Introduction
Ovarian cancer is one of the most common female
genital tumors, with incidence of ovarian cancer lower than that of
cervical and uterine cancer (1). Due
to ovarian cancer being highly malignant, having a poor prognosis
and the highest mortality rate of all female genital tumors, the
five-year survival rate of ovarian cancer is between 20 and 30% in
2013 (2). In addition to these
factors, ovarian cancer is difficult to diagnose and treat, as well
as there being a rising concern with regard to drug resistance
(3). Currently, ovarian cancer is
treated with tumor cytoreductive surgery combined with adjuvant
platinum-based chemotherapy, including cisplatin (4). The majority of patients with ovarian
cancer are sensitive to cisplatin-based chemotherapy initially and
have a high rate of remission in the short term; however, abdominal
or pelvic recurrence is frequent and drug resistance develops
(5,6).
Patients with tumors that progress during or recur within 6 months
of treatment are considered to be cisplatin-resistant (7).
With progress in the field of life sciences,
understanding of the underlying mechanisms of tumor development has
grown from the functional gene level to non-coding RNA. Long
non-coding RNA (lncRNA), which has >200 nucleotide transcripts,
has gained attention in the field of medicine, particularly in
oncology, as a result of their abundance, function and mechanism.
Various lncRNAs have been demonstrated to be involved in the
regulation of tumorigenesis, invasion, metastasis and resistance to
cancer treatments (8,9). Although the study of lncRNA is still in
the initial stages, important observations have been made; these
include the identification of regulator of reprogramming (10) and urothelial cancer-associated 1
(11), which have the ability to
regulate the chemosensitivity of hepatocellular carcinoma. In view
of the current resistance to lncRNAs previously identified to be
dysregulated and associated with chemoresistant ovarian cancer,
further exploration of ovarian cancer drug resistance may reveal
the mechanisms underlying ovarian cancer resistance, and result in
novel methods for the diagnosis and treatment of ovarian
cancer.
The aim of the present study was to further
investigate dysregulated lncRNAs in cisplatin-resistant ovarian
cancer, compared with in cisplatin-sensitive ovarian cancer. This
was conducted to assist understanding of the initiation and
development mechanisms of ovarian cancer, which could be helpful
for discovering potential biomarkers for diagnosis, or novel
therapy targets that could be used in clinical treatment.
Materials and methods
Cell lines and reagents
The cisplatin-sensitive SKOV3 ovarian cancer cell
line and their cisplatin-resistant clones, SKOV3/CDDP, were
obtained from the Chinese Academy of Medical Sciences and Peking
Union Medical College (Beijing, China). Cells were cultured at 37°C
in a humidified atmosphere containing 5% CO2 in
RPMI-1640 medium with 10% (v/v) fetal bovine serum, 100 U/ml
penicillin and 100 U/ml streptomycin (all from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). All reagents were purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), unless stated
otherwise.
Female primary ovarian cancer tissue samples were
obtained from the Gynecology Department of Nanjing Maternal and
Child Health Hospital (Nanjing, China) from January 2015 to January
2016. In total, there were 6 primary cisplatin-resistant ovarian
cancer cases included in the present study, along with 7
age-matched primary cisplatin-sensitive ovarian cancer cases (46
patients with ovarian cancer, 13 patients were treated with
cisplatin; 33 patients were treated with paclitaxel combined with
doxorubicin). All samples were from epithelial ovarian carcinomas,
including 8 serous ovarian carcinomas and 5 mucous type ovarian
carcinomas (3 stage I, 4 stage II, 4 stage III, 2 stage IV)
(12). Once the tissues were
collected, they were washed with 3X RNAlater® (Thermo
Fisher Scientific, Inc.) and put into a freezing tube containing 5X
RNAlater® solution along with liquid nitrogen, which
quick-froze the samples at −70°C. Histopathological diagnoses were
all confirmed as ovarian cancer. Informed consent for the use of
these samples was obtained from each patient. Ethical approval was
obtained from the Nanjing Maternal and Child Health Hospital Ethics
Committee.
Total RNA extraction
Tissue samples and cells were lysed in
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and total RNAs extraction was conducted according to the
manufacturer's protocol. Quantification and a quality check were
performed using Nano-Drop™ and an Agilent 2100
Bio-Analyzer (Agilent Technologies, Inc., Santa Clara, CA, USA),
respectively.
lncRNA expression profiling
For lncRNA expression profiling, 3
cisplatin-resistant ovarian cancer samples and 3
cisplatin-sensitive ovarian cancer samples were profiled using an
Arraystar lncRNA Microarray v3.0 (Arraystar, Inc., Rockville, MD,
USA), as described previously (13).
The RNA was purified from 1 mg total RNA following the removal of
ribosomal RNA (rRNA) using an mRNA-ONLY™ Eukaryotic mRNA
Isolation kit (Epicentre; Illumina, Inc., San Diego, CA, USA).
Following this, the full-length of each sample was amplified and
transcribed into fluorescent RNA by mRNA-ONLY Eukaryotic mRNA
Isolation Kit, Epicentre (Epicentre; Illumina, Inc.) using a random
priming method (14) to prevent bias.
