Introduction
Colorectal cancer (CRC) is one of the most common
digestive malignancies, and it arises through well-defined
sequential multi-step carcinogenesis that transforms normal
glandular epithelium into invasive adenocarcinomas (1,2). The
development of CRC involves genetic and epigenetic modifications.
Aberrant DNA methylation within gene promoters is a primary
mediator of epigenetic inheritance in CRC (3,4).
DNA methylation typically occurs in CpG islands and
it refers to the enzymatic addition of a methyl group to the 5′
position of cytosine by DNA methyltransferases to produce 5-methyl
cytosine. Methylation of CpG islands in the gene promoter region
may induce chromatin conformational modifications that inhibit
access of transcriptional machinery, altering gene expression
levels (5,6). Therefore, promoter methylation is
commonly associated with gene silencing and promoter demethylation
with gene expression (7,8).
There are complex changes of DNA methylation in a
number of carcinomas, and particularly in CRC (9,10).
Numerous genes are aberrantly methylated in CRC patients, including
adenomatous polyposis coli (APC), WNT5A, mutL homolog
1 (MLH1), cyclin-dependent kinase inhibitor 2A
(CDKN2A) and Ras association domain-containing protein 1
(11–15). Aberrant DNA methylation of gene
promoters in CRC is involved in its occurrence, progression,
diagnosis, staging, prognosis and response to chemotherapy
(16).
The protocadherin gamma subfamily A12
(PCDH-γ-A12) gene encodes a cell surface adhesion
protein that serves essential roles in cell-cell and cell-matrix
interactions and tumor metastasis (17,18). The
solute carrier family 19 A 1 (SLC19A1) gene encodes a
membrane protein that is involved in the regulation of
intracellular concentrations of folate (19). SLC19A1 gene mutation is
associated with the risk of CRC (20). The cAMP responsive element binding
protein (CREB) gene encodes a transcription factor that
induces the transcription of genes in response to hormonal
stimulation of the cAMP pathway (21,22).
P300/CREB binding protein genes promote cancer progression in colon
cancer cell lines with microsatellite instability (23). Cylindromatosis (CYLD) encodes a
cytoplasmic protein with three cytoskeletal-associated
protein-glycine-conserved domains, and it regulates cell
proliferation, apoptosis, cell movement and cell differentiation
(24–27). CYLD is downregulated or lost in colon
carcinoma cell lines compared with primary human colonic epithelial
cells. The functional relevant loss of CYLD expression may
contribute to tumor development and progression, and it may provide
a new target for therapeutic strategies (28).
Promoter methylation of the PCDH-γ-A12,
SLC19A1, CREB and CYLD genes has been
demonstrated to regulate their gene expression levels, and
hypermethylation of these promoters has been observed in acute
lymphoblastic leukemia (29), breast
cancer (30,31) and malignant melanoma (32). However, hypermethylation of the
PCDH-γ-A12, SLC19A1, CREB and CYLD
promoters has not been investigated in CRC. In light of the
previous findings, the aim of the present study was to investigate
whether PCDH-γ-A12, SLC19A1, CREB and
CYLD gene promoter methylation contributed to the risk of
CRC.
Materials and methods
Tissue sample collection
In this study, CRC patients who had not received
radiotherapy, chemotherapy, targeted therapy or dendritic
cell/cytokine-induced killer therapy prior to surgery were
recruited between June 2012 and April 2013 (Table I). CRC samples, normal adjacent tissue
samples and matched metastatic lymph node samples were collected at
the time of surgery from 42 primary sporadic CRC patients at the
Department of Gastrointestinal Surgery in the Affiliated Hospital
of Ningbo University, China. Tissues were immediately preserved in
liquid nitrogen at −80°C following removal from the body and stored
at −80°C until use. Normal adjacent tissues were collected from at
least 5 cm away from the edge of the tumor, and there were no
obvious tumor cells, as evaluated by a pathologist. Tumor stage was
determined according to Dukes' staging system, and cellular
differentiation was graded according to Broders' grading system.
Informed consent was given by all subjects. The Human Research
Ethics Committee of Ningbo University approved all aspects of the
study.
 | Table I.Clinical profiles of the colorectal
cancer patients. |
Table I.
Clinical profiles of the colorectal
cancer patients.
|
Characteristics | Subgroup | Patients, n |
|---|
| Gender | Male | 28 |
|
| Female | 14 |
| Age (years) | ≤60 | 16 |
|
| >60 | 26 |
| TNM stage | 1, 2 | 21 |
|
| 3, 4 | 21 |
| Lymph
metastasis | Yes | 21 |
|
| No | 21 |
| Distant
metastasis | Yes | 8 |
|
| No | 34 |
| CEA | ≥5.0 ng/ml | 15 |
|
| <5.0 ng/ml | 27 |
| CA19-9 | ≥37 U/ml | 9 |
|
| <37 U/ml | 33 |
| Tumor location | Colon | 26 |
|
| Rectum | 16 |
|
Differentiation | Poor | 10 |
|
| Moderate | 32 |
|
| Good | 0 |
| Tumor size | <5 cm | 28 |
|
| ≥5 cm | 14 |
| Histological | Adenocarcinoma | 40 |
| classification | Mucinous
adenocarcinoma | 2 |
|
| Undifferentiated
carcinoma | 0 |
DNA isolation and bisulfite
modification
Genomic DNA was isolated using a QIAamp DNA mini kit
(Qiagen GmbH, Hilden, Germany). The concentration and quality of
genomic DNA were determined using the NanoDrop ND-2000
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The concentration of DNA was above 30 ng/µl and the purity of
DNA was at the A260/A280 ratio of 1.7–1.9. DNA was
bisulfite-treated with the EZ DNA Methylation-Gold kit (Zymo
Research, Orange, CA, USA). Following the completion of bisulfite
modification, all unmethylated cytosines in CpG islands were
converted to uracil, while methylated cytosines remained
unchanged.
