Introduction
Monitoring the immunological status of cancer
patients is an important component of treatment decision and
outcome, which requires establishing useful immunological
parameters that can be easily and non-invasively obtained from the
peripheral blood and that reflect both prognosis and the
immunological state. In the search for such markers, we previously
investigated the association of immunological parameters such as
natural killer cells, Th1 cells, Th2 cells, Th1/Th2 balance, Treg
cells, CD57+ T cells, and CD56+ T cells with progression-free
survival (PFS) in patients with advanced gastric carcinomas.
Unexpectedly, we found that CD57+ T cells in the peripheral blood
of advanced gastric cancer patients were an independent poor
prognostic factor (1). A similar
result was previously reported in patients with advanced renal cell
carcinoma, in that those who harbored higher percentages of
CD8+CD57+ lymphocytes in the peripheral blood had shorter overall
survival (OS) (2). Moreover, Focosi
et al (3) reported that CD57+
expression in T lymphocytes is a useful marker for measuring
functional immune deficiency in patients with autoimmune disease,
infectious disease, and cancers. These reports suggest that
CD8+CD57+ T cells play an immunosuppressive role among the CD8+ T
cell population. Treatment with interferon-alpha (IFN-α) tends to
increase the generally low pre-treatment counts of CD8+CD57+ T
cells (4). Accordingly, melanoma
patients with a lower level of CD8+CD57+ T cells in the peripheral
blood before treatment with IFN-α had longer survival longer than
those with a higher level of CD8+CD57+ T cells (5), which indicates that the cytotoxic
subsets of the CD8+CD57+ T cell population may predominate in
melanoma patients. Thus, CD8+CD57+ T cell populations appear to be
composed of both cytotoxic and immunosuppressive subsets. In the
CD8+ T cell differentiation pathway, CD8+CD57+ T cells are regarded
as intermediate-CD8+ T cells (CD27+CD8+CD58+ T cells), which then
differentiate to terminal-CD8+ T cells (CD27-CD8+CD57+ T cells).
The differentiation of intermediate-CD8+ T cells toward cytotoxic
terminal-CD8+ T cells is inhibited by transforming growth
factor-beta, interleukin-10, and programmed cell death-1 in cancer
patients, where the accumulation of incompletely differentiated
CD8+ T cells (likely intermediate-CD8+ T cells) leads to the
uncontrolled progression of malignant cells (1,6,7). Thus, the predominance of cytotoxic over
immunosuppressive CD8+CD57+ T cells may be dependent on the state
of CD8+ T cell differentiation. These findings suggest that it is
important to monitor the dynamic state of CD8+ T cell
differentiation in cancer patients; that is, to determine which
CD8+ T cell population is predominant in the differentiation
pathway.
Therefore, in this study, we investigated the
association of each population in the CD8+ T cell differentiation
pathway with PFS in a series of patients with various types of
cancers in an attempt to identify the parameter that best reflects
the dynamic state of CD8+ T cell differentiation. The results of
this preliminary analysis are expected to provide new clues for
understanding the immunological status of cancer patients, and
improving treatment decisions.
Materials and methods
Patients, sample collection, and
processing
All participants provided written informed consent
before enrollment. The study protocol was approved by the
institutional review board at each participating center (Department
of Gastroenterological Surgery, Graduate School of Medical
Sciences, Kumamoto University). All methods and procedures
associated with this study were conducted in accordance with the
Good Clinical Practice guidelines and conformed to the ethical
principles of the Declaration of Helsinki and local laws. A total
of 100 patients diagnosed with Stage IV carcinomas according to the
unified TNM classification (8) at
Tamana Regional Health Medical Center between July 2013 and March
2016 were enrolled in the study. The patients (45 men and 55 women)
ranged in age from 28 to 96 years (mean 69.0±13.2 years). The
primary organs affected by cancer in these patients were the
stomach (9), colon (23), pancreas
(10), liver (6), bile duct (11), lung (14), breast (9), thyroid (6), ovary (8)
and others (carcinoma of unknown primary) (4). We collected 10-ml peripheral blood
samples from all eligible patients before treatment; patient
eligibility was determined by the presence of histologically and
clinically diagnosed carcinomas.
Antibodies and flow cytometric
analysis (FACS)
The following reagents were used for FACS:
fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (HIT3a),
FITC-conjugated anti-CD57 (NK-1), phycoerythrin (PE)-conjugated
anti-CD27 (M-T271), PE-conjugated anti-FOXP3 (259D/C7),
PE-conjugated anti-CD57 (NK-1), and anti-CD8 PE-Cy7 (RPA-T8) (BD
Biosciences, San Jose, CA, USA). The cells were analyzed on a BD
FACSCalibur flow cytometer and evaluated with BD CellQuest
software. These data were used to divide the patients into two
groups for each parameter based on the proportions of early-CD8+ T
cells, intermediate-CD8+ T cells, terminal-CD8+ T cells, and the
CD57 ratio (terminal-CD8+ T cells/early-CD8+ T cells) to determine
the independent contributions of each marker for reflecting
PFS.
Study endpoints and assessments
The primary endpoint of this study was PFS. PFS was
measured from the date of randomization to the first recurrence or
death regardless of the cause. Tumor assessments were performed
using dynamic computed tomography or magnetic resonance imaging
every 3 months from the baseline for 24 months and every 3–6 months
thereafter. All scans at each site were reviewed by two independent
and blinded radiologists, each with more than 5 years of
experience. In cases of discordance, a third independent
experienced radiologist reviewed the images to reach a consensus.
