Introduction
Lung cancer is the most common cause of
cancer-associated mortality worldwide, and non-small cell lung
cancer (NSCLC) accounts for ~85% of all types of lung cancer
(1). Although lung cancer mortality
has decreased by ~16–20% as a result of screening with low-dose
computed tomography compared with standard chest x-ray, the
majority of lung cancer remains diagnosed at an advanced stage and
frequently has a poor prognosis (2).
Mortality often occurs even in patients with early-stage lung
cancer as a result of recurrent disease and distant metastases
following curative surgical resection. The long-term survival rate
for patients with lung cancer remains low (3). Consequently, prognostic markers other
than the TNM staging system (4) are
necessary in order to improve the prognosis of patients with NSCLC
who have received treatment.
Testes-specific protease 50 (TSP50) is a
testis-specific gene, which encodes a protease-like protein, and is
normally specifically expressed in the spermatocytes of human
testes and is involved in spermatogenesis (5,6). However,
a previous study has observed it to be abnormally activated and
expressed in the majority of breast cancer epithelial cells
(6). This abnormal activation and
expression has been associated with the putative p53-binding site
and several Sp1-binding sites on the TSP50 promoter, which means
that the TSP50 gene maybe negatively regulated by the p53
gene (7). Additional studies have
demonstrated that the overexpression of TSP50 promotes cellular
proliferation and tumorigenesis depending on activation of the
nuclear factor-κB (NF-κB) signaling pathway, by binding to the
NF-κB-nuclear factor of κ light polypeptide gene enhancer in
B-cells inhibitor α (IκBα) complex. This in turn promotes cellular
invasion and metastasis by increasing NF-κB dependent matrix
metalloproteinase-9 (MMP9) expression, thus TSP50 and MMP9 have
been associated with increased rates of metastasis and poorer
survival rate of patients with breast cancer (8,9). In
addition, an increasing number of studies have reported that TSP50
is involved in the progression of other malignant tumors, including
colorectal carcinoma, gastric and cervical cancer (10–12). In
conclusion, these aforementioned results imply that the TSP50 gene
may be an oncogene.
Despite the increasing number of studies
demonstrating that TSP50 is involved in tumorgenesis in various
different types of cancer, the expression state of the TSP50 gene
in NSCLC remains unknown. The present study therefore aimed to
examine the status of the TSP50 protein in specimens of human
NSCLC. The clinicopathological and prognostic significance of TSP50
expression for patients with NSCLC was also assessed.
Materials and methods
Patients and tissue samples
The present retrospective study randomly selected
NSCLC specimens paired with adjacent non-tumorous tissues and
normal lung tissues from patients who had undergone surgical
resection for lung cancer at The First People's Hospital of
Shanghai Jiao Tong University (Shanghai, China), between January
2006 and January 2011. The exclusive criteria were as follows:
Patients who died within 1 month of surgery; patients with history
of second primary tumor; and patients who received neoadjuvant
chemotherapy or radiotherapy. During the study period, tissues from
355 patients who underwent surgical resection at The First People's
Hospital of Shanghai Jiao Tong University were available. In total,
21 cases were excluded due to samples being too small to detect
immunohistochemical staining, 97 were excluded due to incomplete
survival rate data and 39 were excluded according to the exclusion
criteria described above. Therefore, a total of 198 patients were
enrolled in the present retrospective study, and the clinical
follow-up duration of the patients ranged between 2.5 and 98.1
months. The present study was approved by the Ethics Committee of
Shanghai Jiao Tong University School of Medicine (Shanghai, China)
and informed consent was obtained from each patient prior to
surgery.
Surgical specimens were fixed in 4% neutral-buffered
formaldehyde at room temperature for 24 h, and processed for
histopathological and immunohistochemical evaluation.
Clinicopathological factors including age, sex, smoking history,
extent of tumor differentiation, visceral pleural invasion, T
status lymphatic invasion and TNM stage were reviewed for all above
cases. The treatment of patients (surgical procedure and adjuvant
therapy) was also reviewed. Histological diagnosis and grade of
differentiation of the tumors were determined by hematoxylin and
eosin staining according to the World Health Organization criteria
(13) and pathological stage were
classified according to the 7th edition of the American Joint
Committee on Cancer (4). All stage
IIA or higher patients and high-risk stage IB patients with poorly
differentiated tumors or incomplete lymph node resection had
received platinum-based adjuvant chemotherapy or radiotherapy.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue blocks
were cut into sections of ~3–4 µm thickness. Paraffin sections were
routinely deparaffinized with dimethylbenzene after heated at 60°C
for 2.5 h, rehydrated with a graded alcohol series of 100, 90, 80
and 70% concentrations for 5 min separately and heated for antigen
retrieval with 0.01 mol/l citrate buffer solution (pH 6.0) for 25
min at 95°C in a microwave oven, and allowed to cool for 60 min at
room temperature. Endogenous peroxidase was blocked in 3%
H2O2 solution for 10 min at 37°C. Slides were
subsequently blocked with 10% normal goat serum (cat. no. AR0004;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) at room
temperature for 10 min and incubated overnight at 4°C with the
primary rabbit polyclonal antibody against TSP50 (dilution, 1:150;
cat. no. 12574-1-AP; ProteinTech Group, Inc., Chicago, IL, USA).
Slides with PBS served as parallel negative controls and breast
cancer sections incubated with the TSP50 antibody were used as
positive controls. Subsequent to five washing steps with PBS (pH
7.4), slides were incubated with goat anti-rabbit second antibody
conjugated with streptavidin-biotin peroxidase (dilution, 1:1,000;
cat. no. SA1052; Wuhan Boster Biological Technology, Ltd.) at 37°C
for 20 min and visualized with 3,3-diaminobenzidine. The sections
were then counterstained with hematoxylin, washed with tap water
and dehydrated with ethanol washes of 1 min each at increasing
concentrations of 75, 80, 90 and 100%.