The labeled RNAs were hybridized onto the Human lncRNA Array v3.0
(Agilent SureHyb; Agilent Technologies, Inc.). Following washing,
the arrays were scanned using the Agilent lncRNA Microarray Scanner
(Agilent Technologies, Inc.), and the Agilent Feature Extraction
software version 11.0.1.1 (Agilent Technologies, Inc.) was used for
microarray probe signal data collection. Finally, Agilent
GeneSpring GX v12.1 software (Agilent Technologies, Inc.) was
employed to normalize the values. lncRNAs and mRNAs for which at
least 1 out of 2 groups were flagged in ‘present’ or ‘marginal’
were selected for further data analysis.
lncRNA classification pipeline
In order to elucidate the lncRNA expression pattern
in the probe name-centric cisplatin-resistant ovarian cancer gene
expression data, clarification of the lncRNAs represented on the
Affymetrix microarray (Affymetrix; Thermo Fisher Scientific, Inc.)
was conducted via a common lncRNA classification pipeline, using
the following strategies: First, the annotations of the microarray
data involved the probe name, seqname, gene symbol, gene title,
source, chromosome location, sequence and other informative items
for the specific probe set; secondly, the seqname was assigned with
a GENCODE ID, RefSeq database ID and/or Ensembl gene ID. Seqnames
with GENCODE IDs were labeled as ‘Enst.’ Seqnames with Refseq IDs
were labeled as ‘NR_’ (non-coding RNA). Seqnames with Ensembl gene
IDs were labeled as ‘uc’ (www.genome.ucsc.edu). Thirdly, the seqnames obtained
in step 2 were separated by filtering out pseudogenes, rRNAs,
microRNAs and other short RNAs; including transfer RNAs, small
nuclear RNAs and small nucleolar RNAs (15).
Gene ontology (GO) and pathway
analysis
Identification of differentially expressed lncRNAs
was conducted via multiple hypothesis testing [false discovery rate
(FDR<0.05)], fold-change filtering (absolute fold-change
>2.0) and the standard Student's t-test (P<0.05).
Identification of significantly enriched biological terms and
pathways was completed using GO and pathway analysis of
differentially expressed lncRNAs, including antisense lncRNA,
intronic lncRNA, enhancer lncRNA, long intergenic noncoding RNAs
and other lncRNAs. GO terms and pathway enrichment analysis were
based on the Database for Annotation, Visualization and Integrated
Discover (DAVID) bioinformatics resource (david.abcc.ncifcrf.gov; version 6.7) and the result of
pathway enrichment analysis was confirmed by the Kyoto Encyclopedia
of Genes and Genomes (KEGG) online database (www.kegg.jp). Identification of the potential
functions of the lncRNAs that were differentially expressed was
conducted using functional annotation clustering, using DAVID
version 6.7 (david.abcc.ncifcrf.gov) and KEGG (www.kegg.jp), and ranked by enrichment scores.
Validation of differentially expressed
lncRNA by reverse-transcription-quantitative polymerase chain
reaction (RT-qPCR)
Total RNA of sample tissues and cells was extracted
and reverse transcribed into cDNA with random primers by using a
PrimeScript™ Reverse Transcription kit (Takara Bio, Inc., Otsu,
Japan), according to the manufacturer's protocol. A standard qPCR
was performed to confirm the expression levels of differentially
expressed lncRNAs using the Applied Biosystems ViiA 7 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.), following the manufacturer's guidelines. Briefly, the sample
mixtures (Table I) were incubated at
95°C for 10 min for an initial denaturation, followed by 40 PCR
cycles of incubation at 95°C for 15 sec, 60°C for 30 sec and then
72°C for 30 sec. Each sample was performed in triplicate. The
expression levels of lncRNAs were normalized to the internal
control, GAPDH, and then quantified using the 2−∆∆Cq
method (16). The target genes of the
dysregulated lncRNAs were predicted based on the principles of
chromosome location of nearby coding genes and of base-pairing
(17). The aim of this study was to
further explore the dys-regulated lncRNAs in cisplatin resistant
ovarian cancer compared to cisplatin sensitive ovarian cancer,
which may help the understanding of the initiation and development
mechanism of ovarian cancer comprehensively, and probably afford
the potential biomarkers for diagnosis or therapy targets for
clinical treatment. There were 13 patients who treated with
cisplatin in the present study.
 | Table I.Primers for qRT-PCR of LncRNAs. |
Table I.
