Introduction
Cervical cancer is one of the most common
malignancies in women. The incidence is second only to that of
breast cancer, which also poses a serious threat to women's health
(1). In recent years, one study found
that the continuous upgrading of screening methods and the
popularity of the human papillomavirus vaccine had caused the
incidence of both cervical cancer cases and mortality to decline.
Due to the uneven distribution of resources, the incidence is still
rising in developing countries, and the trend may affect younger
patients (2). As molecular biology
research becomes more sophisticated, more and more genes have been
found to be involved in the development of cervical cancer. Finding
new molecular targets for the prevention and treatment of cervical
cancer could have far-reaching implications.
The RUNX3 tumor suppressor gene was discovered a few
years ago. With RUNX1 and RUNX2, it makes up the transcription
factor RUNX family (3). RUNX1 is
associated with hematopoietic function (4), and RUNX2 is an important bone formation
regulator (5). RUNX3 is located on
chromosome 1p36.1 and contains a p1 promoter and p2 promoter
(6). Alkaline phosphatase (ALP), a
marker of BMP9-induced late osteogenic differentiation was enhanced
by the overexpression of RUNX3, whereas it was inhibited by the
knockdown of RUNX3 (7). It was first
reported to be a tumor suppressor gene in gastric epithelial cells
(8), and it was also reported to
absent or mutated in a variety of cancers for hemizygous deletions
(9), protein mislocalization,
epigenetic alterations (10), and
histone modifications (11). Suzuki
et al (12), initially
described the DNA promoter of RUNX3 hypermethylation as a
characteristic of breast cancer. Paradoxically, with the
discoveries reported by Nevadunsky et al (13) and Lee et al (14), found that RUNX3 took on a
growth-stimulating role, which was highly active in ovarian cancer
cells, likewise, it even played an oncogenic role in basal cell
carcinoma, head and neck squamous cell carcinoma (15,16).
Moreover, reports have found that RUNX3 inactivation can be
correlated with advanced tumor stage and negative prognosis
(17), and acts as a crucial early
step in the development of tumors (18,19). To
date, our purpose is to preliminarily explore the expression and
the biological behavior of RUNX3 in cervical cancer.
Materials and methods
Cell culture and reagents
RUNX3 antibody were bought from Santa Cruz
Biotechnology (sc-376543; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). Hce1 and Hela cells, were obtained from Xiangya Medical
College Cancer Institute. The cells were cultured in DMEM high
glucose medium containing 10% fetal bovine serum (FBS) and
penicillin/streptomycin (50 U/ml) at 37°C in 5% CO2. The
HCE cell line is misidentified according to: http://iclac.org/wp-content/uploads/Cross-Contaminations-v8_0.pdf.
The cells of HCE1 in our study names Hunan cervical epithelial cell
line no. 1 (HCE1) was established by Chinese researchers in 2000
and have no possibility of being contaminated by Hela cells.
Construction of plasmids and
transfection
RUNX3 mRNA sequences were picked from Genebank to
design primers. Then PCR amplification was performed and the PCR
product was subjected to 1% agarose gel electrophoresis and the
RUNX3-band was recovered. The recovered RUNX3 fragment and vector
PCDNA3.1 were digested with HindIII and XhoI, and the gel was
recovered by electrophoresis, then ligated with T4 DNA ligase
overnight at 4°C. At last, the ligation product was transformed
into DH5a. After the single clones were picked, the extracted
plasmids were identified and sequenced by double digestion. Then
Hce1 cells were transfected with PCDNA3.1-RUNX3 by
Lipofectamine-2000. The cells with RUNX3 overexpression were named
as ‘Hce1-RUNX3 cells’ in this manuscript.
RNA interference
Homo sapiens RUNX3 mRNA sequence (GenBank accession
no. NM_001031680.2) was used to design RUNX3 siRNA (Guangzhou Ruibo
Biological Technology Co.,Ltd., Guangzhou, China). The following
base pairs of siRNA were used for RUNX3: siRNA 646, (sense)
5′-CCATCACTGTGTTCACCAATT-3′and (antisense)
3′-TTGGTGAACACAGTGATGGTT-5′; siRNA 895, (sense)
5′-CCTCGGAACTGAACCCATTTT-3′ and (antisense)
5′-AATGGGTTCAGTTCCGAGGTT-3′; 100 nM in final concentration; 24 h
after transfection, cells were harvested and used for experiments.
Lipofectamine 2000 was used for siRNA transfections. HeLa cells
were transfected with siRNA by Lipofectamine-2000. The cells with
RUNX3 siRNA were named as ‘HeLa-SiRUNX3 cells’ in this
manuscript.