Adverse events were classified and graded every 2 months according
to the Common Terminology Criteria for Adverse Events (v3.0;
National Cancer Institute, Bethesda, MD, USA) from the time of
consent until the end of the study or drop-out, continuing for at
least 30 days after the last dose of immunotherapy. Multiple events
were counted once for each patient and the most severe event was
summarized.
Statistical analysis
Data are expressed as the mean ± standard deviation.
For statistical analysis, receiver operating characteristic (ROC)
analysis was used to determine the optimal cutoff values for
continuous variables. Comparison of non-normally distributed
variables between groups was performed using the Mann-Whitney U
test. Comparison of categorical variables between two groups was
performed using the χ2 test. The probability of survival
was estimated with the Kaplan-Meier method, and the differences in
survival rates were evaluated by the log-rank test. Multivariate
analysis of prognostic factors was conducted using the Cox
regression model. All statistical analyses were performed using
SPSS v13.0 for Windows (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
Univariate analysis of CD57-related
immune cells in the CD8+ T cell differentiation pathway
Cox proportional-hazards regression analysis was
conducted to determine the factors influencing the PFS rate of
Stage IV cancer patients (Table I).
Based on the univariate analysis of seven variables, early-CD8+ T
cells and intermediate-CD8+ T cells were significantly associated
with a shorter PFS, whereas CD57+ T cells, CD8+CD57+ T cells, and
terminal-CD8+ T cells were significantly correlated with a longer
PFS (Table I). In contrast to the
several reports describing an association of CD8+CD57+ T cells with
poor prognosis, in this study, both CD8+CD57+ T cells and CD57+ T
cells were significantly associated with a longer PFS (Table I). However, these results are in line
with previous reports (6) showing
that intermediate-CD8+ T cells (CD27+CD8+CD57+ T cells) and
terminal-CD8+ T cells (CD27-CD8+CD57+ T cells) were associated with
a shorter and a longer PFS, respectively. In addition, in this
study, early-CD8+ T cells were more significantly associated with a
shorter PFS than were their downstream population of
intermediate-CD8+ T cells. To our knowledge, this is the first
report showing that early-CD8+ T cells are associated with poor
prognosis and may play a major role in establishing the
immunosuppressive state of cancer patients.
 | Table I.Univariate analysis. |
Table I.
Univariate analysis.
| Groups | HR | HR 95% CI | P-value |
|---|
| CD57+ T cells | 0.362 | 0.186–0.704 | 0.003 |
| CD8+CD57+ T
cells | 0.424 | 0.220–0.820 | 0.011 |
| Early-CD8+ T cells
(CD27+CD8+CD57-T cells) | 5.937 | 2.589–13.6 | <0.0001 |
| Intermediate-CD8+ T
cells (CD27+CD8+CD57+ T cells) | 2.325 | 1.216–4.447 | 0.011 |
| Terminal-CD8+ T cells
(CD27-CD8+CD57+ T cells) | 0.154 | 0.072–0.329 | <0.0001 |
| End-CD8+ T cells
(CD27-CD8+CD57-T cells) |
|
| 0.337 |
Early-CD8+ T cells express CD27
differently from terminal-CD8+ T cells
CD27 is a member of the tumor necrosis
factor-receptor superfamily, acting as a co-stimulatory factor, and
CD27 signaling can either improve or suppress T cell function
depending on the level, duration, and timing of the expression of
CD27 ligand (CD70) (9–12). CD27 signaling was found to be mediated
by constitutive CD70 expression in various cancers (9,13–14), which in turn induces FOXP3+ regulatory
CD8+ T cells, leading to the immunosuppressive environment
characteristic of cancer patients. FOXP3+ regulatory CD8+ T cells
have also been reported to inhibit CD8+ T cell differentiation
(15), so that early-CD8+ T cells and
terminal-CD8+ T cells are increased and decreased, respectively,
which is similar to the situation observed in patients with a low
CD57 ratio, as described below. The results of this study suggest
that early-CD8+ T cells (CD27+CD8+CD57-T cells) may play an
immunosuppressive role, possibly by inducing FOXP3+ regulatory
function. This hypothesis requires further detailed investigation
since we did not determine the expression of FOXP3 on the
early-CD8+ T cells in the present study.
CD57 ratio
As shown in Fig. 1,
the terminal-CD8+ T cells showed an inverse correlation to the
early-CD8+ T cells (Spearman correlation coefficient −0.919,
P<0.0001). Therefore, to find a more significant prognostic
factor, we calculated the CD57 ratio (terminal-CD8+ T
cells/early-CD8+ T cells). Indeed, the CD57 ratio was more
significantly associated with a longer PFS compared to
terminal-CD8+ T cells (hazard ratio=0.143; 95% confidence
interval=0.065–0.314; P<0.0001). In the patients with a high
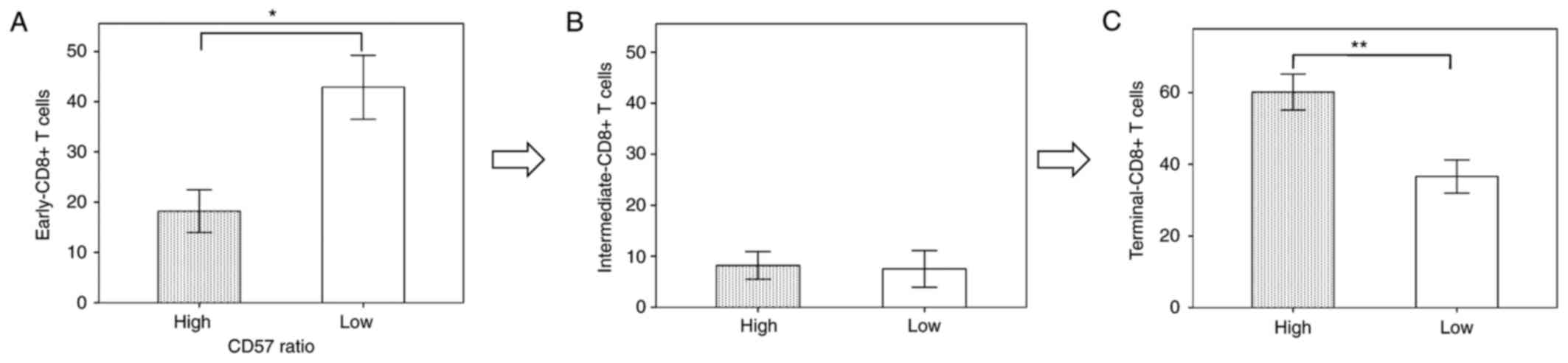
CD57 ratio, early-CD8+ T cells and terminal-CD8+ T cells were
significantly decreased and increased, respectively, as compared to
those with a low CD57 ratio (Fig. 2A and
C), in whom cytotoxic terminal-CD8+ T cells were predominant.
