Introduction
Hepatocellular carcinoma (HCC) is one of the most
difficult types of cancer to treat. The current therapeutic
strategies designed to induce apoptosis are not effective enough to
completely eliminate HCC (1).
Apoptosis-stimulating of p53 protein 2 (ASPP2) is composed of
ankyrin repeats, an SH3 domain, and a proline-rich region (1). ASPP2 binds to p53 through its C-terminus
to stimulate the transactivation function of p53 on the promoters
of pro-apoptotic genes (1). Other
studies also demonstrated that ASPP2 could induce apoptosis in a
p53-independent manner (2–4). Previous results have indicated that
induction of ASPP2 overexpression can promote apoptotic cell death
in hepatoma cells (such as HepG2 or Hep3B), which emphasizes the
value of ASPP2 in treating HCC (3,4).
Autophagy is an evolutionarily conserved pathway
that aids maintenance of cellular homeostasis by targeting proteins
and organelles to lysosomes for degradation (5). ASPP2 overexpression can induce autophagy
by promoting the expression of the transcription factor C/EBP
homologous protein (CHOP) (4).
ASPP2-induced autophagy can induce apoptosis (autophagic apoptosis)
in hepatoma cells in a DNA damage regulated autophagy modulator 1
(DRAM)-dependent manner (4).
Induction of DRAM expression allows for the induction of apoptosis
by autophagy, indicating that DRAM can switch autophagy from being
anti-apoptotic to pro-apoptotic (4).
However, autophagy can serve an anti-apoptotic role as the
inhibition of autophagy can increase the level of apoptosis
(6).
The present study assessed the function of ASPP2
overexpression on inducing autophagy and apoptosis in Huh7.5 cells,
a HCC cell line, and the association between ASPP2-induced
autophagy and apoptosis.
Materials and methods
Cell culture and treatment
Huh7.5 cells were grown in Dulbecco's Modified
Eagle's Medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). DMEM was supplemented with 10% fetal bovine
serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.). Cells were
transfected with plasmids (5 µg) encoding ASPP2, DRAM and green
fluorescent protein-microtubule associated protein 1 light chain 3
(GFP-LC3) by using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h. Small interfering RNAs (siRNAs; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) were used to decrease
the expression of CHOP (cat no. sc-35437) or DRAM (cat no.
sc-96209). The control siRNA (cat no. sc-37007) was also purchased
from Santa Cruz Biotechnology, Inc. siRNA transfections were
performed at 80 nM by reverse transfection with Lipofectamine
RNAiMax (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h,
according to the manufacturer's protocol; 3-methyladenine (3-MA; 10
mM, Santa Cruz Biotechnology, Inc.) was added into the medium of
Huh7.5 cells for 12 h at 37°C to inhibit autophagy. Cells were
grown on glass cover slips for the TUNEL assay.
Western blot analysis
Cell lysates were subjected to western blot
analysis, as described previously (3). Briefly, total cell lysates were
separated by 10% SDS-PAGE, and the separated proteins were
transferred to polyvinylidene difluoride membranes. The protein
blots were blocked with 5% non-fat milk for 1 h at room temperature
and sequentially probed with specific primary antibodies and
horseradish peroxidase-conjugated secondary antibodies. Primary
antibodies and secondary antibodies were used at a dilution of
1:1,000 and 1:5,000, respectively. The detection of specific
proteins on the blots was achieved using a Pierce™ ECL Western
Blotting Substrate (Thermo Fisher Scientific, Inc.), and the
results were captured on ImageQuant LAS 4000 (GE Healthcare,
Chicago, IL, USA) using ImageQuant™ TL 7.0 software (GE
Healthcare). The antibodies for the detection of LC3-I/II, p62, RAC
serine/threonine-protein kinase (AKT), phosphorylated (p)-AKT,
mechanistic target of rapamycin (mTOR), p-mTOR were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA); the antibodies
for detection of β-actin, CHOP, DRAM were purchased form Abcam
(Cambridge, CA, USA). Anti-ASPP2 antibody was purchased from
Sigma-Aldrich (Merck KGaA). Goat anti-Mouse IgG and goat
anti-Rabbit IgG secondary antibodies were purchased from Thermo
Fisher Scientific Inc.
Cell death and apoptosis analysis
Cell death was quantified with the calcein
acetoxymethyl ester (calcein-AM)/propidium iodide (PI) assay
(Thermo Fisher Scientific, Inc.) as previously described (7). Briefly, calcein-AM (1 µg/ml) and PI (1
µg/ml) were added into the supernatant, and the cells were
incubated with calcein-AM/PI for 15 min. Apoptosis was detected
using the terminal deoxynucleotidyl transferase dUTP nick-end
labeling (TUNEL) assay (Promega Corporation, Madison, WI, USA) and
the slides were mounted with 50% glycerol, as described previously
(7). Briefly, at room temperature,
the cells were fixed with 10% paraformaldehyde in PBS for 15 min
and then incubated in 1% Triton X-100/PBS for 5 min. TUNEL
detection solution was dropped onto the glass cover slips, and the
cells were incubated at 37°C in the dark for 60 min. Nuclear
staining with DAPI was conducted for 5 min at room temperature
following rinsing with PBS. Finally, the slips were mounted with
50% glycerol following rinsing with PBS. The images of the
calcein-AM/PI and TUNEL assays were obtained with an Olympus IX71
inverted fluorescence microscope (magnification, ×20; Olympus
Corporation, Tokyo, Japan). A quantitative cell death/apoptosis
analysis was performed by counting >1,000 cells in each
examination.
