Introduction
Malignant tumor is able to elicit host immune
response (1–4). However, according to the cancer
immunoediting theory, subpopulations of genetically heterogeneous
neoplastic cells evolve to escape immune surveillance and thrive
(5). During recent decades, numerous
experimental and clinical studies have demonstrated that tumor
cells surviving from immune surveillance acquire various (6) capacities to change constitution and/or
function of immune and stromal cells at the tumor site, creating
topical immunosuppressive milieu, thus the concept of tumor
microenvironment (TME) was proposed (7), which could partly explain why and how
tumor cells avoid antitumor immune responses. The specific
mechanisms involved in TME are highly complicated, including tumor
cell exploiting co-inhibitory signaling molecules through
interaction with immune cells, secretion of immunosuppressive
cytokines, recruitment of immune regulatory cells [regulatory T
lymphocyte (Treg) and myeloid-derived suppressor cell (MDSC)],
inducing cancer-related fibroblast and tumor-associated macrophage,
and other unidentified pathways (6,8–10). Notably, a number of these mechanisms
are important self-protective measures from tissue damage caused by
excessive immune reaction (11,12). Based
on these findings, a number of monoclonal antibody agents were
developed which benefit many patients with malignant tumors
(13–15). To date, the critical role of TME in
altering the biological behaviors of tumors have been established,
and it is proposed that genetic alterations in neoplastic cells as
well as the host immune system affect tumorigenesis and progression
(16).
Moreover, experimental evidence suggests that
primary tumor lesions are able to exert systemic effects by various
means. For example, a number of studies demonstrate that the
systemic effects of the tumor are able to form so-called
pre-metastatic niches in distant tissues or organs, and also
facilitate its own progression (17–22). By
contrast, some studies indicate that the systemic effects of the
tumor are able to inhibit metastasis (23,24). In
spite of these inconsistent findings, it has been demonstrated that
the tumor is able to exhibit systemic effects. Therefore, it is
hypothesized since tumor cells are able to reshape immune and
stromal cell profile at the tumor site, the systemic effects of the
tumor may adapt peripheral immune components to create a pro-tumor
peripheral immune environment. Nevertheless, at present, there is a
lack of direct evidence for peripheral immune profile in tumor
patients.
The present authors are particularly interested in
tumor metastasis, which is also the most common type of relapse for
the majority of malignancies following complete resection and
adjuvant therapies, particularly for lung cancer, which has one of
the highest cancer-associated mortalities and incidence among
malignant tumors according to recent statistics (25). Numerous patients with small primary
malignant lesions develop metastasis, which suggests that
metastasis is a relatively unique process inproportionate with the
topical progression of the tumor (26).
At present, there is limited information regarding
why and how tumor cells can be safely transported through the blood
or lymphatic vessel to another organ or lymph node without being
captured and killed by circulating immune components. Another
question is how latent micrometastasis develop to be clinically
detectable. Theoretically, if the peripheral immune system were
robust, it would be highly probable for metastatic tumor cells in
circulation to be eliminated. Therefore, the present authors
hypothesize that the peripheral immune environment may be adapted
in a way so the metastatic cells can evade from the immune
response.
In the present study, a preliminary study was
performed to characterize the profile of the peripheral circulating
immune system in patients with non-small cell lung cancer (NSCLC)
and healthy controls by assaying the proportion of lymphocyte
subpopulations and the levels of a number of tumor-associated
cytokines. Furthermore, comparisons were also performed between
NSCLC patients with and without metastasis.
Materials and methods
The present study was conducted following Human
Experimentation Review and approved by Research Ethics Committee of
the General Hospital of People's Liberation Army and the General
Hospital of Beijing Command. Informed consent was obtained from all
patients enrolled in the present study, and information from the
patients was protected. From April 2015 to December 2015, 48
eligible patients with NSCLC, who were admitted to either the
General Hospital of Beijing Command (Beijing, China) or the General
Hospital of People's Liberation Army (Beijing, China), were
included in the present study. Patient age ranged from 42–72 years,
with the mean age of 56. The inclusion criteria were as follows: i)
Having a clinical diagnosis of lung cancer; ii) being newly
diagnosed without receiving any antitumor therapy; iii) without
acute or chronic inflammatory disease during study; iv) without
suffering from immunodeficiency condition; v) without suffering
from immune-related disease; vi) without a history of long-term
drug therapy that may affect immunity. The exclusion criteria were
as follows: i) Pathological diagnosis of benign disease or small
cell lung cancer (SCLC); ii) without pathological diagnosis; iii)
incomplete examinations and iv) the presence of other concomitant
malignancy. A total of 21 patients admitted to General Hospital of
Beijing Command were also included as the control group: Two
patients were pathologically diagnosed as lung hamartoma, one
patient was pathologically diagnosed as costal fibrous dysplasia,
and 18 patients were diagnosed as congenital chest wall deformity.
The inclusion criteria for the control group were as follows: i)
Without any type of malignant tumor; ii) without acute or chronic
inflammatory disease during study; iii) without suffering from
immunodeficiency disorders; iv) without suffering from
immune-related disease and v) without a history of long-term drug
therapy that may affect immunity. All participants were divided
into two groups: NSCLC group and control group. Additionally, the
NSCLC group was further divided into two subgroups: Subgroup I
(NSCLC at stage I; n=17) and subgroup II (NSCLC with metastasis;
n=31). In subgroup I, 16 patients were at pathological stage I, and
1 patient was at clinical stage I. In subgroup II, lymph node
metastasis was pathologically confirmed, and distant metastasis was
confirmed with imaging.
Peripheral blood and serum sample
Peripheral venous blood were obtained from patients
in the morning and stored separately in heparin-coated and
non-coagulated tubes. The blood samples were transferred
immediately to the laboratory. Serum was aliquoted by
centrifugation (200 × g for 10 min) at room temperature, then
stored at −80°C for subsequent assay to detect the level of
cytokines.
Flow cytometric assay of lymphocyte
subpopulations
The reagents used for immunostaining were as
follows: Fluorescein isothiocyanate (FITC) or phycoerythrin cyanin
(PC)7-conjugated anti-cluster of differentiation (CD)3 (20 µl;
catalog no. 6607100) PC5-conjugated anti-CD4 (10 µl; catalog no.
H07752), FITC-conjugated anti-CD8 (20 µl; catalog no. H07756),
phycoerythrin (PE)-conjugated anti-CD25 (20 µl; catalog no.