Methylation-specific polymerase chain
reaction (MSP) and bisulfite sequencing
The methylated and unmethylated primers (Table II) were designed using the Primer
Premier 6.0 program (Premier Biosoft International, Palo Alto, CA,
USA). MSP was performed in a total volume of 20 µl containing 2 µl
bisulfite modified DNA, 1 µmol each of forward and reverse primers,
10 µl Premix Taq (Takara Biotechnology Co., Ltd., Dalian, China)
and 7 µl double-distilled water with the following cycling
parameters: 10 min of denaturation at 95°C followed by 55 cycles of
30 sec at 95°C, 45 sec at 72°C and a final extension for 10 min at
72°C. Polymerase chain reaction (PCR) products were then loaded and
electrophoresed on 2% agarose gels, stained with ethidium bromide,
and visualized under UV illumination. In order to confirm the
result of methylation- and unmethylation-specific PCR, PCR products
randomly obtained from the group were sequenced bidirectionally by
Invitrogen (Thermo Fisher Scientific, Inc.) with the same primers
used for MSP.
 | Table II.List of all primers used. |
Table II.
List of all primers used.
| Gene | Subgroup | Sense (5′-3′) | Antisense
(5′-3′) | Size (bp) |
|---|
|
PCDH-γ-A12 | M |
ATTAAGGTGGTGGCGGTGGAT |
GACGCCGACGCTCCTATCAA | 449 |
|
| U |
AAGGTGGTGGTGGTGGATAG |
ACCAACACTCCTATCAAAC | 443 |
| SLC19A1 | M |
TTGTTGTAGCGGTGTTGGAAGG |
TCCGCCGCAACCTACGAAT | 361 |
|
| U |
TTTGTTGTAGTGGTGTTGGAAG |
TTCCACCACAACCTACAAAT | 363 |
| CREB | M |
CGGCGGTTAAGAGTAGAGTTA |
GCGTCACTCACCAACACT | 492 |
|
| U |
TGGTGGTTAAGAGTAGAGTTA |
TCACTCACCAACACTCCAC | 489 |
| CYLD | M |
AGTTGGTGGTAGCGTAGCG |
CATTCACTAACCTCGAACGA | 495 |
|
| U |
TGGTGGTAGTGTAGTGTTT |
TCACTAACCTCAAACAACA | 489 |
Statistics
Statistical analysis was performed using the
Statistical Package for the Social Sciences (SPSS) statistical
software package (version 16.0; SPSS, Inc., Chicago, IL, USA), and
the results were obtained using GraphPad Prism version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). All analyses were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
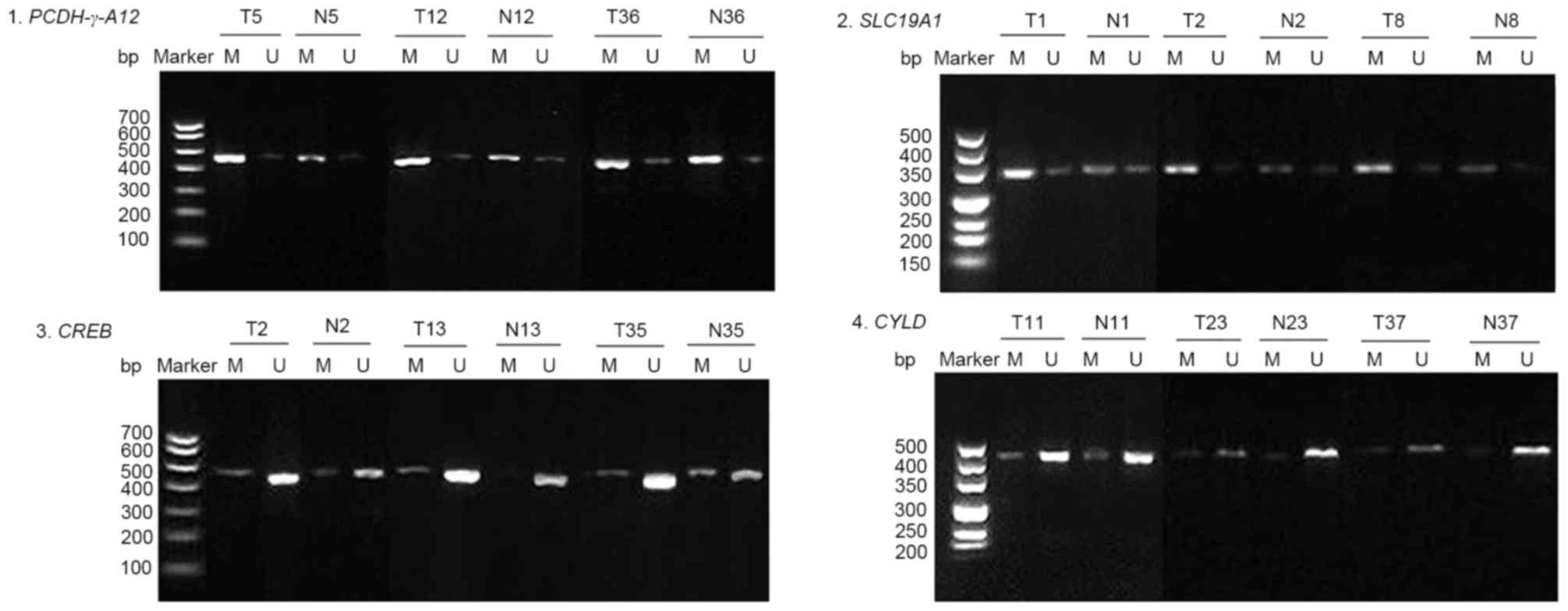
Methylation rates of promoters in CRC
vs
normal tissues
A total of 42 pairs of CRC and adjacent normal
tissues were examined, and representative results of the agarose
gel electrophoresis were selected (Fig.