Statistical analysis
Data in the present study are the results of at
least three independent experiments and are expressed as the mean ±
standard deviation. One-way analysis of variance followed by
Tukey's multiple comparison test was used to evaluate the
differences between the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Overexpression of ASPP2 induces
autophagy in Huh7.5 cells
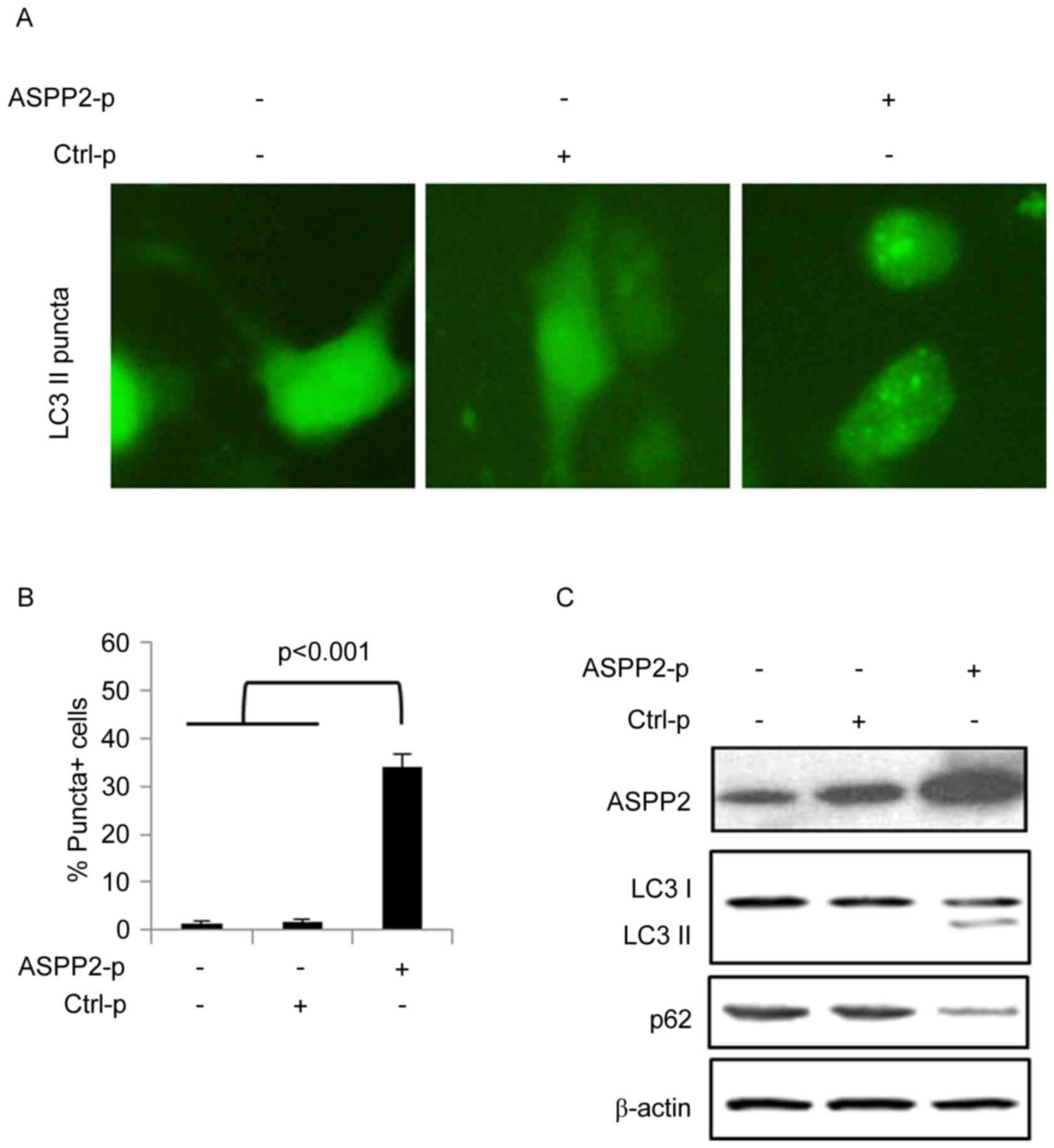
Huh7.5 cells were co-transfected with plasmids
encoding GFP-LC3 and ASPP2 (ASPP2-p) for 48 h. A vector plasmid was
used as a control for ASPP2 (Ctrl-p). An immunofluorescence assay
determined that ASPP2 overexpression induced the development of
GFP-LC3-II puncta-positive cells, indicating that ASPP2
overexpression induces autophagy in Huh7.5 cells (Fig. 1A and B). Huh7.5 cells were transfected
with ASPP2-p or Ctrl-p for 48 h and then western blotting
determined that ASPP2 overexpression also induced the development
of LC3-II and reduced the expression of p62 (Fig. 1C). These data indicated that ASPP2
overexpression induced autophagy in Huh7.5 cells.