H07774), PC7-conjugated anti-CD19 (10 µl; catalog no. IM3628),
PE-conjugated anti-CD56 (20 µl; catalog no. H07788) and
PE-conjugated anti-CD16 (20 µl; catalog no. H07766) (all from
Beckman Coulter, Inc., Brea, CA, USA).
Cell staining
Blood samples were stained according to the
manufacturer's instructions, and the following panels were
designed: i) CD8-FITC/CD25-PE/CD45-ECD/CD4-PC5/CD3-PC7 and ii)
CD3-FITC/CD16+56-PE/CD45-ECD/CD14-PC5/CD19-PC7.
Quantification by flow cytometry
Following staining, the blood samples were assayed
using a 5-colored uni-laser flow cytometer (FC500; Beckman Coulter,
Inc.). Data analysis was undertaken using the CXP software (Beckman
Coulter, Inc., Brea, CA, USA. The combinations of antibodies used
for analysis are follows: CD3+ for T lymphocytes, CD19+ for B
lymphocytes, CD3+CD4-CD8- for double-negative T lymphocytes (DN T
cells), CD3+CD4+CD25+ for activated T lymphocytes, CD3+CD4+CD25
high roughly for Treg, CD3+CD16+CD56+ for natural killer T cells
and CD3-CD16+CD56+ for natural killer cells.
Flow cytometric assay of serum
cytokines
Serum cytokine levels [including interferon (IFN)-γ,
tumor necrosis factor (TNF)-α, transcription growth factor (TGF)-β,
interleukin (IL)-2, IL-4, IL-6, IL-10 and IL-17A] were assayed by
using the commercially available Aimplex human Th1/Th2/Th17plex
assay kit and the human TGF-β assay kit, from AimPlex Biosciences,
(Pomona, CA, USA) and Beijing Quantobio Biotechnology Co., Ltd.
(Beijing, Chin), respectively, following the manufacturer's
instructions. Quantitation measurements were performed by a
4-colored uni-laser flow cytometer (FACSCalibur; BD Biosciences,
Franklin Lakes, NJ, USA). FCAP Array software (version 3.0) was
used to process data. Standard curves for each type of cytokine
were generated with manufacturer-supplied reference analytes.
Statistical analysis
The data are presented as the mean or mean ±
standard deviation. To compare the proportion of lymphocytes and
subpopulations between groups, Student's t-test or Wilcoxon
rank-sum test were used. Similarly, Student-test or Wilcoxon
rank-sum test was used to compare concentrations of cytokines
between groups. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using the SPSS software (version 13; SPSS, Inc., Chicago,
IL, USA).
Results
General characteristics of the
participants
There were 48 eligible NSCLC patients participating
the present study. Among them, 30 cases were male, 18 cases were
female. A total of 29 cases were diagnosed with adenocarcinoma, 14
cases with squamous cell carcinoma, 3 cases were adenosquamous
carcinoma and 2 cases with large cell carcinoma (Table I).
 | Table I.Basic information of
