Introduction
Neuroendocrine neoplasms are a group of
heterogeneous tumors that originate from peptidergic neurons and
neuroendocrine cells (1). With the
widespread use of immunohistochemical techniques and electron
microscopy in the pathological diagnosis of tumors, neuroendocrine
tumors (NETs) have been found not only in endocrine but also in
non-endocrine organs, such as the gastrointestinal tract, pancreas,
gall bladder and lung. (2). The
incidence of NETs in the gastrointestinal tract was reported to be
45% in the small intestine, 20% in the rectum, 17% in the appendix,
11% in the colon, and 7% in the stomach (2).
NETs of the gastrointestinal tract often spread to
the liver, while primary hepatic NETs (PHNETs), first described by
Edmondson in 1958, are very rare (3).
Existing reports about PHNETs are mostly of small sample sizes, and
the clinicopathological characteristics and prognostic factors of
PHNETs are still unclear (4–7). Here, we collected and classified the
clinicopathological features of 28 PHNETs patients [9 in Wuhan
Union Hospital (Wuhan, China), 15 in Tongji Hospital (Wuhan,
China), and 4 in Hubei Cancer Hospital (Wuhan, China)] and
discussed whether the related clinicopathological indicators can be
used to improve the prognostic analysis of PHNETs.
Materials and methods
Ethical approval
Ethical approval was requested and obtained from the
Medical Ethics Committee of Tongji Medical College (Wuhan, China).
Written informed consent was obtained from all participants.
Clinical data collection
We collected liver tissue specimens by excision or
needle biopsy from 32 patients who were diagnosed with PHNETs in
Wuhan Union Hospital, Tongji Hospital, and Hubei Cancer Hospital
between 2012 and 2017. Among them, 28 patients were of pathological
and follow-up data integrity, and thus eligible for
histopathological and prognostic analysis. The clinicopathological
features of all patients were reviewed and analyzed. Cases were
examined by imaging, histopathological and immunohistochemical
analysis, and long-term postoperative follow-ups, including
ultrasound, enhanced chest computed tomography (CT), and upper and
lower gastrointestinal endoscopic examination, were conducted. No
primary extrahepatic lesions were found, so all patients were
eventually diagnosed with PHNETs. We confirm that we obtained
informed consent of all 28 patients.
Observation indexes
Clinical indicators for observation: Tumor size
(≤5/>5 cm) and treatment (radical surgery/others). Biochemical
indicators: Alpha fetoprotein (AFP; ≤400/>400 ug/l),
carcinoembryonic antigen (CEA; ≤5/l/>5 ug/l), carbohydrate
antigen 125 (CA125; ≤35/>35 ug/ml), ALT (≤40/>40 U/l),
aspartate aminotransferase (AST; ≤40/>40 U/l), albumin
(≤35/>35 G/l), and hemoglobin (HB; ≤110/>110 G/l).
Pathological indicators: Tumor grading (G1, G2/G3), Ki-67 PI
(≤20/>20%), and chromogranin A (CGA; -/+; Fig. 1).
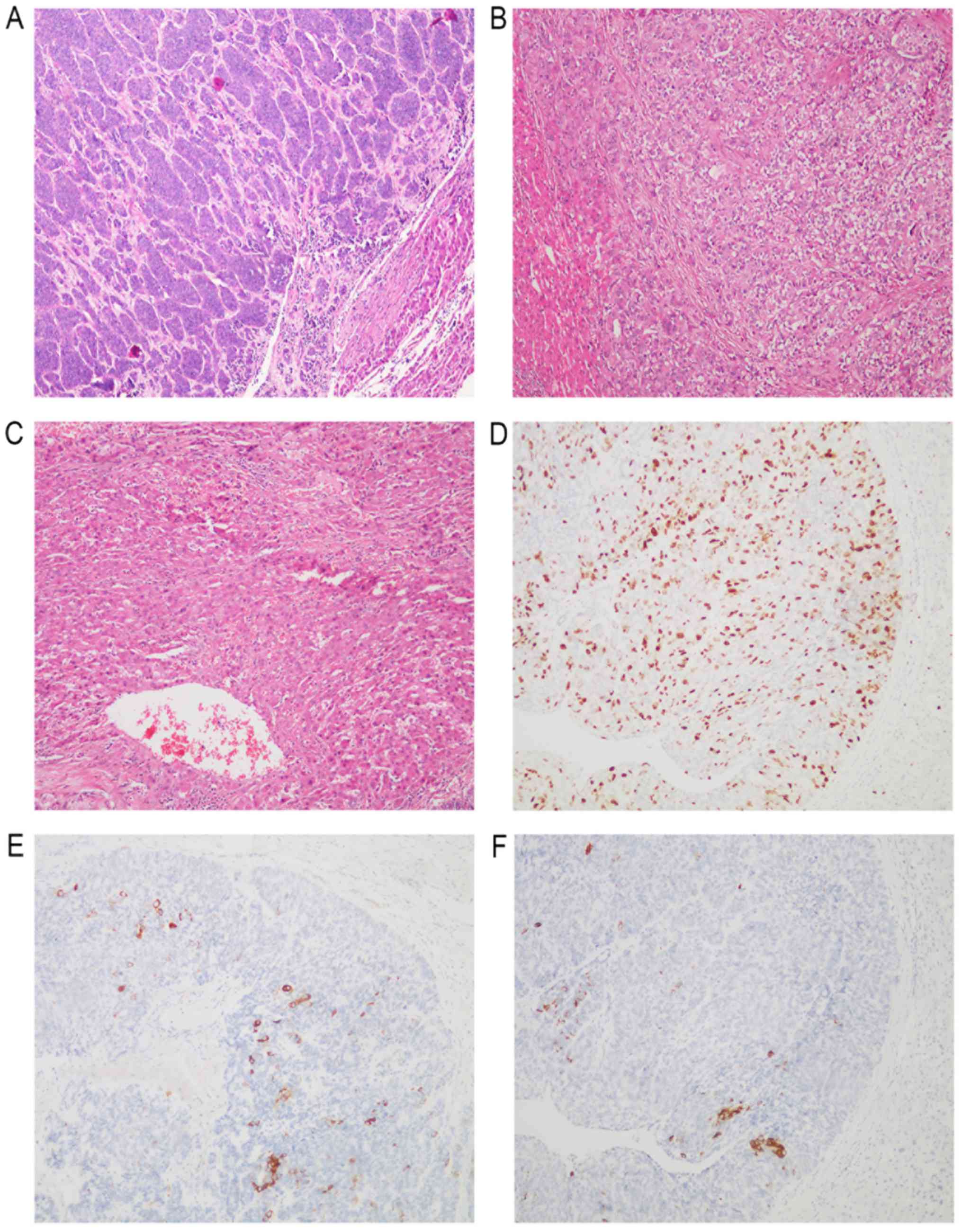 | Figure 1.Histological findings in liver tumors.
(A) NET G1, histological examination showed that the tumor cells
were arranged into trabeculae, gland bubbles or gyrus. The nucleus
was centered. Small and round, with relatively uniform size. (B)