ASPP2 overexpression induces autophagy
by inhibiting AKT/mTOR pathway, but not by inducing the expression
of CHOP or DRAM, in Huh7.5 cells
ASPP2 overexpression can induce autophagy by
inducing the expression of CHOP and DRAM (4). In the present study, ASPP2
overexpression did not increase the expression of CHOP and DRAM in
Huh7.5 cells (Fig. 2A). Additionally,
knockdown of CHOP or DRAM using siRNAs did not significantly affect
the level of GFP-LC3-II puncta-positive Huh7.5 cells following
transfection with ASPP2-p for 48 h (Fig.
2B and C). Thus, in the present study, CHOP and DRAM were not
involved in ASPP2-induced autophagy. Mechanistic target of
rapamycin (mTOR) has been identified to be a negative regulator of
autophagy development (8). When mTOR
is phosphorylated by AKT, it is activated and then inhibits
autophagy development. Here, western blot analysis determined that
ASPP2 overexpression significantly reduced the levels of p-AKT and
p-mTOR, indicating that ASPP2 overexpression induced autophagy
development by inhibiting the activation of the AKT-mTOR pathway in
Huh7.5 cells (Fig. 2A).
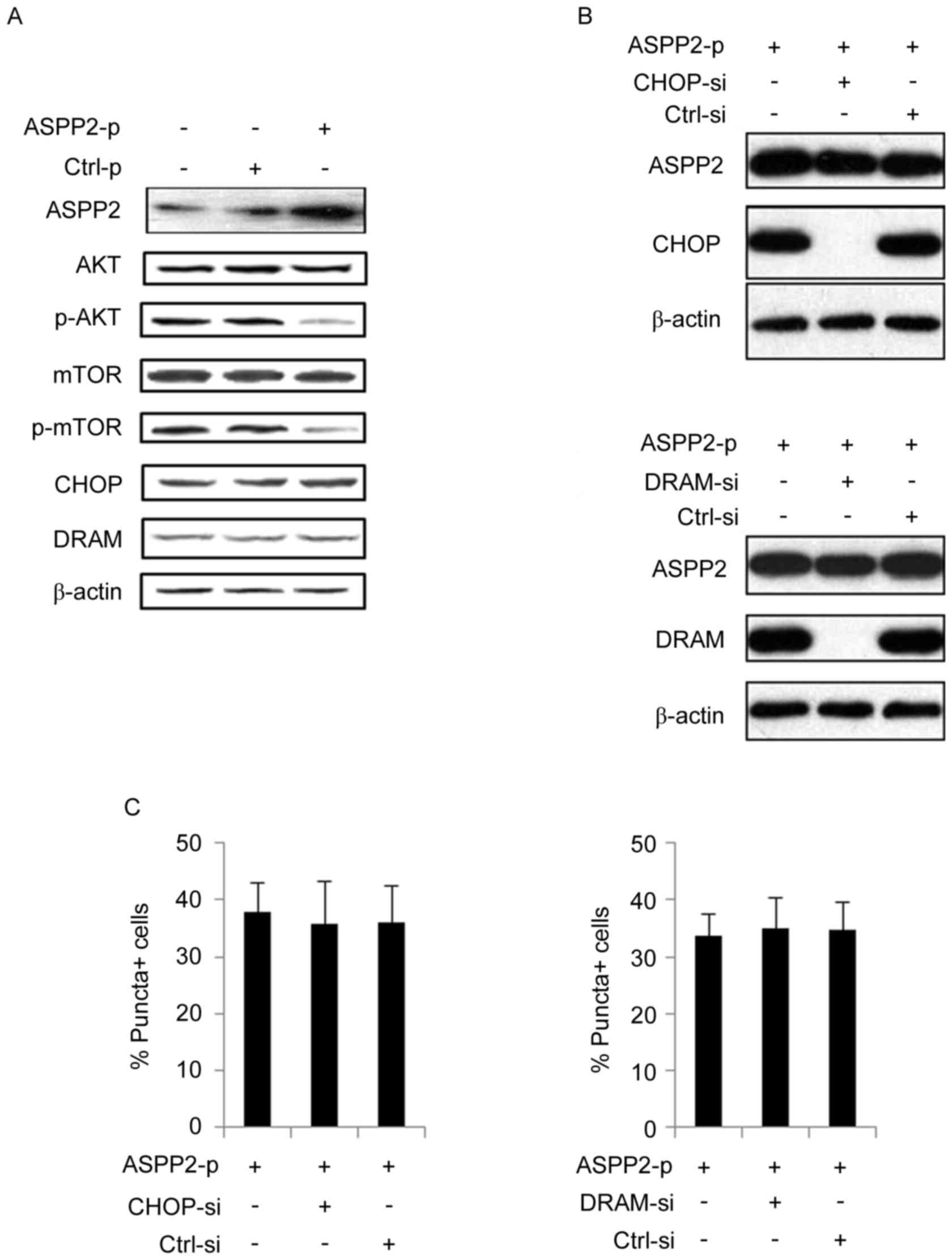 | Figure 2.ASPP2 overexpression inactivates the
AKT/mTOR pathway and CHOP/DRAM has no effect on ASPP2-induced
autophagy. (A) Hun7.5 cells were transfected with ASPP2-p or Ctrl-p
for 48 h. Western blotting detection with indicated antibodies. (B)
Huh7.5 cells were co-transfected with siRNAs to knock down
expression of CHOP/DRAM (upper panel, CHOP-si; lower panel,
DRAM-si) and ASPP2-p for 48 h. Western blotting was used to detect
the effect of siRNAs treatment on knocking down of CHOP and DRAM.