Evaluation of immunohistochemical
staining
Two experienced pathologists who were blind to
clinical information independently evaluated all TSP50 stained
slides. Staining intensities of individual cells were graded as
follows: 0, no staining; 1, weak; 2, medium; and 3, strong. The
percentages of positive tumor cells in the field were calculated as
follows: Score 0, negative staining; score 1, 0–10%; score 2,
10–50%; score 3, 51–75%; and score 4, 75–100%. The final score was
the sum of the staining intensity score and percentage staining
score. The final scores were then defined as: 0, negative
expression; 1–3, weakly positive expression; 4–5, moderate
expression; and 6–7, strong positive expression. The scores from
all NSCLC samples were then divided into two groups: Low expression
(scores 0–3); and high expression (scores 4–7).
Assessment and imaging of IHC was performed using a
Leica DM6000B light microscope equipped with Leica DFC
Cameras-Image Acquisition System software [version 4.5.0; Leica
Microsystems (Schweiz) AG, Heerbrugg, Switzerland].
Statistical analysis
Disease free survival (DFS) was defined as the time
from surgical resection to recurrence, and overall survival (OS) as
the time from surgical resection to mortality relative to disease
recurrence. Associations between TSP50 protein expression and
clinicopathological parameters were assessed using the Pearson's
χ2 test or Fisher's exact test for the categorical
variables. To analyze the follow-up data, DFS and OS curves were
generated using the Kaplan-Meier method and differences in survival
curves was analyzed using the log-rank test. Multivariate analysis
was performed using the Cox's proportional hazards regression model
on all significant characteristics determined from univariate
analysis. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS software version 21.0 for Windows (IBM SPSS, Armonk, NY,
USA).
Results
Patients clinical characteristics
Among the 198 patients, 110 were males and 88 were
females, with a mean age of 66.3±10.19 years (range, 22–80 years),
and 95 patients were current or former smokers while 103 patients
had never smoked. In total, 116 patients (59%) were classified as
having early stage NSCLC (stage I or II) and 82 patients (41%) were
classified as advanced stage (stage III and IV). A total of 81
patients (41%) had positive lymph nodes, 53 patients (27%) were
staged as T3 or T4, 64 (32%) had well-differentiated tumors and 98
(50%) had moderately-differentiated tumors. A total of 90 patients
(45%) were positive for visceral pleural invasion, and 124 patients
(63%) received adjuvant chemotherapy or radiotherapy subsequent to
surgery. All specimens consisted of 106 patients with
adenocarcinoma (54%) and 92 patients with non-adenocarcinoma (46%;
77 squamous carcinoma and 15 adenosquamous carcinoma). The specific
clinical characteristics of patients included in the present study
are summarized in Table I.
 | Table I.TSP50 expression and
clinicopathological parameters of NSCLC cases. |
Table I.
TSP50 expression and
clinicopathological parameters of NSCLC cases.
|
|
| TSP50 expression
(%) |
|
|---|
|
|
|
|
|
|---|
| Characteristics | No. of patients
(%) | Low expression
(negative and weak) | High expression
(medium and strong) | P-value |
|---|
| Total | 198 (100) | 122 (62) | 76 (38) |
|
| Age, years |
|
|
| 0.367 |
|
<60 | 73 (37) | 42 (22) | 31 (15) |
|
|
>60 | 125 (63) | 80 (40) | 45 (23) |
|
| Sex |
|
|
| 0.414 |
| Male | 110 (56) | 65 (33) | 45 (23) |
|
|
Female | 88 (44) | 57 (29) | 31 (15) |