Western blot analysis
Whole cell lysates containing 30 µg of total
cellular proteins were analyzed by western blot. The antibodies
used include RUNX3 antibody (1:400) and goat anti-mouse HRP labeled
antibody, which were purchased from Santa Cruz Biotechnology, Inc.;
GAPDH antibody (1:4,000) was purchased from Hangzhou Sanjian
Company. We analyzed the molecular weight and grayscale values of
the target bands with the Gelpro 32 analysis software processing
system and calculated the RUNX3/GAPDH ratio.
Immunofluorescence
The cells were fixed in 2.5% paraformaldehyde for 30
min at room temperature and then permeated with 0.2% Triton-X for
15 min. Subsequently, the cells were blocked with 3% BSA/PBS for 30
min and incubated overnight at 4°C with RUNX3. After washing, the
cells were incubated with secondary anti-mouse IgG at room
temperature for 1 h. Nuclei of cells were stained with 1 µg/ml of
RUNX3, DAPI, and Merger.
Wound healing experiment
Hce-1 cells were overexpressed with RUNX3 and Hela
cells were transfected into 24-well plates with siRUNX3. After the
cells were cultured and grown to 100% confluence, they were
scratched with a 10 µl pipette tip (time 0), washed with PBS to
remove isolated cells, and incubated with complete growth medium.
The cells migrated to the injured area and were photographed after
48 h with an inverted microscope.