1). The results revealed that the methylation rates of
PCDH-γ-A12, SLC19A1, CREB and CYLD promoters
in CRC were 83.33% (35/42), 78.57% (33/42), 26.19% (11/42) and
14.29% (6/42), while the methylation rates of these promoters in
normal tissues were 57.14% (24/42), 45.24% (19/42), 11.90% (5/42)
and 11.90% (5/42). PCDH-γ-A12 and SLC19A1 gene
promoters were more frequently methylated in CRC tissues than in
normal tissues (83.33% vs. 57.14%, P=0.009 and 78.57% vs. 42.54%,
P=0.002), while there was no significant difference in methylation
rates of CREB and CYLD gene promoters between CRC
tissues and normal tissues (26.19% vs. 11.90%, P=0.095 and 14.29%
vs. 11.90%, P=0.746; Table
III).
 | Table III.Methylation status of
PCDH-γ-A12, SLC19A1, CREB and CYLD genes in
colorectal cancer and normal tissues. |
Table III.
Methylation status of
PCDH-γ-A12, SLC19A1, CREB and CYLD genes in
colorectal cancer and normal tissues.
| Gene | Group | Total | M | U | M% | χ2 | P-value |
|---|
|
PCDH-γ-A12 | Cases | 42 | 35 | 7 | 83.33 | 6.891 | 0.009 |
|
| Controls | 42 | 24 | 18 | 57.14 |
|
|
| SLC19A1 | Cases | 42 | 33 | 9 | 78.57 | 9.894 | 0.002 |
|
| Controls | 42 | 19 | 23 | 45.24 |
|
|
| CREB | Cases | 42 | 11 | 31 | 26.19 | 2.779 | 0.095 |
|
| Controls | 42 | 5 | 37 | 11.90 |
|
|
| CYLD | Cases | 42 | 6 | 36 | 14.29 | 0.105 | 0.746 |
|
| Controls | 42 | 5 | 37 | 11.90 |
|
|
Methylation rates of promoters in
lymph vs. non-lymph metastasis CRC tissues
In addition, the methylation rates of
PCDH-γ-A12, SLC19A1, CREB and CYLD promoters
in lymph metastasis CRC tissues were 100.00% (21/21), 95.24%
(20/21), 33.33% (7/21) and 19.05% (4/21), while the methylation
rates of these promoters in non-lymph metastasis CRC tissues were
66.67% (14/21), 61.90% (13/21), 19.05% (4/21) and 9.52% (2/21).
PCDH-γ-A12 and SLC19A1 gene promoters were
more frequently methylated in lymph metastasis CRC tissues than
non-lymph metastasis CRC tissues (100.00% vs. 66.67%, P=0.013% and
95.24% vs. 61.90%, P=0.024), while there was no significant
difference in the methylation rate of CREB and CYLD
gene promoters between lymph metastasis CRC tissues and non-lymph
metastasis CRC tissues (33.33% vs. 19.05%, P=0.292 and 19.05% vs.
9.52%, P=0.659; Table IV).
 | Table IV.Methylation status of PCDH-γ-A12,
SLC19A1, CREB and CYLD genes in lymph metastasis and
non-lymph metastasis colorectal cancer tissues. |
Table IV.
Methylation status of PCDH-γ-A12,
SLC19A1, CREB and CYLD genes in lymph metastasis and
non-lymph metastasis colorectal cancer tissues.