Primers for qRT-PCR of LncRNAs.
| Seqname | Primers
(5′-3′) |
|---|
|
Enst00000435726 | F:
GGAGGTCACTCTCAACACCC |
|
| R:
CAGAGGAGATGAAAGCCATAGA |
|
Enst00000585612 | F:
GGAAAGCCTTTAGCCATCGT |
|
| R:
TTCAGGTAGTTGCTTCACATCC |
|
Enst00000566734 | F:
AGGACGGTCAGTCATCCTTT |
|
| R:
ATCTTCAGGCACAAAAACCCA |
|
Enst00000453783 | F:
GCAGTGCTTGGAGATTGGGA |
|
| R:
TTCATGAGCCCCACACACAA |
| NR_023915 | F:
GCCTACCTGTGGTCTCTTGG |
|
| R:
ACCTCTTTGTGGCCATCACC |
| RP11_697E22.2 | F:
GAAAGAGGGTTTCCGTGCCA |
|
| R:
CGCCACCCTTGGGGTATTT |
| uc010jub | F:
CCAGCAGCCCTCTGGGAA |
|
| R:
AGAAAGGCTGGGCTGAAGTG |
| tcons_00008505 | F:
CTGGGCAACAAGTCCACAGA |
|
| R:
TTAGACCGTCATGGCGGAAG |
| GAPDH | F:
GGTGAAGGTCGGAGTCAACG |
|
| R:
CAAAGTTGTCATGGATGHACC |
Statistical analysis
Values are expressed as the mean ± standard
deviation from at least three independent experiments. The
differences in lncRNA expression levels were determined by analysis
of variance and multiple hypothesis testing followed by the false
discovery rate method as a post-hoc test. The sensitivity and
specificity were analyzed according to the standard formulas
(14). All P-values were two-sided
and a value of P<0.05 was considered to indicate a statistically
significant difference. Computer-based calculations were conducted
using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
Results
Differential lncRNA expression
profiles between cisplatin-resistant ovarian cancer and
cisplatin-sensitive ovarian cancer
In the present study, the expression levels of
lncRNAs in 3 cisplatin-resistant ovarian cancer and 3 age-matched
cisplatin-sensitive ovarian cancer samples (enough to screen out
the dysregulated lncRNAs) were detected via a high-throughput
microarray technique. The patients with tumors that progressed
during or recurred within 6 months of the treatment were considered
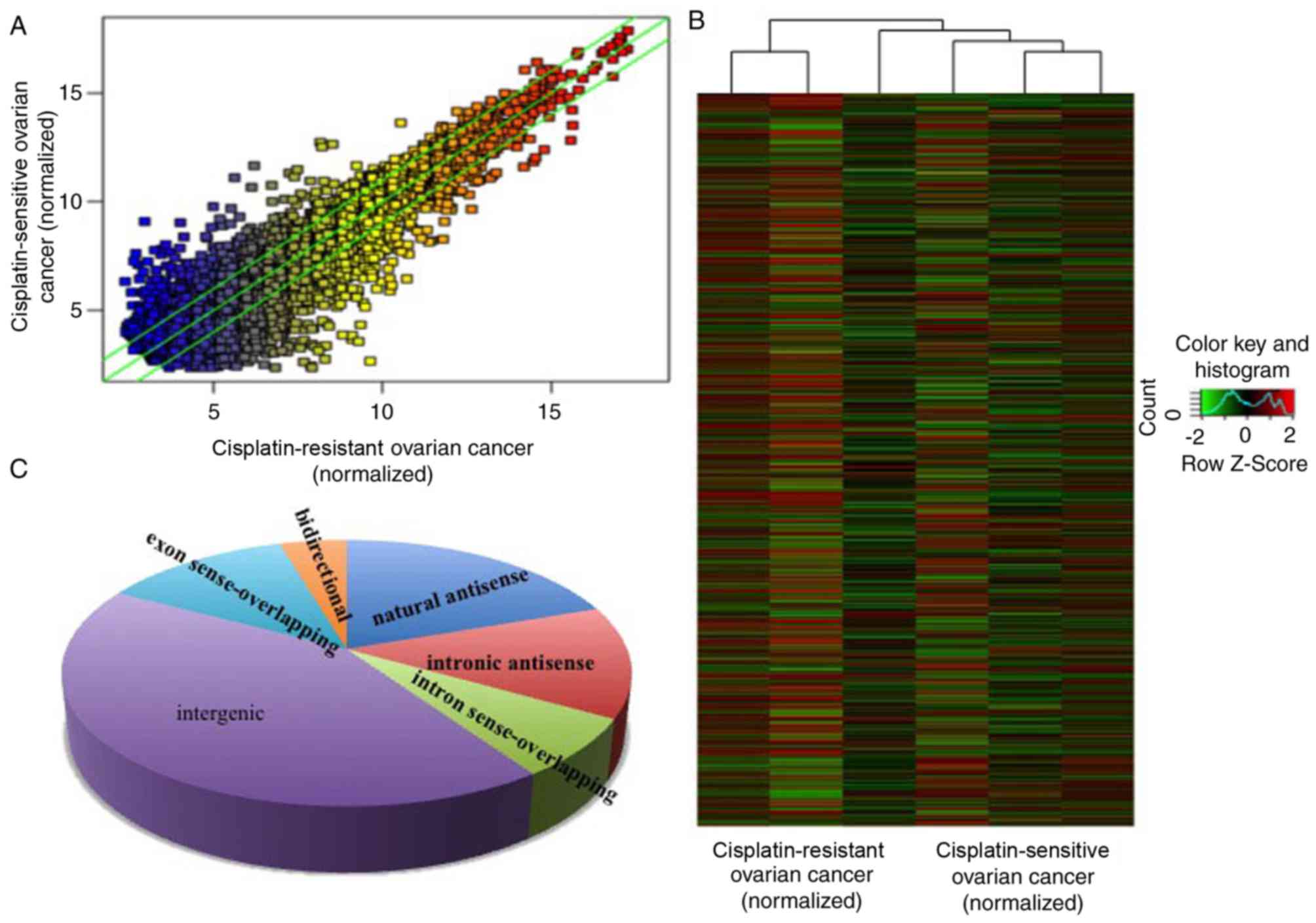
cisplatin-resistant. The results of the microarray revealed that
there were 823 upregulated and 765 downregulated lncRNAs in
cisplatin-resistant ovarian cancer, compared with
cisplatin-sensitive ovarian cancer (Fig.
1A and B) with fold-change filtering (absolute fold-change
>2.0), significant difference identified using a Student's
t-test (P<0.05) and multiple hypothesis testing (FDR<0.05).
According to the nearby coding genes, these differentially
expressed lncRNAs included 312 natural antisense, 216 intronic
antisense, 114 intron sense-overlapping, 673 intergenic, 201 exon
sense-overlapping and 72 bidirectional lncRNAs (Fig. 1C).
Go and pathway analysis of
differentially expressed lncRNAs
To explore the potential functions of the
dysregulated lncRNAs in cisplatin-resistant ovarian cancer, the
target genes of the lncRNAs were predicted based on the principles
of chromosome location of nearby coding genes and of base-pairing
(14). Following this, GO analysis
was conducted for the lncRNAs and target genes. The GO project
(www.geneontology.org) primarily covers 3
areas, including the biological process, molecular function and
cellular component, and provides controlled annotations to describe
the gene and gene products attributed to any organism (11). The GO-analyzed results indicated that
these gene products were primarily located within membrane-bound
organelles, extracellular regions, intracellular membrane-bound
organelles, cytoplasm, intracellular regions and intracellular,
among other locations (Fig. 2A). The
genes were predicted to be enriched in the biological processes
associated with the cell cycle, namely, regulation of the mitotic
cell cycle, cell division, M phase of the mitotic cycle, regulation
of the cell cycle process and regulation of the cell cycle, among
other processes (Fig. 2B). The
molecular functions of these genes included organic cyclic compound
binding, protein binding (interaction, selectively and
non-covalently, with any protein or protein complex), binding
(selective, non-covalent, often stoichiometric, interaction of a
molecule with one or more specific sites on another molecule) and
ion binding (Fig. 2C). Furthermore,
the pathway analysis demonstrated that these gene products
participated in several signaling pathways in humans, including
mitogen-activated protein kinase (MAPK) signaling, protein process
in the endoplasmic reticulum, Parkinson's disease, endocytosis,
ubiquitin mediated proteolysis, p53 signaling pathway, spliceosome,
cell cycle, oocyte meiosis and proteasome (Fig. 2D).