|
| Smoking history |
|
|
| 0.301 |
|
Smoker | 95 (48) | 55 (28) | 40 (20) |
|
| Never
smoked | 103 (52) | 67 (34) | 36 (18) |
|
| Tumor
differentiation |
|
|
| 0.012 |
| Well | 64 (32) | 46 (23) | 18 (9) |
|
|
Moderately | 98 (50) | 61 (31) | 37 (19) |
|
|
Poorly | 36 (18) | 15 (8) | 21 (10) |
|
| Visceral pleural
invasion |
|
|
| 0.455 |
|
Absent | 90 (45) | 58 (29) | 32 (16) |
|
|
Present | 108 (55) | 64 (33) | 44 (22) |
|
| T status |
|
|
| 0.004 |
|
T1+T2 | 145 (73) | 98 (50) | 47 (23) |
|
|
T3+T4 | 53 (27) | 24 (12) | 29 (15) |
|
| Lymphatic
invasion |
|
|
| 0.245 |
|
Positive | 81 (41) | 46 (24) | 35 (17) |
|
|
Negative | 117 (59) | 76 (38) | 41 (21) |
|
| TNM stage |
|
|
| 0.026 |
|
I+II | 116 (59) | 79 (40) | 37 (19) |
|
|
III+IV | 82 (41) | 43 (22) | 39 (19) |
|
| Surgical
procedure |
|
|
| 0.196 |
|
Pneumonectomy | 11 (6) | 6 (3) | 5 (3) |
|
|
Lobectomy | 125 (63) | 83 (42) | 42 (21) |
|
| Wedge
resection | 62 (31) | 33 (17) | 29 (14) |
|
| Histological
types |
|
|
| 0.431 |
|
ADC | 106 (54) | 68 (35) | 38 (19) |
|
|
Non-adenocarcinoma | 92 (46) | 54 (27) | 38 (19) |
|
| Adjuvant
therapy |
|
|
| 0.277 |
|
Yes | 124 (63) | 80 (41) | 44 (22) |
|
| No | 74 (37) | 42 (21) | 32 (16) |
|
TSP50 is highly expressed in
NSCLC
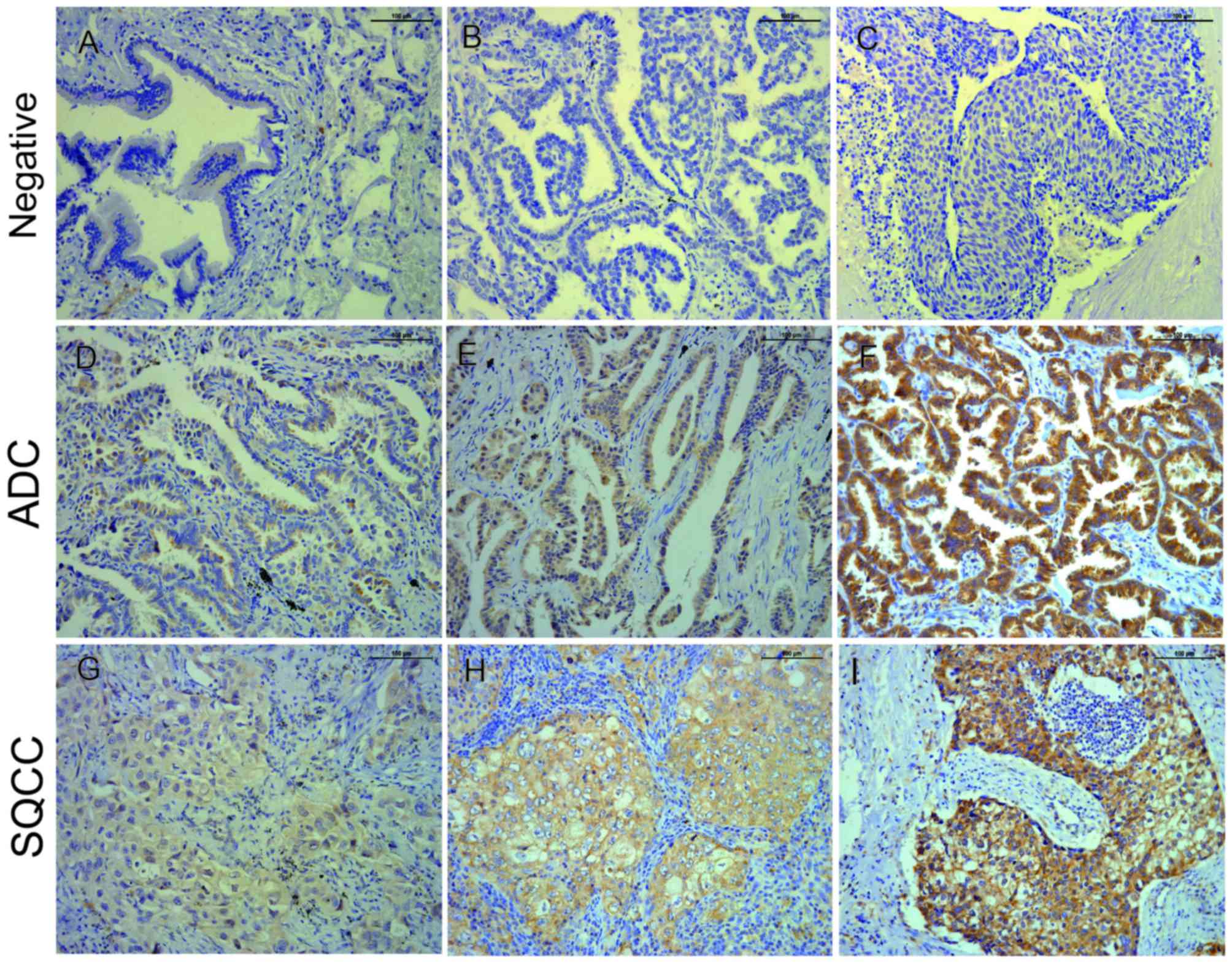
The present study detected 198 cases of
paraffin-embedded NSCLC with matched adjacent non-tumor tissues, as
well as 15 cases of paraffin-embedded normal lung tissues via
immunohistochemical staining. Stained cells were recognized as
positive when cytoplasmic staining was identified. The low
expression (negative and weak protein expression combined) rate of
TSP50 in NSCLC tissues was 61%. Meanwhile, the high expression
(moderate and strong protein expression combined) rate of TSP50 in
NSCLC tissues was 39% (Table II),
which was significantly increased compared with the rates in either
adjacent non-tumor or normal lung tissues, respectively
(P<0.001; Fig. 1, Table II).
 | Table II.TSP50 immunohistochemical staining
for protein expression in NSCLC, adjacent non-tumorous and normal
tissues. |
Table II.
TSP50 immunohistochemical staining
for protein expression in NSCLC, adjacent non-tumorous and normal
tissues.
|
|
| TSP50 protein
expression |
|
|---|
|
|
|
|
|
|---|
| Tissue sample | No. of cases | Negative (%) | Weak (%) | Medium (%) | Strong (%) | P-value |
|---|
| NSCLC tissues | 198 | 64
(32) | 58 (29) | 61 (31) | 15 (8) | <0.001 |
| Adjacent
non-tumor | 198 | 177 (89) | 19 (10) | 2 (1) | 0
(0) |
|
| Normal lung
tissues | 15 | 15
(100) | 0 (0) | 0 (0) | 0
(0) |
|
Association between TSP50 expression
and clinicopathological characteristics
The association between TSP50 expression and the
clinicopathological characteristics of patients with NSCLC is
summarized in Table I. The results
demonstrated that the expression of TSP50 was correlated with tumor
differentiation (P=0.012), and that high expression of TSP50 was
more frequent among poorly differentiated tumors compared with well
or moderately differentiated tumors [18 and 32 (P=0.003), 18 and
50% (P=0.033), respectively]. In addition, high expression of TSP50
was more frequently observed in advanced stage (III and IV) samples
compared with early stage (I and II) specimens (41 and 59%,
respectively; P=0.026). Similar results were observed for tumor
status, with high expression levels of TSP50 more frequently
observed in late tumor status (T3+T4) compared with in early tumor
status (T1+T2) specimens (54 and 32%, respectively; P=0.004).