(C) Huh7.5 cells were co-transfected with siRNAs to knock down
CHOP/DRAM (left panel, CHOP-si; right panel, DRAM-si) and ASPP2-p
for 48 h. Immunofluorescence assay was used to detect the level of
GFP-LC3-II-positive cells. The values represent the mean ± standard
deviation of three independent experiments. ASPP2,
apoptosis-stimulating protein of p53; AKT, RAC
serine/threonine-protein kinase; mTOR, mechanistic target of
rapamycin; Ctrl-p, control plasmid; siRNA, small interfering RNA;
CHOP, C/EBP homologous protein; DRAM, DNA damage regulated
autophagy modulator 1; CHOP-si, siRNA targeting CHOP; GFP-LC3-II,
green fluorescent protein-tagged microtubule-associated protein
1A/1B light chain 3B. |
Autophagy impairs the function of
ASPP2 overexpression on inducing apoptotic cell death in Huh7.5
cells
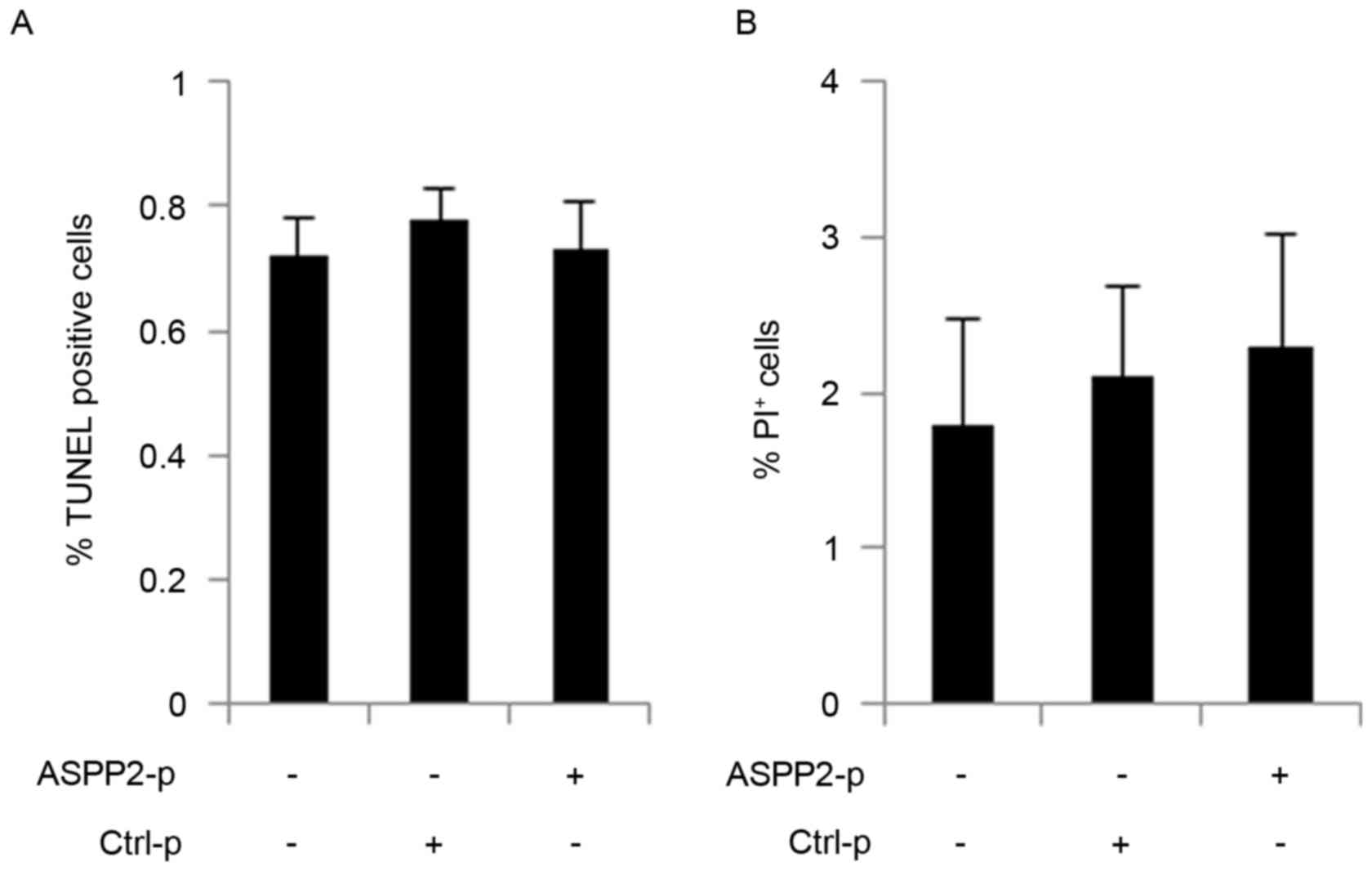