Transwell assay
Hce-1 cells were overexpressed with RUNX3 and Hela
cells were transfected with SiRUNX3, respectively. The
polycarbonate film was coated with 1 mg/ml matrigel gum (dissolved
in serum-free DMEM medium) and incubated at 37°C for 1 h.
Homogeneous cells (2.0×104) suspended in 100 µl of
complete medium were inoculated in the upper chamber of the
transwell unit, 700 µl of a high-sugar medium containing 10% FBS
was added to the lower chamber as a chemical attractant. These were
allowed to invade for 48 h at 37°C in a CO2 incubator.
Then the cells above the membrane were removed and the cells
migrating through the membrane to the lower chamber were fixed with
75% ethanol and stained with 0.5% crystal violet.
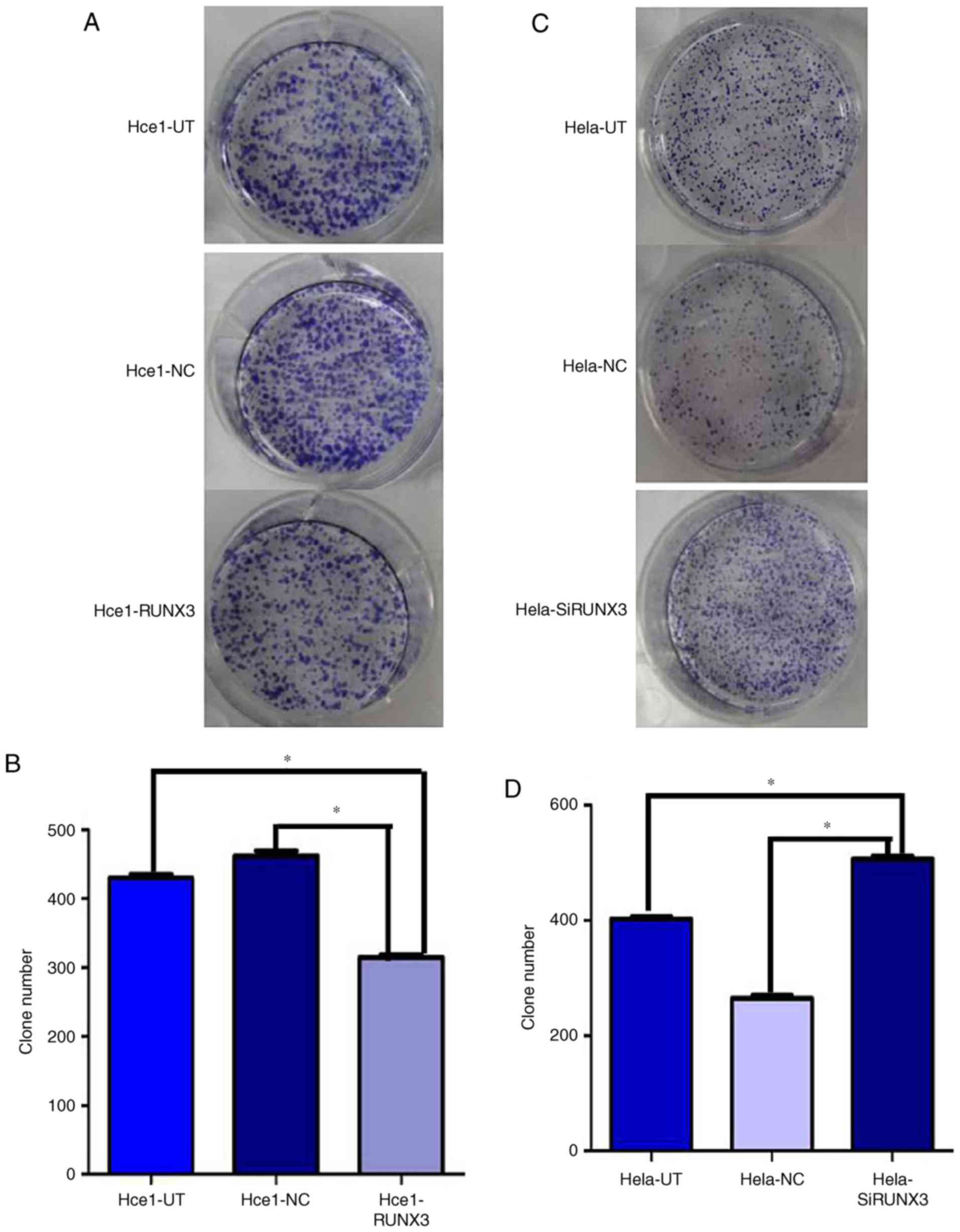
Colony formation experiment
After 24 h of transfection, the cells were seeded at
a density of 300/ml in 6-well plates. Colonies were allowed to grow
for 2 weeks. The culture medium was discarded and washed twice with
PBS. Then the cells were fixed in methanol for 15 min and stained
with Giemsa solution for 20 min. Lastly, the clones (more than 10
cells) were counted under a microscope.
Statistical analysis
All statistical analyses were done using SPSS 17.0
for windows. The data are presented as the mean ± SD. The
statistical significance of differences was determined by Student's
