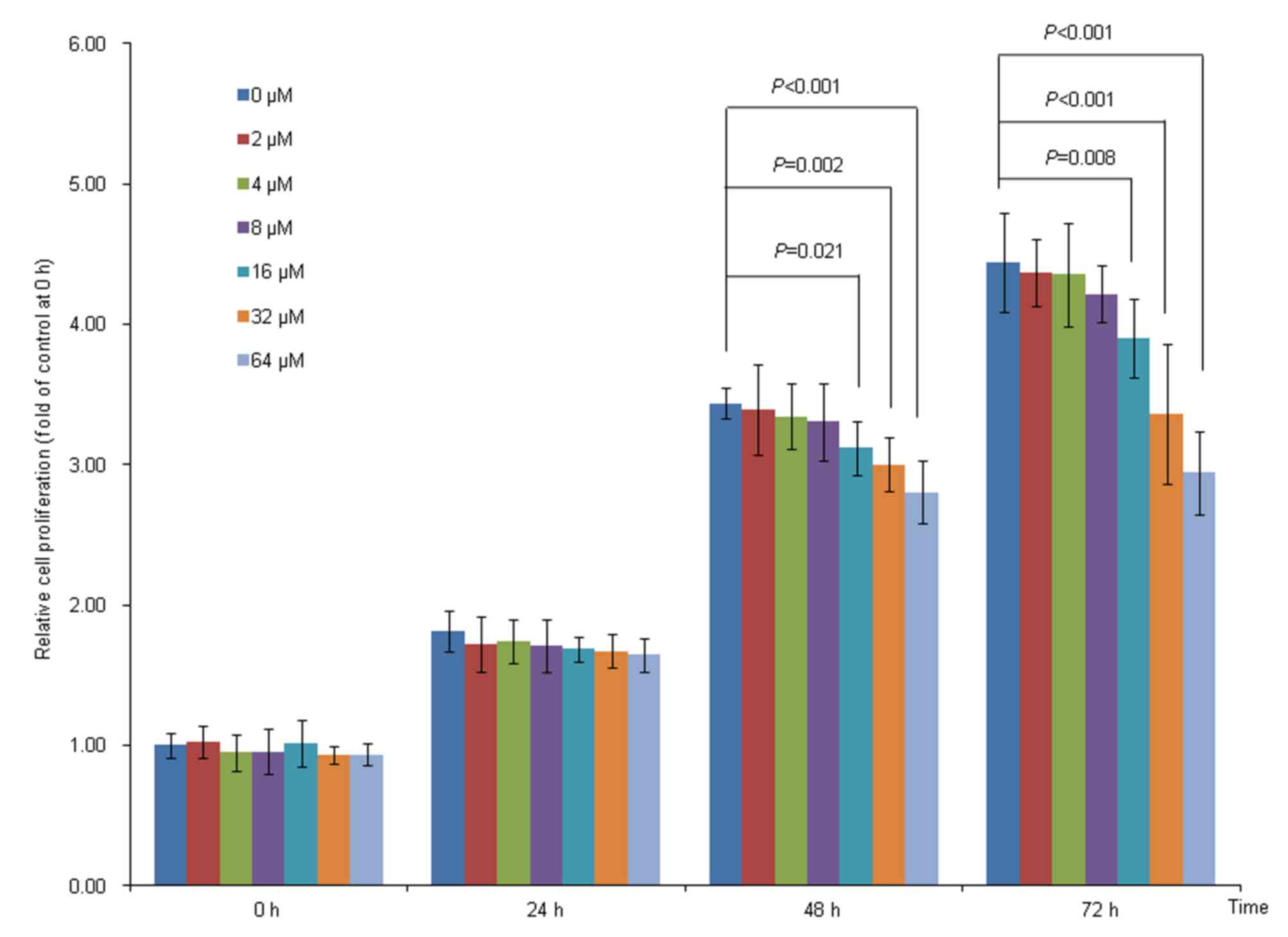
The effects of BaP treatment on Hep-G2
cell proliferation
The results of the present study demonstrated that
Hep-G2 cell proliferation was significantly suppressed by 16, 32
and 64 µM BaP treatment at 48 and 72 h, compared with the solvent
control (Fig. 1). When the
concentration of BaP treatment was <8 µM, the cell proliferation
was not significantly affected (Fig.
1). Therefore, the highest dose of BaP treatment did not exceed
8 µM for the subsequent experiments, as it had no obvious toxicity
to cells at this concentration.