participants. |
Table I.
Basic information of
participants.
|
| NSCLC group | Control group |
|---|
|
|
|
|
|---|
| Parameters | Subgroup I | Subgroup II | CCWD | LH | CFD |
|---|
| Gender |
|
|
|
|
|
|
Male | 8 | 22 | 14 |
| 1 |
|
Female | 9 | 9 | 4 | 2 |
|
| Pathology |
|
|
|
|
|
|
Adenocarcinoma | 12 | 17 |
|
|
|
|
Squamous cell carcinoma | 3 | 11 |
|
|
|
|
Adenosquamous carcinoma | 2 | 1 |
|
|
|
|
Large-cell lung carcinoma | 0 | 2 |
|
|
|
| Metastasis |
|
|
|
|
|
|
N1-N2 | 0 | 14 |
|
|
|
| N3 or
distant organ metastasis | 0 | 17 |
|
|
|
Distribution of lymphocyte subpopulations as
determined by flow cytometric analysis (Fig. 1A-D). Lymphocyte subsets between the
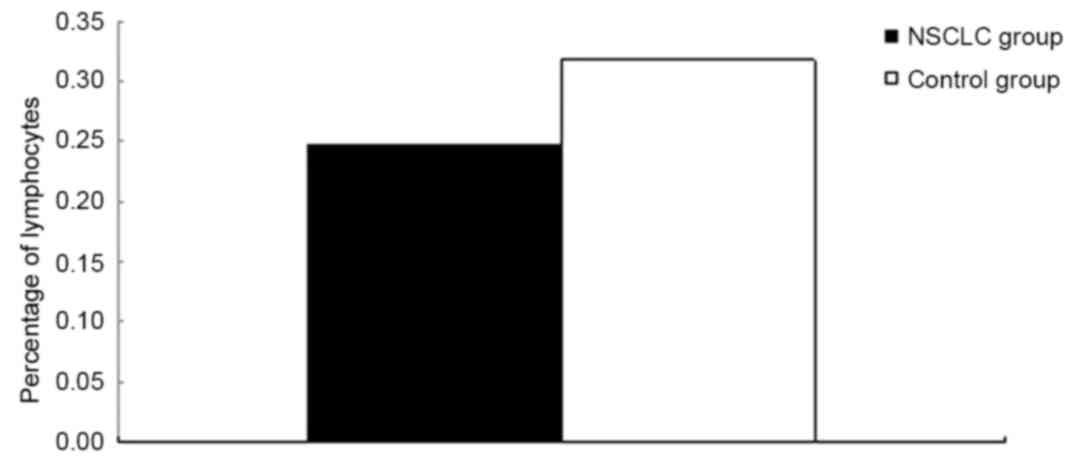
NSCLC group and the control group were compared (Table II). The results indicated that the
percentage of lymphocytes in the NSCLC group was significantly
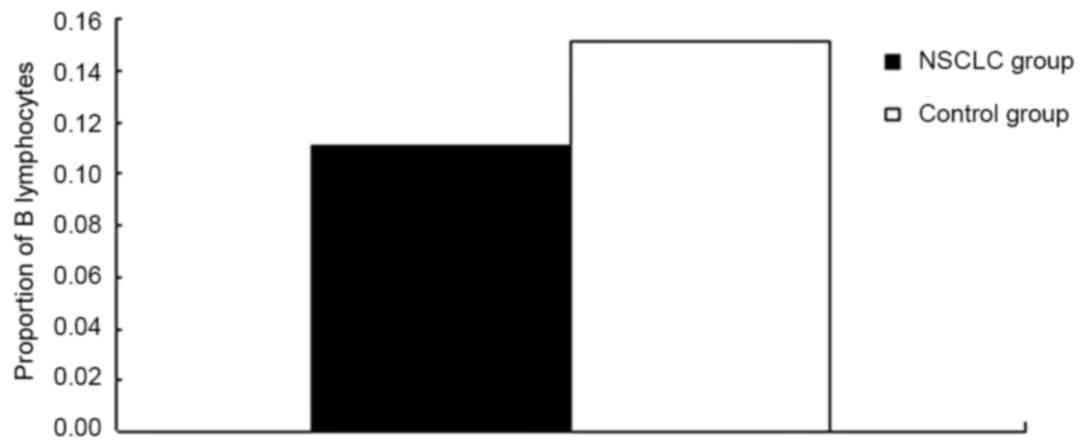
lower compared with the control group (P=0.008; Fig. 2). Additionally, the proportion of B
cells (CD19+) among lymphocytes in the NSCLC group was
significantly lower compared with the control group (P<0.0001;
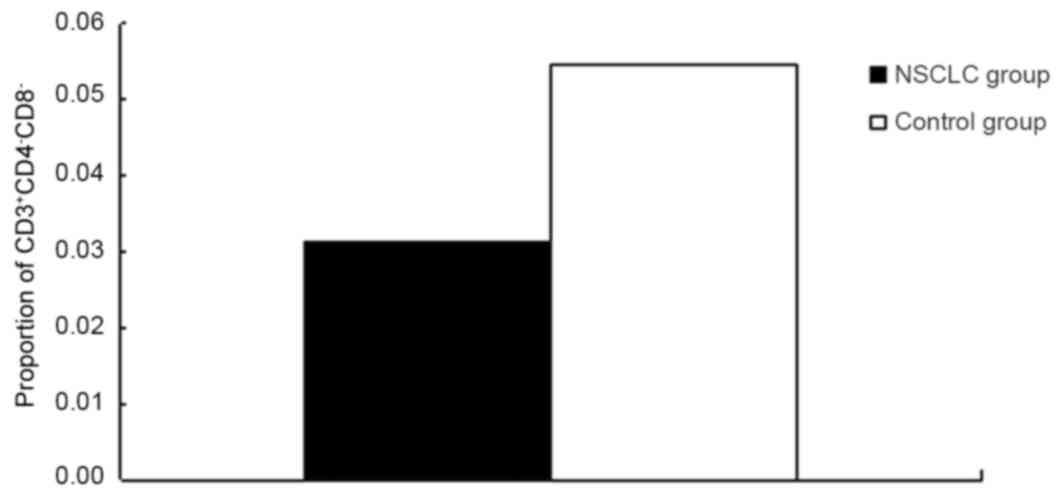
Fig. 3 and Table II). The proportion of DN T cells
(CD3+CD4-CD8-) among lymphocytes in the NSCLC group was
significantly lower compared with the control group (P=0.001;
Fig. 4 and Table II). However, there were no
significant differences in the proportion of other subpopulations
assayed (Table II). The ratio of
CD4+/CD8+ cells was not significantly different between the NSCLC
group and the control group (Table
II).
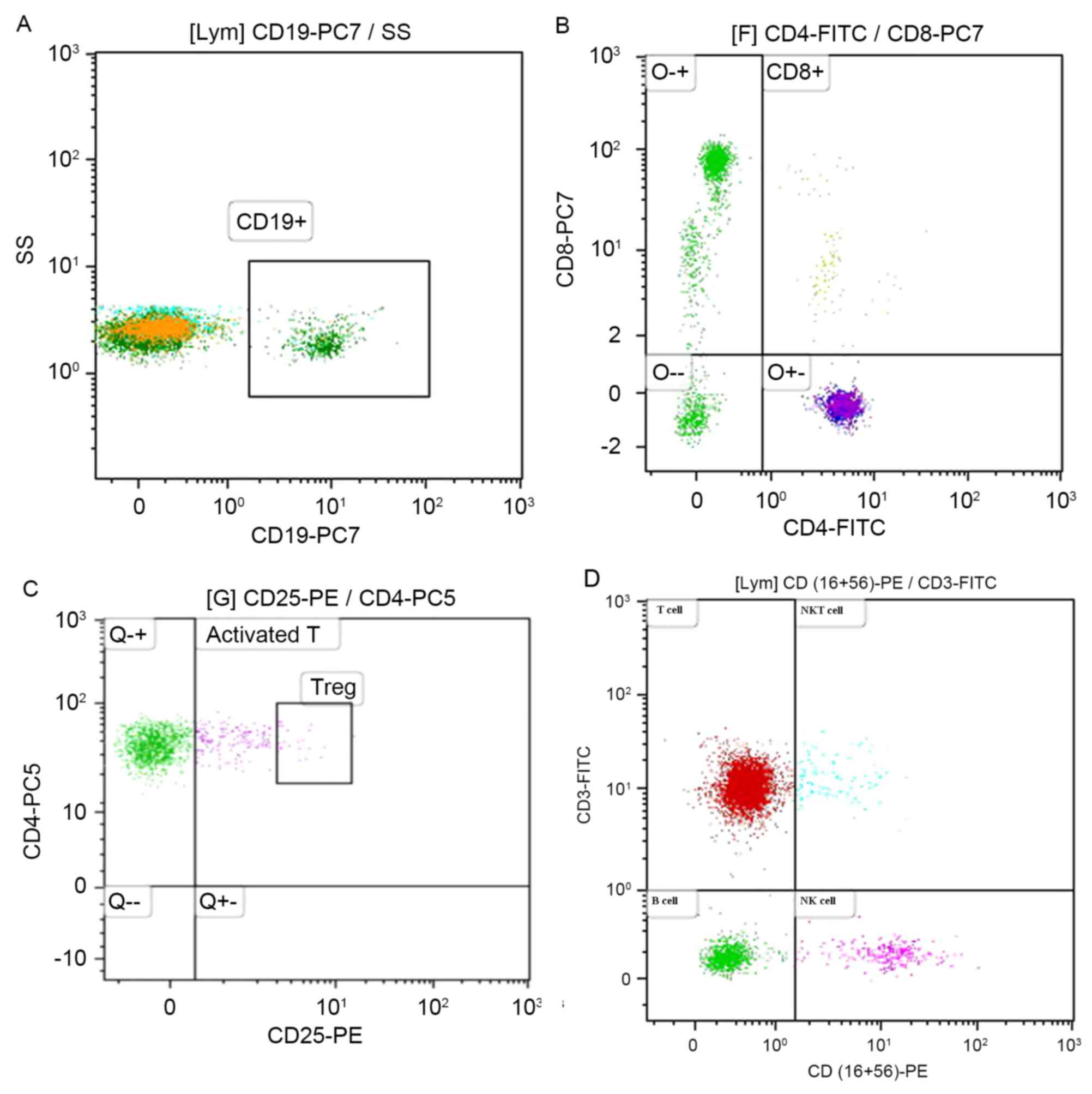 | Figure 1.Distribution of lymphocyte subsets
from peripheral blood as determined by flow cytometry. (A)
Expression of CD19, and B lymphocyte is indicated as CD19+. (B)
Co-expression of CD4 and CD8 by CD3+ lymphocyte. A distinct subset
of CD4-CD8- is shown in the lower left quadrant. (C) Co-expression
of CD4 and CD25 by CD3+ lymphocyte. The CD4+CD25+ subset in the
upper left quadrant is indicated as activated CD4+ T lymphocyte,
and a relatively distinct subset of CD4+CD25+ expressing high CD25
is identified as Treg. (D) Co-expression of CD3 and CD16+56, A
subset of CD3+CD16+CD56+ in the upper right quadrant is indicated
as NKT cells, A subset of CD3-CD16+CD56+ in the lower right
quadrant is indicated as NK cells. CD, cluster of differentiation;
FITC, fluorescein isothiocyanate; lym, lymphocyte; NK cells,
natural killer cells; NKT, natural killer T lymphocytes; PC,
phycoerythrin cyanin; PE, phycoerythrin; Treg, regulatory T
lymphocyte. |
 | Table II.Comparison of lymphocyte subsets
between the NSCLC group and the control group. |
Table II.