| Gene | Subgroup | Total | M | U | M% | χ2 | P-value |
|---|
| PCDH-γ-A12 | Cases | 21 | 21 | 0 | 100.00 | 6.171 | 0.013 |
|
| Controls | 21 | 14 | 7 | 66.67 |
|
|
| SLC19A1 | Cases | 21 | 20 | 1 | 95.24 | 5.091 | 0.024 |
|
| Controls | 21 | 13 | 8 | 61.90 |
|
|
| CREB | Cases | 21 | 7 | 14 | 33.33 | 1.109 | 0.292 |
|
| Controls | 21 | 4 | 17 | 19.05 |
|
|
| CYLD | Cases | 21 | 4 | 17 | 19.05 | 0.194 | 0.659 |
|
| Controls | 21 | 2 | 19 | 9.52 |
|
|
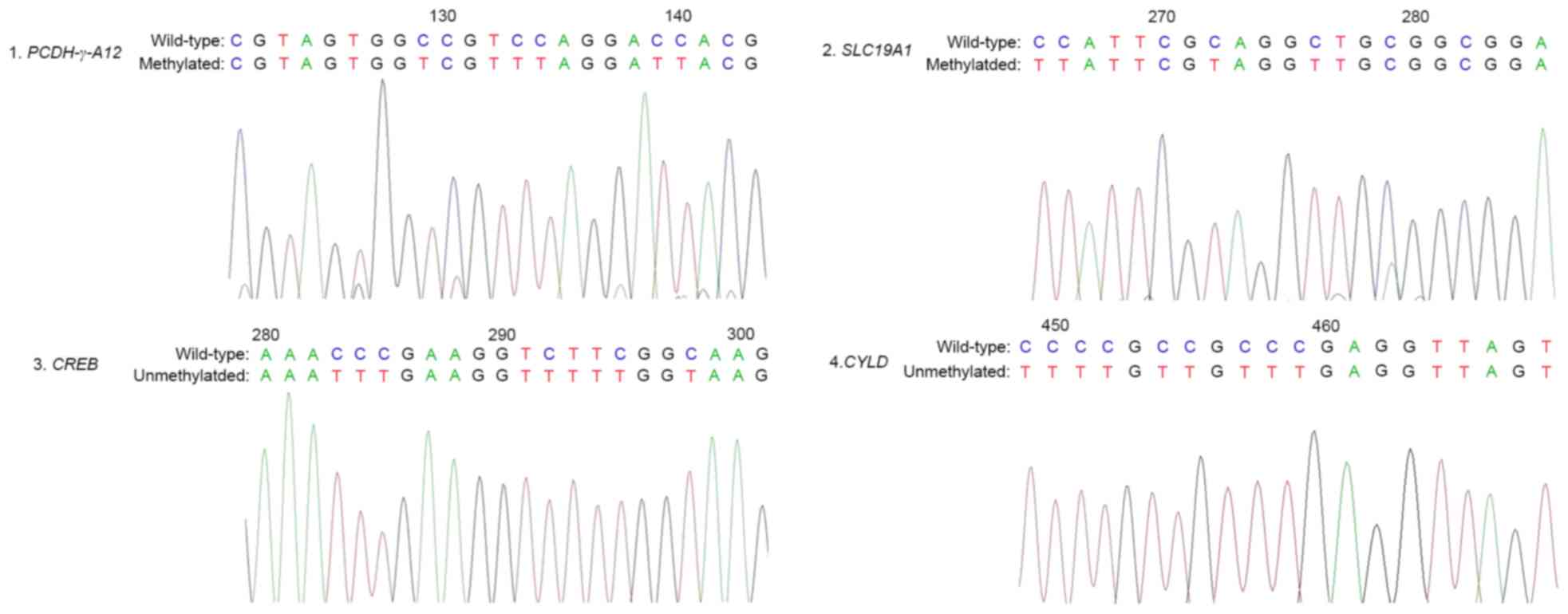
Bisulphite sequencing of PCDH-γ-A12,
SLC19A1, CREB and CYLD genes
In order to confirm the results of the PCR-based
methylation analysis describe above, high-resolution bisulfite
genomic sequencing was performed in the stochastic samples derived
from the methylation PCR experiments. In agreement with the MSP
results, CpG dinucleotides of the PCDH-γ-A12 and
SLC19A1 promoters in the samples demonstrated extensive
hypermethylation, whereas the CREB and CYLD promoters
were unmethylated at these CpG dinucleotides (Fig. 2).
Correlation between methylation status
of promoters and clinicopathological factors
The correlation between the methylation status of
the PCDH-γ-A12, SLC19A1, CREB and CYLD gene
promoters and the clinicopathological characteristics of CRC is
shown in Table V. There was no
significant difference in clinicopathological factors, including
sex, age, tumor-node-metastasis stage, lymph node status,
metastasis status, tumor location, differentiation status, tumor
size and histological grade. There was also no correlation between
the methylation status of the PCDH-γ-A12, SLC19A1,
CREB and CYLD gene promoters and the serum levels of
carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9
(CA19-9).
 | Table V.Association between PCDH-γ-A12,
SLC19A1, CREB and CYLD methylation in CRC serum and
clinicopathological features. |
Table V.
Association between PCDH-γ-A12,
SLC19A1, CREB and CYLD methylation in CRC serum and
clinicopathological features.