In contrast, in the patients with a low CD57 ratio, early-CD8+ T
cells and terminal-CD8+ T cells were significantly increased and
decreased, respectively, as compared to those with a high CD57
ratio (Fig. 2A and C), in whom
immunosuppressive early-CD8+ T cells were predominant. These
results suggest that the CD57 ratio can accurately reflect the
degree of predominance of cytotoxic vs. immunosuppressive
populations. Thus, we propose that the CD57 ratio can serve as a
useful immune parameter to display the dynamic status of CD8+ T
cell differentiation.
Multivariate analyses and PFS
To determine the independent value and relative risk
of these prognostic factors, we performed a multivariate analysis
of the six possible determinants (CD57+ T cells, CD8+CD57+ T cells,
early-CD8+ T cells, intermediate-CD8+ T cells, terminal-CD8+ T
cells, and CD57 ratio) using the Cox regression model. The results
confirmed that the CD57 ratio was an independent PFS predictor of
Stage IV cancer patients (Table
II).
 | Table II.Multi-variate analysis. |
Table II.
Multi-variate analysis.
| Variable | HR | HR 95% CI | P-value |
|---|
| Univariate
analysis |
|
|
|
| CD57+ T
cells | 0.362 | 0.186–0.704 | 0.003 |
| CD8+CD57+
T cells | 0.424 | 0.220–0.820 | 0.011 |
|
Early-CD8+ T cells
(CD27+CD8+CD57-T cells) | 5.937 | 2.589–13.6 | <0.0001 |
|
Intermediate-CD8+ T cells
(CD27+CD8+CD57+ T cells) | 2.325 | 1.216–4.447 | 0.011 |
|
Terminal-CD8+ T cells
(CD27-CD8+CD57+ T cells) | 0.154 | 0.072–0.329 | <0.0001 |
| CD57
ratio | 0.143 | 0.065–0.314 | <0.0001 |
| Multi-variate
analysis |
|
|
|
| CD57
ratio | 0.143 | 0.065–0.314 | <0.0001 |
The univariate analysis results showed that the
proportions of early-CD8+ T cells, intermediate-CD8+ T cells,
terminal-CD8+ T cells, and the CD57 ratio had a more significant
influence on the PFS rate than the other possible determinants, but
not on the Overall Survival (OS). We then divided the Stage IV
cancer patients into groups based on the proportions of each of
these markers with cutoff values of 19.1, 8.3, 33.7, and 21.8%,
respectively, as determined using the ROC curve (sensitivity of 80,
54.1, 68, and 88.9%; specificity of 68, 71.4, 100, and 75%; area
under the curve of 0.748, 0.587, 0.788, and 0.819; P=0.024, 0.146,
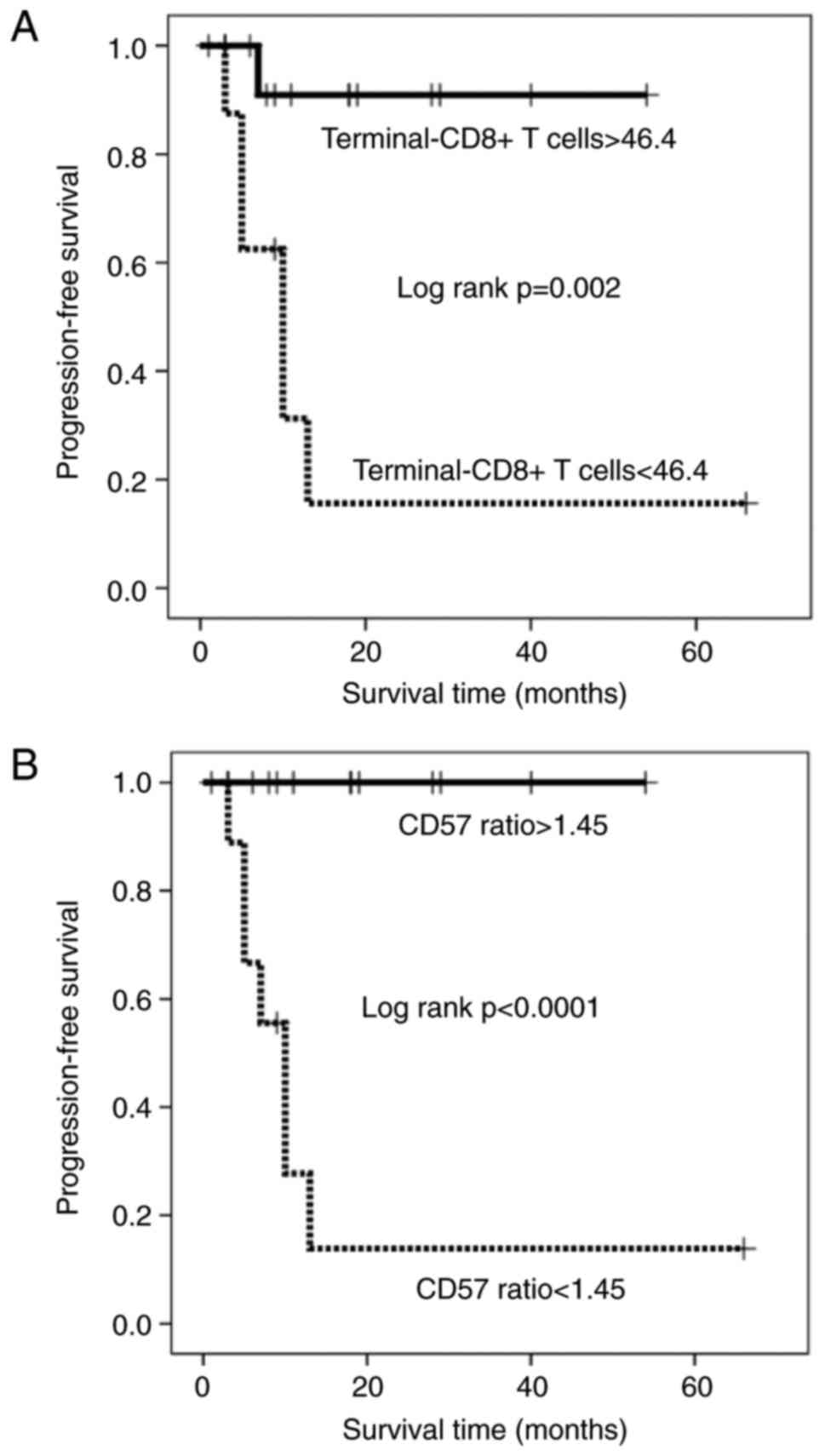