Comparison of lymphocyte subsets
between the NSCLC group and the control group.
| Lymphocyte
subsets | NSCLC group (mean ±
SD) | Control group |
P-valuea |
|---|
| Lymphocyte | 0.247±0.09 | 0.318±0.117 | 0.008 |
|
CD3+ | 0.699±0.092 | 0.722±0.055 | 0.288 |
|
CD3+CD4+ | 0.410±0.08 | 0.384±0.078 | 0.222 |
|
CD3+CD8+ | 0.267±0.074 | 0.285±0.062 | 0.346 |
|
CD3+CD4−CD8− | 0.032±0.018 | 0.054±0.034 | 0.001 |
|
CD3+CD4+CD25+ | 0.094±0.053 | 0.103±0.033 | 0.076 |
|
CD3+CD4+CD25high | 0.002±0.003 | 0.001±0.002 | 0.059 |
|
CD19+ | 0.110±0.044 | 0.152±0.047 | <0.0001 |
|
CD3+CD16+CD56+ | 0.080±0.057 | 0.081±0.091 | 0.291 |
|
CD3−CD16+CD56+ | 0.168±0.098 | 0.123±0.040 | 0.080 |
|
CD4+/CD8+ | 1.681±0.639 | 1.449±0.572 | 0.159 |
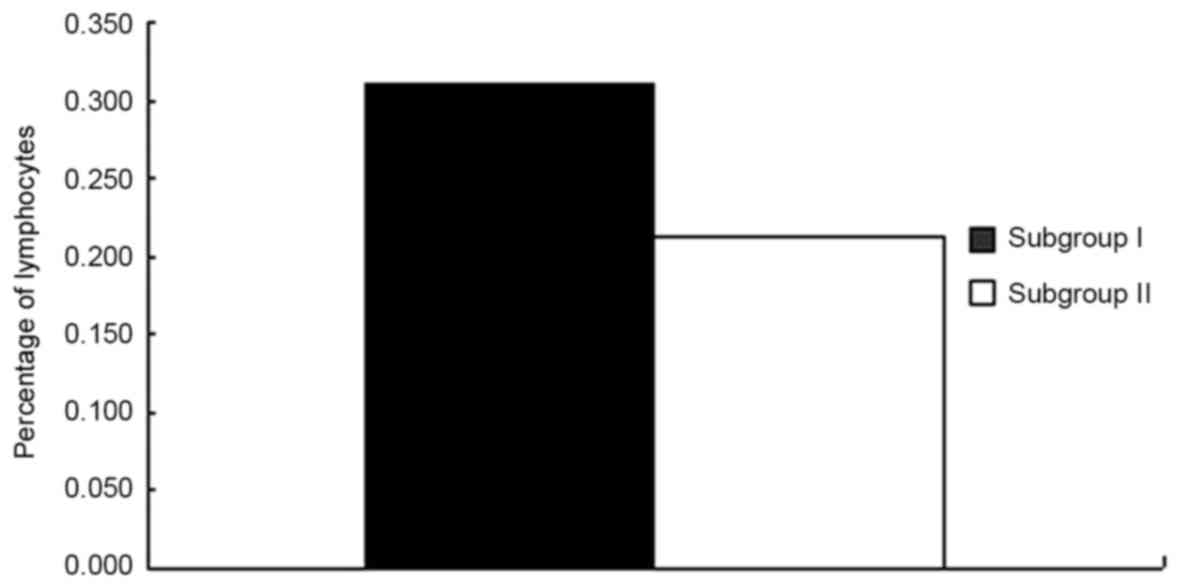
Subsequently, comparisons between subgroups I and II
were performed (Table III). The
percentage of lymphocytes in subgroup I was significantly higher
compared with subgroup II (P<0.0001; Fig. 5 and Table
III). However, there were no significant differences in the
proportion of other assayed subpopulations between subgroups I and
II. There were also no significant differences between the two
groups in the proportions of CD3+CD4+CD25+, CD3+CD4+CD25high and
CD3+CD4+ cells (Table III).
 | Table III.Comparison of lymphocyte subsets
between subgroups I and II. |
Table III.