However, the Pearson's χ2 test and Fisher's exact test
identified no significant association between TSP50 expression and
age, sex, smoking history, visceral pleural invasion, lymphatic
invasion, surgical procedure, histological types or adjuvant
therapy.
TSP50 high-level expression predicts
poor survival rates in NSCLC patients
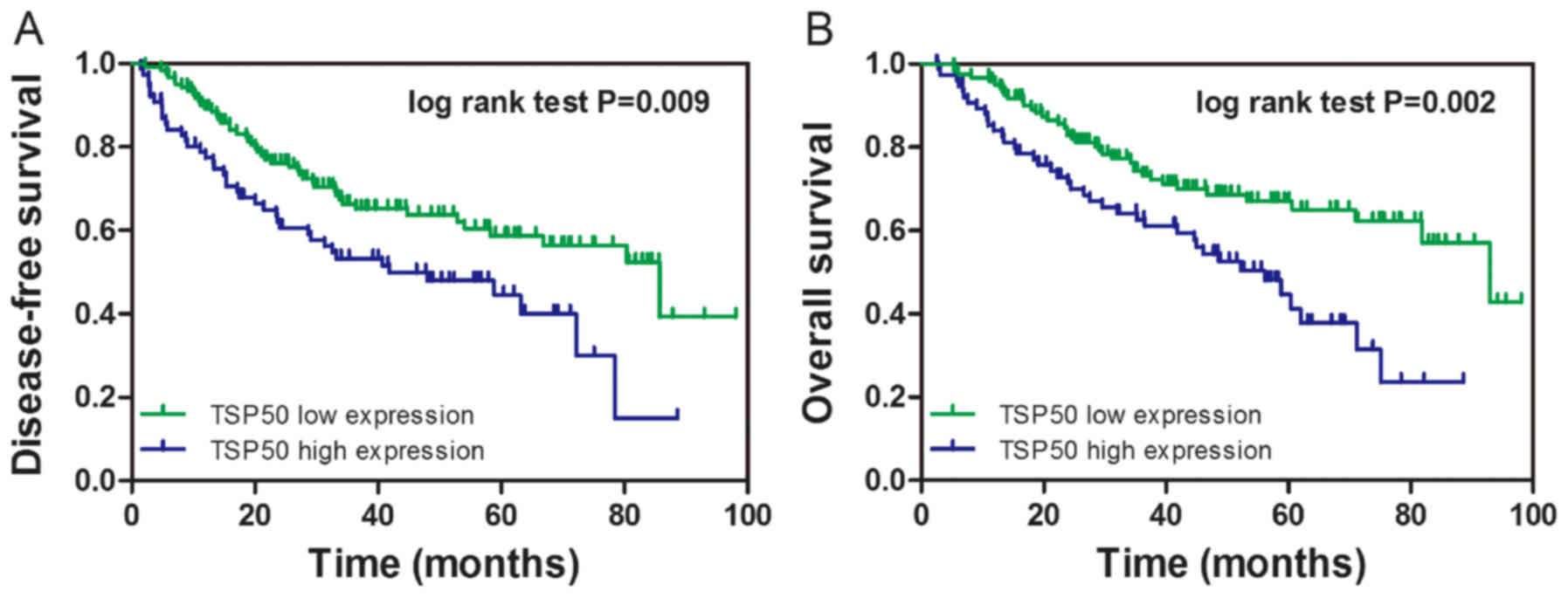
To investigate the prognostic value of TSP50
expression in NSCLC, the present study constructed a survival curve
via the Kaplan-Meier method and analyzed the association between
TSP50 expression and patient survival rate using the log-rank test.
It was revealed that patients with high TSP50 expression had lower
DFS (log-rank=6.876, P=0.009) and OS (log-rank=9.609, P=0.002)
rates compared within patients with low TSP50 expression (Fig. 2A and B, respectively).
TSP50 is an independent prognostic
factor in SCLC via the Cox's proportional hazards regression
model
The median DFS for all study patients was 38.0
months. By univariate analysis, it was identified that in patients
with NSCLC, a poorer DFS was associated with smoking history
(HR=1.592, P=0.031), lymph node metastasis (HR=1.658, P=0.019) and
an advanced (III and IV) TNM stage (HR=1.649, P=0.021). In
addition, high TSP50 expression was significantly associated with a
poorer DFS (33.7 vs. 41.0 months, HR=1.751, P=0.010). Multivariate
survival analysis was performed using the Cox's proportional
hazards regression model for all significant variables in the
univariate survival analysis. This multivariate analysis revealed
that smoking history (HR=1.548, P=0.044), lymph node invasion
(HR=1.587, P=0.033), TNM stage (HR=1.610, P=0.030) and TSP50
expression (HR=1.590, P=0.034) were independent prognostic
indicators of DFS for patients with NSCLC (Table III).
 | Table III.Univariate and multivariate Cox's
proportional hazard models for DFS. |
Table III.