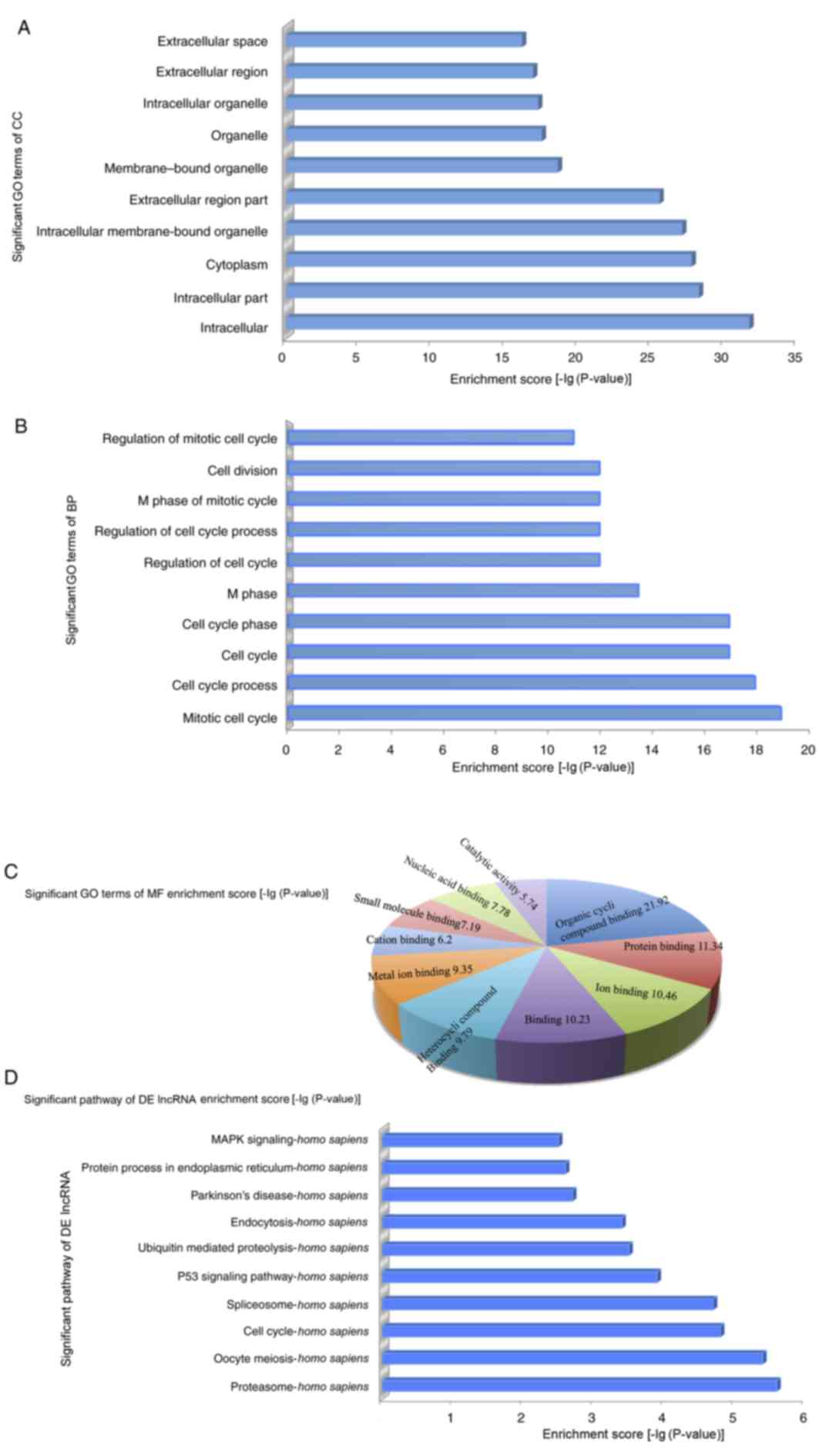 | Figure 2.In order to explore the potential
functions of dysregulated lncRNAs in cisplatin-resistant ovarian
cancer, GO and pathway analysis were performed. (A) The GO analysis
data demonstrated that gene products were primarily located on the
membrane-bound organelles, extracellular regions, intracellular
membrane-bound organelles, cytoplasm, intracellular and
intracellular region. (B) Genes were predicted to be enriched in
the following biological processes: Regulation of the mitotic cell
cycle, cell division, M phase of the mitotic cycle, regulation of
the cell cycle process and regulation of the cell cycle. (C) The
molecular functions of these genes, including organic cyclic
compound binding, protein binding, binding and ion binding. (D)
Pathway analysis demonstrated that gene products were involved in
several signaling pathways in humans. GO, gene ontology; DE,
differently expressed; MF, molecular function; MAPK,
mitogen-activated protein kinase; lncRNA, long non-coding RNA. |
Discovery of cisplatin-resistant
ovarian cancer-associated lncRNAs
In the present study, the expression levels of those
dysregulated lncRNAs were validated not only in a sample pool of 13
patients (46 patients with ovarian cancer; 13 patients were treated
with cisplatin; 33 patients were treated with paclitaxel combined