two-tailed t-test for two groups, and one-way ANOVA followed by
Student Newman Keuls post hoc test for multiple groups. P-values of
<0.05 were considered to indicate a statistically significant
difference. All the cell experiments were repeated at least three
times.
Results
Expression of RUNX3 in cervical cancer
cell lines
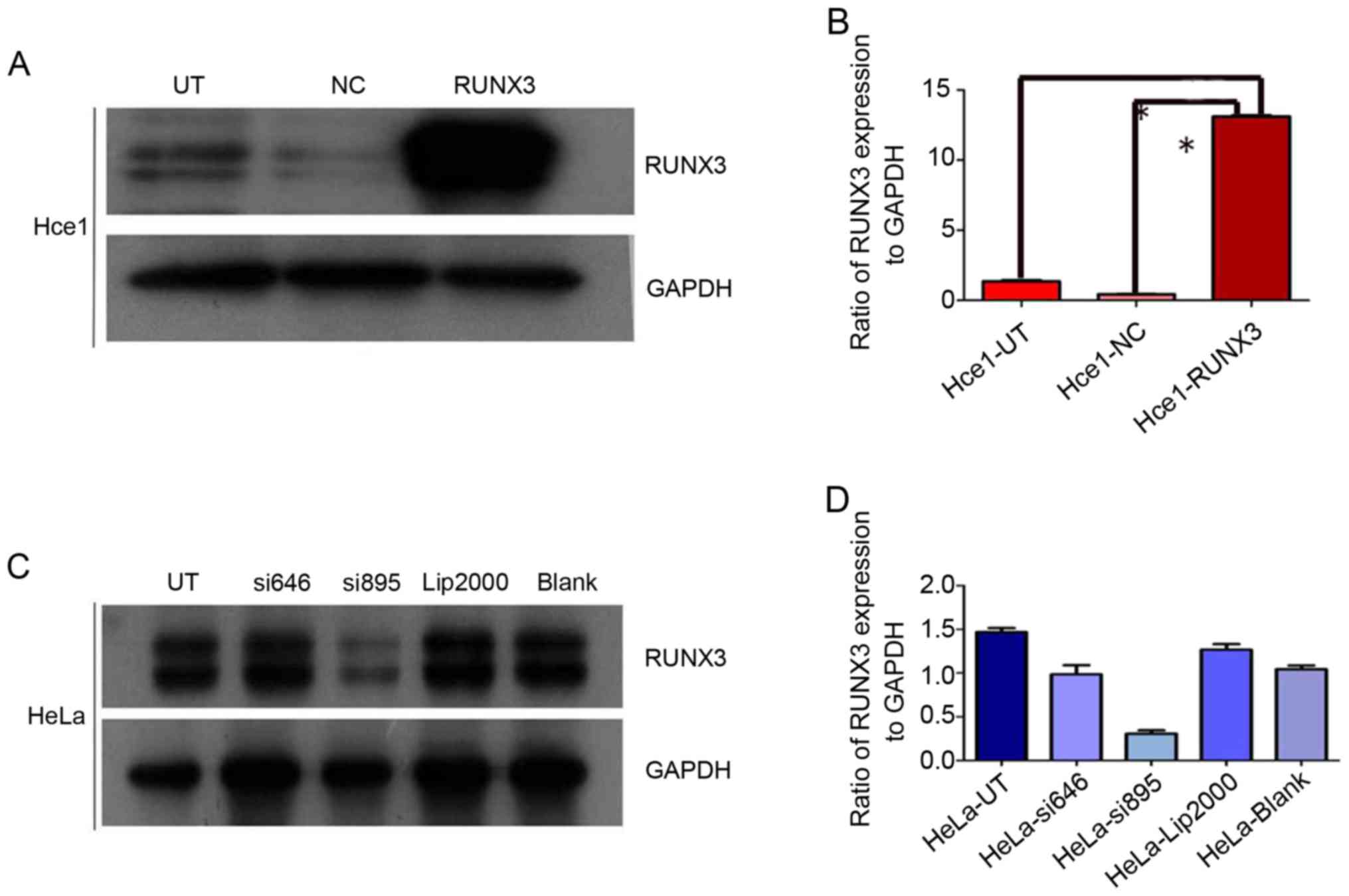
The efficiency of exogenous RUNX3 expression and
RUNX3 siRNA in cervical cancer cells was verified by western blot
analysis. As shown in picture 1, the protein of forced RUNX3
expression was markedly higher than in controls. However, the
vector group (NC) and untreated group (UT) exhibited few
differences (P>0.05; Fig. 1A). The
ratios of RUNX3/GAPDH were 13.1±1.2, 1.4±0.2, and 0.4±0.1
(P<0.05; Fig. 1B). RUNX3 gene was
successfully knocked down by si895 in Hela cells (Fig. 1C) and the ratio of RUNX3/GAPDH was
0.3±0.1, which was markedly lower than in Hela-UT (1.4±0.2) group
(P<0.05; Fig. 1D).
Subcellular location of RUNX3 in
cervical cancer cells
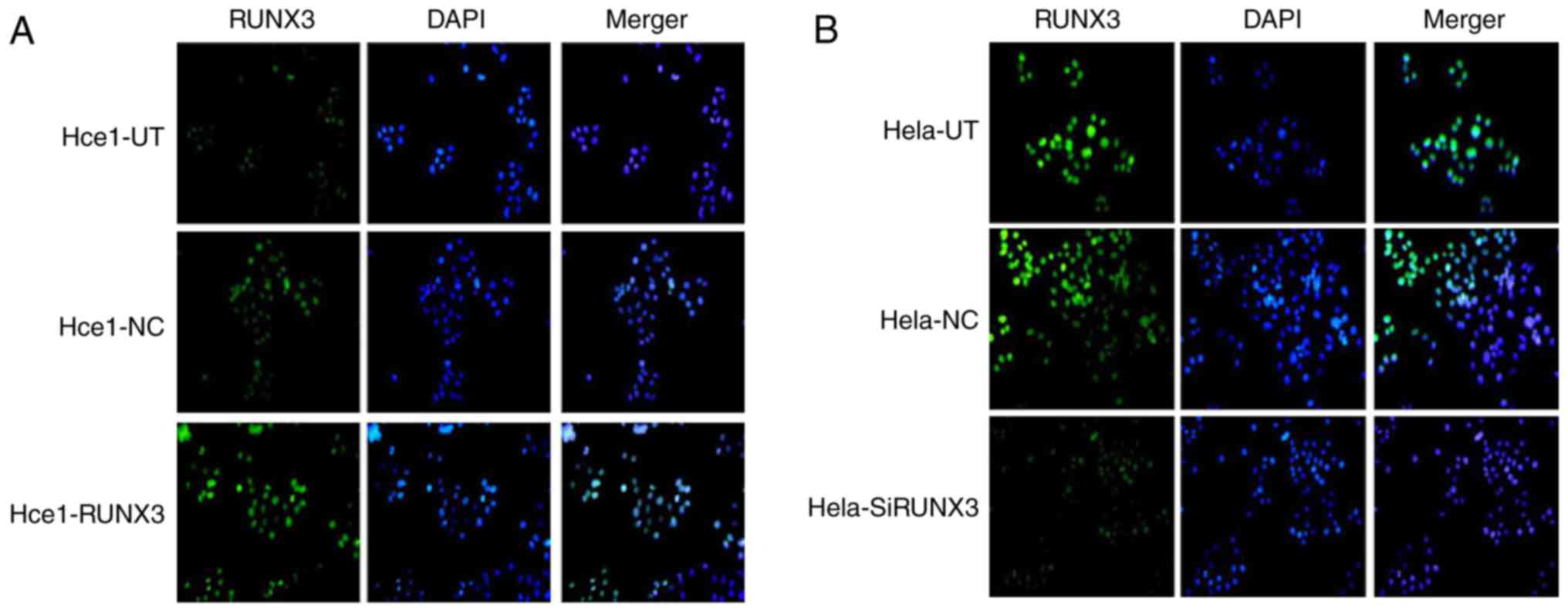
We next examined the subcellular localization of
RUNX3 in cervical cells using immunofluorescence. As shown in
Fig. 2A and B, RUNX3 clearly
demonstrated a strong nuclear localization in Hce1-RUNX3 cells and
Hela-UT cells, but in other groups, the expression levels of RUNX3
were visibly decreased.