Univariate and multivariate Cox's
proportional hazard models for DFS.
|
|
| Disease-free
survival (DFS) |
|---|
|
|
|
|
|---|
|
Characteristics | No. of patients
(%) | Median DFS
(months) | Univariate analysis
adjusted HR (95% CI) | Univariate analysis
P-value | Multivariate
analysis adjusted HR (95% CI) | Multivariate
analysis P-value |
|---|
| All | 198 (100) | 38.0 |
|
|
|
|
| Age, years |
|
|
| 0.455 |
| NA |
|
<60 | 73 (37) | 36.2 | 0.848
(0.551–1.306) |
| NA |
|
|
>60 | 125 (63) | 39.1 |
|
|
|
|
| Sex |
|
|
| 0.167 |
| NA |
|
Male | 110 (56) | 35.9 | 1.354
(0.881–2.079) |
| NA |
|
|
Female | 88 (44) | 40.7 |
|
|
|
|
| Smoking
history |
|
|
| 0.031 |
| 0.044 |
|
Smoker | 95 (48) | 34.5 | 1.592
(1.043–2.430) |
| 1.548
(1.011–2.370) |
|
| Never
smoked | 103 (52) | 41.2 |
|
|
|
|
| Tumor
differentiation |
|
|
| 0.059 |
| NA |
|
Well | 64 (32) | 39.7 | 0.751
(0.558–1.011) |
| NA |
|
|
Moderately | 98 (50) | 37.1 |
|
|
|
|
|
Poorly | 36 (18) | 37.5 |
|
|
|
|
| Visceral pleural
invasion |
|
|
| 0.586 |
| NA |
|
Absent | 90 (45) | 39.1 | 1.125
(0.736–1.719) |
| NA |
|
|
Present | 108 (55) | 37.5 |
|
|
|
|
| T status |
|
|
| 0.067 |
| NA |
| T1+
T2 | 145 (73) | 39.5 | 1.523
(0.972–2.387) |
| NA |
|
| T3+
T4 | 53 (27) | 34.8 |
|
|
|
|
| Lymphatic
invasion |
|
|
| 0.019 |
| 0.033 |
|
Positive | 81 (41) | 32.9 | 1.658
(1.087–2.530) |
| 1.587
(1.038–2.424) |
|
|
Negative | 117 (59) | 41.9 |
|
|
|
|
| TNM stage |
|
|
| 0.021 |
| 0.030 |
| I +
II | 116 (59) | 40.5 | 1.649
(1.078–2.522) |
| 1.610
(1.047–2.474) |
|
| III +
IV | 82 (41) | 35.0 |
|
|
|
|
| Surgical
procedure |
|
|
| 0.145 |
| NA |
|
Pneumonectomy | 11 (6) | 45.2 | 0.753
(0.514–1.103) |
| NA |
|
|
Lobectomy | 125 (63) | 39.1 |
|
|
|
|
| Wedge
resection | 62 (31) | 35.9 |
|
|
|
|
| Histological
types |
|
|
| 0.295 |
| NA |
|
ADC | 106 (54) | 39.2 | 0.798
(0.524–1.216) |
|
|
|
|
Non-adenocarcinoma | 92 (46) | 36.7 |
|
| NA |
|
| Adjuvant
therapy |
|
|
| 0.338 |
|
|
| No | 124 (63) | 40.4 | 1.244
(0.796–1.944) |
| NA | NA |
|
Yes | 74 (37) | 37.0 |
|
|
|
|
| TSP50
expression |
|
|
| 0.010 |
| 0.034 |
|
Low | 122 (62) | 41.0 | 1.751
(1.146–2.677) |
| 1.590
(1.035–2.441) |
|
|
High | 76 (38) | 33.7 |
|
|
|
|
The median OS for all study subjects was 42.3
months. Univariate analysis demonstrated that late tumor status
(T3+T4; HR=1.651, P=0.037), lymph node metastasis (HR=1.889,
P=0.005), advanced stage (III and IV; HR=2.119, P=0.001), and high
TSP50 expression (38.2 vs. 45.2, P=0.002) were significantly
associated with poorer OS. Additional multivariate analysis
demonstrated that lymph node invasion (HR=1.849, P=0.007), TNM
stage (HR=1.975, P=0.003) and TSP50 expression (HR=1.814, P=0.010)
proved to be independent prognostic indicators of OS for NSCLC
patients (Table IV).
 | Table IV.Univariate and multivariate Cox's
proportional hazard models for OS. |
Table IV.
Univariate and multivariate Cox's
proportional hazard models for OS.