Huh7.5 cells were transfected with ASPP2-p for 48 h
and then TUNEL and Calcein AM/PI assays were used to detect
apoptosis and cell death, respectively. Notably, the TUNEL and
Calcein AM/PI assays determined that ASPP2 overexpression could not
induce apoptosis and cell death in Huh7.5 cells (Fig. 3A and B). Autophagy has been identified
to be an anti-apoptotic factor in cells, so whether ASPP2-induced
autophagy could impair the pro-apoptotic function of ASPP2 was
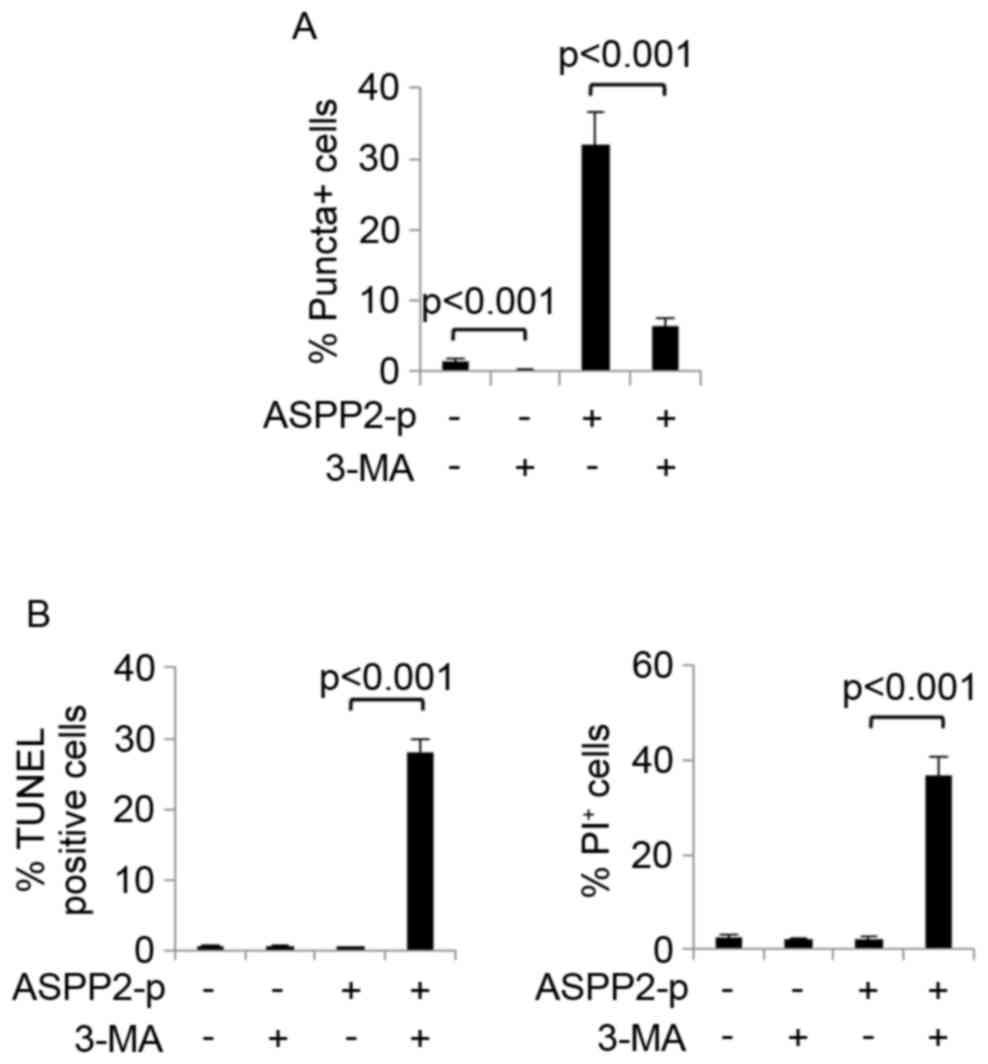
investigated. An autophagy inhibitor, 3-MA, was used to inhibit
autophagy development in Huh7.5 cells with or without transfection
with ASPP2-p. It was determined that 3-MA treatment successfully
inhibited basal and ASPP2-induced autophagy in Huh7.5 cells as
demonstrated by a significant decrease of GFP-LC3-II
puncta-positive cells (Fig. 4A).