|
|
|
|
PCDH-γ-A12 | SLC19A1 | CREB | CYLD |
|---|
|
|
|
|
|
|
|
|
|---|
|
Characteristics | Subgroup | Patient, n | M | U | P-value | M | U | P-value | M | U | P-value | M | U | P-value |
|---|
| Gender | Male | 28 | 25 | 3 | 0.306 | 21 | 7 | 0.690 | 7 | 21 | 1 | 4 | 24 | 1.000 |
|
| Female | 14 | 10 | 4 |
| 12 | 2 |
| 4 | 10 |
| 2 | 12 |
|
| Age (years) | ≤60 | 16 | 14 | 2 | 0.887 | 10 | 6 | 0.109 | 4 | 12 | 1 | 3 | 13 | 0.846 |
|
| >60 | 26 | 21 | 5 |
| 23 | 3 |
| 7 | 19 |
| 3 | 23 |
|
| TNM stage | 1, 2 | 21 | 18 | 3 | 1.000 | 17 | 4 | 1.000 | 6 | 15 | 0.547 | 2 | 19 | 0.659 |
|
| 3, 4 | 21 | 17 | 4 |
| 16 | 5 |
| 5 | 16 |
| 4 | 17 |
|
| Lymph
metastasis | Yes | 21 | 21 | 0 | 0.013 | 20 | 1 | 0.024 | 7 | 14 | 0.292 | 4 | 17 | 0.756 |
|
| No | 21 | 14 | 7 |
| 13 | 8 |
| 4 | 17 |
| 2 | 19 |
|
| Distant
metastasis | Yes | 8 | 7 | 1 | 1.000 | 8 | 0 | 0.245 | 4 | 4 | 0.209 | 3 | 5 | 0.128 |
|
| No | 34 | 28 | 6 |
| 25 | 9 |
| 7 | 27 |
| 3 | 31 |
|
| CEA | ≥5.0 ng/ml | 15 | 12 | 3 | 1.000 | 10 | 5 | 0.313 | 3 | 12 | 0.754 | 3 | 12 | 0.742 |
|
| <5.0 ng/ml | 27 | 23 | 4 |
| 23 | 4 |
| 8 | 19 |
| 3 | 24 |
|
| CA19-9 | ≥37 U/ml | 9 | 7 | 2 | 1.000 | 6 | 3 | 0.600 | 3 | 6 | 0.903 | 2 | 7 | 0.818 |
|
| <37 U/ml | 33 | 28 | 5 |
| 27 | 6 |
| 8 | 25 |
| 4 | 29 |
|
| Tumor location | Colon | 26 | 21 | 5 | 0.887 | 23 | 3 | 0.109 | 8 | 18 | 0.618 | 3 | 23 | 0.846 |
|
| Rectum | 16 | 14 | 2 |
| 10 | 6 |
| 3 | 13 |
| 3 | 13 |
|
|
Differentiation | Poor | 10 | 9 | 1 | 0.871 | 9 | 1 | 0.570 | 4 | 6 | 0.468 | 2 | 8 | 0.941 |
|
| Moderate | 32 | 26 | 6 |
| 24 | 8 |
| 7 | 25 |
| 4 | 28 |
|
|
| Good | 0 | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| Tumor size | <5 cm | 28 | 23 | 5 | 1.000 | 22 | 6 | 1.000 | 7 | 21 | 1 | 3 | 25 | 0.640 |
|
| ≥5 cm | 14 | 12 | 2 |
| 11 | 3 |
| 4 | 10 |
| 3 | 11 |
|
| Histological
classification | Adenocarcinoma | 40 | 33 | 7 | 1.000 | 31 | 9 | 1.000 | 10 | 30 | 1 | 4 | 36 | 0.558 |
|
| Mucinous
adenocarcinoma | 2 | 2 | 0 |
| 2 | 0 |
| 1 | 1 |
| 1 | 1 |
|
|
| Undifferentiated
carcinoma | 0 | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
Discussion
Cancer develops through a multi-step process which
results from the progressive accumulation of genetic and epigenetic
alterations (33). Epigenetic
modifications, which have a fundamental role in the regulation of
gene expression, involve DNA methylation, specific histone
modifications and non-coding RNA interventions (34,35). As
one of the main epigenetic modifications, DNA methylation of
promoters often downregulates gene transcription, while DNA
demethylation of promoters activates gene expression. DNA
methylation-mediated tumor suppressor gene silencing may contribute
to tumor progression (7,36). Aberrant DNA methylation of gene
promoters has become a promising biomarker for the early diagnosis
of diseases (37–41).
In the colon, aberrant DNA methylation arises
extremely early, initially in normal-appearing mucosa, and it may
be part of the age-associated field defects observed in sporadic
CRC (42). Hypermethylation in CpG
islands has been demonstrated to be a novel mechanism of tumor
suppressor gene silencing (7,8). A number of genes have now been
demonstrated to be hypermethylated in colorectal tumors, including
APC (11), MLH1
(43) and O6-methylguanine
DNA methyltransferase (44). For
example, the inactivation of the cyclin-dependent kinase inhibitor
P16/CDKN2A/INK4a by methylation leads to the
disruption of cell-cycle regulation and potentially provides a
growth advantage to affected cells (45).
PCDH-γ-A12 is a member of the
protocadherin γ gene cluster, which includes 22 genes divided into
3 subfamilies (subfamily A, B and C) (46). The exon of PCDH-γ-A12
encodes the extracellular region, which includes six cadherin
ectodomains and a transmembrane region. These cadherin-like cell
adhesion proteins most likely serve a critical role in the
establishment and function of specific cell-cell connections in the
brain and cancer (18). The
hypermethylation of PCDH-γ-A12 induces the
downregulation of PCDH-γ-A12 gene transcription by
rendering the chromatin structure inaccessible to the transcription
machinery in a variety of tumors including bladder cancer, breast
cancer, acute lymphoblastic leukemia and non-small cell lung cancer
(17,29,47,48). The
present study in CRC provides new evidence for the contribution of
PCDH-γ-A12 promoter hypermethylation to the
occurrence and metastasis of CRC.
SLC19A1 encodes a membrane protein that is a
transporter of folate, and is involved in the regulation of
intracellular concentrations of folate. SLC19A1 is also a major
transporter of antifolate drugs used for certain types of cancer
chemotherapy, including methotrexate (MTX) (30). The expression of SLC19A1 is
downregulated following exposure to MTX in breast cancer, and a
reverse correlation was identified between the promoter methylation
and mRNA levels of SLC19A1. A variant of the SLC19A1 gene is
associated with metastatic colorectal cancer (20). The present study in CRC adds new
evidence for the contribution of SLC19A1 promoter
hypermethylation to the occurrence and metastasis of CRC.