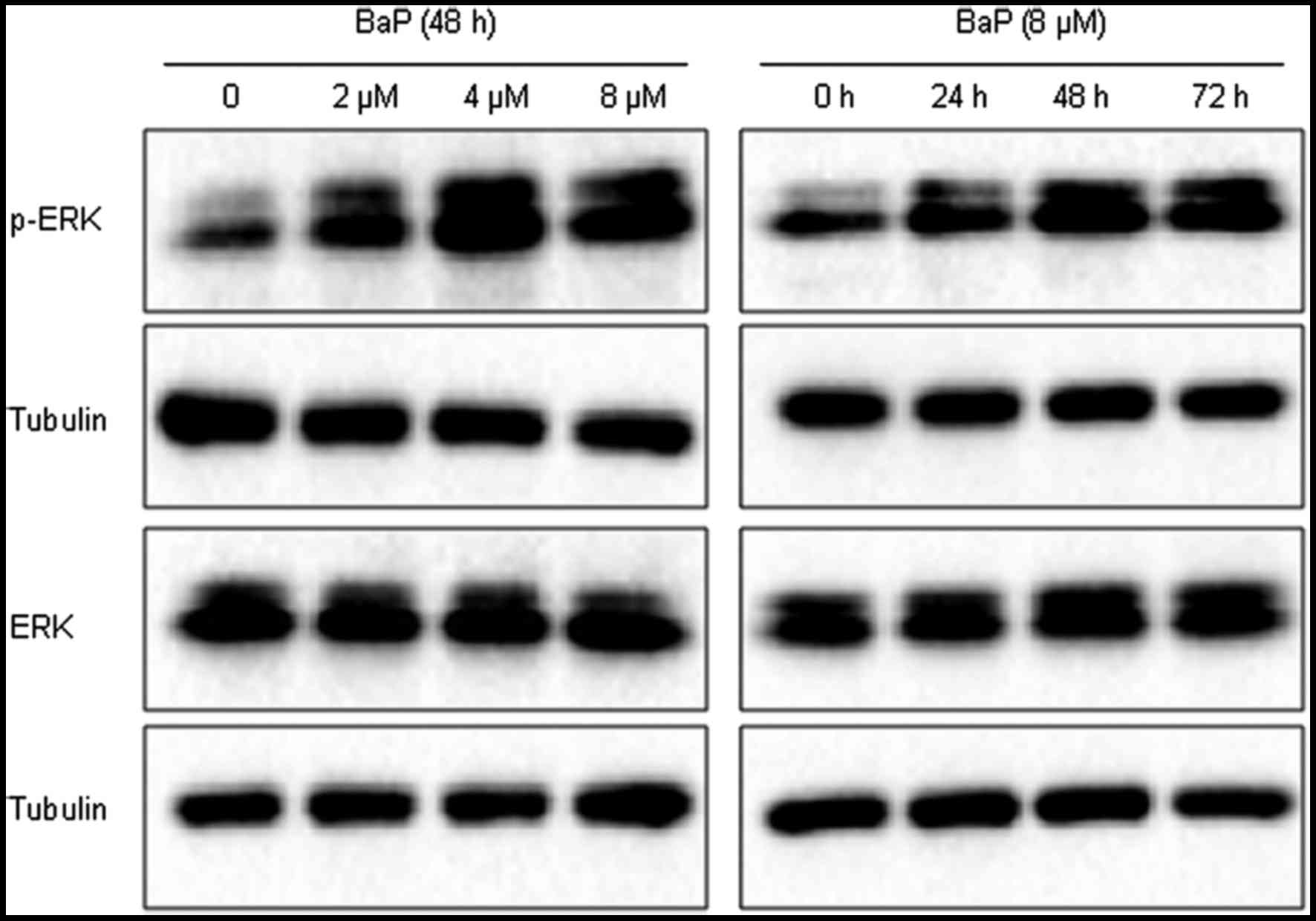
The effects of BaP treatment on the
expression of p-ERK
The results of the present study demonstrated that
the expression of p-ERK protein was markedly increased in the
BaP-treated groups (2, 4 and 8 µM), compared with the solvent
control group (Fig. 2). Furthermore,
BaP exposure (8 µM) markedly increased the expression of p-ERK
protein at 24, 48 and 72 h, compared with the solvent control
(Fig. 2). BaP treatment had no
obvious effects of total ERK expression, and tubulin was used as
loading control.
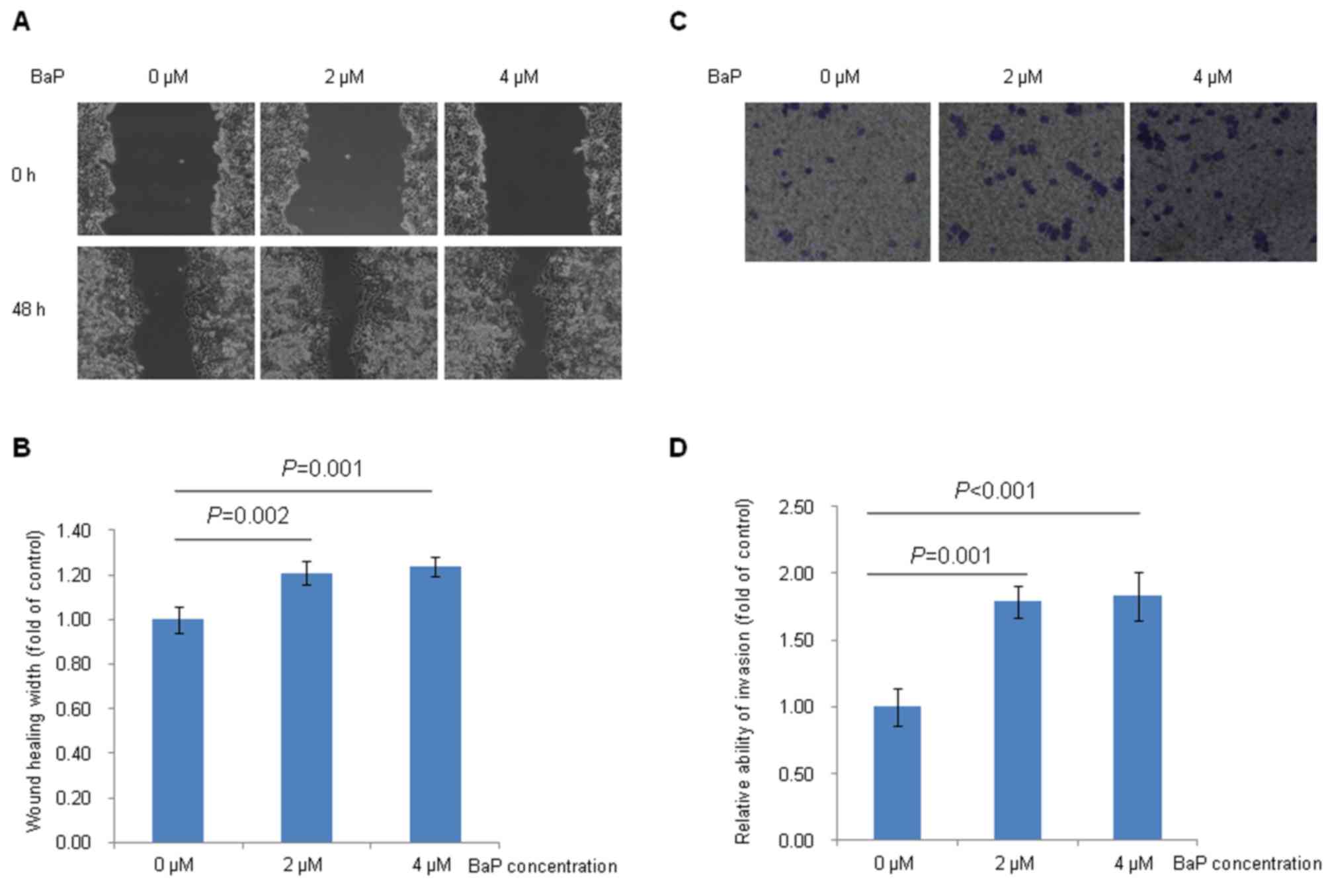
The effects of BaP treatment on Hep-G2
cell migration and invasion
Fig. 3 demonstrated
the effects of BaP treatment on Hep-G2 cell migration and invasion
in vitro. The results demonstrated that Hep-G2 cells treated
with BaP (2 and 4 µM) migrated more quickly to heal the scratched
wounds than the cells treated with DMSO (Fig. 3A and B). Furthermore, the results of
the present study indicated that Hep-G2 cells treated with BaP (2
and 4 µM) had an enhanced ability to invade through the Matrigel
matrix, compared with cells treated with DMSO (Fig. 3C and D).
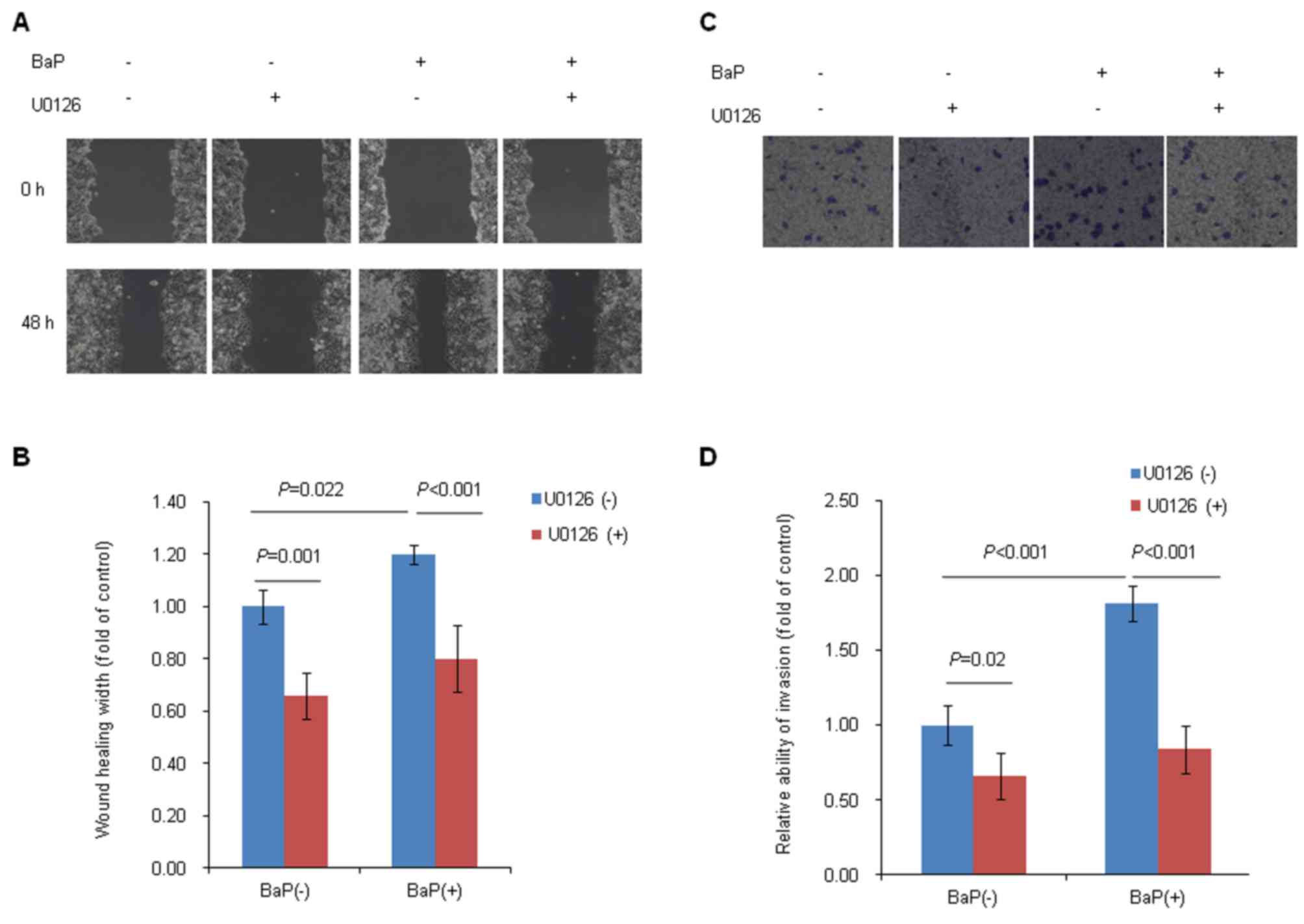