Comparison of lymphocyte subsets
between subgroups I and II.
| Lymphocyte
subsets | Subgroup I | Subgroup II |
P-valuea |
|---|
| Lymphocyte | 0.309±0.094 | 0.214±0.066 | <0.001 |
|
CD3+ | 0.716±0.106 | 0.689±0.084 | 0.354 |
|
CD3+CD4+ | 0.434±0.062 | 0.397±0.087 | 0.125 |
|
CD3+CD8+ | 0.254±0.088 | 0.275±0.065 | 0.196 |
|
CD3+CD4−CD8− | 0.034±0.019 | 0.030±0.019 | 0.360 |
|
CD3+CD4+CD25+ | 0.086±0.025 | 0.098±0.025 | 0.875 |
|
CD3+CD4+CD25high | 0.002±0.000 | 0.002±0.004 | 0.883 |
|
CD19+ | 0.106±0.025 | 0.112±0.052 | 0.931 |
|
CD3+CD16+CD56+ | 0.063±0.048 | 0.090±0.061 | 0.070 |
|
CD3−CD16+CD56+ | 0.163±0.110 | 0.170±0.095 | 0.813 |
|
CD4+/CD8+ | 1.889±0.590 | 1.567±0.644 | 0.059 |
Levels of cytokines
In the present study, the levels of eight types of
cytokines, including IL-2, IL-4, IL-6, IL-10, IL-17A, TNF-α, TGF-β
and IFN-γ were analyzed. Comparisons were performed for each of the
eight cytokines between the NSCLC group and the control group. The
results indicated that the levels of IL-6 in the NSCLC group were
significantly higher compared with the control group (0.008)
(Table IV). However, there were no
significant differences for other cytokines (Table IV). Subsequently, comparisons were
performed in the levels of cytokines between subgroups I and II,
and no significant differences were identified (Table IV).
 | Table IV.Comparison of the levels of cytokines
between the NSCLC group and control group |
Table IV.
Comparison of the levels of cytokines
between the NSCLC group and control group
| Cytokines | NSCLC group
(pg/ml) | Control group
(pg/ml) |
P-valuea |
|---|
| IL-2 | 1.07±0.827 | 1.40±0.426 | 0.083 |
| IL-4 | 1.38±1.151 | 1.75±0.872 | 0.093 |
| IL-6 | 6.01±3.292 | 3.86±2.184 | 0.008 |
| IL-10 | 5.25±1.721 | 5.28±1.584 | 0.988 |
| IL-17A | 2.01±1.226 | 1.99±0.435 | 0.081 |
| TNF-α | 0.35±0.379 | 0.35±0.301 | 0.652 |
| TGF-β | 55,920±15,692 | 56,224±10,178 | 0.864 |
| IFN-γ | 3.57±2.050 | 3.83±2.404 | 0.675 |
Discussion
Malignant tumor remains one of the biggest threats
to human health. According to statistics, the total number of
cancer-associated mortalities worldwide in was 8.2 million, and the
number of newly diagnosed cases was 14.1 million (25). Although intensive research has been
conducted to unravel tumorigenesis and to identify novel
therapeutic approaches and as a result enormous progress has been
made in knowledge and clinical management, there remains to be
questions regarding the underlying mechanisms of tumor.
Molecular and biological studies revealed that
neoplastic cells are genetically unstable and heterogeneous, which
account for complexity and diversity of tumorigenesis and its
biological behaviors. Host immune response targeting malignant
tumor in patients and animal models have long been observed
(1–3,27). As
conventional therapeutic modalities are mostly concerned with
prolonging the survival time of patients with marked toxic side
effects, various types of immunotherapy have been attempted for
decades (28). During the recent
decade, the breakthrough finding of TME made tumor immunology
another focus for investigation in tumor biology.
It is now clear that tumor cells have evolved to
acquire various capacities to alter the topical milieu of tumor
tissues, and to facilitate proliferation and invasion (29). The complicated mechanisms remain to be
completely elucidated, and mechanisms that have been established
includes exploitation of co-inhibitory checkpoint molecules through
interaction between tumor cells and immune effector cells,
recruitment of immune suppressive cells (Tregs and MDSCs),
secretion of inhibitory cytokines and other agents, and reshaping
the function of stromal and immune cells (6,8–10,30). These
findings provide new strategy and targets for immunotherapy, and
newly developed monoclonal agents based on these findings have
achieved good clinical effects (13,14).
Since TME has a critical role in the biological
behavior of tumor, it has been proposed that the involvement of the
immune system is equally important in tumor development. Recently,
evidence suggested that tumor cells were not only able to
manipulate and reform local environment, but also exert systemic
influence through tumor-derived cytokines and microvesicles. It was
demonstrated that the systemic effects of the tumor could
compromise distant tissues and organs so as to facilitate
metastasis, and promote tumor growth (16). Nevertheless, there were also a number
of studies (31) with inconsistent
results (24). These seemingly
contradictory findings suggest the complexity of the systemic
effects of tumors.
Theoretically since tumors are able to exert
systemic effects, it is highly likely that they may adapt to the
peripheral environment to facilitate progression and metastasis
(32). Increasing evidence suggest
that tumor is a systemic disease in that topical alteration within
tumor tissue is closely associated with its systemic effect, for
example the recruitment of Tregs into tumor site is accompanied by
increased levels of Tregs in the peripheral blood (33,34).
In the present study, the changes in the proportions
of peripheral lymphocytes and subpopulations were analyzed. The
findings indicated that the percentage of lymphocytes in the NSCLC
group was significantly lower compared with the control group
(P=0.008). This is in accordance with results in other types of
malignant tumor (35,36).
To date, the reason or specific mechanisms for
lymphopenia in malignant tumor is unclear. Ray-Coquard et al
(37) proposed that a decreased
lymphocyte count might reflect immunosuppressive condition in the
tumor-bearing host, which suggest that the host tends to have an
inadequate immunological reaction. A decreased lymphocyte count
might also be a consequence of lympholysis caused by cytokines
produced by tumor cells in the case of lymphoma (37).
The present study hypothesized that decreased
lymphocyte count in tumor-bearing host is caused by tumor lesion,
which is supported by evidence that elimination of tumor lesion by
tumor antigen vaccination treatment is able to normalize decreased
lymphocyte frequency (37). The
results of the present study indicated that the percentage of
lymphocytes in NSCLC with metastasis is significantly lower
compared with the percentage in early stage NSCLC, which also
support the hypothesis that decreased lymphocyte is associated with
tumor progression. In addition, since tumor with metastasis is
indicative of poor prognosis, the findings of the present study
support that lymphopenia is an independent prognostic factor for
overall and progression-free survival in cancer (37). However, the specific underlying
mechanisms of how tumor affects the proportion of peripheral
lymphocytes require further studies.