NET G2, histological examination showed that the tumor cells were
arranged in the adenocarcinoma or cerebral gyrus, and the core was
larger than NET G1, and moderately atypical. (C) NEC G3,
histological examination showed that the tumor cells were arranged
in large nests or parenchyma, without organs, and the nuclei were
round, elliptical, irregular or spindle, and can be seen in giant
cells of the tumor. (D) Immunohistochemistry revealed the tumor
cells were positive for Ki-67. (E) Immunohistochemistry revealed
the tumor cells were positive for synaptophysin. (F)
Immunohistochemistry revealed the tumor cells were positive for
chromorgranin A (magnification, ×100). NET, neuroendocrine tumor;
G1-3, tumor grades 1–3; NEC, neuroendocrine carcinoma. |
Follow-up results
28 patients were followed up. Follow-up time ranged
from 2 to 50 months (mean, 18.7 months; median survival time, 18.0
months). No tumor recurrence was found in any of the cases who
received a liver transplantation until the end of the follow up.
One case had no diarrhea after treatment and survived tumor-free
for 16 months. Of the 18 dead cases, 15 were neuroendocrine
carcinoma (NEC) and 3 were NET G2. One case of NEC died after a
follow-up period of two months. The rest of cases were followed up
for more than 5 months.
WHO classification (2010) of tumors of the digestive
system. NET classification criteria were as follows: NET G1:
Mitotic figure <2/10 HPF and/or PI ≤2%; NET G2: Mitotic figure
(2–20)/l0 HPF and/or PI 3–20%; and NEC/G3: Mitotic figure >20/10
HPF and/or Ki-67 PI >20% (8).
Statistical analysis
SPSS statistical software (version 18.0) was used
for statistical processing. The indexes age, sex, tumor size, tumor
grade, AFP, CEA, CA125, ALT, albumin, HB, CGA, surgery received,
and Ki-67 PI were compared between NET (G1, G2) and NEC (G3)
applying the Mann-Whitney and Pearson chi-square tests. Variance
analysis of the effects of the pathologic indexes on the prognosis
was performed by the Kaplan-Meier survival curve and the log-rank
test; P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinicopathological data
Among the 28 patients, 15 were male and 13 were
female, corresponding to a male/female ratio of 1.15:1.0. Patients
were aged between 32 and 76 years (mean=53 years). Sixteen patients
had clinical symptoms, with 15 patients having epigastric
discomfort as their first symptom and one having diarrhea. For the
rest 12 patients, no obvious clinical symptoms, except hepatoncus,
were found during physical examination (Fig. 2). Five patients had a history of
hepatitis B. Auxiliary examinations showed that 1 case (3.5%) had
an elevated serum AFP, information of 2 cases (7.1%) were not
available, and the rest of cases were in the normal range. For CEA,
1 case (3.5%) had an elevated serum CEA, information of 2 cases
(7.1%) were not available, and the rest of cases were in the normal
range. For CA199, 4 cases (14.3%) had an elevated serum CA199,
information of 2 cases (7.1%) were not available, and the rest of
cases were in the normal range. For CA125, 3 cases (10.6%) had an
elevated serum CA125, information of two cases (7.1%) were not
available, and the rest of the cases were in the normal range.