Increased ERK activity is required for
BaP-induced Hep-G2 cell migration and invasion
As described earlier, BaP treatment induced the
expression of p-ERK protein and enhanced the migration and invasion
abilities of Hep-G2 cells. To further investigate whether or not
ERK signaling serves an important role in the BaP-induced migration
and invasion of Hep-G2 cells, wound-healing and Transwell invasion
assays were performed in the presence of BaP and a known ERK
inhibitor, U0126. The results data demonstrated that the ERK
inhibitor, U0126, significantly inhibited the migration and
invasion of Hep-G2 cells. As demonstrated in Fig. 4A, the scratched wound width was
markedly wider in the U0126-treated group (+) than that in the
U0126-untreated group (−) at 48 h (Fig.
4A). When the migration ability was represented as the healing
width, it was observed that the healing width was markedly
decreased in U0126-treated group (+) as compared with
U0126-untreated group (−) at 48 h (Fig.
4B). The number of invaded cells was markedly decreased in
U0126-treated group (+), compared with the U0126-untreated group
(−) (Fig. 4C and D). Furthermore, the
results of the present study demonstrated that blocking of ERK
activation abolished the abilities of the migration (Fig. 4A and B) and invasion (Fig. 4C and D) of Hep-G2 cells induced by
treatment with BaP, which suggested that Hep-G2 cell migration and
invasion induced by BaP treatment may be mediated by ERK
activation.
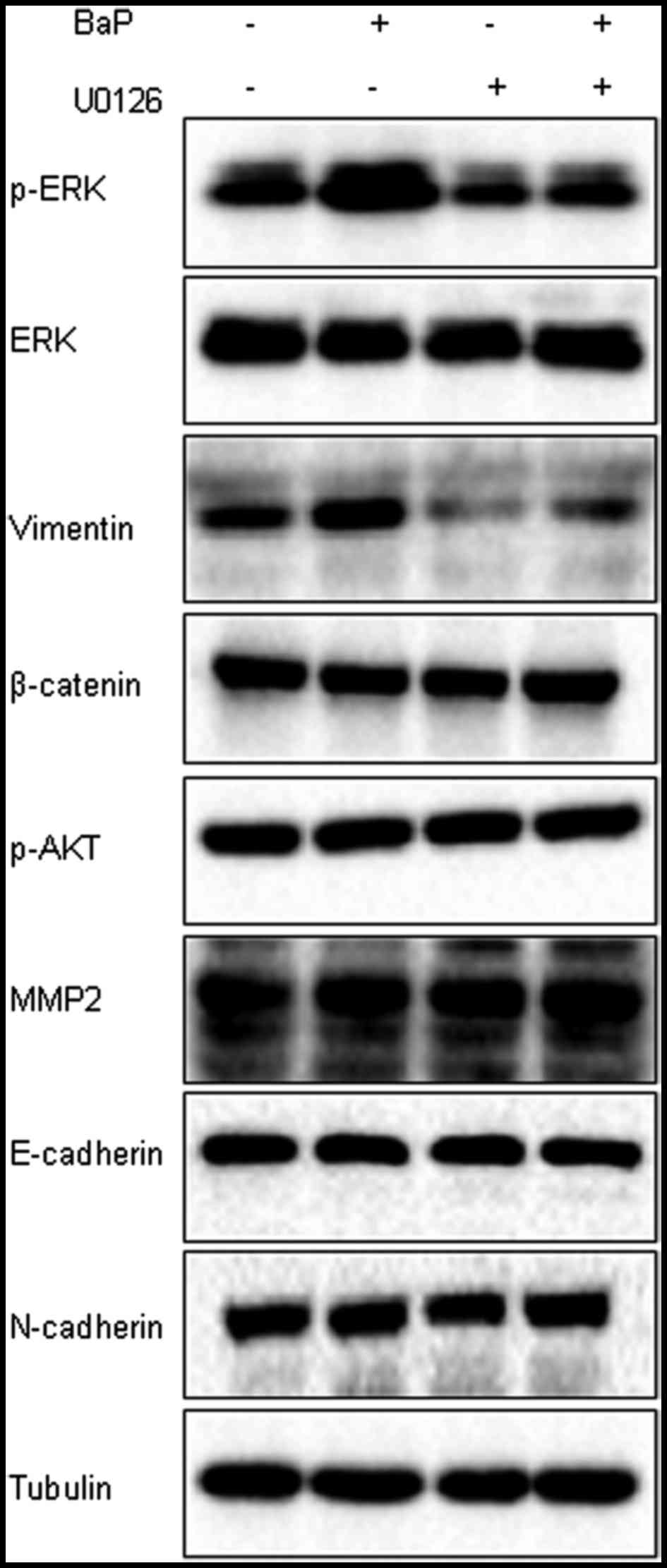
BaP-induced vimentin protein
expression is modulated by ERK activation
Several proteins, including matrix metalloproteinase
2 (MMP2), p-Akt, E-cadherin, N-cadherin and vimentin serve
important roles in the invasion and metastasis of liver cancer
(31–34). Whether or not these proteins were
involved in the downstream targets of ERK signaling in Hep-G2 cells
treated with BaP was subsequently investigated. The expression of
these proteins in the presence of BaP and the ERK inhibitor, U0126,
was detected. The results demonstrated that the increased
expression of vimentin protein was observed in the BaP treatment
group, compared with the solvent control, which was inhibited by
treatment with U0126 (Fig. 5). No
effect on the expression of proteins, including β-catenin, p-Akt,
MMP2, E-cadherin and N-cadherin in Hep-G2 cells was observed
followed treatment with BaP (Fig. 5).