0.009, and 0.005, respectively). The Kaplan-Meier survival curve
revealed that patients with a high percentage of terminal-CD8+ T
cells and those with a high CD57 ratio had a significantly longer
PFS duration than those with a low percentage of terminal-CD8+ T
cells and those with a low CD57 ratio, respectively (Kaplan-Meyer
log-rank P<0.0001; Fig. 3A and B).
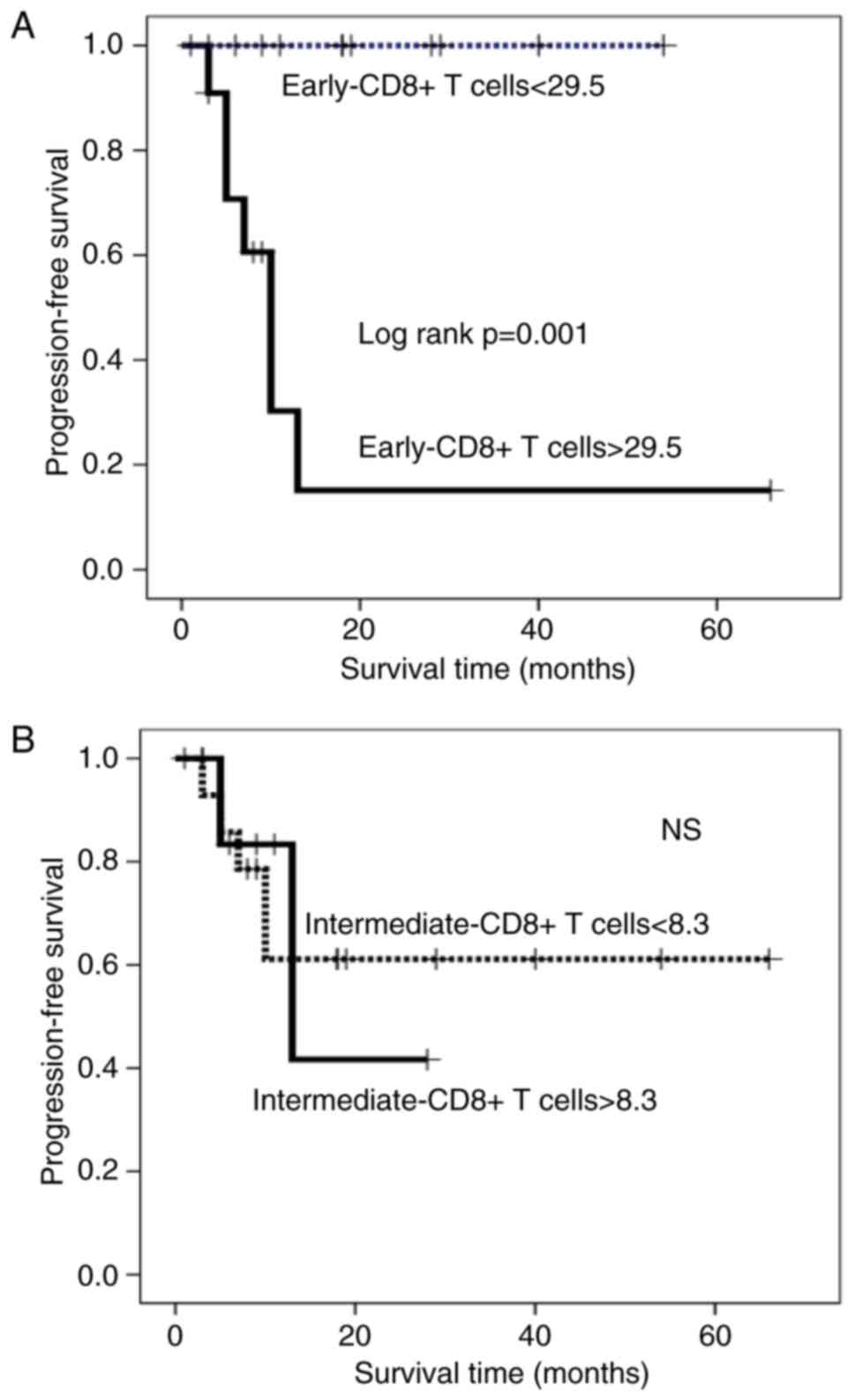
In contrast, patients with higher percentages of early- and
intermediate-CD8+ T cells had a significantly shorter PFS duration
than those with lower percentages (Kaplan-Meyer log-rank
P<0.0001; Fig. 4A and B). The
median of PFS was 10 months in the patients with low-CD57 ratio, 8
months in the patients with low-terminal-CD8+ T cells, and 11
months in the patients with low-early-CD8+ T cells. However, the
two-year survival rate of patients with a high percentage of early-
and intermediate-CD8+ T cells was 11.1 and 28.1%, respectively,
suggesting that early-CD8+ T cells are more strongly associated
with a state of immunosuppression in Stage IV cancer patients than
are intermediate-CD8+ T cells. These results that terminal-CD8+ T
cells and early-CD8+ T cells may have cytotoxic and