In the present study, it was observed that the
proportion of CD3+CD4-CD8-cells, a poorly known subpopulation in
the peripheral blood, was significantly lower compared with the
control group (P=0.001), which has not been reported in any types
of tumor previously. CD3+CD4-CD8-lymphocytes are also known as DN T
cell with αβT-cell receptor (TCR) or γδTCR. The
CD3+CD4-CD8-subpopulation is very small in number and represents
1–3% of peripheral mononuclear cells. CD3+CD4-CD8-cells are mainly
distributed in the peripheral blood and lymph nodes (38,39). A
previous study demonstrated that this novel subset of T cell might
have a role in autoimmune disease, transplantation, viral infection
and malignant tumor by exerting different functions (40). DN T cells are able to suppress CD4+
and CD8+ T cell-mediated response by eliminating effector T cells
in murine models via the combination of Fas/Fas ligand or
perforin/granzyme secretion, or suppressing the proliferation of
activated T cells in humans via cell-cell interactions (41). Due to immunosuppressive properties of
DN T cells, DN T cell has been proposed as a novel therapeutic
target for autoimmune disease and transplantation. Studies have
demonstrated that DN T cells are able to enhance the survival of
organ allografts and xenografts (42). In human infections caused by the human
immunodeficiency virus and Simian immunodeficiency virus, DN T
cells are able to exert T helper cell-like functions in
compensation for very low levels of CD4+ T cells (43).
The roles of DN T cells in tumor have been gradually
unraveled. Young et al (44)
demonstrated that isolated DN T cells are able to kill lymphoma A20
cells in vitro, and prevent lymphoma cell growth in a mouse
model (44). Merims et al
(45) proposed a novel approach to
expand DN T cells isolated from leukemia patients in vitro,
and the results indicated that expanded DN T cells were able to
kill leukemia blast cells isolated from patients in vitro
via a perforin-dependent mechanism (45). Additionally, Voelkl et al
(46) identified a DN T cell clone
capable of killing melanoma cell isolated from a patient.
The findings of the present study suggest that tumor
cells might decrease the proportion of peripheral DN T cells by an
unidentified mechanism in order to create a favorable peripheral
environment for distant organ metastasis since DN T cells are able
to kill tumor cells directly in the absence of CD8+ cells. If this
finding is verified in future studies, DN T cells may be a
promising therapeutic target for clinic prevention and control of
metastasis. Further studies on the capability of DN T cells in the
killing of NSCLC tumor cells would provide important insight.
Notably, the present study also observed that the
proportion of peripheral B lymphocytes in the NSCLC group was
significantly lower compared with the in control group
(P<0.0001), which has not previously been reported in any type
of tumor. Except for its common function of antigen presentation
and antibody production or secretion, the role of B lymphocytes in
tumor has long been observed (47–49). Many
studies using murine models demonstrated that B cells were able to
markedly suppress antitumor immunity in various types of tumor. In
the B cell deficient mice (BCDM) model, slow growth or regression
of implanted tumors was associated with indicators of antitumor
immune responses, including dense infiltration of CD4+ and CD8+ T
cells in the tumor bed, increased Th1 response and enhanced
Cytotoxic T lymphocyte-mediated cytotoxity against tumor cells
(48). By contrast, tumor growth
restored when B cells were transplanted into BCDM or wild-type mice
(48–50).
A subset of B cells was recognized with
immunosuppressive function, namely B regulatory cells (Breg)
(51). Breg has been identified with
different phenotypes in different settings. Studies indicated that
B regs were able to induce primary CD4+ T cell differentiation into
the Th1/Th2 type (52). However, the
mechanism and the specific conditions that enable B reg cells to
exert this function are unclear. Moreover, B regs have been
demonstrated to be able to promote the conversion of naive CD4+ T
cells into Tregs, Tregs have been established to exert an important
immunosuppressive role in TME. A number of studies support that the
observation that the effect of Bregs in tumor may be mediated by
the conversion of Tregs (53). In
tumor models, Bregs have been observed to infiltrate tumor tissues.
Tumor infiltrating Bregs [(TIL, tumor-infiltrating
lymphocytes)-Bregs] are able to express various immunosuppressive
molecules, which may mediate their immunosuppressive effect
(53). However, the role of Bregs in
tumor remains controversial since studies indicate that TIL-Breg is
associated with improved survival (54), and some studies indicated that B cells
may have a protective against tumor (55). Based on these studies, it is
hypothesized that the preliminary findings of the present study
indicate that B cells may be recruited into tumor tissues.
Circulating cytokine is closely associated with
systemic and local immune status in disease, such as cancer. In the
present study, it was observed that the level of IL-6 in the NSCLC
group was significantly higher compared with the control group
(P=0.008), whereas the proportions of the other 7 cytokines,
including IFN-γ, TNF-α, TGF-β, IL-2, IL-4, IL-10 and IL-17A, were
not significantly different between the NSCLC group and the control
group. In addition, the levels of none of the cytokines was
significantly different between subgroups I and II. Previous in
vitro experiments demonstrated that IL-6 may have a dual role
in antitumor immunity. IL-6 is able to promote tumor growth through
downstream mediators and help sustain immunosuppressive milieu in
TME (56). Additionally, IL-6 is also
an important mediator of T cell recruitment to lymph nodes and
tumor site, and skewing the conversion of CD4+ T cells from Tregs
to a Th17 phenotype (56). Lippitz
(57) also concluded that circulating
IL-6 level is elevated in cancer patients and is also correlated
with poor prognosis (50). Moreover,
Lippitz (57) proposed that systemic
cytokine cascade is characteristic of cancer (50).
The authors of the present study support the
hypothesis that systemic cytokine changes are closely associated
with tumor progression, which may be regulated by the tumor
considering tumor cells are able to secret various pro-tumor
cytokines. Although, in the present study, significant changes in
the levels of cytokines were not observed, which is inconsistent
with the analysis of Lippitz (57),
the reason may be due to a relatively smaller sample size used in
the present study. The present authors support the hypothesis that
systemic cytokine cascade exists in patients with tumors, which
reflects tumor stage and host immune status. Furthermore, these
changes in the level of cytokines may be potential targets for
immunotherapy.
In conclusion, it was observed in the present study
that in NSCLC patients, the proportion of lymphocytes and two
subpopulations (CD3+CD4-CD8- and CD19+) were significantly
different between NSCLC patients and healthy controls.
The level of circulating IL-6 in NSCLC patients was
also significantly higher in the NSCLC group compared with healthy
controls. These preliminary results support the hypothesis that
peripheral immune system is adapted by tumor lesion, and a further
question is whether if it is a strategy adopted by tumor cells in
order to facilitate progression and metastasis. Further studies
which focus on the role of peripheral B cells and DN T cells in
tumor may determine if these cells have a role in
immunosurveillance and provide a novel strategy for
immunotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL analyzed the data and was a major contributor in
writing the manuscript. XC made substantial contribution to the
conception and design of study and gave the final approval of the
version to be published. JZ had major role in designing the study.