Additionally, the expression of additional proteins, including
TGFB-induced factor (TGIF), p-STAT3, Twist1 and STIM1, was also
detected in Hep-G2 cells induced by BaP, but BaP treatment did not
have any notable effects on the expression of these proteins (data
not shown).
Discussion
It has been reported that several key molecular
events are implicated in BaP-induced hepato-carcinogenesis.
Bolotina et al (35)
demonstrated that BaP-dependent activation of transcript factors
nuclear factor (NF)-κB and activator protein 1 (AP-1) was
associated with tumor promotion in hepatoma cell cultures. Cui
et al (36) demonstrated that
pregnane X receptor regulated the AhR/CYP1A1 pathway and protected
liver cells from BaP-induced DNA damage. Marrone et al
(37) reported that treatment of
HepaRG cells with BaP resulted in specific changes in the
expression of microRNAs compared with their non-carcinogenic
analogues. Souza et al (38)
demonstrated that BaP and its major metabolites deregulated
metastatic markers via non-genotoxic and genotoxic mechanisms and
activated the inflammatory pathway (NF-κB signaling and
cytokine-cytokine receptor interaction) in Hep-G2 cells. BaP also
induced strong repression of genes involved in cholesterol and
fatty acid biosynthesis in Hep-G2 cells (38).
Previous studies have documented the association
between BaP exposure and cancer cell invasion and metastasis. Ueng
et al (39) reported that BaP
treatment enhanced the invasive ability of lung cancer CL5 cells
in vitro. Wang et al (30) indicated that BaP treatment may promote
A549 lung cancer cell migration and invasion. Miller et al
(40) reported that BaP treatment
increased the invasion abilities of human breast cancer MDA-MB-231
cells. The present study observed that BaP treatment enhanced the
abilities of migration and invasion in liver cancer Hep-G2 cells
in vitro. The results of the present study are consistent
with previous observations (16). Ba
et al (16) observed that
chronic BaP exposure was able to promote liver cancer cell
migration and invasion in vivo and in vitro. Taken
together, the results of the present study indicated that exposure
to BaP had effects on liver cancer metastasis and progression.
Numerous studies have demonstrated that ERK
signaling is involved in BaP-induced carcinogenesis. For example,
Patten Hitt et al (41)
demonstrated that BaP activated ERK, which was involved in cell
proliferation, in colon adenocarcinoma HT29 cells (41). Wang et al (42) reported that BaP-induced cell cycle
progression occurred via the ERK-induced checkpoint kinase 1
pathway activation in human lung cancer cells. Kometani et
al (43) reported that the EGFR
tyrosine kinase inhibitor reduced the cellular proliferation and
the level of phosphorylation of ERK1/2, which is a downstream
signal of the EGFR in BaP-treated A549 cells. The present study
observed that BaP treatment increased the level of p-ERK protein
expression in Hep-G2 cells, and the ERK inhibitor, U0126, blocked
BaP-induced Hep-G2 cell migration and invasion, which suggested
that ERK activation may mediate BaP-induced migration and invasion
in Hep-G2 cells. Two previous studies have demonstrated that
elevated ERK activity is required for BaP-induced migration and
invasion in human breast cancer MDA-MB-231 and MCF-7 cells
(29,44). Taken together, the results of the
present study and previous studies suggested that ERK signaling
served an important role in the migration and invasion of different
types of cancer cells induced by BaP.
Vimentin, a type III intermediate filament protein,
is expressed in mesenchymal cells. Vimentin is often used as a
marker of liver cancer metastasis. Hu et al (45) reported that overexpression of vimentin
was significantly associated with liver cancer metastasis. Wei
et al (46) reported that
silencing of glucose-regulated protein 78 enhanced liver cancer
cell migration through regulation of vimentin. Wang et al
(47) demonstrated that long
non-coding RNA AOC4P suppressed liver cancer metastasis by
enhancing vimentin degradation and inhibiting
epithelial-mesenchymal transition. The results of a study
undertaken by Dong et al (34)
indicated that osteopontin promoted epithelial-mesenchymal
transition of liver cancer cells through regulating vimentin. The
present study observed that BaP treatment increased the expression
of vimentin protein, which was attenuated by inhibition of ERK
activity.