Roles of RUNX3 in migration and
invasion of cervical cancer cells
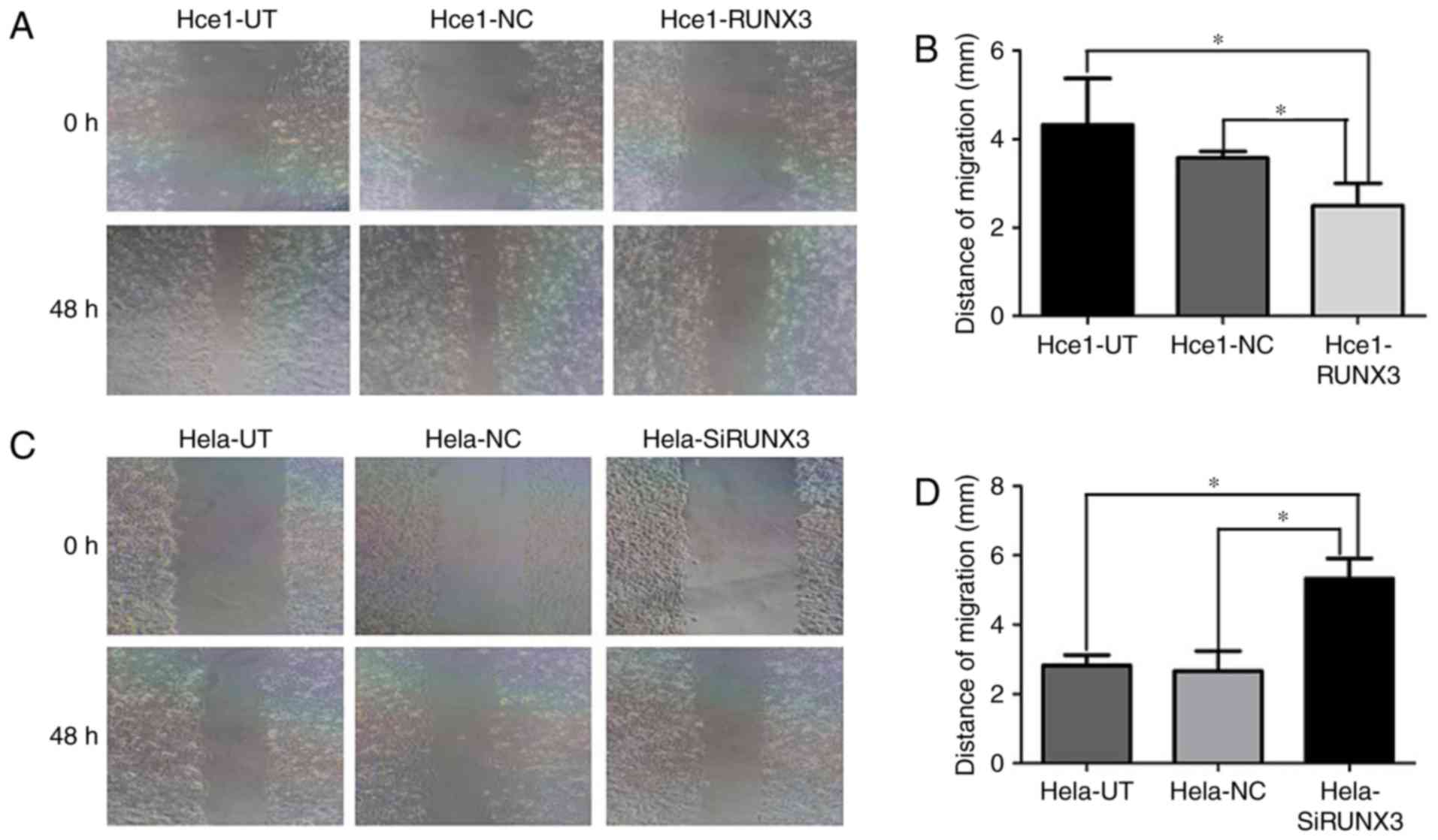
To establish the biological roles of RUNX3 in
cervical cancer cells, wound healing assay was carried out to
assess cell migration and Transwell assay was set up to investigate
effects of RUNX3 on cell invasion. As shown in Fig. 3, Hce1-RUNX3 cells migrated a shorter
distance (2.2±0.7) than in the Hce1-UT (4.2±1.4) and Hce1-NC groups
(3.7±0.3) (P<0.05; Fig. 3 A and
B). Conversely, Hela-SiRUNX3 cells migrated a longer distance
(5.1±0.6) than in the Hela-UT (2.7±0.3) and Hela-NC groups
(2.6±0.6) (P<0.05; Fig. 3C and
D).