immunosuppressive function, respectively, are only preliminary
data, so that we should perform further experiments to examine the
expression of cytotoxic markers in terminal-CD8+ T cells and of
immunosuppressive markers such as PD-1 and FOXP3 in early-CD8+ T
cells.
The results in this study obtained from the cancer
patients with different origin may be deficient in credibility
because tumors with different origin have very different biology.
We selected the 23 patients with colon carcinomas from all patients
and performed the same analysis as to PFS. The Kaplan-Meier
survival curve revealed that patients with a high percentage of
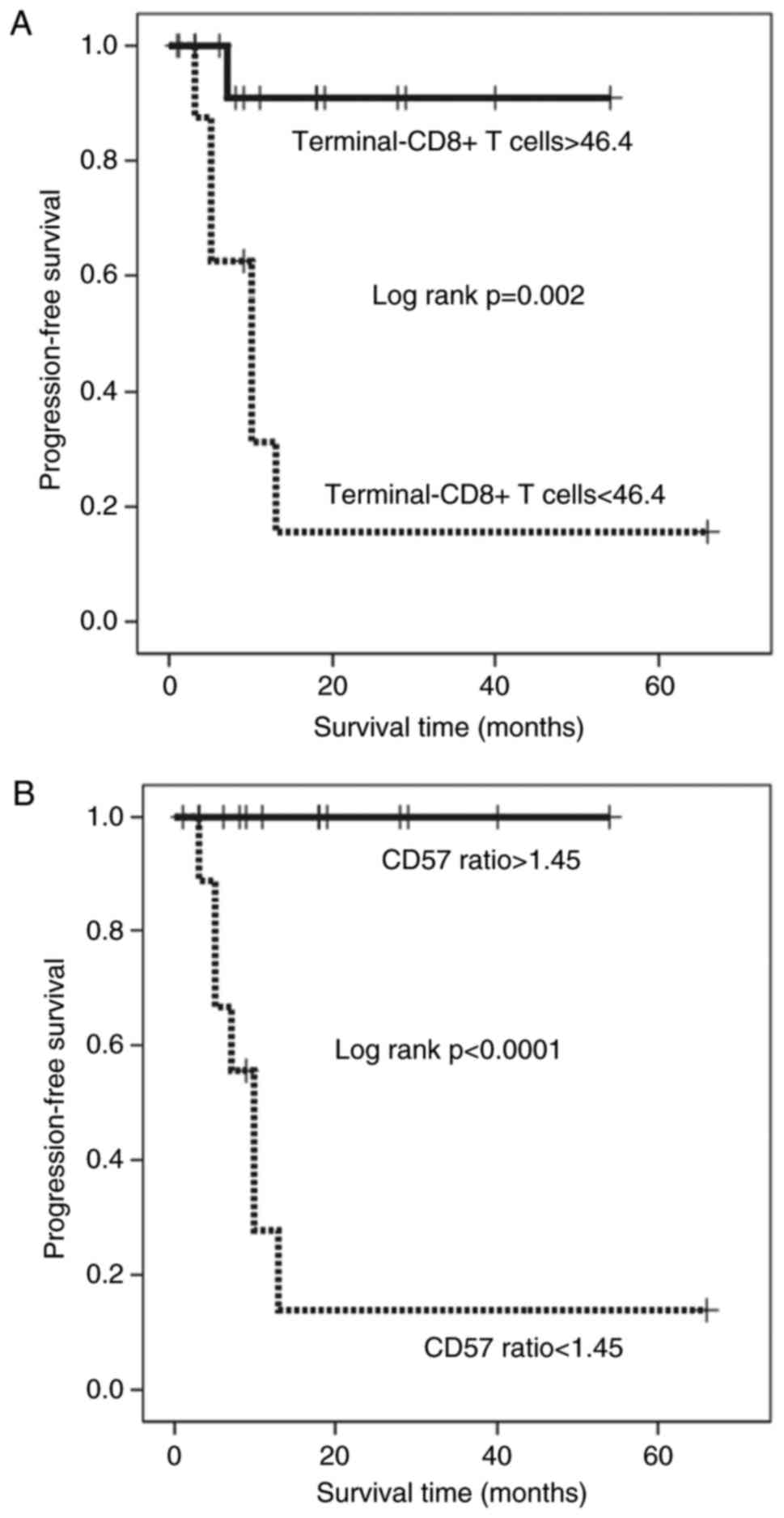
terminal-CD8+ T cells and those with a high CD57 ratio had a
significantly longer PFS duration than those with a low percentage
of terminal-CD8+ T cells and those with a low CD57 ratio,
respectively (Kaplan-Meyer log-rank P=0.002 and P<0.0001;
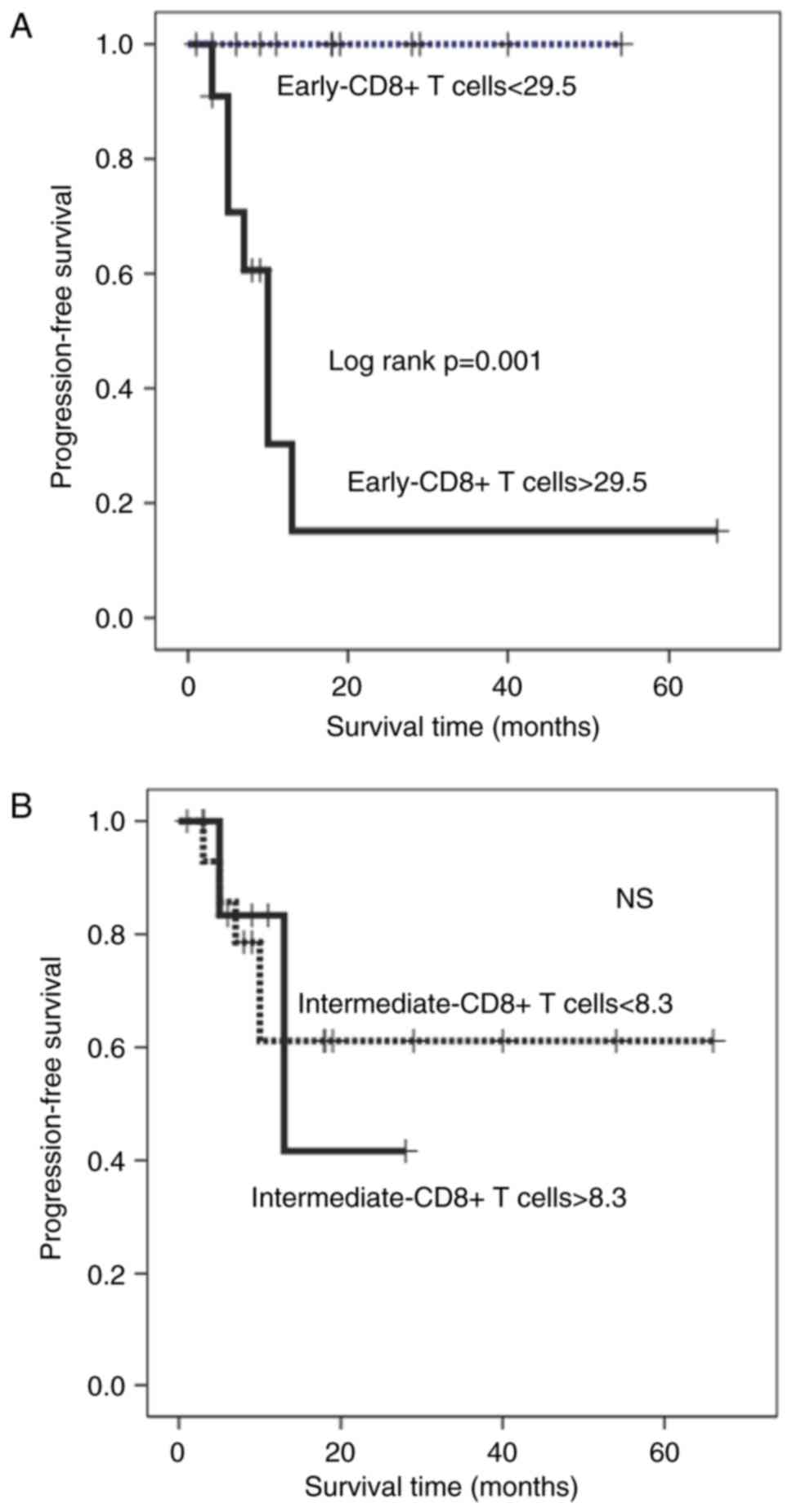
Fig. 5A and B). In contrast, patients
with higher percentages of early-CD8+ T cells had a significantly
shorter PFS duration than those with lower percentages
(Kaplan-Meyer log-rank P=0.001; Fig.