There are several limitations to the present study.
To begin with, Hep-G2 cells were treated with BaP (2, 4 and 8 µM)
for 48 h, which may be different from long-term exposure to low
concentrations of BaP for humans under normal living conditions.
Furthermore, although the results of the present study demonstrated
that BaP exposure increased the expression of p-ERK at times
between 24 and 72 h, while the effects at times between 0 and 24 h
were not investigated and require investigation in future studies.
Additionally, the ERK inhibitor was revealed to attenuate
BaP-induced vimentin protein expression. However, the underlying
mechanisms regarding how p-ERK regulates vimentin protein
expression were not fully addressed in the present study and should
be a focus of future studies. Furthermore, the potential role of
ERK in BaP-induced migration and invasion was only investigated at
the cellular level. Further studies on this topic based on animal
experiments and population should be performed to verify the
results of the present study.
The Hep-G2 cell line was originally thought to be a
hepatocellular carcinoma cell line, but was later revealed to
derive from a hepatoblastoma (28),
and is most frequently used to study the invasion and metastasis of
liver cancer (48–52). In the present study, the Hep-G2 cell
line was used to investigate the potential role of p-ERK in
BaP-induced invasion and migration of liver cancer cells. Although
this cell line was reported to be misidentified as hepatocellular
carcinoma cell, this issue is unlikely to affect the outcomes of
the present study.
In summary, to the best of our knowledge, the
present study was the first to demonstrate that BaP exposure
promoted Hep-G2 cell migration and invasion by upregulating p-ERK
protein expression. The present study, in part, enriched
understanding of the mechanisms on the increased risk of liver
cancer metastasis among people who are exposed to BaP.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
U1404815).
Availability of data and materials
The datasets generated/analyzed during the present
study are included in the published article.
Authors' contributions
YW contributed to study design, implementation,
experiments, data analysis, and manuscript writing. YW, TP and LL
contributed to western blot analysis. YW, LL, and HW contributed to
the cell proliferation assay, wound healing assay and invasion
assay. HY and DZ contributed to data analysis.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans: Some non-heterocyclic polycyclic
aromatic hydrocarbons and some related exposures. IARC Monogr Eval
Carcinog Risks Hum. 92:1–853. 2010.PubMed/NCBI
|
|
2
|
He XZ, Chen W, Liu ZY and Chapman RS: An
epidemiological study of lung cancer in Xuan Wei County, China:
Current progress. Case-control study on lung cancer and cooking
fuel. Environ Health Perspect. 94:9–13. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshimoto T, Inoue T, Iizuka H, Nishikawa
H, Sakatani M, Ogura T, Hirao F and Yamamura Y: Differential
induction of squamous cell carcinomas and adenocarcinomas in mouse
lung by intratracheal instillation of benzo(a)pyrene and charcoal
powder. Cancer Res. 40:4301–4307. 1980.PubMed/NCBI
|
|
4
|
Senthilnathan P, Padmavathi R, Magesh V
and Sakthisekaran D: Chemotherapeutic efficacy of paclitaxel in
combination with Withania somnifera on benzo(a)pyrene-induced
experimental lung cancer. Cancer Sci. 97:658–664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson KE, Kadlubar FF, Kulldorff M,
Harnack L, Gross M, Lang NP, Barber C, Rothman N and Sinha R:
Dietary intake of heterocyclic amines and benzo(a)pyrene:
Associations with pancreatic cancer. Cancer Epidemiol Biomarkers
Prev. 14:2261–2265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li D, Day RS, Bondy ML, Sinha R, Nguyen
NT, Evans DB, Abbruzzese JL and Hassan MM: Dietary mutagen exposure
and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev.
16:655–661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ronco AL, De Stefani E, Correa P,
Deneo-Pellegrini H, Boffetta P, Acosta G and Mendilaharsu M:
Dietary benzo[a]pyrene, alcohol drinking, and risk of breast
cancer: A case-control study in Uruguay. Asian Pac J Cancer Prev.
12:1463–1467. 2011.PubMed/NCBI
|
|
8
|
Roe FJ and Waters MA: Induction of
hepatoma in mice by carcinogens of the polycyclic hydrocarbon type.
Nature. 214:299–300. 1967. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vesselinovitch SD, Kyriazis AP,
Mihailovich N and Rao KV: Conditions modifying development of
tumors in mice at various sites by benzo(a)pyrene. Cancer Res.
35:2948–2953. 1975.PubMed/NCBI
|
|
10
|
Su Y, Zhao B, Guo F, Bin Z, Yang Y, Liu S,
Han Y, Niu J, Ke X, Wang N, et al: Interaction of benzo[a]pyrene
with other risk factors in hepatocellular carcinoma: A case-control
study in Xiamen, China. Ann Epidemiol. 24:98–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abe S, Nemoto N and Sasaki M:
Sister-chromatid exchange induction by indirect
mutagens/carcinogens, aryl hydrocarbon hydroxylase activity and
benzo[alpha]pyrene metabolism in cultured human hepatoma cells.