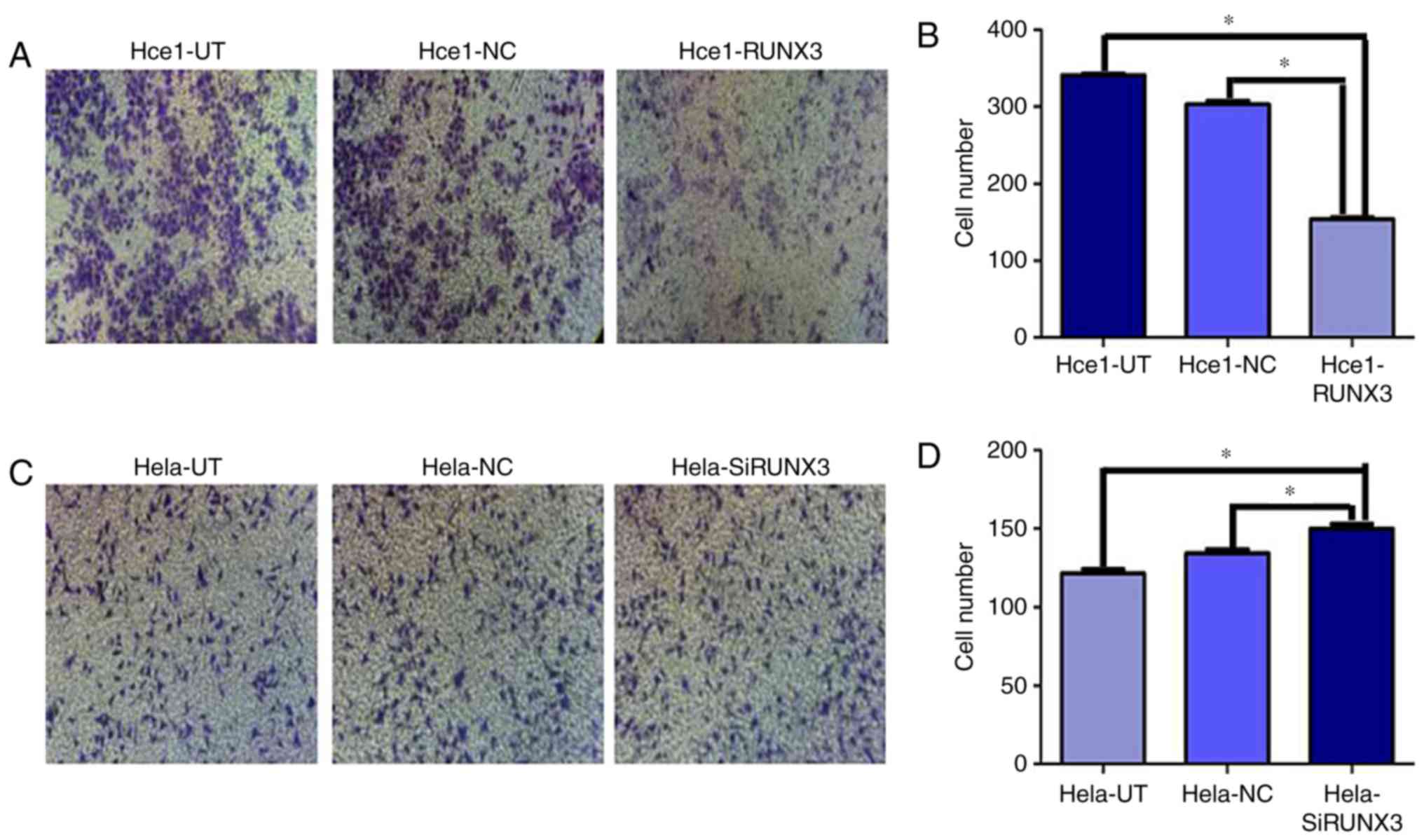
Hce1-RUNX3 cells still showed much lower penetration
ability (153±9) through the matrigel-coated membrane than in the
Hce1-UT (340±14) and Hce1-NC groups (304±12) (P<0.05). However,
compared with the Hela-UT (121±6) and Hela-NC groups (130±8), the
invasion ability of Hela-SiRUNX3 cell was enhanced (155±9)
(P<0.05; Fig. 4A-D). All of these
results collectively suggested that RUNX3 overexpression inhibited
cervical cancer cell migration and invasion. However, inhibition of
RUNX3 expression promoted migration and invasion of cervical cancer
cells.