Twenty-two cases were single tumors (78.6%), and 6 cases were
multiple tumors (21.4%; Table I). All
cases (100%, 28/28) were immunohistochemically positive for
synaptophysin. Among these cases were 20 cases (71.4%) in the G3
group, and 14 cases (50%) received the radical operation.
 | Table I.Main demographic, biochemical and
clinical characteristics of the 28 primary hepatic neuroendocrine
tumor patients. |
Table I.
Main demographic, biochemical and
clinical characteristics of the 28 primary hepatic neuroendocrine
tumor patients.
| Variable | Unit | Value |
|---|
| Age | Years | 53 (32–76) |
| Sex | Male | 15 (53.5%) |
| Albumin | g/l | 39 (25–49) |
| ALT | U/l | 83 (11–834) |
| AST | U/l | 63 (16–159) |
| PHNET diameter | cm | 6.5 (1.5–18) |
| AFP | ng/ml | 56 (2–1499) |
| HB | g/l | 123 (70–169) |
| Treatment | Radical
operation | 14 (50%) |
| Histological | G3 | 20 (71.4%) |
| differentiation |
|
|
Classification and related prognostic
indicators
The 28 cases of PHNETs were divided into the groups
NET G1 (3 cases), NET G2 (5 cases), and NEC G3 (20 cases). For each
group, the indexes age, sex, tumor size, tumor grade, AFP, CEA,
CA125, ALT, albumin, HB, CGA, as well as surgery received were
detected, and correlation analysis was performed applying the
Mann-Whitney or Pearson chi-square test (Table II). Then, these clinicopathological
features and the prognosis were tested by single factor analysis.
The groups NET G1 and NET G2 were combined into one group and
compared with the NEC G3 group. The survival curve showed that the
survival rate of the NET (G1, G2) group was higher than that of the
NEC (G3) group (P<0.05). The average survival time was 37.3±5.3
months for the NET group and 14.9±2.5 months for the NEC group.
Cases of Ki-67 PI ≤20% were compared with cases of Ki-67 PI
>20%, and the survival curve showed that the group of Ki-67 PI
≤20% had a higher total survival rate than the group of Ki-67 PI
>20% (P<0.05). The average survival time was 36.1±5.2 months
for the group of Ki-67 PI ≤20% and 12.0±1.7 months for the group of
Ki-67 PI >20%. The cases of ALT ≤40 U/l were compared with the
cases of ALT >40 U/l, and the survival curve showed that the
group of ALT ≤40 U/l had a higher total survival rate than the
group of ALT >40 U/l (P<0.05). The average survival time was
26.7±4.2 months for the group of ALT ≤40 U/l and 12.4±1.7 months
for the group of ALT >40 U/l. Cases of AST ≤40 U/l were compared
with cases of AST >40 U/l, and the survival curve showed that
the group of AST ≤40 U/l had a higher total survival rate than the
group of AST >40 U/l (P<0.05). The average survival time was
28.7±5.0 months for the group of AST ≤40 U/l and 14.4±2.4 months
for the group of AST >40 U/l. Cases of CA125 ≤35 ug/ml were
compared with cases of CA125 >35 ug/ml, and the survival curve
showed that the group of CA125 ≤35 ug/ml had a higher total
survival rate than the group of CA125 >35 ug/ml (P<0.05). The
average survival time was 24.5±3.8 months for the group of CA125
≤35 ug/ml and 8.3±3.8 months for the group of CA125 >35 ug/ml.
Cases of HB ≤110 G/l were compared with cases of HB >110 G/l,
and the survival curve showed that the group of HB >110 G/l had
a higher total survival rate than the group of HB ≤110 G/l
(P<0.05). The average survival time was 25.5±3.7 months for the
HB >110 G/l group and 10.5±2.7 months for the HB ≤110 G/l group.
Cases who had received a surgical operation were compared with
those who had not received any surgical operation, and the survival
curve showed that the operated group had a higher total survival
rate than the non-operated group (P<0.05). The average survival
time was 31.3±6.1 months for the operated group and 15.3±2.1 months
for the non-operated group. The survival time is not correlated to
sex, age (≤50 or >50), tumor size (≤5 or >5 cm), and the
immunohistochemical result of CGA (negative or positive; P>0.05;
Fig. 3). All clinicopathological
features that had a positive result in the single-factor analysis
were further tested by multiple-factor analysis, revealing that
only Ki-67 had a correlation with prognosis. To sum up, testing the
effects of various clinicopathological indicators on the prognosis
showed that tumor group, Ki-67 PI, CA125, ALT, AST, HB, and whether
surgery had been received can significantly affect the survival
rate of patients, demonstrating that these indicators have
important roles in evaluating the severity and prognosis of PHNETs.