Next, TUNEL and Calcein AM/PI assays determined that ASPP2
overexpression significantly induced apoptosis and cell death when
autophagy was inhibited by 3-MA treatment in Huh7.5 cells (Fig. 4B). These data indicated that, at least
in Huh7.5 cells, autophagy impaired the function of ASPP2
overexpression on inducing apoptotic cell death.
Overexpression of DRAM recovers the
function of ASPP2 on inducing apoptotic cell death in Huh7.5
cells
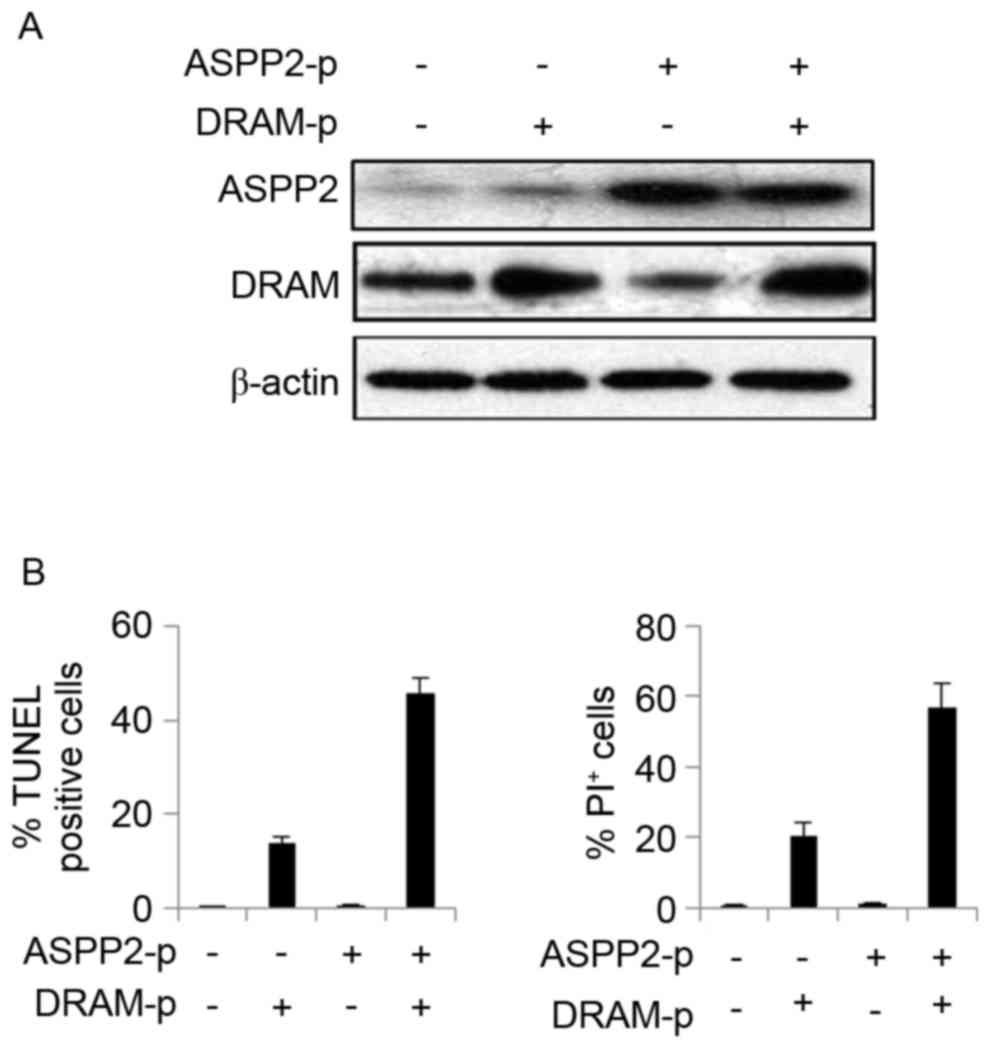
It was previously demonstrated that the expression
of DRAM could give autophagy the ability to induce apoptosis
(4). In the present study, Huh7.5
cells were co-transfected with plasmids encoding ASPP2 and DRAM for
48 h to induce the overexpression of ASPP2 and DRAM (Fig. 5A). The results of the TUNEL and
Calcein AM/PI assays revealed that overexpression of ASPP2 and DRAM
significantly induced apoptotic cell death in Huh7.5 cells,
indicating that overexpression of DRAM recovered the pro-apoptotic
function of ASPP2 (Fig. 5B). These
data indicated that induction of DRAM expression could improve the
function of ASPP2 on inducing apoptosis, which may aid the
treatment of HCC.