|
|
| OS |
|---|
|
|
|
|
|---|
|
Characteristics | No. of patients
(%) | Median OS
(months) | Univariate analysis
adjusted HR (95% CI) | Univariate analysis
P-value | Multivariate
analysis adjusted HR (95% CI) | Multivariate
analysis P-value |
|---|
| All | 198 (100) | 42.3 |
|
|
|
|
| Age, years |
|
|
| 0.521 |
| NA |
|
<60 | 73 (37) | 40.0 | 0.862
(0.547–1.358) |
| NA |
|
|
>60 | 125 (63) | 43.7 |
|
|
|
|
| Sex |
|
|
| 0.177 |
| NA |
|
Male | 110 (56) | 40.0 | 1.363
(0.869–2.139) |
| NA |
|
|
Female | 88 (44) | 45.6 |
|
|
|
|
| Smoking
history |
|
|
| 0.058 |
| NA |
|
Smoker | 95 (48) | 38.6 | 1.535
(0.985–2.391) |
| NA |
|
| Never
smoked | 103 (52) | 45.7 |
|
|
|
|
| Tumor
differentiation |
|
|
| 0.311 |
| NA |
|
Well | 64 (32) | 44.6 |
|
|
|
|
|
Moderately | 98 (50) | 41.1 | 0.851
(0.623–1.162) |
| NA |
|
|
Poorly | 36 (18) | 41.5 |
|
|
|
|
| Visceral pleural
invasion |
|
|
| 0.067 |
| NA |
|
Absent | 90 (45) | 44.1 | 1.536
(0.971–2.430) |
| NA |
|
|
Present | 108 (55) | 41.3 |
|
|
|
|
| T status |
|
|
| 0.037 |
| 0.965 |
| T1+
T2 | 145 (73) | 44.1 | 1.651
(1.032–2.641) |
| 1.012
(0.604–1.695) |
|
| T3+
T4 | 53 (27) | 38.3 |
|
|
|
|
| Lymphatic
invasion |
|
|
| 0.005 |
| 0.007 |
|
Positive | 81 (41) | 36.6 | 1.889
(1.213–2.942) |
| 1.849
(1.186–2.884) |
|
|
Negative | 117 (59) | 46.6 |
|
|
|
|
| TNM stage |
|
|
| 0.001 |
| 0.003 |
| I +
II | 116 (59) | 45.1 | 2.119
(1.351–3.324) |
| 1.975
(1.255–3.108) |
|
| III +
IV | 82 (41) | 39.0 |
|
|
|
|
| Surgical
procedure |
|
|
| 0.378 |
| NA |
|
Pneumonectomy | 11 (6) | 47.7 |
|
| NA |
|
|
Lobectomy | 125 (63) | 43.8 | 0.834
(0.556–1.249) |
|
|
|
| Wedge
resection | 62 (31) | 40.0 |
|
|
|
|
| Histological
types |
|
|
| 0.275 |
| NA |
|
ADC | 106 (54) | 43.8 | 0.782
(0.503–1.216) |
| NA |
|
|
Non-adenocarcinoma | 92 (46) | 40.7 |
|
|
|
|
| Adjuvant
therapy |
|
|
| 0.256 |
| NA |
| No | 124 (63) | 43.8 | 1.317
(0.819–2.118) |
| NA |
|
|
Yes | 74 (37) | 41.8 |
|
|
|
|
| TSP50
expression |
|
|
| 0.002 |
| 0.010 |
|
Low | 122 (62) | 45.2 | 1.999
(1.279–3.124) |
| 1.814
(1.156–2.846) |
|
|
High | 76 (38) | 38.2 |
|
|
|
|
Discussion
TSP50 is a human testis-specific gene, which encodes
a 50-kDa serine (Ser) protease-like protein, and was isolated from
a fragment of hypomethylated DNA from human breast cancer cells
using the methylation sensitive representational difference
analysis technique (14). Although
the TSP50 gene product shares a similar enzymatic structure with
numerous Ser proteases, it is possible to classify it as a novel
protease for its two critical catalytic triads, which have a
substitution of threonine (Thr) at the Ser residue site, which
makes it different from traditional Ser proteases (5,15,16). Normally, TSP50 is expressed only in
germ cells in human testes and is overexpressed in different types
of human cancers, including breast and cervical cancer and
laryngocarcinoma (17). Currently,
TSP50 is also considered to be a cancer/testis antigen (CTA), which
are immunogenic proteins (18). CTAs
are a large family of ~140 members, a number of which have been
reported to be overexpressed in lung cancer, including
melanoma-associated antigen (MAGE)-3, NY-SAR-35, NY-ESO-1, MAGE-1,
cancer testis-7, cancer/testis antigen 2 and MAGE-10 (19,20). Grah
et al (21) reported that a
statistically higher immunohistological expression rate of
MAGE-A3/4 was observed in squamous cell carcinoma. The
aforementioned study also revealed that there was a statistically
significant correlation between MAGE-1 expression in adenocarcinoma
and the presence of tumor necrosis and a significant correlation
between NY-ESO-1 expression and positive lymph nodes in
adenocarcinoma (21).
As little has been reported regardingTSP50
expression in patients with NSCLC, the present study performed IHC
staining of 198 NSCLC specimens and revealed that the expression
rate of the TSP50 protein was significantly increased compared with
either adjacent non-tumor or normal lung tissues, suggesting that
TSP50 may function as an oncogene and may be involved in the
progression of NSCLC. Although weak and medium expression of TSP50
was observed in certain adjacent non-tumorous tissues in the
present study, a possible explanation for this discrepancy is that
these adjacent non-tumorous tissues belong to atypical hyperplasia
tissues, the extent of which may determine the irrelative low-level
expression. In addition, the present study analyzed the
relationship between TSP50 expression and clinicopathological
features of patients with NSCLC and identified that high-level
TSP50 expression was significantly correlated with poor tumor
differentiation (P=0.012), late tumor status (P=0.004) and a late
pathologic stage (P=0.026). In contrast, no relationship was
identified between the expression of TSP50 and other clinical
factors, including age, sex, smoking history, visceral pleural
invasion, lymphatic invasion location subtype or surgical
procedure, histological types and adjuvant therapy (P>0.05).
These results confirmed that TSP50, as with other CTAs, is involved
in NSCLC progression and metastasis. In addition, the results of
the present study were also consistent with previous findings that
suggested TSP50 as a potential oncogene, which has elevated
expression levels in numerous other types of cancer.