Especially Ki-67 PI, which can be an independent prognostic factor.
The effects of tumor size, AFP, CEA, CGA, and albumin on severity
evaluation and prognosis were limited (Table III).
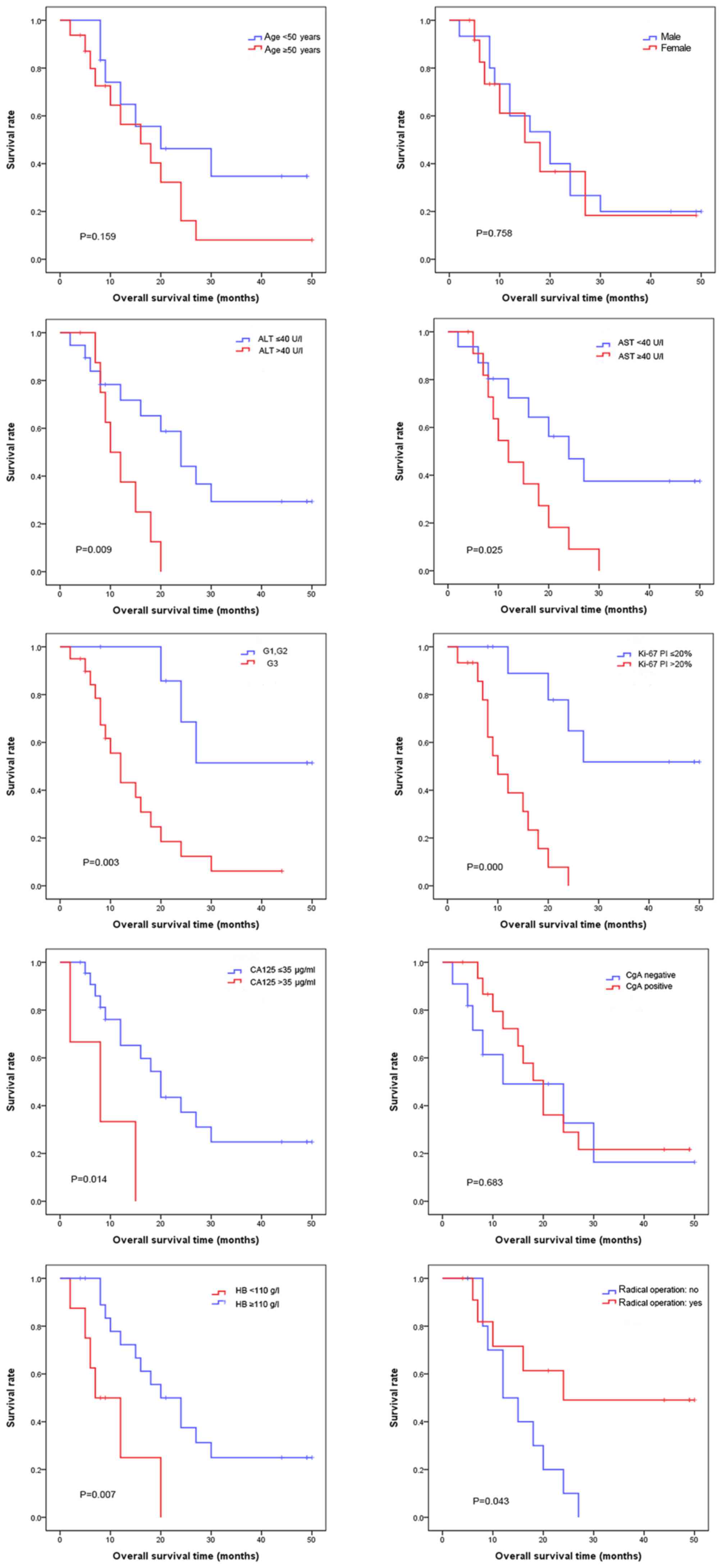 | Figure 3.Patients were divided into low and
high age groups, male and female groups, and CGA negative and
positive groups; and the survival analysis was conducted
respectively, showing no significant difference in overall survival
rate (P>0.05). At the same time, the patients were divided into
the ALT normal and abnormal groups, AST normal and abnormal groups,
net (G1, G2) and NEC (G3) groups, Ki-67 PI >20% and Ki-67 PI
≤20% groups, CA125 abnormal and normal groups, HB abnormal and
normal groups, and the radical operation and others groups. Then
the survival analysis was conducted respectively, revealing that
the overall survival rate is significantly different (P<0.05).