Discussion
ASPP2, an inducer of apoptosis, has been reported to
inhibit the growth of hepatoma cells in vitro and in
vivo (9). The present study
demonstrated that ASPP2 overexpression failed to induce apoptosis
in Huh7.5 cells, as ASPP2-induced autophagy impaired the function
of ASPP2 on inducing apoptosis. These results are different from
previous results, which demonstrated that ASPP2
overexpression-induced autophagy could induce apoptosis in HCC
Hep1-6, HepG2 and Hep3B cell lines in a CHOP- and DRAM-dependent
manner. We hypothesize that the different background of HCC cell
lines is a critical factor that affects the function of
ASPP2-induced autophagy on inducing or inhibiting apoptosis.
Previous studies demonstrated that ASPP2 is an
autophagy inhibitor that impairs the formation of the autophagosome
membrane by interacting with Atg5 (10,11).
However, a previous study also demonstrated that ASPP2 could induce
autophagy development by increasing CHOP expression. CHOP reduces
the level of B-cell lymphoma-2 (Bcl-2) and then reduces the
formation of Bcl-2-Beclin-1 complexes, which contributes to the
increase of free Beclin-1 in the cytoplasm and the initiation of
autophagy (4). In the present study,
although CHOP is not involved in ASPP2-induced autophagy, it was
identified that inactivation of the AKT/mTOR pathway also
contributes to ASPP2-induced autophagy, indicating that in
different situations ASPP2 can induce autophagy through activation
of different mechanisms.
DRAM has been identified to induce apoptosis in an
autophagy dependent or independent manner (4). In the present study, although DRAM is
not involved in ASPP2-induced autophagy, overexpression of DRAM
recovers the function of ASPP2 on inducing apoptotic cell death in
Huh7.5 cells. To the best of our knowledge, the mechanism by which
DRAM induces apoptosis or autophagic apoptosis remains unclear;
however, the data in the present study strongly indicated that
elucidation of the mechanisms by which DRAM induces apoptosis is
critical for treating tumors (4,12).
Autophagy is regarded to serve dual roles on cell
death. Autophagy is reported to prevent cells from cell death
signals, including nutrition depletion or organelle damage
(5). Autophagy could degrade certain
cytoplasmic redundant organelle or proteins to provide nutrition
for cells (5). The autophagy-mediated
degradation of impaired organelles, including uncoupled
mitochondria, eliminates the large production of pro-apoptotic
inducers, preventing pro-apoptotic factor-initiated cell death
(13). However, other studies have
demonstrated that autophagy can also be a pro-apoptotic factor,
since the inhibition of autophagy reduces the level of apoptosis
and the promotion of autophagy has an opposite result (4,12,14). In the present study, ASPP2-induced
autophagy had an anti-apoptotic role and the induction of autophagy
was associated with the inactivation of the AKT/mTOR pathway. In
fact, although autophagy and apoptosis are involved in maintaining
cellular homeostasis and the two physiological functions are
regarded to be closely associated with each other, the mechanism by
which autophagy induces apoptosis remains, to the best of our
knowledge, unclear. The data generated in the present study
indicated that the different autophagy-inducing signals may
determine the different roles of autophagy on apoptosis; for
example, CHOP/DRAM-induced autophagy induces apoptosis, but
inactivation of AKT/mTOR pathway induces an anti-apoptotic
autophagy.
mTOR is a major target of AKT. When mTOR is
activated by AKT via phosphorylation, activated mTOR will inhibit
autophagy (15). Previous studies
indicate that inhibition of mTOR function by its inhibitors
promotes autophagy development and reduces the sensitivity of cells
to cell death signals (16,17). Up to now, the mechanism by which ASPP2
inactivates the AKT/mTOR pathway remains unclear, because, to the
best of our knowledge, few studies have investigated the associated
between the AKT/mTOR pathway and ASPP2. A previous study
demonstrated that ASPP2 could bind to AKT in nucleus (3). In the present study, although it is not
known whether the interaction between of ASPP2 and AKT could affect
the activation of AKT, the data indicated that ASPP2 had the
ability to suppress the function of AKT, and overexpression of
ASPP2 might be benefit for treating certain tumors with high AKT
activation levels.