Certain studies have focused on the correlation
between colorectal cancer clinical features and the methylation of
certain genes, including p15, APC and E-cadherin,
suggesting that the inactivation of certain tumor suppressor genes
through aberrant promoter methylation of CpG islands may serve a
role in the development of colorectal cancer (49,50).
Multiple methylation pathways may be involved in the tumorigenesis
of CRC and associated with the aggressiveness of clinical disease
(37). In the present study, the
correlation between the methylation of PCDH-γ-A12,
SLC19A1, CREB and CYLD and colorectal cancer
clinical features was examined. However, no significant correlation
was identified between PCDH-γ-A12, SLC19A1,
CREB and CYLD methylation and the clinical features,
which may be due to the lack of power in the samples used.
CEA is a member of a family of cell surface
glycoproteins that are excessively produced in the majority of
human colorectal carcinomas (51).
CEA measurement is mainly used as a tumor marker to monitor
colorectal carcinoma treatment, to identify recurrences following
surgical resection and to localize cancer spread through
measurement of biological fluids (52,53).
CA19-9 is a useful tumor-associated antigen for the serological
detection of colorectal carcinomas, and may be used to monitor
patients with advanced colorectal carcinomas (54). One aim of the present study was to
observe whether the status of PCDH-γ-A12, SLC19A1,
CREB and CYLD promoter methylation had a correlation
with the serum level of CEA and CA19-9. However, no significant
correlation was observed between PCDH-γ-A12, SLC19A1,
CREB and CYLD promoter methylation and the serum
level of CEA and CA19-9. This may imply that aberrant methylation
of PCDH-γ-A12, SLC19A1, CREB and CYLD combined
with conventional tumor markers could serve as complementary
markers in the diagnosis of CRC. However, further study is
necessary to confirm this hypothesis.
In conclusion, PCDH-γ-A12 and
SLC19A1 promoters, but not CREB and CYLD
promoters, are hypermethylated and contribute to the occurrence and
metastasis of colorectal cancer. These findings may provide a new
direction in the detection and treatment of CRC. Future research is
required to determine the detailed mechanisms of how the
PCDH-γ-A12 and SLC19A1 genes contribute to the
risk of CRC.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Natural
Science Foundation of Zhejiang Province (LY15H160015 and
LS14H26001), the K. C. Wong Magna Fund in Ningbo University, the
Science and Technology Innovation Team of Ningbo (2011B82014), the
Specialized Research Fund for the Social Development of Hangzhou
(20160533B21) and the Scientific Innovation Fund of the Affiliated
Hospital of Hangzhou Normal University.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
MY and SD designed the research. CZ, JL, TH and CC
conducted the experiments. CZ, QH and HJ analyzed the data. The
manuscript was drafted by CZ and SD. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Human Research Ethics
Committee of Ningbo University and written informed consent was
obtained from all participants.
Consent for publication
All participants provided written informed consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bardhan K and Liu K: Epigenetics and
colorectal cancer pathogenesis. Cancers (Basel). 5:676–713. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lao VV and Grady WM: Epigenetics and
colorectal cancer. Nat Rev Gastroenterol Hepatol. 8:686–700. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hinoue T, Weisenberger DJ, Lange CP, Shen
H, Byun HM, Van Den Berg D, Malik S, Pan F, Noushmehr H, van Dijk
CM, et al: Genome-scale analysis of aberrant DNA methylation in
colorectal cancer. Genome Res. 22:271–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hellman A and Chess A: Gene body-specific
methylation on the active X chromosome. Science. 315:1141–1143.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jones PA: Functions of DNA methylation:
Islands, start sites, gene bodies and beyond. Nat Rev Genet.
13:484–492. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang CJ, Zhou JX, Liu J, Ma ZY, Zhang SW,
Dou K, Huang HW, Cai T, Liu R, Zhu JK, et al: The splicing
machinery promotes RNA-directed DNA methylation and transcriptional
silencing in Arabidopsis. EMBO J. 32:1128–1140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwitalla S, Ziegler PK, Horst D, Becker
V, Kerle I, Begus-Nahrmann Y, Lechel A, Rudolph KL, Langer R,
Slotta-Huspenina J, et al: Loss of p53 in enterocytes generates an
inflammatory microenvironment enabling invasion and lymph node
metastasis of carcinogen-induced colorectal tumors. Cancer Cell.
23:93–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amatu A, Sartore-Bianchi A, Moutinho C,
Belotti A, Bencardino K, Chirico G, Cassingena A, Rusconi F,
Esposito A, Nichelatti M, et al: Promoter CpG island
hypermethylation of the DNA repair enzyme MGMT predicts clinical
response to dacarbazine in a phase II study for metastatic
colorectal cancer. Clin Cancer Res. 19:2265–2272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gay LJ, Mitrou PN, Keen J, Bowman R,
Naguib A, Cooke J, Kuhnle GG, Burns PA, Luben R, Lentjes M, et al:
Dietary, lifestyle and clinicopathological factors associated with
APC mutations and promoter methylation in colorectal cancers from
the EPIC-Norfolk study. J Pathol. 228:405–415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rawson JB, Mrkonjic M, Daftary D, Dicks E,
Buchanan DD, Younghusband HB, Parfrey PS, Young JP, Pollett A,
Green RC, et al: Promoter methylation of Wnt5a is associated with
microsatellite instability and BRAF V600E mutation in two large
populations of colorectal cancer patients. Br J Cancer.