with doxorubicin), but also in cisplatin-resistant ovarian cancer
cells, SKOV3/CDDP, and cisplatin-sensitive ovarian cancer cells,
SKOV3. The differentially expressed lncRNAs were selected by
fold-change filtering (absolute fold-change >2.0), Student's
t-test (P<0.05) and multiple hypothesis testing (FDR<0.05),
and at least 1 out of 2 groups were flagged as ‘present’ or
‘marginal’ following lncRNA expression profiling. Finally, it was
identified that 62 lncRNAs exhibited significant differential
expression levels in cisplatin-resistant ovarian cancer, compared
with in cisplatin-sensitive ovarian cancer controls. Of these 62
dysregulated lncRNAs, 38 lncRNAs were demonstrated to be
upregulated and 24 lncRNAs were downregulated. The RT-qPCR results
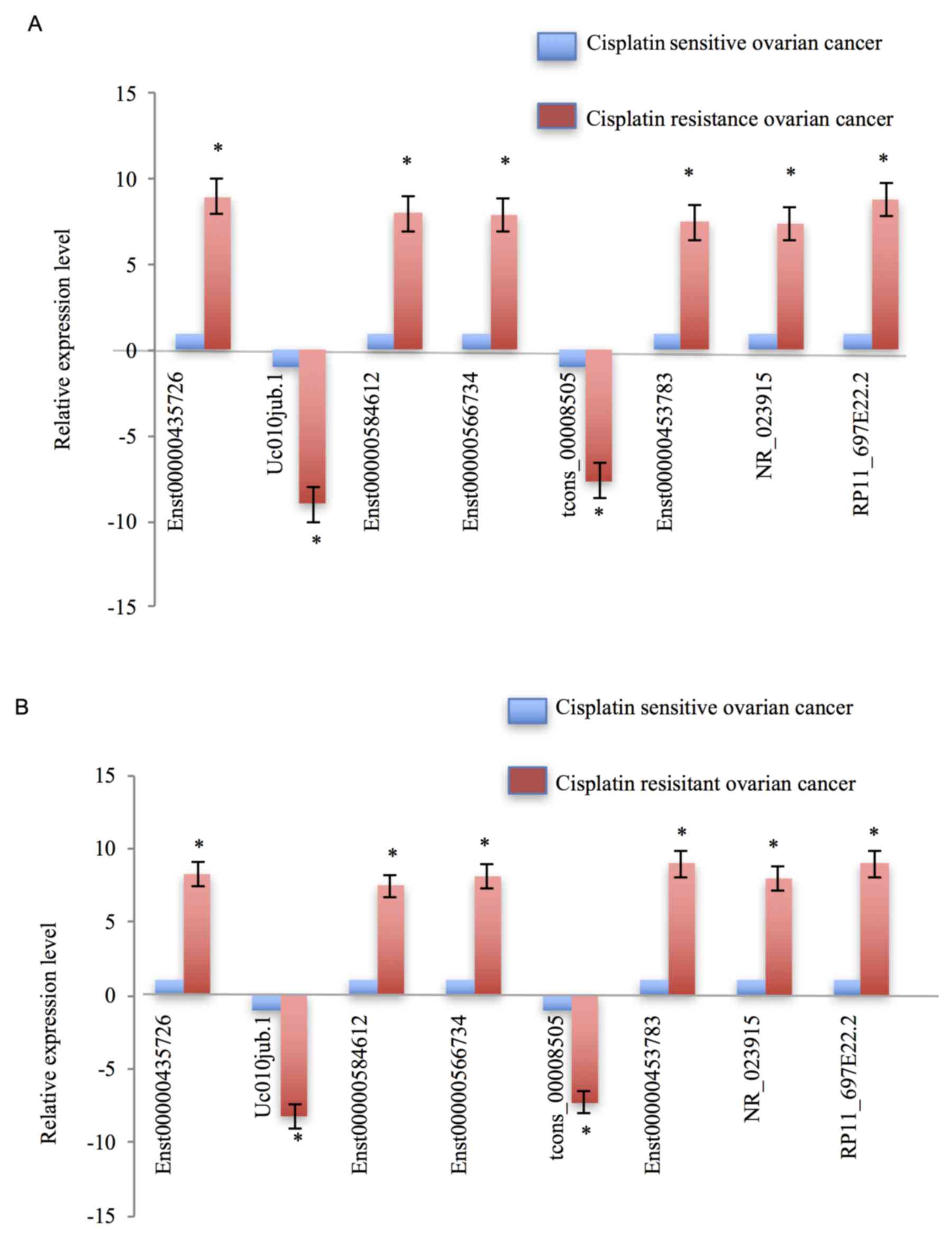
demonstrated that, compared with cisplatin-sensitive ovarian cancer
tissues, Enst0000435726, Enst00000585612, Enst00000566734,
Enst00000453783, NR_023915 and RP11_697E22.2 were markedly
upregulated in cisplatin-resistant ovarian cancer tissues; however,
uc010jub.1 and tcons_00008505 were notably downregulated (Fig. 3A). The expression patterns of these
eight dysregulated lncRNAs in cisplatin-resistant ovarian cancer
cells, compared with the cisplatin-sensitive ovarian cancer cells,
appeared concordant with the results from the tissue samples
(Fig. 3B).