Role of RUNX3 expression in colony
formation
The role of RUNX3 as a tumor suppressor gene was
assessed by a test of colony formation assay. Hce1-RUNX3 cells
formed 318±18 colonies, while the Hce1-UT and Hce1-NC groups formed
433±16 and 460±21 colonies, respectively (P<0.05; Fig. 5A and B). Conversely, Hela-SiRUNX3
cells had 509±19 colonies, indicating dramatically greater colony
forming ability than the Hela-UT and Hela-NC groups, which formed
402±14 and 267±12 colonies respectively (P<0.05; Fig. 5C and D). In this way, these results
suggest that stable forced RUNX3 expression can inhibit the ability
of cervical cancer cells to form colonies, and silencing RUNX3
expression was found to enhance colony formation by Hela cells.
Discussion
The HCE cell line is misidentified according to:
http://iclac.org/wp-content/uploads/Cross-Contaminations-v8_0.pdf,
but the cell of HCE1 we used in our study names Hunan cervical
epithelial cell line no. 1 (HCE1) was established from 5 carcinoma
specimens of hysterectomy by Chinese researchers in 2000, which has
been passaged more than 80 times, obviously different with HCE cell
line mentioned in the data which reported in 1981 (20). Hence, the HCE1 cells in our study
definitely not consistant with what the data mentioned. During the
experiment, we strictly adhere to the rules of cell culture, the
morphology and growth characteristics are quite difference between
HCE1 and Hela cells. HCE1 cells have no possibility of being
contaminated by Hela cells.
So far, only a few reports have addressed the
biological behavior of RUNX3 in tumorigenesis of cervical cancer.
The mechanisms by which RUNX3 acts in cervical cancer have not been
reported. Transcription factor of RUNX3, which is a downstream
effector of TGF-β, acts on the TGF-β receptor type II (21) or the downstream protein SMADs, and
then promotes the proliferation, apoptosis, angiogenesis, and
invasion of tumor cells through TGF-β signal transduction pathway
(22–24), which might explain its wide
involvement in tumorigenesis, including that of cervical
cancer.
In the present study, the recombination plasmid of
PCDNA3.1-RUNX3 was constructed and successfully transfected into
Hce1 cells. The expressive efficiency of RUNX3 in Hce1-RUNX3 cells
and Hela-SiRUNX3 cells were examined by western blot which showed
significantly increases in Hce1-RUNX3 cells and markedly decreased
in Hela-SiRUNX3 cells. Our results demonstrated that high levels of
RUNX3 expression could effectively inhibit the migration and
invasion of cervical cancer cells, compared with control group in
Hce1 cells. However, down-regulation of RUNX3 expression promoted
migration and invasion by Hela cells. Moreover, colony formation
numbers of cervical cancer cells decreased dramatically after
transfection with RUNX3. In this way, our study showed that RUNX3
might play an important role in inhibiting the proliferation,
migration, and invasion of cervical cancer cells.
Collectively, these results show that RUNX3 plays
significant roles in the development of cervical cancer, and it
might be a promising strategy for cervical cancer therapy. Further
study of the underlying mechanisms and signaling pathways of RUNX3
in cervical cancer may help improve the prognosis of patients with
cervical cancer.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
81302245).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PF contributed to analysis and manuscript
preparation. ZL and CZ performed the data analyses and wrote the
manuscript. MD helped perform the analysis with constructive
discussions.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lertkhachonsuk AA, Yip CH, Khuhaprema T,
Chen DS, Plummer M, Jee SH, Toi M and Wilailak S: Asian Oncology
Summit 2013: Cancer prevention in Asia: Resource-stratified
guidelines from the Asian Oncology Summit 2013. Lancet Oncol.
14:e497–e507. 2005. View Article : Google Scholar
|
|
2
|
Mohanty G and Ghosh SN: Risk factors for
cancer of cervix, status of screening and methods for its
detection. Arch Gynecol Obstet. 291:247–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ito Y: RUNX genes in development and
cancer: Regulation of viral gene expression and the discovery of
RUNX family genes. Adv Cancer Res. 99:33–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hug BA, Ahmed N, Robbins JA and Lazar MA:
A chromatin immunoprecipitation screen reveals protein kinase Cbeta
as a direct RUNX1 target gene. J Biol Chem. 279:825–830. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito Y and Miyazono K: RUNX transcription
factors as key targets of TGF-beta superfamily signaling. Curr Opin
Genet Dev. 13:43–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bangsow C, Rubins N, Glusman G, Bernstein
Y, Negreanu V, Goldenberg D, Lotem J, Ben-Asher E, Lancet D,
Levanon D and Groner Y: The RUNX3 gene-sequence, structure and
regulated expression. Gene. 279:221–232. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Feng Q, Ji C, Liu X, Li L and Luo
J: RUNX3 plays an important role in mediating the BMP9-induced
osteogenic differentiation of mesenchymal stem cells. Int J Mol
Med. 40:1991–1999. 2017.PubMed/NCBI
|
|
8
|
Li QL, Ito K, Sakakura C, Fukamachi H,
Inoue Ki, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, et al: Causal
relationship between the loss of RUNX3 expression and gastric
cancer. Cell. 109:113–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen LF: Tumor suppressor function of
RUNX3 in breast cancer. J Cell Biochem. 113:1470–1477.