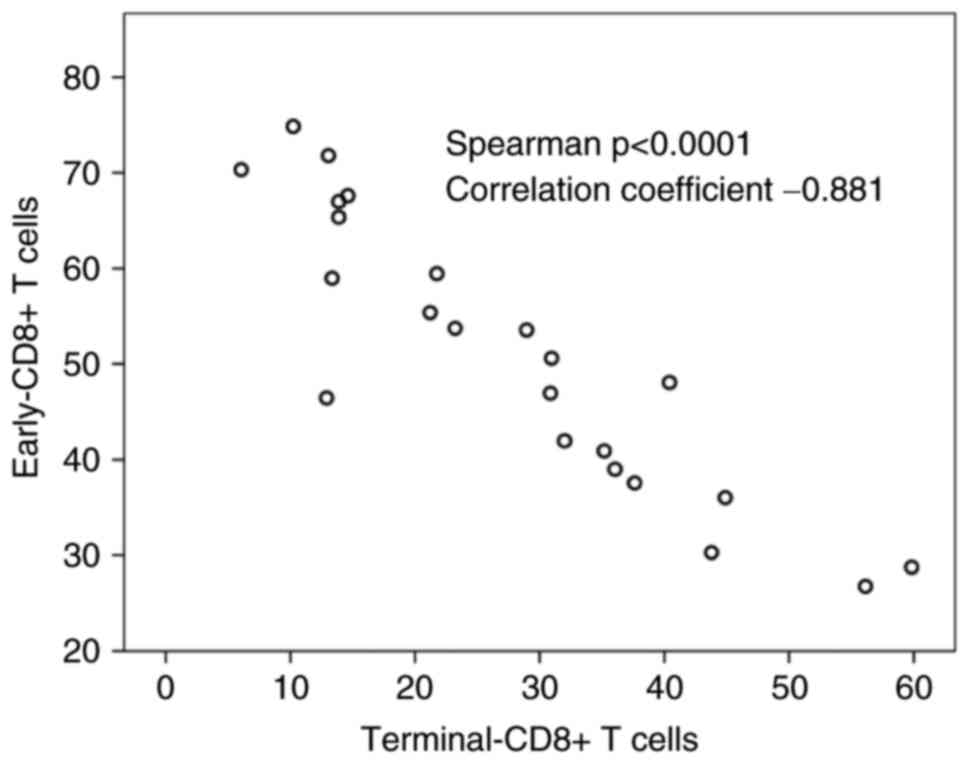
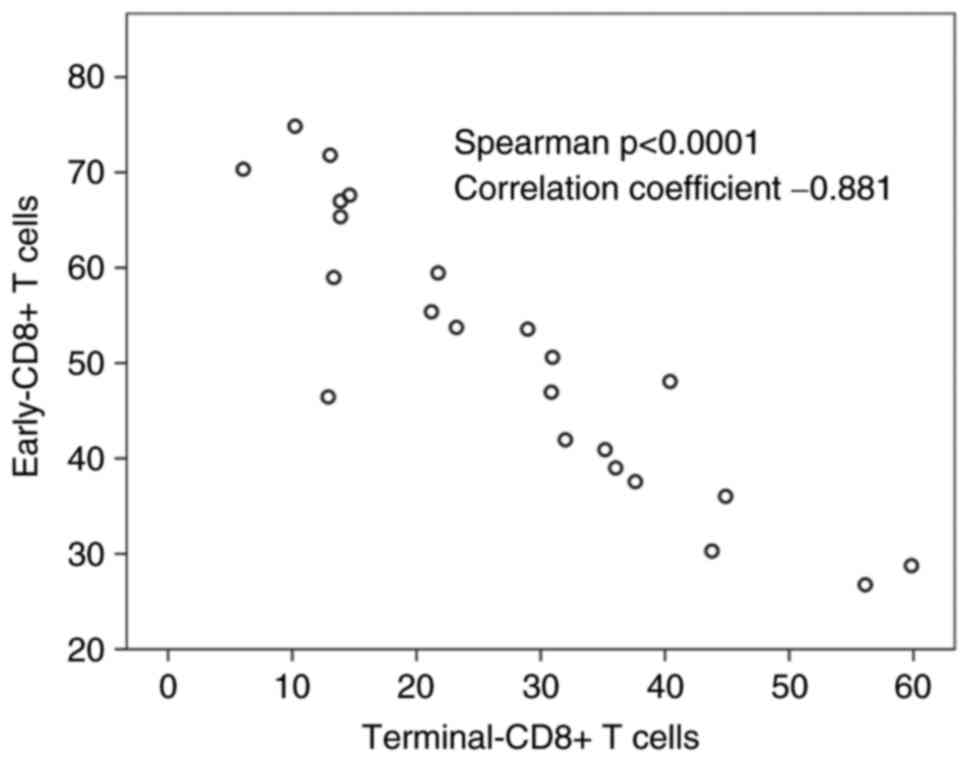
6A). Terminal-CD8+ T cells showed a significant reverse
correlation to early-CD8+ T cells (correlation coefficient=−0.881
Spearman P<0.0001) (Fig. 7). The
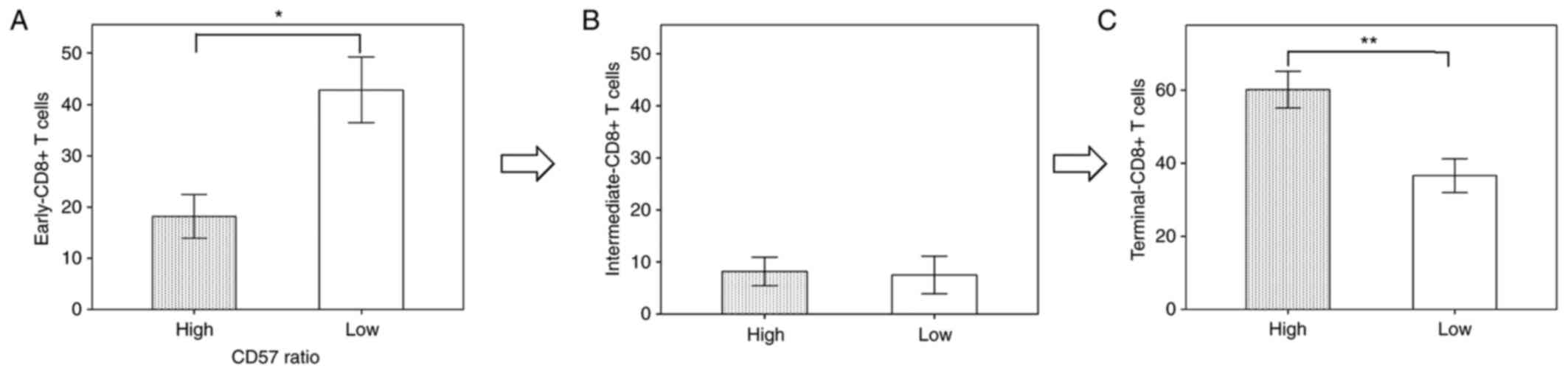
percentage of terminal-CD8+ T cells was higher in the patients with
high-CD57 ratio than in those with low-CD57 ratio, whereas the
percentage of early-CD8+ T cells was higher in the patients with
low-CD57 ratio than in those with high-CD57 ratio (Fig. 8). These results obtained from the
colon cancer patients also lead to the same conclusion that the
CD57 ratio appears to be the most powerful immunological prognostic
parameter obtained from the peripheral blood, precisely reflecting
the state of CD8+ T cell-differentiation.