ALT, alanine aminotransferase; AST, aspartate aminotransferase;
NEC, neuroendocrine carcinoma; G1-3, tumor grades 1–3; CA125,
carbohydrate antigen 125; HB, hemoglobin; PI, Ki-67 positive index;
CGA, chromogranin A. |
 | Table II.Correlations of histological grade
with clinicopathological characteristics. |
Table II.
Correlations of histological grade
with clinicopathological characteristics.
|
| Histological
grade |
|
|---|
|
|
|
|
|---|
| Parameters | G1/G2 | G3 | P-value |
|---|
| Age, years |
|
| 0.629 |
| ≤50 | 4 | 8 |
|
|
>50 | 4 | 12 |
|
| Sex |
|
| 0.811 |
| Male | 4 | 11 |
|
|
Female | 4 | 9 |
|
| CA125, µg/ml |
|
| 0.220 |
| ≤35 | 8 | 15 |
|
|
>35 | 0 | 3 |
|
|
Unknown | 0 | 2 |
|
| Tumor size, cm |
|
| 0.793 |
| ≤5 | 3 | 7 |
|
|
>5 | 3 | 9 |
|
|
Unknown | 2 | 4 |
|
| AFP, µg/l |
|
| 0.497 |
| ≤400 | 8 | 17 |
|
|
>400 | 0 | 1 |
|
|
Unknown | 0 | 2 |
|
| Number of tumor
lesions |
|
| 0.466 |
|
Single | 7 | 15 |
|
|
Multiple | 1 | 5 |
|
| ALT, U/l |
|
| 0.021a |
|
≤40 | 8 | 11 |
|
|
>40 | 0 | 9 |
|
| AST, U/l |
|
| 0.004b |
|
≤40 | 8 | 8 |
|
|
>40 | 0 | 12 |
|
| HB, g/l |
|
| 0.159 |
|
≤110 | 1 | 8 |
|
|
>110 | 7 | 12 |
|
| Treatment |
|
| 0.094 |
| Radical
operation | 2 | 12 |
|
|
Others | 6 | 8 |
|
| Albumin, g/l |
|
| 0.053 |
|
≤35 | 0 | 7 |
|
|
>35 | 8 | 13 |
|
 | Table III.Cox proportional hazard regression
analysis of patients' overall survival. |
Table III.
Cox proportional hazard regression
analysis of patients' overall survival.
|
| Univariable | Multivariable |
|---|
|
|
|
|
|---|
| Variable | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 1.156 | 0.451–2.967 | 0.763 | – | – | – |
| Age, years (>50
vs. ≤50) | 1.928 | 0.748–4.972 | 0.174 | – | – | – |
| ALT, U/l (>40
vs. ≤40) | 3.648 | 1.276–10.429 | 0.016a | 1.021 | 0.291–47.353 | 0.312 |
| AST, U/l (>40
vs. ≤40) | 2.730 | 1.079–6.909 | 0.034a | 3.564 | 0.006–1.110 | 0.059 |
| HB, g/l (>110
vs. ≤110) | 0.256 | 0.088–0.745 | 0.012a | 1.620 | 0.110–1.599 | 0.203 |
| Ki-67 PI (>20%
vs. ≤20%) | 8.980 | 2.439–33.064 | 0.001b | 26.069 | 3.967–171.323 | 0.001b |
| CGA (positive vs.
negative) | 0.824 | 0.319–2.132 | 0.690 | – | – | – |
| Tumor diameter, cm
(>5 vs. ≤5) | 0.791 | 0.275–2.270 | 0.662 | – | – | – |
| Treatment (radical
operation vs. others) | 0.269 | 0.093–0.779 | 0.015a | 3.176 | 0.020–1.203 | 0.075 |
| CA125, µg/ml
(>35 vs. ≤35) | 4.639 | 1.191–18.064 | 0.027a | 1.566 | 0.507–21.748 | 0.212 |
Discussion
PHNETs accounts for 0.3–4.0% of the NETs, while
reports of PHNETs are mainly of individual cases, so there is very
little understanding of the clinicopathological features and the
biological behavior of this disease (8–11).