2012.PubMed/NCBI
|
|
10
|
Vogiatzi P, De Falco G, Claudio PP and
Giordano A: How does the human RUNX3 gene induce apoptosis in
gastric cancer? Latest data, reflections and reactions. Cancer Biol
Ther. 5:371–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SH, Kim J, Kim WH and Lee YM: Hypoxic
silencing of tumor suppressor RUNX3 by histone modification in
gastric cancer cells. Oncogene. 15:184–194. 2009. View Article : Google Scholar
|
|
12
|
Suzuki M, Shigematsu H, Shames DS, Sunaga
N, Takahashi T, Shivapurkar N, Iizasa T, Frenkel EP, Minna JD,
Fujisawa T and Gazdar AF: DNA methylation-associated inactivation
of TGFbeta-related genes DRM/Gremlin, RUNX3 and HPP1 in human
cancers. Br J Cancer. 93:1029–1037. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nevadunsky NS, Barbieri JS, Kwong J,
Merritt MA, Welch WR, Berkowitz RS and Mok SC: RUNX3 protein is
overexpressed in human epithelial ovarian cancer. Gynecol Oncol.
112:325–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee CW, Chuang LS, Kimura S, Lai SK, Ong
CW, Yan B, Salto-Tellez M, Choolani M and Ito Y: RUNX3 functions as
an oncogene in ovarian cancer. Gynecol Oncol. 122:410–417. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsunematsu T, Kudo Y, Iizuka S, Ogawa I,
Fujita T, Kurihara H, Abiko Y and Takata T: RUNX3 has an oncogenic
role in head and neck cancer. PLoS One. 4:e58922009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kudo Y, Tsunematsu T and Takata T:
Oncogenic role of RUNX3 in head and neck cancer. J Cell Biochem.
112:387–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai KW, Koh KX, Loh M, Tada K, Subramaniam
MM, Lim XY, Vaithilingam A, Salto-Tellez M, Iacopetta B and Ito Y:
MicroRNA-130b regulates the tumour suppressor RUNX3 in gastric
cancer. Eur J Cancer. 46:1456–1463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng F, Wu J, Zhao S, Luo Q, Tang Q, Yang
L, Li L, Wu W and Hann SS: Baicalein increases the expression and
reciprocal interplay of RUNX3 and FOXO3a through crosstalk of AMPKα
and MEK/ERK1/2 signaling pathways in human non-small cell lung
cancer cells. J Exp Clin Cancer Res. 34:412015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YS, Lee JW, Jang JW, Chi XZ, Kim JH,
Li YH, Kim MK, Kim DM, Choi BS, Kim EG, et al: RUNX3 inactivation
is a crucial early event in the development of lung adenocarcinoma.
Cancer Cell. 24:603–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu SF, Zhu HC, Gu FH, Huang BY and Liu HY:
Characteristics of an established cervical carcinoma cell line
HCE1. Hunan Yi Ke Da Xue Xue Bao. 25:532–534. 2000.(In Chinese).
PubMed/NCBI
|
|
21
|
Ito K, Lim AC, Salto-Tellez M, Motoda L,
Osato M, Chuang LS, Lee CW, Voon DC, Koo JK, Wang H, et al: RUNX3
attenuates beta-catenin/T cell factors in intestinal tumorigenesis.
Cancer Cell. 14:226–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang B, Du J, Li Y, Tang F, Wang Z and He
S: EZH2 elevates the proliferation of human cholangiocarcinoma
cells through the downregulation of RUNX3. Med Oncol. 31:2712014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Subramaniam MM, Chan JY, Yeoh KG, Quek T,
Ito K and Salto-Tellez M: Molecular pathology of RUNX3 in human
carcinogenesis. Biochim Biophys Acta. 1796:315–331. 2009.PubMed/NCBI
|
|
24
|
Chen F, Liu X, Bai J, Pei D and Zheng J:
The emerging role of RUNX3 in cancer metastasis (Review). Oncol
Rep. 35:1227–1236. 2016. View Article : Google Scholar : PubMed/NCBI
|