Liu et al (11)
and Zheng et al (10) reported
that high expression levels of TSP50 are associated with more
aggressive malignancy and poor prognosis in human gastric cancer
and colorectal carcinoma, particularly for patients with
early-stage colorectal carcinoma tumors. Grah et al
(21) observed that there was a
significantly improved survival rate in patients with
adenocarcinoma who had positive expression of the CTA MAGE-A3/4
(P=0.012). With regard to survival rate, the present study also
observed that patients with NSCLC with high-level TSP50 expression
had lower DFS (P=0.009) and OS rates (P=0.002) compared with
patients with low-level TSP50 expression. Although subgroup
analysis failed to reveal a significant difference in survival
rates in early-stage patients (P=0.184), a trend was still
observed. An increase in sample numbers may aid with the
identification of an association. In addition, multivariate
survival analysis demonstrated that TSP50 expression was a
significant independent hazard factor for DFS in patients with
NSCLC along with smoking history, pathological stage and lymphatic
invasion and for OS along with pathological stage and lymphatic
invasion. These results demonstrated that TSP50 has the potential
to be an effective predictor of poor prognosis. To the best of our
knowledge, this is the first study demonstrating that TSP50
expression is associated with clinical characteristics and poor
prognosis in patients with NSCLC.
Previous studies, along with the present study, have
suggested the use of TSP50 as a potential novel biomarker for the
prognostic evaluation and as a potential molecular target in
various types of tumors (10,11). Therefore, the molecular mechanism of
how TSP50 affects tumor progression needs to be understood. Zhang
(22) revealed that the knockdown of
TSP50 may cause the inhibition of cellular proliferation,
migration, and invasion of laryngocarcinoma Hep2 cells. Yuan et
al (12) reported that the
overexpression of TSP50 promoted proliferation and migration of
cervical cancer cells, depending on its threonine protease activity
and its interactions with tumor necrosis factor-α-induced NF-κB. To
delineate the involvement of TSP50 in cellular proliferation, Li
et al (23) constructed and
characterized a mutant TSP50 (TSP50 T310A) whose mutation
significantly decreased TSP50-induced cellular proliferation in
vitro and abolished the tumorigenicity of TSP50 in nude mice.
These results revealed that the T310A mutation prevented
interaction between TSP50 and the NF-κB-IκBα complex, which is
necessary for TSP50 to perform its function in cellular
proliferation. Despite these previous studies increasing the
understanding of the molecular mechanism by which TSP50 affects
tumor progression, there remain multiple functions and mechanisms
by which TSP50 is involved in tumorigenesis, which have not yet
been elucidated. As the NF-κB pathway has been demonstrated to be
associated with TSP50 in numerous tumorigenesis pathways, it has
been speculated that TSP50 may perform an oncogenic function in
lung tumorigenesis through similar or more complicated signaling
pathways. However, additional in vitro and in vivo
studies are required to clarify this.
In addition, previous results demonstrated that by
enhancing tumor cell survival, CTAs may decrease the effectiveness
of treatment with cytotoxic agents, indicating that CTAs may be
important in treatment responses to cytotoxic or growth inhibitory
anti-cancer drugs (24). As for
TSP50, Zhou et al (25)
reported that TSP50 encodes a Testis-Specific Protease and it is
negatively regulated by p53, knockdown of TSP50 resulted in greater
sensitivity to doxorubicin-induced apoptosis in P19 murine
embryonal carcinoma cells and that activation of caspase-3 was
involved in this process. CTAs expressed in the normal testis and
in different types of human cancers not only exhibit
characteristics important for tumorigenesis, but also represent
promising targets for immunotherapy (20). Locally advanced, good performance
status patients with NSCLC may be offered concurrent chemotherapy,
radical radiotherapy and/or surgery, with a PFS rate of ~8 months
and a <15% 5-year survival rate (26). Therefore, immunotherapeutic strategies
for the treatment of NSCLC are currently in clinical trials, and
involve increasing tumor immunogenicity by using cancer vaccines to
augment tumor-immune recognition and overcoming tumor
immunosuppression by using immune checkpoint inhibitors, which
demonstrated promising prospects (27). The present results demonstrated that
high-level TSP50 expression was significantly correlated with late
tumor status (P=0.004) and late pathological stage (P=0.026).
Therefore, additional investigations to determine the prognostic
value of TSP50 in other clinical settings, including advanced NSCLC
treated with targeted treatments, immunotherapy, or combined with
chemotherapy would facilitate clinical use of this novel
protein.
In conclusion, TSP50 is overexpressed in NSCLC. The
present study proposed that TSP50 may be an independent prognostic
factor and a novel therapeutic target for NSCLC. Additional
investigations are required to fully elucidate the specific
molecular mechanisms behind the function of TSP50 in NSCLC, and to
demonstrate the prognostic value of TSP50 in a clinical
setting.
Acknowledgements
The authors would like to thank Dr Hua-Mei Tang and
Dr Jun Lin (Department of Pathology, Shanghai First People's
Hospital, Shanghai, China) for their evaluation of the IHC
data.
Funding
The present study was supported by grants from The
National Natural Science Foundation of China (General Program;
grant no. 81372521).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL, WLQ and BWS conceived and designed the
experiments. WLQ and BWS performed the experiments, and BWS, YDH
and HYH were responsible for clinical data collection and
follow-up. QL, WLQ, BWS, HMT and JL analyzed the data and QL, WLQ
and BWS contributed reagents, materials and analysis tools. WLQ and
BWS wrote the manuscript and all authors have read and approved the
final version of manuscript.
Ethics approval and consent to
participate
All procedures performed were in conformity to the
ethical standards of the institutional and national committee on
human experimentation and with the 1964 Helsinki declaration and
its later amendments. Ethical approval was given by the medical
ethics committee of Shanghai First People's Hospital with the
following reference number: 2013KY036.