Diagnosis of PHNETs depends on three dispensable consecutive
stages, the preoperative, intraoperative, and postoperative stage
(12). Pathology is the gold standard
in NET diagnosis, but it is impossible to distinguish a primary
from a metastatic tumor only based on the pathological morphology
(11). Therefore, comprehensive and
careful intraoperative examinations as well as long-term
postoperative follow-ups are essential to detect tiny extrahepatic
primary lesions. Follow-up examinations include gastrointestinal
endoscopy, abdominal ultrasound, thoracic abdominal CT and MRI. In
our study, most of the 28 patients had received CT and MRI
examinations, which had similar characteristics. We chosed a
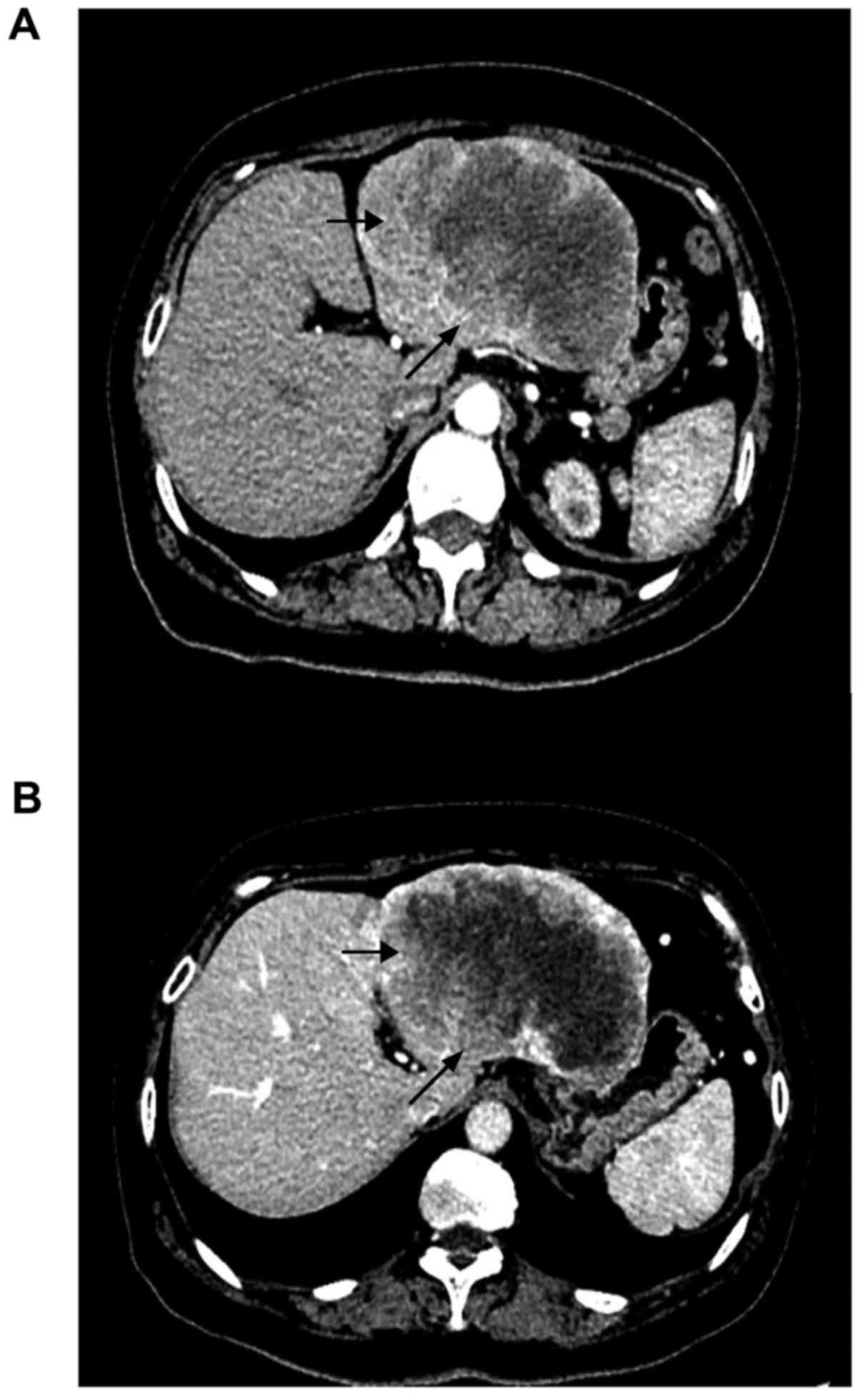
patient that had been performed CT scan in Fig. 2, which was representative. We had
obtained patient consent for publication of the CT images shown in
this manuscript and we had delieved all the imformations of the
patient in Fig. 2.
Some researchers put forward that PHNETs diagnosis
should focus on the comprehensive examination of NETs in the small
intestine, colorectum, bronchus, lung, gall bladder, and pancreas,
because the tiny lesions in these tissues may metastasize to the
liver (4,12–14). It is
not easy to find tiny primary lesions during the process of
diagnosis, but postoperative clinical follow-ups and auxiliary
examinations are very helpful to detect these potential primary
lesions.
In the past, PHNETs has been divided into two
categories, carcinoid tumors and NECs. According to the diagnostic
criteria of carcinoid tumors, the 28 cases of this study could not
be classified as classical carcinoid tumors; therefore, we named
them NETs. It is reported that there was no significant difference
in the participation of men and women (15). In our study, slightly more men than
women participated (1.15:1.0), mostly middle-aged and elderly
people (average age=53 years) with single tumors (78.6%), which was
consistent with previous literature (4). Our results showed that the average
survival time of the NET (G1, G2) group was 37.3 months and that of
the NEC group was 14.9 months. The total survival rate of the NET
(G1, G2) group was significantly higher than that of the NEC group,
indicating that using the WHO classification (2010) to classify
PHNETs is reasonable and feasible (16). Some PHNETs are low-grade malignancies,
which have a slow clinical progress, but some PHNETs have
characteristics like invasive growth, relapse, and even distant
metastasis. One case (NET G1, Ki-67 PI=3%, single tumor, radical
operation) with a 50-months follow-up showed no tumor recurrence
until the end of the follow-up. One case (poorly differentiated,
Ki-67 PI about 90%, large patchy necrosis) in G3 died after a
follow-up of two months. The pathological features of this patient
indicated that the PHNETs was highly malignant, with rapid
development and poor prognosis (15).