Our results suggest that the CD57 ratio is a
convenient and powerful marker for predicting the prognosis of
Stage IV cancer patients given that it was a better independent
prognostic factor than terminal-CD8+ T cells, i.e., cytotoxic
effector cells, and is easily calculated by the data obtained from
peripheral blood samples. Further, the CD57 ratio could predict the
dynamic state of CD8+ T cell differentiation (measurement of the
predominance of cytotoxic vs. immunosuppressive CD8+ T cells). We
also revealed that early-CD8+ T cells (CD27+CD8+CD57-T cells) are
the more important immunosuppressive population in patients with
Stage IV carcinomas compared to intermediate-CD8+ T cells, which
are only preliminary data, so that we should perform further
experiments to examine the expression of immunosuppressive markers
such as PD-1 and FOXP3 in early-CD8+ T cells. The adoption of these
markers can help to improve personalized medicine for late-stage
cancer patients, leading to their optimum treatment.
References
|
1
|
Akagi J and Baba H: Prognostic value of
CD57(+) T lymphocytes in the peripheral blood of patients with
advanced gastric cancer. Int J Clin Oncol. 13:528–535. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Characiejus D, Pasukoniene V, Kazlauskaite
N, Valuckas KP, Petraitis T, Mauricas M and Den Otter W: Predictive
value of CD8highCD57+ lymphocyte subset in interferon therapy of
patients with renal cell carcinoma. Anticancer Res. 22:3679–3683.
2002.PubMed/NCBI
|
|
3
|
Focosi D, Bestagno M, Burrone O and
Petrini M: CD57+ T lymphocytes and functional immune deficiency. J
Leuloc Biol. 87:107–116. 2010. View Article : Google Scholar
|
|
4
|
Dondi E, Roué G, Yuste VJ, Susin SA and
Pellegrini S: A dual role of IFN-alpha in the balance between
proliferation and death of human CD4+ T lymphocytes during primary
response. J Immunol. 173:3740–3747. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Characiejus D, Pasukoniene V,
Jonusauskaite R, Azlauskaite N, Aleknavicius E, Mauricas M and
Otter WD: Peripheral blood CD8high CD57+ lymphocyte levels may
predict outcome in melanoma patients treated with adjuvant
interferon-alpha. Anticancer Res. 28:1139–1142. 2008.PubMed/NCBI
|
|
6
|
Wu RC, Hwu P and Radvanyi LG: New insights
on the role of CD8(+)CD57(+) T-cells in cancer. Oncoimmunology.
1:954–956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brenchley JM, Karandikar NJ, Betts MR,
Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles
SA, Connors M, et al: Expression of CD57 defines replicative
senescence and antigen-induced apoptotic death of CD8+ T cells.
Blood. 101:2711–2720. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sobin LH and Wittekind CH: UICC TNM
Classification of malignant tumours. John Wiley and Sons; New York:
1997
|
|
9
|
Papagno L, Spina CA, Marchant A, Salio M,
Rufer N, Little S, Dong T, Chesney G, Waters A, Easterbrook P, et
al: Immune activation and CD8+ T-cell differentiation towards
senescence in HIV-1 infection. PLoS Biol. 2:E202004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nolte MA, van Olffen RW, van Gisbergen KP
and van Lier RA: Timing and tuning of CD27-CD70 interactions: The
impact of signal strength in setting the balance between adaptive
responses and immunopathology. Immunol Rev. 229:216–231. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matter M, Odermatt B, Yagita H, Nuoffer JM
and Ochsenbein AF: Elimination of chronic viral infection by
blocking CD27 signaling. J Exp Med. 203:2145–2155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Penaloza-MacMaster P, Rasheed Ur A, Iyer
SS, Yagita H, Blazar BR and Ahmed R: Opposing effects of CD70
costimulation during acute and chronic lymphocytic choriomeningitis
virus infection of mice. J Virol. 85:6168–6174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borst J, Hendriks J and Xiao Y: CD27 and
CD70 in T cell and B cell activation. Curr Opin Immunol.
17:275–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wischhusen J, Jung G, Radovanovic I, Beier
C, Steinbach JP, Rimner A, Huang H, Schulz JB, Ohgaki H, Aguzzi A,
et al: Identification of CD70-mediated apoptosis of immune effector
cells as a novel immune escape pathway of human glioblastoma.
Cancer Res. 62:2592–2599. 2002.PubMed/NCBI
|
|
15
|
Claus C, Riether C, Schürch C, Matter MS,
Hilmenyuk T and Ochsenbein AF: CD27 signaling increases the
frequency of regulatory T cells and promotes tumor growth. Cancer
Res. 72:3664–3676. 2012. View Article : Google Scholar : PubMed/NCBI
|