GX and YS made substantial contribution in analysis and
interpretation of the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by Research Ethics Committee
of the General Hospital of People's Liberation Army (approval no.
S2015-02-11). All patients provided informed consent to participate
this study.
Consent for publication
Informed consent was obtained from all patients
included in this study for publication of the associated data and
the accompanying image.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
TME
|
tumor microenvironment
|
|
MDSC
|
myeloid-derived suppressor cell
|
|
Treg
|
regulatory T lymphocyte
|
|
SCLC
|
small cell lung cancer
|
|
IFN-γ
|
interferon-γ
|
|
TNF-α
|
tumor necrosis factor-α
|
|
TGF-β
|
transcription growth factor-β
|
|
SD
|
standard deviation
|
References
|
1
|
Gross L: Intradermal immunization of C3H
mice against a sarcoma that originated in an animal of the same
line. Cancer Res. 3:326–333. 1943.
|
|
2
|
Prehn RT and Main JM: Immunity to
methylcholanthrene-induced sarcomas. J Natl Cancer Inst.
18:769–778. 1957.PubMed/NCBI
|
|
3
|
Hewitt HB, Blake ER and Walder AS: A
critique of the evidence for active host defence against cancer,
based on personal studies of 27 murine tumours of spontaneous
origin. Br J Cancer. 33:241–259. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klein G and Klein E: Immune surveillance
against virus-induced tumors and nonrejectability of spontaneous
tumors: Contrasting consequences of host versus tumor evolution.
Proc Natl Acad Sci USA. 74:2121–2125. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi
Y, Hu G and Sun Y: New horizons in tumor microenvironment biology:
Challenges and opportunities. BMC Med. 13:452015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Swartz MA, Iida N, Yull FE, Roberts EW,
Sangaletti S, Wong MH, Yull FE, Coussens LM and DeClerck YA: Tumor
microenvironment complexity: Emerging roles in cancer therapy.
Cancer Res. 72:2473–2480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lindau D, Gielen P, Kroesen M, Wesseling P
and Adema GJ: The immunosuppressive tumour network: Myeloid-derived
suppressor cells, regulatory T cells and natural killer T cells.
Immunology. 138:105–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Obeid E, Nanda R, Fu YX and Olopade OI:
The role of tumor-associated macrophages in breast cancer
progression (Review). Int J Oncol. 43:5–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiga K, Hara M, Nagasaki T, Sato T,
Takahashi H and Takeyama H: Cancer-associated fibroblasts: Their
characteristics and their roles in tumor growth. Cancer (Basel).
7:2443–2458. 2015. View Article : Google Scholar
|
|
11
|
Anderson AC, Joller N and Kuchroo VK:
Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized
functions in immune regulations. Immunity. 44:989–1004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smigiel KS, Srivastava S, Stolley JM and
Campbell DJ: Regulatory T-cell homeostasis: Steady-state
maintenance and modulation during inflammation. Immunol Rev.
259:40–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ott PA, Hodi FS and Robert C: CTLA-4 and
PD-1/PD-L1blockade: New immunotherapeutic modalities with durable
clinical benefit in melanoma patients. Clin Cancer Res.
19:5300–5309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Romano E and Romero P: The therapeutic
promise of disrupting the PD-1/PD-L1 immune checkpoint in cancer:
Unleashing the CD8 T cell mediated anti-tumor activity results in
significant, unprecedented clinical efficacy in various solid
tumors. J Immunother Cancer. 3:152015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitchem JB, Brennan DJ, Knolhoff BL, Belt
BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L,
Piwnica-Worms D, et al: Targeting tumor-infiltrating macrophages
decreases tumor-initiating cells, relieves immunosuppression and
improves chemotherapeutics responses. Cancer Res. 73:1128–1141.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McAllister SS and Weinberg RA: The
tumour-induced systemic environment as a critical regulator of
cancer progression and metastasis. Nat Cell Biol. 16:717–727. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiratsuka S, Nakamura K, Iwai S, Murakami
M, Itoh T, Kijima H, Shipley JM, Senior RM and Shibuya M: MMP9
induction by vascular endothelial growth factor receptor-1 is
involved in lung-specific metastasis. Cancer Cell. 2:289–300. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hiratsuka S, Watanabe A, Aburatani H and
Maru Y: Tumour-mediated upregulation of chemoattractants and
recruitment of myeloid cells predetermines lung metastasis. Nat
Cell Biol. 8:1369–1375. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Erler JT, Bennewith KL, Cox TR, Lang G,
Bird D, Koong A, Le QT and Giaccia AJ: Hypoxia-induced lysyl
oxidase is a critical mediator of bone marrow cell recruitment to
form the premetastatic niche. Cancer Cell. 15:35–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim S, Takahashi H, Lin WW, Descargues P,
Grivennikov S, Kim Y, Luo JL and Karin M: Carcinoma-produced
factors activate myeloid cells through TLR2 to stimulate
metastasis. Nature. 457:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sceneay J, Chow MT, Chen A, Halse HM, Wong
CS, Andrews DM, Sloan EK, Parker BS, Bowtell DD, Smyth MJ and
Möller A: Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+
immune suppressor cells and compromises NK cell cytotoxicity in the
premetastatic niche. Cancer Res. 72:3906–3911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang SY, Halvorsen OJ, Gravdal K,
Bhattacharya N, Lee JM, Liu NW, Johnston BT, Johnston AB, Haukaas
SA, Aamodt K, et al: Prosaposin inhibits tumor metastasis via
paracrine and endocrine stimulation of stromal p53 and Tsp-1. Proc
Natl Acad Sci USA. 106:12115–12120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Granot Z, Henke E, Comen EA, King TA,
Norton L and Benezra R: Tumor entrained neutrophils inhibit seeding
in the premetastatic lung. Cancer Cell. 20:300–314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurusu Y, Yamashita J and Ogawa M:
Detection of circulating tumor cells by reverse
transcriptasepolymerase chain reaction in patients with resectable
non-small-cell lung cancer. Surgery. 126:820–826. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grabenbauer GG, Lahmer G, Distel L and
Niedobitek G: Tumor-infiltrating cytotoxic T cells but not
regulatory T cells predict outcome in anal squamous cell carcinoma.
Clin Cancer Res. 12:3355–3360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pietras K and Ostman A: Hallmarks of
cancer: Interactions with the tumor stroma. Exp Cell Res.