Discussion
Mortality rates associated with ovarian cancer are
the highest out of the female reproductive system tumors,
demonstrating a serious threat to physical and mental health in
females (4–6). Currently, the primary clinical treatment
for ovarian cancer is a combination of platinum-based chemotherapy
and cytoreductive surgery; however, nearly 70% of patients with
ovarian cancer relapse and become multidrug resistant within 6
months (3,4). Improving the survival rate of patients
with ovarian cancer is a global concern (18). The mechanism of ovarian cancer
resistance involves a variety of genes and a number of different
signaling pathways, including the following aspects: Affecting the
effective concentration of intracellular changes, including the
multidrug resistance gene; expression of the multidrug resistance
protein; expression of the lung resistance associated protein
(19,20) and affecting drug targets, including
β-tubulin expression changes, the cytoskeleton protein gene and the
abnormal expression of compartment of uncoupling receptor and
ligand (21); DNA damage repair
abnormalities, including DNA mismatch repair gene, topoisomerase
gene and other changes in the levels of expression or interaction
abnormalities (22,23); apoptosis-associated genes, including
TP53, survivin, caspase, B cell lymphoma 2, and other gene
regulation abnormalities associated with resistance to ovarian
cancer (24,25). Although, targeted drugs have been
developed in response to a number of the aforementioned mechanisms
(26,27), they do not induce a fundamental change
in the resistance to ovarian cancer.
Since the human-genome project, lncRNAs have gained
attention due to their regulation of histone acetylation, gene
methylation, post-transcription translation and other biological
processes (28–30). Recently, numerous lncRNAs have been
demonstrated to serve critical functions in regulating the
physiological behavior of malignant cancer types, including breast,
ovarian, gastric and lung cancer, among others. Additionally,
lncRNAs have been demonstrated to regulate cancer cell viability,
apoptosis, invasion and metastasis (31–37).
Although dysregulated lncRNAs between cisplatin-resistant and
cisplatin-sensitive ovarian cancer tissues have been identified
(38,39), there remains limited knowledge
regarding the differentially expressed lncRNAs. The aim of the
present study was to improve the understanding of lncRNA expression
levels in cisplatin-resistant ovarian cancer.
The results of the microarray assay in the present
study revealed dysregulated lncRNAs between cisplatin-resistant and
cisplatin-sensitive ovarian cancer tissues, including 312 natural
antisense and 673 intergenic (40,41). The
data indicate that the resistance behavior of cisplatin-resistant
ovarian cancer is potentially associated with these differentially
expressed lncRNAs. In order to predict the potential function of
these dysregulated lncRNAs, GO analysis was conducted. It was
determined that lncRNA regulates several biological processes,
including regulation of the mitotic cell cycle, cell division and M
phase of the mitotic cycle, which are closely associated with the
drug resistance (42) of cancer. The
potential functions were classified into 10 categories through
analysis of the target gene pool, namely those involving protein
binding, binding, heterocyclic compound binding, cation binding,
catalytic activity, ion binding, small molecule binding, nucleic
acid binding, metal ion binding and organic cyclic compound
binding. Notably, it was demonstrated that the dysregulated lncRNAs
exhibited binding activity; therefore, these dysregulated lncRNAs
may serve important functions in biological processes through
regulating the cell cytoskeleton. Furthermore, pathway analysis
indicated that these dysregulated lncRNAs mainly participated in
signaling pathways in humans, including MAPK signaling, protein
process in endoplasmic reticulum, Parkinson's disease, endocytosis,
ubiquitin mediated proteolysis, p53 signaling pathway, spliceosome,
cell cycle, oocyte meiosis, proteasome and ubiquitin-mediated
proteolysis, which have been well studied in the initiation and
development of ovarian cancer (32,34,42). The
association between oocyte development and cisplatin-resistant
ovarian cancer occurrence were investigated. These differentially
expressed lncRNAs partially indicated that the function in
cisplatin-resistant ovarian cancer corresponded with
cisplatin-sensitive ovarian cancer tissues, and that these lncRNAs
may be potential biomarkers for diagnosis, or therapeutic targets
for cisplatin-resistant ovarian cancer therapy.
The expression levels of these dysregulated lncRNAs
were confirmed in a 13-sample pool, in order to avoid heterogeneity
of cisplatin-resistant ovarian cancer and individual differences.
The differentially expressed lncRNAs were selected as
aforementioned. were identified. The expression levels of all 8
dysregulated lncRNAs were confirmed separately in SKOV3 and
SKOV3/CDDP cells, and the results were consistent with the results
from the tissue samples.
RP11-697E22.2 is an lncRNA that targets the gene
hepatocyte nuclear factor 1β. As with all of the 6 lncRNAs, which
were observed to be upregulated in the present study, it is an
intergenic lncRNA (43). Recently,
intergenic and antisense lncRNAs have been demonstrated to regulate
cell behavior in a variety of different types of cancer (44–46). The
complex mechanisms underlying the function and large quantity of
lncRNAs available have resulted in lncRNAs being a popular area of
study. These dysregulated lncRNAs identified in the present study
are associated with cisplatin-resistant ovarian cancer and may be
potential novel biomarkers for diagnosis, or affordable potential
targets for the individual therapy of patients with
cisplatin-resistant ovarian cancer in the future.
Although the differentially expressed lncRNAs
between cisplatin-resistant ovarian cancer and age-matched
cisplatin-sensitive ovarian cancer tissues were explored, these
lncRNAs may not be associated with ovarian cancer resistance. The
data demonstrated the differences in the lncRNA expression profile
between cisplatin-resistant and paired cisplatin-sensitive ovarian
cancer, and the eight aforementioned lncRNAs may be potential
targets for individual therapy. The dysregulated lncRNAs in ovarian
cell lines were validated and the results were in accordance with
the results from the tissues. The aim of the present study was to
identify the differentially expressed lncRNAs between
cisplatin-resistant and cisplatin-sensitive ovarian cancer.
Investigations into lncRNA target genes are potential avenues of
further study. Additionally, validation was performed only in SKOV3
cells, and was a limitation of the present study. Considering the
multiple mechanisms underlying drug resistance in ovarian cancer
chemotherapy, tissues collected from the other 33 patients may
assist in validating the dysregulated lncRNAs in an increased
number of ovarian cancer tissues and cells, including
paclitaxel-resistant tissues and cells, in the future. It is
necessary to validate these results in larger cohorts and
additional cell lines, and further studies should aim to
investigate the underlying mechanism.