Consent for publication
The study participants provided consent for the data
to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Herbst RS, Heymach JV and Lippman SM: Lung
Cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldstraw P: The 7th edition of TNM in
lung cancer: What now? J Thorac Oncol. 4:671–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan LM, Shan JD, De Risi D, Broome J,
Lovecchio J, Gal D, Vinciguerra V and Xu HP: Isolation of a novel
gene, TSP50, by a hypomethylated DNA fragment in human breast
cancer. Cancer Res. 59:3215–3221. 1999.PubMed/NCBI
|
|
6
|
Shan J, Yuan L, Xiao Q, Chiorazzi N,
Budman D, Teichberg S and Xu HP: A possible protease in human
testes, is activated in breast cancer epithelial cells1. Cancer
Res. 62:290–294. 2002.PubMed/NCBI
|
|
7
|
Xu H, Shan J, Jurukovski V, Yuan L, Li J
and Tian K: TSP50 encodes a testis-specific protease and is
negatively regulated by p53. Cancer Res. 67:1239–1245. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song ZB, Bao YL, Zhang Y, Mi XG, Wu P, Wu
Y, Yu CL, Sun Y, Zheng LH and Huang YX: Testes-specific protease 50
(TSP50) promotes cell proliferation through the activation of the
nuclear factor κB (NF-κB) signalling pathway. Biochem J.
436:457–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song ZB, Ni JS, Wu P, Bao YL, Liu T, Li M,
Fan C, Zhang WJ, Sun LG, Huang YX and Li YX: Testes-specific
protease 50 promotes cell invasion and metastasis by increasing
NF-kappaB-dependent matrix metalloproteinase-9 expression. Cell
Death Dis. 6:e17032015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng L, Xie G, Duan G, Yan X and Li Q:
High Expression of Testes-specific protease 50 is associated with
poor prognosis in colorectal carcinoma. PLoS One. 6:e222032011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu F, Cao Q, Liu N, Li C, You C, Liu C,
Xue L and Luo R: Overexpression of testes-specific protease 50
(TSP50) predicts poor prognosis in patients with gastric cancer.
Gastroenterol Res Pract. 2014:4982462014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan J, Wu C, Huang M, Zhou J, Ben W and
Zhang G: TSP50 depends on its threonine protease activity and its
interactions with TNF-α-induced NF-κB for its role in human
cervical tumorigenesis. Cell Biochem Biophys. 71:891–896. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maggiore C, Mulè A, Fadda G, Rossi ED,
Lauriola L, Vecchio FM and Capelli A: Histological classification
of lung cancer. Rays. 29:353–355. 2004.PubMed/NCBI
|
|
14
|
Wang M, Bao YL, Wu Y, Yu CL, Meng XY,
Huang YX, Sun Y, Zheng LH and Li YX: Basic FGF Downregulates TSP50
expression via the ERK/Sp1 Pathway. J Cell Biochem. 111:75–81.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song ZB, Liu B, Li YY, Wu P, Bao YL, Huang
YX, Wu Y, Sun LG, Yu CL, Sun Y, et al: The catalytic triad of
testes-specific protease 50 (TSP50) is essential for its function
in cell proliferation. Cell Signal. 26:2266–2275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei J, Liu Y, Yang S, Xu J, Kong H, Han B,
Bao Y, Wu Y, Yin W, Li W, et al: Screening of single-chain variable
fragments against TSP50 from a phage display antibody library and
their expression as soluble proteins. J Biomol Screen. 11:546–552.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu YL and Sun YN: Down-regulation of
testes-specific protease 50 induces apoptosis in human
laryngocarcinoma HEp2 cells in a NF-κB-mediated pathway. Mol Biol.
41:7743–7747. 2014.
|
|
18
|
Kosaka-Suzuki N, Suzuki T, Pugacheva EM,
Vostrov AA, Morse HC III, Loukinov D and Lobanenkov V:
Transcription factor BORIS (Brother of the Regulator of Imprinted
Sites) directly induces expression of a cancer-testis antigen,
TSP50, through regulated binding of BORIS to the Promoter. J Biol
Chem. 286:27378–27388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng YH, Wong EW and Cheng CY:
Cancer/testis, (CT) antigens, carcinogenesis and spermatogenesis.
Spermatogenesis. 1:209–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim YD, Park HR, Song MH, Shin DH, Lee CH,
Lee MK and Lee SY: Pattern of cancer/testis antigen expression in
lung cancer patients. Int J Mol Med. 29:656–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grah JJ, Katalinic D, Juretic A, Santek F
and Samarzija M: Clinical significance of immunohistochemical
expression of cancer/testis tumor-associated antigens (MAGE-A1,
MAGE-A3/4, NY-ESO-1) in patients with non-small cell lung cancer.
Tumori. 100:60–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XP: Depression of testes-specific
protease 50 (TSP50) inhibits cell proliferation and induces
apoptosis in laryngocarcinoma. Tumour Biol. 35:10781–10788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li YY, Bao YL, Song ZB, Sun LG, Wu P,
Zhang Y, Fan C, Huang YX, Wu Y, Yu CL, et al: The threonine
protease activity of testes-specific protease 50 (TSP50) is
essential for its function in cell proliferation. PLoS One.
7:e350302012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gjerstorff MF, Andersen MH and Ditzel HJ:
Oncogenic cancer/testis antigens: Prime candidates for
immunotherapy. Oncotarget. 6:15772–15787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou L, Bao YL, Zhang Y, Wu Y, Yu CL,
Huang YX, Sun Y, Zheng LH and Li YX: Knockdown of TSP50 inhibits
cell proliferation and induces apoptosis in P19 cells. IUBMB Life.
62:825–832. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–94.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mostafa AA and Morris DG: Immunotherapy
for lung cancer: Has it finally arrived? Front Oncol. 4:2882014.
View Article : Google Scholar : PubMed/NCBI
|