316:1324–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carlini MJ, De Lorenzo MS and Puricelli L:
Cross-talk between tumor cells and the microenvironment at the
metastatic niche. Curr Pharm Biotechnol. 12:1900–1908. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McAllister SS, Gifford AM, Greiner AL,
Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander
BL, Repasky EA and Weinberg RA: Systemic endocrine instigation of
indolent tumor growth requires osteopontin. Cell. 133:994–1005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2014. View
Article : Google Scholar
|
|
34
|
Jóźwicki W, Brożyna AA, Siekiera J and
Slominski AT: Frequency of CD4+CD25+Foxp3+ cells in peripheral
blood in relation to urinary bladder cancer malignancy indicators
before and after surgical removal. Oncotraget. 7:11450–11462.
2016.
|
|
35
|
Fogar P, Sperti C, Basso D, Sanzari MC,
Greco E, Davoli C, Navaglia F, Zambon CF, Pasquali C, Venza E, et
al: Decreased total lymphocyte counts in pancreatic cancer: An
index of adverse outcome. Pancreas. 32:22–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahmad SS, Akhtar K, Verma AK, Mallik AZ
and Siddiqui SA: Total peripheral lymphocyte count in malignant
tumors: An index of prognostication. J Med Sci. 12:24–28. 2012.
View Article : Google Scholar
|
|
37
|
Ray-Coquard I, Cropet C, Van Glabbeke M,
Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P,
Labidi I, et al: Lymphopenia as a prognostic factor for overall
survival in advanced carcinomas, sarcomas, and lymphomas. Cancer
Res. 69:5383–5391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fischer K, Voelkl S, Heymann J, Przybylski
GK, Mondal K, Laumer M, Kunz-Schughart L, Schmidt CA, Andreesen R
and Mackensen A: Isolation and characterization of human
antigen-specific TCR alpha beta+ CD4 (-)CD8- double-negative
regulatory T cells. Blood. 105:2828–2835. 2015. View Article : Google Scholar
|
|
39
|
Thomson CW, Lee BP and Zhang L:
Double-negative regulatory T cells: Non-conventional regulators.
Immunol Res. 35:163–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Priatel JJ, Utting O and Teh HS:
TCR/self-antigen interactions drive double-negative T cell
peripheral expansion and differentiation into suppressor cells. J
Immunol. 167:6188–6194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Voelkl S, Gary R and Mackensen A:
Characterization of the immunoregulatory function of human TCR-αβ+
CD4-CD8- double-negative T cells. Eur J Immunol. 41:739–748. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ligocki AJ and Niederkorn JY: Advances on
Non-CD4+Foxp3+T regulatory cells: CD8+, Type1, and double negative
t regulatory cells in organ transplantation. Transplantation.
99:1553–1559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sundaravaradan V, Mir KD and Sodora DL:
Double-negative T cells during HIV/SIV infections: Potential pinch
hitters in the T cell lineup. Curr Opin HIV AIDS. 7:164–171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Young KJ, Kay LS, Phillips MJ and Zhang L:
Antitumor activity mediated by double-negative T cells. Cancer Res.
63:8014–8021. 2003.PubMed/NCBI
|
|
45
|
Merims S, Li X, Joe B, Dokouhaki P, Han M,
Childs RW, Wang ZY, Gupta V, Minden MD and Zhang L: Anti-leukemia
effect of ex vivo expanded DNT cells from AML patients: A potential
novel autologous T-cell adoptive immunotherapy. Leukemia.
25:1415–1422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Voelkl S, Moore TV, Rehli M, Nishimura MI,
Mackensen A and Fischer K: Characterization of MHC class-I
restricted TCR+ CD4-CD8-double negative T cells recognizing the
gp100 antigen from a melanoma patient after gp100 vaccination.
Cancer Immunol Immunother. 58:709–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qin Z, Richter G, Schüler T, Ibe S, Cao X
and Blankenstein T: B cells inhibit induction of T cell-dependent
tumor immunity. Nat Med. 4:627–630. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shah S, Divekar AA, Hilchey SP, Cho HM,
Newman CL, Shin SU, Nechustan H, Challita-Eid PM, Segal BM, Yi KH
and Rosenblatt JD: Increased rejection of primary tumors in mice
lacking B cells: Inhibition of antitumor CTL and TH1 cytokine
responses by B cells. Int J Cancer. 117:574–586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee-Chang C, Bodogai M, Martin-Montalvo A,
Wejksza K, Sanghvi M, Moaddel R, de Cabo R and Biragyn A:
Inhibition of breast cancer metastasis by resveratrol-mediated
inactivation of tumor-evoked regulatory B cells. J Immunol.
191:4141–4151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Y, Eliav Y, Shin SU, Schreiber TH,
Podack ER, Tadmor T and Rosenblatt JD: B lymphocyte inhibition of
anti-tumor response depends on expansion of Treg but is independent
of B-cell IL-10 secretion. Cancer Immunol Immunother. 62:87–89.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cunningham RC: Autoimmunity in primary
immune deficiency: Taking lessons from our patients. Clin Exp
Immunol. 164 Suppl 2:S6–S11. 2011. View Article : Google Scholar
|
|
52
|
Harris DP, Haynes L, Sayles PC, Duso DK,
Eaton SM, Lepak NM, Johnson LL, Swain SL and Lund FE: Reciprocal
regulation of polarized cytokine production by effector B and T
cells. Nat Immunol. 1:4752000. View
Article : Google Scholar : PubMed/NCBI
|
|
53
|
Olkhanud PB, Damdinsuren B, Bodogai M,
Gress RE, Sen R, Wejksza K, Wersto RP and Biragyn A: Tumor evoked
regulatory B cells promote breast cancer metastasis by converting
resting CD4+ T cells to T-regulatory cells. Cancer Res.
71:3505–3515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Milne K, Köbel M, Kalloger SE, Barnes RO,
Gao D, Gilks CB, Watson PH and Nelson BH: Systematic analysis of
immune infiltrates in high-grade serous ovarian cancer reveals
CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One.
4:e64122009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kobayashi T, Hamaguchi Y, Hasegawa M,
Fujimoto M, Takehara K and Matsushita T: B cells promote tumor
immunity against B16F10 melanoma. Am J Pathol. 184:3120–3129. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fisher DT, Appenheimer MM and Evans SS:
The two faces of IL-6 in the tumor microenvironment. Semin Immunlo.
26:38–47. 2014. View Article : Google Scholar
|
|
57
|
Lippitz BE: Cytokine patterns in patients
with cancer: A systematic review. Lancet Oncol. 14:e218–e228. 2013.
View Article : Google Scholar : PubMed/NCBI
|