Mutat Res. 109:83–90. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cherpillod P and Amstad PA:
Benzo[a]pyrene-induced mutagenesis of p53 hot-spot codons 248 and
249 in human hepatocytes. Mol Carcinog. 13:15–20. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SY, Lee SM, Ye SK, Yoon SH, Chung MH
and Choi J: Benzo[a]pyrene-induced DNA damage and p53 modulation in
human hepatoma HepG2 cells for the identification of potential
biomarkers for PAH monitoring and risk assessment. Toxicol Lett.
167:27–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Delgado ME, Haza AI, Arranz N, Garcia A
and Morales P: Dietary polyphenols protect against N-nitrosamines
and benzo(a)pyrene-induced DNA damage (strand breaks and oxidized
purines/pyrimidines) in HepG2 human hepatoma cells. Eur J Nutr.
47:479–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian M, Zhao B, Zhang J, Martin FL, Huang
Q, Liu L and Shen H: Association of environmental benzo[a]pyrene
exposure and DNA methylation alterations in hepatocellular
carcinoma: A Chinese case-control study. Sci Total Environ.
541:1243–1252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ba Q, Li J, Huang C, Qiu H, Li J, Chu R,
Zhang W, Xie D, Wu Y and Wang H: Effects of benzo[a]pyrene exposure
on human hepatocellular carcinoma cell angiogenesis, metastasis,
and NF-κB signaling. Environ Health Perspect. 123:246–254.
2015.PubMed/NCBI
|
|
17
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Osaki LH and Gama P: MAPKs and signal
transduction in the control of gastrointestinal epithelial cell
proliferation and differentiation. Int J Mol Sci. 14:10143–10161.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Zhao GD, Shi Z, Qi LL, Zhou LY and
Fu ZX: The Ras/Raf/MEK/ERK signaling pathway and its role in the
occurrence and development of HCC. Oncol Lett. 12:3045–3050. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang S and Liu G: Targeting the
Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol Lett.
13:1041–1047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mehdizadeh A, Somi MH, Darabi M and
Jabbarpour-Bonyadi M: Extracellular signal-regulated kinase 1 and 2
in cancer therapy: A focus on hepatocellular carcinoma. Mol Biol
Rep. 43:107–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Liu Q, Wu M, Li M, Ding H, Shan X,
Liu J, Tao T, Ni R and Chen X: GAB2 promotes cell proliferation by
activating the ERK signaling pathway in hepatocellular carcinoma.
Tumour Biol. 37:11763–11773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ito Y, Sasaki Y, Horimoto M, Wada S,
Tanaka Y, Kasahara A, Ueki T, Hirano T, Yamamoto H, Fujimoto J, et
al: Activation of mitogen-activated protein kinases/extracellular
signal-regulated kinases in human hepatocellular carcinoma.
Hepatology. 27:951–958. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmitz KJ, Wohlschlaeger J, Lang H,
Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR,
Schmid KW and Baba HA: Activation of the ERK and AKT signalling
pathway predicts poor prognosis in hepatocellular carcinoma and ERK
activation in cancer tissue is associated with hepatitis C virus
infection. J Hepatol. 48:83–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang L, Yan Q, Fang S, Liu M, Li Y, Yuan
YF, Li Y, Zhu Y, Qi J, Yang X, et al: Calcium-binding protein 39
promotes hepatocellular carcinoma growth and metastasis by
activating extracellular signal-regulated kinase signaling pathway.
Hepatology. 66:1529–1545. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dang Z, Shangguan J, Zhang C, Hu P, Ren Y,
Lv Z, Xiang H and Wang X: Loss of protocadherin-17 (PCDH-17)
promotes metastasis and invasion through hyperactivation of
EGFR/MEK/ERK signaling pathway in hepatocellular carcinoma. Tumour
Biol. 37:2527–2535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
29
|
Guo J, Xu Y, Ji W, Song L, Dai C and Zhan
L: Effects of exposure to benzo[a]pyrene on metastasis of breast
cancer are mediated through ROS-ERK-MMP9 axis signaling. Toxicol
Lett. 234:201–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Zhai W, Wang H, Xia X and Zhang C:
Benzo(a)pyrene promotes A549 cell migration and invasion through
up-regulating twist. Arch Toxicol. 89:451–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Shen M, Lu P, Li X, Zhu S and Yue
S: NEDD9 may regulate hepatocellular carcinoma cell metastasis by
promoting epithelial-mesenchymal-transition and stemness via
repressing Smad7. Oncotarget. 8:1714–1724. 2017.PubMed/NCBI
|
|
32
|
Wang C, Ruan P, Zhao Y, Li X, Wang J, Wu
X, Liu T, Wang S, Hou J, Li W, et al: Spermidine/spermine
N1-acetyltransferase regulates cell growth and metastasis via
AKT/β-catenin signaling pathways in hepatocellular and colorectal
carcinoma cells. Oncotarget. 8:1092–1109. 2017.PubMed/NCBI
|
|
33
|
Ye Y, Long X, Zhang L, Chen J, Liu P, Li
H, Wei F, Yu W, Ren X and Yu J: NTS/NTR1 co-expression enhances
epithelial-to-mesenchymal transition and promotes tumor metastasis
by activating the Wnt/β-catenin signaling pathway in hepatocellular
carcinoma. Oncotarget. 7:70303–70322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong Q, Zhu X, Dai C, Zhang X, Gao X, Wei
J, Sheng Y, Zheng Y, Yu J, Xie L, et al: Osteopontin promotes
epithelial-mesenchymal transition of hepatocellular carcinoma
through regulating vimentin. Oncotarget. 7:12997–13012. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bolotina NA, Gasparian AV, Dubovaja TK,
Evteev VA and Kobliakov VA: Benzo[a]pyrene-dependent activation of
transcription factors NF-kappaB and AP-1 related to tumor promotion
in hepatoma cell cultures. Biochemistry (Mosc). 72:552–557. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cui H, Gu X, Chen J, Xie Y, Ke S, Wu J,
Golovko A, Morpurgo B, Yan C, Phillips TD, et al: Pregnane X
receptor regulates the AhR/Cyp1A1 pathway and protects liver cells
from benzo-[α]-pyrene-induced DNA damage. Toxicol Lett. 275:67–76.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marrone AK, Tryndyak V, Beland FA and
Pogribny IP: MicroRNA responses to the genotoxic carcinogens
aflatoxin B1 and Benzo[a]pyrene in human HepaRG cells. Toxicol Sci.