Acknowledgements
The present study was supported by the Science and
Technology Development Fund of Shanghai Pudong New Area (grant no.
PKJ2016-Y32). The authors wish to thank Kang Chen Bio-tech Ltd.,
(Shanghai, China) for consultation in the present study.
Funding
The present study was supported by the Science and
Technology Development Fund of Shanghai Pudong New Area (grant no.
PKJ2016-Y32).
Availability of data and materials
All data and materials are availabile.
Author's contributions
QL was responsible for collecting and analyzing data
and writing the article. JZha was responsible for the collection of
tissue samples. JZho, BLY and PPL were responsible for the cell
experiments. LC and LJ were responsible for the tissue experiments.
HL was responsible for analyzing the data and amending the
article.
Ethics approval and consent to
participate
Informed consent for the use of these samples was
obtained from each patient. Ethical approval was obtained from the
Nanjing Maternal and Child Health Hospital Ethics Committee.
Consent for publication
Written informed consent for publication was
obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mo L, Pospichalova V, Huang Z, Murphy SK,
Payne S, Wang F, Kennedy M, Cianciolo GJ, Bryja V, Pizzo SV and
Bachelder RE: Ascites increases expression/function of multidrug
resistance proteins in ovarian cancer cells. PLoS One.
10:e01315792015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khrunin AV, Khokhrin DV, Moisseev AA,
Gorbunova VA and Limborska SA: Pharmacogenomic assessment of
cisplatin-based chemotherapy outcomes in ovarian cancer.
Pharmacogenomics. 15:329–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Jiang K, Qiu X, Li M, Hao Q, Wei L,
Zhang W, Chen B and Xin X: Overexpression of CXCR4 is significantly
associated with cisplatin-based chemotherapy resistance and can be
a prognostic factor in epithelial ovarian cancer. BMB Rep.
47:33–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khrunin A, Ivanova F, Moisseev A, Khokhrin
D, Sleptsova Y, Gorbunova V and Limborska S: Pharmacogenomics of
cisplatin-based chemotherapy in ovarian cancer patients of
different ethnic origins. Pharmacogenomics. 13:171–178. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu S, Fu GB, Tao Z, OuYang J, Kong F,
Jiang BH, Wan X and Chen K: MiR-497 decreases cisplatin resistance
in ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget.
6:26457–26471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang J, Ahmad A and Sarkar FH: The role of
MicroRNAs in breast cancer migration, invasion and metastasis. Int
J Mol Sci. 13:13414–13437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi K, Yan IK, Kogure T, Haga H and
Patel T: Extracellular vesicle-mediated transfer of long non-coding
RNA ROR modulates chemosensitivity in human hepatocellular cancer.
FEBS Open Bio. 4:458–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: Long non-coding RNA UCA1 increases chemoresistance of
bladder cancer cells by regulating Wnt signaling. FEBS J.
281:1750–1758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brun JL, Feyler A, Chêne G, Saurel J, Brun
G and Hocké C: Long-term results and prognostic factor in patients
with epithelial ovarian cancer. Gynecol Oncol. 78:21–27. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Fu Z, Ji C, Gu P, Xu P, Yu N, Kan
Y, Wu X, Shen R and Shen Y: Systematic gene microarray analysis of
the lncRNA expression profiles in human uterine cervix carcinoma.
Biomed Pharmacother. 72:83–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv M, Xu P, Wu Y, Huang L, Li W, Lv S, Wu
X, Zeng X, Shen R, Jia X, et al: LncRNAs as new biomarkers to
differentiate triple negative breast cancer from non-triple
negative breast cancer. Oncotarget. 7:13047–13059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Sun S, Pu JK, Tsang AC, Lee D,
Man VO, Lui WM, Wong ST and Leung GK: Long non-coding RNA
expression profiles predict clinical phenotypes in glioma.
Neurobiol Dis. 48:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spurlock CF III, Tossberg JT, Guo Y,
Collier SP, Crooke PS III and Aune TM: Expression and functions of
long noncoding RNAs during human T helper cell differentiation. Nat
Commun. 6:69322015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Odening KE, Li W, Rutz R, Laufs S,
Fruehauf S, Fishelson Z and Kirschfink M: Enhanced complement
resistance in drug-selected P-glycoprotein expressing
multi-drug-resistant ovarian carcinoma cells. Clin Exp Immunol.
155:239–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Connor R, O'Leary M, Ballot J, Collins
CD, Kinsella P, Mager DE, Arnold RD, O'Driscoll L, Larkin A,
Kennedy S, et al: A phase I clinical and pharmacokinetic study of
the multi-drug resistance protein-1 (MRP-1) inhibitor sulindac, in
combination with epirubicin in patients with advanced cancer.
Cancer Chemother Pharmacol. 59:79–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obata H, Yahata T, Quan J, Sekine M and
Tanaka K: Association between single nucleotide polymorphisms of
drug resistance-associated genes and response to chemotherapy in
advanced ovarian cancer. Anticancer Res. 26:2227–2232.
2006.PubMed/NCBI
|
|
22
|
Scartozzi M, De Nictolis M, Galizia E,
Carassai P, Bianchi F, Berardi R, Gesuita R, Piga A, Cellerino R
and Porfiri E: Loss of hMLH1 expression correlates with improved
survival in stage III–IV ovarian cancer patients. Eur J Cancer.
39:1144–1149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muenyi CS, States VA, Masters JH, Fan TW,
Helm CW and States JC: Sodium arsenite and hyperthermia modulate
cisplatin-DNA damage responses and enhance platinum accumulation in
murine metastatic ovarian cancer xenograft after hyperthermic
intraperitoneal chemotherapy (HIPEC). J Ovarian Res. 4:92011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Metzinger DS, Taylor DD and Gercel-Taylor
C: Induction of p53 and drug resistance following treatment with
cisplatin or paclitaxel in ovarian cancer cell lines. Cancer Lett.