These data showed that PHNETs is a heterogeneous tumor, and it is
crucial to classify the PHNETs for guiding clinical prognosis and
treatment according to its biological behaviors like slow growth,
obvious malignancy, and even metastasis. In the G3 group, tumor
tissues were mainly poorly differentiated, Ki-67 PI was high, and
the necrosis was often combined. These pathological indicators may
be correlated with the prognosis of PHNETs. At the same time,
prognosis of patients with abnormal liver function indexes, such as
ALT, was worse than for patients with normal liver function
indexes, demonstrating that these clinical biochemical indexes may
be correlated with the prognosis of PHNETs. In the NET group (G1,
G2), more cases showed high expression of Ki-67 than in the NEC
group, although still 3 cases had a lower expression of Ki-67. This
was similar to the ALT distribution, suggesting that this
classification system can predict the severity of tumors to a
certain extent, but has some limitations when the grading simply
bases on ALT and Ki-67 PI, and the application of other
pathological indexes for classification needs further
investigation. The two groups did not exhibit any significant
differences in tumor size, AFP, CEA, CGA, and albumin. Significant
differences in these pathological indicators showed that it is
reasonable and feasible to classify PHNETs into NET G1, NET G2, and
NEC according to the WHO (2010) classification standard for
neuroendocrine neoplasms of the digestive system. Furthermore, this
classification can help to predict the severity and prognosis of
PHNETs (17). However, although the
WHO classification is for these cases identical, the prognosis
still exhibits differences. Therefore, whether other pathological
indicators have an effect on the prognosis and more accurate
evaluation criteria are required needs to be further discussed.
Regarding the effects of pathological indicators on the prognosis,
the G1, G2 groups had a significantly higher survival rate than the
G3 group, prompting that tumor grading has a guiding effect on
prognosis. Grading can be used as an effective indicator of the
prognosis for the cases where only a small quantity of tumor cells
is available, e.g., for needle biopsy. The group of Ki-67 PI ≤20%
had a higher total survival rate than the group of Ki-67 PI >20%
(P<0.05), suggesting that the expression level of Ki-67 has a
guiding effect on the prognosis. The group of AST (ALT) ≤40 U/l had
a higher total survival rate than the group of AST (ALT) >40 U/l
(P<0.05), suggesting that the levels of liver transaminases have
a guiding effect on prognosis. The group of HB >110 G/l had a
higher total survival rate than the group of HB ≤110 G/l
(P<0.05), indicating that the anemia status has a guiding effect
on prognosis. However, liver enzyme levels and low HB values may
reflect not cause but result of the malignancy of the PHNETs in
regards to the elevated liver enzyme levels and low HB values
exhibited by malignant tumors. The group of CA125 ≤35 ug/ml had a
higher total survival rate than the group of CA125 >35 ug/ml
(P<0.05), indicating that an abnormal rise of CA125 has a
guiding effect on prognosis. The operated group had a higher total
survival rate than the non-operated group (P<0.05), suggesting
that receiving a radical operation has a guiding effect on
prognosis. The guiding effects of tumor size, AFP, CEA, CGA, and
albumin on the prognosis were limited. It is revealed in this study
that poor tumor grading, high expression of Ki-67, poor liver
function, and anemia are the main characteristics of malignant
tumors: Tumor grading of G3, high expression of Ki-67, poor liver
function, anemia, abnormal level of CA125, and lack of radical
operation (18,19) are correlated with shorter survival
time and poor prognosis. Moreover, the high expression of Ki-67 is
an independent prognostic factor. All results support that these
pathological indicators can help to predict the severity and
prognosis of PHNETs.
Acknowledgements
The authors would like to thank Dr Qiu-Shuang Wang
and Dr Xiao-meng Dai (Tongji Medical College) for their
suggestions.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY and JH conceived and designed the study. RC and
MQ analyzed and interpreted the patient data. RC was a major
contributor in writing the manuscript. YC, TZ and XH contributed
towards the acquisition of data, and data analysis and
interpretation. YL, WS and TX helped with the acquisition of data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was requested and obtained from the
Medical Ethics Committee of Tongji Medical College of Huazhong
University of Science and Technology. Written informed consent was
obtained from all participants.
Consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PHNETs
|
primary hepatic neuroendocrine
tumors
|
|
CA125
|
carbohydrate antigen 125
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
HB
|
hemoglobin
|
|
PI
|
Ki-67 positive index
|
|
CT
|
computed tomography
|
|
AFP
|
α-fetoprotein
|
|
CEA
|
carcinoembryonic antigen
|
|
CGA
|
chromogranin A
|
|
NEC
|
neuroendocrine carcinoma
|
|
NET
|
neuroendocrine tumor
|
|
HPF
|
high power field
|
References
|
1
|
Song JE, Kim BS and Lee CH: Primary
hepatic neuroendocrine tumor: A case report and literature review.
World J Clin Cases. 4:243–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang K, Cheng YS, Yang JJ, Jang X and Guo
JX: Primary hepatic neuroendocrine tumor with multiple liver
metastases: A case report with review of the literature. World J
Gastroentero. 21:3132–3138. 2015. View Article : Google Scholar
|
|
3
|
Camargo ÉS, Viveiros Mde M, Neto Corrêa
IJ, Robles L and Rezende MB: Primary hepatic carcinoid tumor: Case
report and literature review. Einstein (Sao Paulo). 12:505–508.
2014.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwao M, Nakamuta M, Enjoji M, Kubo H,
Fukutomi T, Tanabe Y, Nishi H, Taguchi KI, Kotoh K and Nawata H:
Primary hepatic carcinoid tumor: Case report and review of 53
cases. Med Sci Monit. 7:746–750. 2001.PubMed/NCBI
|
|
5
|
Kellock T, Tuong B, Harris AC and Yoshida
E: Diagnostic imaging of primary hepatic neuroendocrine tumors: A
case and discussion of the literature. Case Rep Radiol.
2014:1564912014.PubMed/NCBI
|
|
6
|
Li R, Tang CL, Yang D, Zhang XH, Cai P, Ma
KS, Guo DY and Ding SY: Primary hepatic neuroendocrine tumors:
Clinical characteristics and imaging features on contrast-enhanced
ultrasound and computed tomography. Abdom Radiol (NY).
41:1767–1775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morishita A, Yoneyama H, Nomura T,
Sakamoto T, Fujita K, Tani J, Miyoshi H, Haba R and Masaki T:
Primary hepatic neuroendocrine tumor: A case report. Mol Clin
Oncol. 4:954–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang YQ, Xu F, Yang JM and Huang B:
Primary hepatic neuroendocrine carcinoma: Clinical analysis of 11
cases. Hepatobiliary Pancreat Dis Int. 9:44–48. 2010.PubMed/NCBI
|
|
9
|
Chen Z, Xiao HE, Ramchandra P and Huang
HJ: Imaging and pathological features of primary hepatic
neuroendocrine carcinoma: An analysis of nine cases and review of
the literature. Oncol Lett. 7:956–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pedrassa BC, da Rocha EL, Kierzenbaum ML,
Bormann RL, Francisc VV and D'Ippolito G: Uncommon hepatic tumors:
Iconographic essay-Parte 2. Radiol Bras. 47:374–379. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yalav O, Ülkü A, Akçam TA, Demiryürek H
and Doran F: Primary hepatic neuroendocrine tumor: Five cases with
different preoperative diagnoses. Turk J Gastroenterol. 23:272–278.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang LX, Liu K, Lin GW and Jiang T:
Primary hepatic neuroendocrine tumors: Comparing CT and MRI
features with pathology. Cancer Imaging. 15:132015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gravante G, De Liguori Carino N, Overton
J, Manzia TM and Orlando G: Primary carcinoids of the liver: A
review of symptoms, diagnosis and treatments. Dig Surg. 25:364–368.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rocca A, Calise F, Marino G, Montagnani S,
Cinelli M, Amato B and Guerra G: Primary giant hepatic
neuroendocrine carcinoma: A case report. Int J Surg. 1 12
Suppl:S218–S221. 2014. View Article : Google Scholar
|
|
16
|
Cho CS, Labow DM, Tang L, Klimstra DS,
Loeffler AG, Leverson GE, Fong Y, Jarnagin WR, D'Angelica MI, Weber
SM, et al: Histologic grade is correlated with outcome after
resection of hepatic neuroendocrine neoplasms. Cancer. 113:126–134.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang LM, An SL and Wu JX: Diagnosis and
therapy of primary hepatic neuroendocrine carcinoma: Clinical
analysis of 10 cases. Asian Pac J Cancer Prev. 15:2541–2546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sutton R, Doran HE, Williams EMI, Vors J,
Vinjamuri S, Evans J, Campbell F, Raraty MG, Ghaneh P, Hartley M,
et al: Surgery for midgut carcinoid. Endocr Relat Cancer.
10:469–481. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fenwick SW, Wyatt JI, Toogood GJ and Lodge
JP: Hepatic resection and transplantation for primary carcinoid
tumors of the liver. Ann Surg. 239:210–219. 2004. View Article : Google Scholar : PubMed/NCBI
|