149:496–502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Souza T, Jennen D, van Delft J, van
Herwijnen M, Kyrtoupolos S and Kleinjans J: New insights into
BaP-induced toxicity: Role of major metabolites in transcriptomics
and contribution to hepatocarcinogenesis. Arch Toxicol.
90:1449–1458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ueng TH, Chang YL, Tsai YY, Su JL, Chan
PK, Shih JY, Lee YC, Ma YC and Kuo ML: Potential roles of
fibroblast growth factor-9 in the benzo(a)pyrene-induced invasion
in vitro and the metastasis of human lung adenocarcinoma. Arch
Toxicol. 84:651–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miller ME, Holloway AC and Foster WG:
Benzo-[a]-pyrene increases invasion in MDA-MB-231 breast cancer
cells via increased COX-II expression and prostaglandin E2 (PGE2)
output. Clin Exp Metastasis. 22:149–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hitt Patten E, DeLong MJ and Merrill AH
Jr: Benzo(a)pyrene activates extracellular signal-related and p38
mitogen-activated protein kinases in HT29 colon adenocarcinoma
cells: Involvement in NAD(P)H:quinone reductase activity and cell
proliferation. Toxicol Appl Pharmacol. 183:160–167. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang BY, Wu SY, Tang SC, Lai CH, Ou CC, Wu
MF, Hsiao YM and Ko JL: Benzo[a]pyrene-induced cell cycle
progression occurs via ERK-induced Chk1 pathway activation in human
lung cancer cells. Mutat Res. 773:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kometani T, Yoshino I, Miura N, Okazaki H,
Ohba T, Takenaka T, Shoji F, Yano T and Maehara Y: Benzo[a]pyrene
promotes proliferation of human lung cancer cells by accelerating
the epidermal growth factor receptor signaling pathway. Cancer
Lett. 278:27–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Castillo-Sanchez R, Villegas-Comonfort S,
Galindo-Hernandez O, Gomez R and Salazar EP: Benzo-[a]-pyrene
induces FAK activation and cell migration in MDA-MB-231 breast
cancer cells. Cell Biol Toxicol. 29:303–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu L, Lau SH, Tzang CH, Wen JM, Wang W,
Xie D, Huang M, Wang Y, Wu MC, Huang JF, et al: Association of
Vimentin overexpression and hepatocellular carcinoma metastasis.
Oncogene. 23:298–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wei PL, Kuo LJ, Wang W, Lin FY, Liu HH,
How T, Ho YS, Huang MT, Wu CH and Chang YJ: Silencing of
glucose-regulated protein 78 (GRP78) enhances cell migration
through the upregulation of vimentin in hepatocellular carcinoma
cells. Ann Surg Oncol. 19 Suppl 3:S572–S579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang TH, Lin YS, Chen Y, Yeh CT, Huang YL,
Hsieh TH, Shieh TM, Hsueh C and Chen TC: Long non-coding RNA AOC4P
suppresses hepatocellular carcinoma metastasis by enhancing
vimentin degradation and inhibiting epithelial-mesenchymal
transition. Oncotarget. 6:23342–23357. 2015.PubMed/NCBI
|
|
48
|
Xie X, Zhu H, Zhang J, Wang M, Zhu L, Guo
Z, Shen W and Wang D: Solamargine inhibits the migration and
invasion of HepG2 cells by blocking epithelial-to-mesenchymal
transition. Oncol Lett. 14:447–452. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen C, Xue Y, Zhang D, Xu W, Xu H, Yao H,
Pei D and Gu Y: Short hairpin RNA silencing of TGF-βRII and FZD-7
synergistically suppresses proliferation and metastasis of
hepatocellular carcinoma cells. Oncol Lett. 11:2039–2046. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He S, Lin J, Yu S and Sun S: Upregulation
of PREX2 promotes the proliferation and migration of hepatocellular
carcinoma cells via PTEN-AKT signaling. Oncol Lett. 11:2223–2228.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu Z, Wang J, Mao Y, Zou B and Fan X:
MicroRNA-101 suppresses migration and invasion via targeting
vascular endothelial growth factor-C in hepatocellular carcinoma
cells. Oncol Lett. 11:433–438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao X, Yang W, Pei F, Ma W and Wang Y:
Downregulation of matrix metalloproteinases contributes to the
inhibition of cell migration and invasion in HepG2 cells by sodium
valproate. Oncol Lett. 10:531–535. 2015. View Article : Google Scholar : PubMed/NCBI
|