236:302–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raspollini MR, Amunni G, Villanucci A,
Castiglione F, Rossi Degl'Innocenti D, Baroni G, Paglierani M and
Taddei GL: HER-2/neu and bcl-2 in ovarian carcinoma:
Clinicopathologic, immunhistochemical and molecular study in
patients with shorter and longer survival. Appl Immunohistochem Mol
Morphol. 14:181–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dao MD, Alwan LM, Gray HJ, Tamimi HK, Goff
BA and Liao JB: Recurrence patterns after extended treatment with
bevacizumab for ovarian, fallopian tube, and primary peritoneal
cancers. Gynecol Oncol. 130:295–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhillon S: Bevacizumab combination
therapy: A review of its use in patients with epithelial ovarian,
fallopian tube, or primary peritoneal cancer. BioDrugs. 27:375–392.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen YA and Aravin AA: Non-coding RNAs in
transcriptional regulation: The review for current molecular
biology reports. Curr Mol Biol Rep. 1:10–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Latos PA, Pauler FM, Koerner MV, Şenergin
HB, Hudson QJ, Stocsits RR, Allhoff W, Stricker SH, Klement RM,
Warczok KE, et al: Airn transcriptional overlap, but not its lncRNA
products, induces imprinted Igf2r silencing. Science.
338:1469–1472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li H, Yu B, Li J, Su L, Yan M, Zhu Z and
Liu B: Overexpression of lncRNA H19 enhances carcinogenesis and
metastasis of gastric cancer. Oncotarget. 5:2318–2329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin G, Sun J, Isaacs SD, Wiley KE, Kim ST,
Chu LW, Zhang Z, Zhao H, Zheng SL, Isaacs WB and Xu J: Human
polymorphisms at long non-coding RNAs (lncRNAs) and association
with prostate cancer risk. Carcinogenesis. 32:1655–1659. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arase M, Horiguchi K, Ehata S, Morikawa M,
Tsutsumi S, Aburatani H, Miyazono K and Koinuma D: Transforming
growth factor-β-induced lncRNA-Smad7 inhibits apoptosis of mouse
breast cancer JygMC(A) cells. Cancer Sci. 105:974–982. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang K, Long B, Zhou LY, Liu F, Zhou QY,
Liu CY, Fan YY and Li PF: CARL lncRNA inhibits anoxia-induced
mitochondrial fission and apoptosis in cardiomyocytes by impairing
miR-539-dependent PHB2 downregulation. Nat Commun.
5:35962014.PubMed/NCBI
|
|
35
|
Yang L, Lin C, Jin C, Yang JC, Tanasa B,
Li W, Merkurjev D, Ohgi KA, Meng D, Zhang J, et al:
lncrna-dependent mechanisms of androgen receptor-regulated gene
activation programs. Nature. 500:598–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang K, Liu CY, Zhou LY, Wang JX, Wang M,
Zhao B, Zhao WK, Xu SJ, Fan LH, Zhang XJ, et al: APF lncRNA
regulates autophagy and myocardial infarction by targeting
miR-188-3p. Nat Commun. 6:67792015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi SJ, Wang LJ, Yu B, Li YH, Jin Y and
Bai XZ: LncRNA-ATB promotes trastuzumab resistance and
invasion-metastasis cascade in breast cancer. Oncotarget.
6:11652–11663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen X, Xie B, Ma Z, Yu W, Wang W, Xu D,
Yan X, Chen B, Yu L, Li J, et al: Identification of novel long
non-coding RNAs in triple-negative breast cancer. Oncotarget.
6:21730–21739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen C, Li Z, Yang Y, Xiang T, Song W and
Liu S: Microarray expression profiling of dysregulated long
non-coding RNAs in triple-negative breast cancer. Cancer Biol Ther.
16:856–865. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Necsulea A, Soumillon M, Warnefors M,
Liechti A, Daish T, Zeller U, Baker JC, Grützner F and Kaessmann H:
The evolution of lncRNA repertoires and expression patterns in
tetrapods. Nature. 505:635–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang X, Chen Z, Mishra AK, Silva A, Ren W,
Pan Z and Wang JH: Chemotherapy-induced differential cell cycle
arrest in B cell lymphomas affects their sensitivity to Wee1
inhibition. Haematologica: Haematol. 2017:1759922017.
|
|
43
|
Hajarnis SS, Patel V, Aboudehen K,
Attanasio M, Cobo-Stark P, Pontoglio M and Igarashi P:
Transcription factor hepatocyte nuclear factor-1β (HNF-1β)
regulates MicroRNA-200 expression through a long Noncoding RNA. J
Biol Chem. 290:24793–24805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cui W, Qian Y, Zhou X, Lin Y, Jiang J,
Chen J, Zhao Z and Shen B: Discovery and characterization of long
intergenic non-coding RNAs (lincRNA) module biomarkers in prostate
cancer: An integrative analysis of RNA-Seq data. BMC Genomics. 16
Suppl 7:S32015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu Y, Wang J, Qian J, Kong X, Tang J, Wang
Y, Chen H, Hong J, Zou W, Chen Y, et al: Long noncoding RNA GAPLINC
regulates CD44-dependent cell invasiveness and associates with poor
prognosis of gastric cancer. Cancer Res. 74:6890–6902. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu Y, Liu H, Shi X, Yao Y, Yang W and Song
Y: The long non-coding RNA HNF1A-AS1 regulates proliferation and
metastasis in lung adenocarcinoma. Oncotarget. 6:9160–9172.
2015.PubMed/NCBI
|