Taken together, the results of the present study
revealed a mechanism by which hepatoma cells could escape from
ASPP2-induced apoptosis. These data may prove valuable for the
future study of tumor therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
National Natural Science Foundation of China (grant nos. 81402556,
81773168 and 81272266), International Cooperation and Exchanges
NSFC (no. 81361120401), The capital health research and development
of special foundation (no. 2014-1-1151), and the foundation of
Beijing Institute of Hepatology (nos. BJIH-01602 and BJIH-01715),
Tumor Invasion and Metastasis Mechanism Research Key Laboratory
Open Fund of Beijing2015 (no. 2015ZLQX05).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DL and RL contributed equally to this work, both
completed the majority of the laboratory work. XG and LP assisted
DL and RL to complete the rest of the laboratory work. DC and KL
designed the project. YZ assisted DC and KL to design the
experimental work. KL completed the writing of this article.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bergamaschi D, Samuels Y, O'Neil NJ,
Trigiante G, Crook T, Hsieh JK, O'Connor DJ, Zhong S, Campargue I,
Tomlinson ML, et al: iASPP oncoprotein is a key inhibitor of p53
conserved from worm to human. Nat Genet. 33:162–167. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song B, Bian Q, Zhang YJ, Shao CH, Li G,
Liu AA, Jing W, Liu R, Zhou YQ, Jin G and Hu XG: Downregulation of
ASPP2 in pancreatic cancer cells contributes to increased
resistance to gemcitabine through autophagy activation. Mol Cancer.
14:1772015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu K, Jiang T, Ouyang Y, Shi Y, Zang Y,
Li N, Lu S and Chen D: Nuclear EGFR impairs ASPP2-p53
complex-induced apoptosis by inducing SOS1 expression in
hepatocellular carcinoma. Oncotarget. 6:16507–16516.
2015.PubMed/NCBI
|
|
4
|
Liu K, Shi Y, Guo X, Wang S, Ouyang Y, Hao
M, Liu D, Qiao L, Li N, Zheng J and Chen D: CHOP mediates
ASPP2-induced autophagic apoptosis in hepatoma cells by releasing
Beclin-1 from Bcl-2 and inducing nuclear translocation of Bcl-2.
Cell Death Dis. 5:e13232014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shirakabe A, Ikeda Y, Sciarretta S,
Zablocki DK and Sadoshima J: Aging and autophagy in the heart. Circ
Res. 118:1563–1576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin L and Baehrecke EH: Autophagy, cell
death, and cancer. Mol Cell Oncol. 2:e9859132015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu K, Lou J, Wen T, Yin J, Xu B, Ding W,
Wang A, Liu D, Zhang C, Chen D and Li N: Depending on the stage of
hepatosteatosis, p53 causes apoptosis primarily through either
DRAM-induced autophagy or BAX. Liver Int. 33:1566–1574.
2013.PubMed/NCBI
|
|
8
|
Tan VP and Miyamoto S: Nutrient-sensing
mTORC1: Integration of metabolic and autophagic signals. J Mol Cell
Cardiol. 95:31–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao J, Wu G, Bu F, Lu B, Liang A, Cao L,
Tong X, Lu X, Wu M and Guo Y: Epigenetic silence of
ankyrin-repeat-containing, SH3-domain-containing, and
proline-rich-region-containing protein 1 (ASPP1) and ASPP2 genes
promotes tumor growth in hepatitis B virus-positive hepatocellular
carcinoma. Hepatology. 51:142–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Wang XD, Lapi E, Sullivan A, Jia
W, He YW, Ratnayaka I, Zhong S, Goldin RD, Goemans CG, et al:
Autophagic activity dictates the cellular response to oncogenic
RAS. Proc Natl Acad Sci USA. 109:13325–13330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen R, Wang H, Liang B, Liu G, Tang M,
Jia R, Fan X, Jing W, Zhou X, Wang H, et al: Downregulation of
ASPP2 improves hepatocellular carcinoma cells survival via
promoting BECN1-dependent autophagy initiation. Cell Death Dis.
7:e25122016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu K, Shi Y, Guo XH, Ouyang YB, Wang SS,
Liu DJ, Wang AN, Li N and Chen DX: Phosphorylated AKT inhibits the
apoptosis induced by DRAM-mediated mitophagy in hepatocellular
carcinoma by preventing the translocation of DRAM to mitochondria.
Cell Death Dis. 5:e10782014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flores-Toro JA, Go KL, Leeuwenburgh C and
Kim JS: Autophagy in the liver: Cell's cannibalism and beyond. Arch
Pharm Res. 39:1050–1061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodríguez ME, Cogno IS, Sanabria Milla LS,
Morán YS and Rivarola VA: Heat shock proteins in the context of
photodynamic therapy: Autophagy, apoptosis and immunogenic cell
death. Photochem Photobiol Sci. 15:1090–1102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chagin AS: Effectors of mTOR-autophagy
pathway: Targeting cancer, affecting the skeleton. Curr Opin
Pharmacol. 28:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu L and Brink M: mTOR, cardiomyocytes and
inflammation in cardiac hypertrophy. Biochim Biophys Acta.
1863:1894–1903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng XT, Wu ZH, Wei Y, Dai JJ, Yu GF,
Yuan F and Ye LC: Induction of autophagy by salidroside through the
AMPK-mTOR pathway protects vascular endothelial cells from
oxidative stress-induced apoptosis. Mol Cell Biochem. 425:125–138.
2017. View Article : Google Scholar : PubMed/NCBI
|