104:1906–1912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakagawa H, Nuovo GJ, Zervos EE, Martin EW
Jr, Salovaara R, Aaltonen LA and de la Chapelle A: Age-related
hypermethylation of the 5′ region of MLH1 in normal colonic mucosa
is associated with microsatellite-unstable colorectal cancer
development. Cancer Res. 61:6991–6995. 2001.PubMed/NCBI
|
|
14
|
Shima K, Nosho K, Baba Y, Cantor M,
Meyerhardt JA, Giovannucci EL, Fuchs CS and Ogino S: Prognostic
significance of CDKN2A (p16) promoter methylation and loss of
expression in 902 colorectal cancers: Cohort study and literature
review. Int J Cancer. 128:1080–1094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oliveira C, Velho S, Domingo E, Preto A,
Hofstra RM, Hamelin R, Yamamoto H, Seruca R and Schwartz S Jr:
Concomitant RASSF1A hypermethylation and KRAS/BRAF mutations occur
preferentially in MSI sporadic colorectal cancer. Oncogene.
24:7630–7634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coppedè F: Epigenetic biomarkers of
colorectal cancer: Focus on DNA methylation. Cancer Lett.
342:238–247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu Y, Lemon W, Liu PY, Yi Y, Morrison C,
Yang P, Sun Z, Szoke J, Gerald WL, Watson M, et al: A gene
expression signature predicts survival of patients with stage I
non-small cell lung cancer. PLoS Med. 3:e4672006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morishita H and Yagi T: Protocadherin
family: Diversity, structure, and function. Curr Opin Cell Biol.
19:584–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stanislawska-Sachadyn A, Mitchell LE,
Woodside JV, Buckley PT, Kealey C, Young IS, Scott JM, Murray L,
Boreham CA, McNulty H, et al: The reduced folate carrier (SLC19A1)
c.80G>A polymorphism is associated with red cell folate
concentrations among women. Ann Hum Genet. 73:484–491. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang L, Zhang T, Xie C, Liao X, Yu Q,
Feng J, Ma H, Dai J, Li M, Chen J, et al: SLCO1B1 and SLC19A1 gene
variants and irinotecan-induced rapid response and survival: A
prospective multicenter pharmacogenetics study of metastatic
colorectal cancer. PLoS One. 8:e772232013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shaywitz AJ and Greenberg ME: CREB: A
stimulus-induced transcription factor activated by a diverse array
of extracellular signals. Annu Rev Biochem. 68:821–861. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Altarejos JY and Montminy M: CREB and the
CRTC co-activators: Sensors for hormonal and metabolic signals. Nat
Rev Mol Cell Biol. 12:141–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ionov Y, Matsui S and Cowell JK: A role
for p300/CREB binding protein genes in promoting cancer progression
in colon cancer cell lines with microsatellite instability. Proc
Natl Acad Sci USA. 101:1273–1278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wickstrom SA, Masoumi KC, Khochbin S,
Fassler R and Massoumi R: CYLD negatively regulates cell-cycle
progression by inactivating HDAC6 and increasing the levels of
acetylated tubulin. EMBO J. 29:131–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Donnell MA, Perez-Jimenez E, Oberst A,
Ng A, Massoumi R, Xavier R, Green DR and Ting AT: Caspase 8
inhibits programmed necrosis by processing CYLD. Nat Cell Biol.
13:1437–1442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao J, Sun L, Huo L, Liu M, Li D and Zhou
J: CYLD regulates angiogenesis by mediating vascular endothelial
cell migration. Blood. 115:4130–4137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alameda JP, Fernandez-Acenero MJ,
Moreno-Maldonado R, Navarro M, Quintana R, Page A, Ramirez A, Bravo
A and Casanova ML: CYLD regulates keratinocyte differentiation and
skin cancer progression in humans. Cell Death Dis. 2:e2082011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hellerbrand C, Bumes E, Bataille F,
Dietmaier W, Massoumi R and Bosserhoff AK: Reduced expression of
CYLD in human colon and hepatocellular carcinomas. Carcinogenesis.
28:21–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taylor KH, Pena-Hernandez KE, Davis JW,
Arthur GL, Duff DJ, Shi H, Rahmatpanah FB, Sjahputera O and
Caldwell CW: Large-scale CpG methylation analysis identifies novel
candidate genes and reveals methylation hotspots in acute
lymphoblastic leukemia. Cancer Res. 67:2617–2625. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang R, Li WW, Hoang BH, Kim H, Banerjee
D, Kheradpour A, Healey JH, Meyers PA, Bertino JR and Gorlick R:
Quantitative correlation between promoter methylation and messenger
RNA levels of the reduced folate carrier. BMC Cancer. 8:1242008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Demura M and Bulun SE: CpG dinucleotide
methylation of the CYP19 I.3/II promoter modulates cAMP-stimulated
aromatase activity. Mol Cell Endocrinol. 283:127–132. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Massoumi R, Kuphal S, Hellerbrand C, Haas
B, Wild P, Spruss T, Pfeifer A, Fassler R and Bosserhoff AK:
Down-regulation of CYLD expression by Snail promotes tumor
progression in malignant melanoma. J Exp Med. 206:221–232. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiba T, Marusawa H and Ushijima T:
Inflammation-associated cancer development in digestive organs:
Mechanisms and roles for genetic and epigenetic modulation.
Gastroenterology. 143:550–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mossman D and Scott RJ: Long term
transcriptional reactivation of epigenetically silenced genes in
colorectal cancer cells requires DNA hypomethylation and histone
acetylation. PLoS One. 6:e231272011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lopez-Serra P and Esteller M: DNA
methylation-associated silencing of tumor-suppressor microRNAs in
cancer. Oncogene. 31:1609–1622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McGarvey KM, Greene E, Fahrner JA,
Jenuwein T and Baylin SB: DNA methylation and complete
transcriptional silencing of cancer genes persist after depletion
of EZH2. Cancer Res. 67:5097–5102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee BB, Lee EJ, Jung EH, Chun HK, Chang
DK, Song SY, Park J and Kim DH: Aberrant methylation of APC, MGMT,
RASSF2A, and Wif-1 genes in plasma as a biomarker for early
detection of colorectal cancer. Clin Cancer Res. 15:6185–6191.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Richards KL, Zhang B, Sun M, Dong W,
Churchill J, Bachinski LL, Wilson CD, Baggerly KA, Yin G, Hayes DN,
et al: Methylation of the candidate biomarker TCF21 is very
frequent across a spectrum of early-stage nonsmall cell lung
cancers. Cancer. 117:606–617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang D, Hong Q, Shen Y, Xu Y, Zhu H, Li
Y, Xu C, Ouyang G and Duan S: The diagnostic value of DNA
methylation in leukemia: A systematic review and meta-analysis.
PLoS One. 9:e968222014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang D, Shen Y, Dai D, Xu Y, Xu C, Zhu H,
Huang T and Duan S: Meta-analyses of methylation markers for
prostate cancer. Tumour Biol. 35:10449–10455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sui X, Wang D, Geng S, Zhou G, He C and Hu
X: Methylated promoters of genes encoding protocadherins as a new
cancer biomarker family. Mol Biol Rep. 39:1105–1111. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kondo Y and Issa JP: Epigenetic changes in
colorectal cancer. Cancer Metastasis Rev. 23:29–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Malhotra P, Anwar M, Kochhar R, Ahmad S,
Vaiphei K and Mahmood S: Promoter methylation and
immunohistochemical expression of hMLH1 and hMSH2 in sporadic
colorectal cancer: A study from India. Tumour Biol. 35:3679–3687.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pietrantonio F, Perrone F, de Braud F,
Castano A, Maggi C, Bossi I, Gevorgyan A, Biondani P, Pacifici M,
Busico A, et al: Activity of temozolomide in patients with advanced
chemorefractory colorectal cancer and MGMT promoter methylation.
Ann Oncol. 25:404–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Herman JG, Merlo A, Mao L, Lapidus RG,
Issa JP, Davidson NE, Sidransky D and Baylin SB: Inactivation of
the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA
methylation in all common human cancers. Cancer Res. 55:4525–4530.
1995.PubMed/NCBI
|
|
46
|
Yagi T: Clustered protocadherin family.
Dev Growth Differ 50 Suppl. 1:S131–S140. 2008. View Article : Google Scholar
|
|
47
|
Reinert T, Modin C, Castano FM, Lamy P,
Wojdacz TK, Hansen LL, Wiuf C, Borre M, Dyrskjot L and Orntoft TF:
Comprehensive genome methylation analysis in bladder cancer:
Identification and validation of novel methylated genes and
application of these as urinary tumor markers. Clin Cancer Res.
17:5582–5592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tommasi S, Karm DL, Wu X, Yen Y and
Pfeifer GP: Methylation of homeobox genes is a frequent and early
epigenetic event in breast cancer. Breast Cancer Res. 11:R142009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin SY, Yeh KT, Chen WT, Chen HC, Chen ST,
Chiou HY and Chang JG: Promoter CpG methylation of tumor suppressor
genes in colorectal cancer and its relationship to clinical
features. Oncol Rep. 11:341–348. 2004.PubMed/NCBI
|
|
50
|
Lind GE, Thorstensen L, Lovig T, Meling
GI, Hamelin R, Rognum TO, Esteller M and Lothe RA: A CpG island
hypermethylation profile of primary colorectal carcinomas and colon
cancer cell lines. Mol Cancer. 3:282004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Benchimol S, Fuks A, Jothy S, Beauchemin
N, Shirota K and Stanners CP: Carcinoembryonic antigen, a human
tumor marker, functions as an intercellular adhesion molecule.
Cell. 57:327–334. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ding W, Wang J, Wang F, Wang G, Wu Q, Ju
S, Cong H and Wang H: Serum sAPRIL: A potential tumor-associated
biomarker to colorectal cancer. Clin Biochem. 46:1590–1594. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Baek JY, Yeo HY, Chang HJ, Kim KH, Kim SY,
Park JW, Park SC, Choi HS, Kim DY and Oh JH: Serpin B5 is a
CEA-interacting biomarker for colorectal cancer. Int J Cancer.
134:1595–1604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Petrioli R, Licchetta A, Roviello G,
Pascucci A, Francini E, Bargagli G, Conca R, Miano ST, Marzocca G
and Francini G: CEA and CA19.9 as early predictors of progression
in advanced/metastatic colorectal cancer patients receiving
oxaliplatin-based chemotherapy and bevacizumab. Cancer Invest.
30:65–71. 2012. View Article : Google Scholar : PubMed/NCBI
|