Introduction
Gastric cancer is the fourth most common cause of
cancer-associated mortality worldwide and accounts for ~10% of the
annual incidences of cancer-associated mortality (1,2). Almost
two-thirds gastric cancer cases occur in less developed regions
(3). Research has been performed to
improve the clinical outcomes of gastric cancer; this has included
improvement in large-scale nationwide screening processes, radical
surgery, chemotherapy and radiation therapy (4–6). Despite
medical progress, Eastern Asia has the highest gastric cancer
mortality rate worldwide (7). The
primary cause of mortality due to gastric cancer is metastasis,
which is often resistant to conventional therapy (8). Peritoneal metastasis is one of the main
causes of gastric cancer progression and accounts for the majority
of gastric cancer-induced mortalities (9). Therefore, the development of novel
strategies to prevent the peritoneal metastasis of gastric cancer
is required.
Members of the phosphatase of regenerating liver
(PRL) protein family have been associated with numerous cancer
types (10,11), and PRL-3 has been identified as a
potentially critical biomarker of gastric cancer (12). A previous study demonstrated that
PRL-3 expression was significantly increased in primary gastric
cancer with peritoneal metastasis compared with the corresponding
primary gastric cancer without metastasis (13). Additionally, PRL-3 has been
demonstrated to induce the proliferation, invasion, migration,
tumorigenesis and metastasis of malignant neoplasms (14,15),
including gastric cancer (16).
The mechanisms by which PRL-3 promotes the motility,
metastasis and invasion of gastric cancer cells are not fully
understood. Dysregulated phosphatidylinositol 3-kinase (PI3K)/RAC
serine/threonine-protein kinase (AKT) signaling has been
demonstrated to cause abnormal cell growth and cellular
transformation in malignant neoplasms, including gastric cancer
(17–19). PRL-3 regulates PTEN expression and
signals through PI3K/AKT to promote the epithelial-mesenchymal
transition (20). Furthermore, PRL-3
has been demonstrated to promote the peritoneal metastasis of
gastric cancer through PI3K/AKT signaling in vitro (21). However, these results have not been
confirmed at a whole-animal level. To analyze the in vivo
roles of PRL-3 and PI3K/AKT signaling, a nude mouse model of
peritoneal metastasis was established and treated with LY294002
(LY), a potent and specific PI3K inhibitor (22), to test whether inhibition of the
PI3K/AKT signaling pathway prevents PRL-3-mediated progression of
tumorigenesis in vivo.
Materials and methods
Cell lines and animals
The human gastric adenocarcinoma cancer SGC7901 cell
line was purchased from the Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in DMEM (Biological Industries, Kibbutz
Beit-Haemek, Israel) supplemented with 10% fetal bovine serum (FBS;
Shanghai Li Rui Biological Technology Co., Ltd., Shanghai, China)
and penicillin/streptomycin, and maintained in a humidified
atmosphere with 5% CO2 at 37°C. Specific pathogen-free
male BALB/c-nu mice (n=24, 5 weeks old, weighing 16–17 g) were
purchased from the Hunan Slack King of Laboratory Animal Co., Ltd.
(Changsha, China) [Production permit no. SCXK (XIANG) 2013-0004].
All mice were housed in the same environment (room temperature was
maintained at 26±2°C, relative humidity was kept between 40 and
60%, and the light regimen was a 12/12-h dark: light cycle) and
allowed free access to water and food. Animal experiments were
performed in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals, and the present
study was approved by the Ethics Committee of Nanchang University
(Nanchang, China).
Cell proliferation analysis
To analyze SGC7901 cell proliferation, a Cell
Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's instructions.
SGC7901 cells were plated into 96-well plates and incubated for 24
h with different concentrations of LY294002 (0, 5, 10, 20 or 40 µM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Following a 24-h
incubation at 37°C in 5% CO2, 10 µl CCK-8 was added to
each well, and incubated for 4 h at 37°C. The absorbance values
were detected at 450 nm using an enzyme-labeled instrument (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Generating a stable EGFP-PRL-3/SGC7901
cell line
EGFP-PRL-3 plasmid construction was performed as
previously described (21). The
EGFP-PRL-3 vector was transfected into SGC7901 cells using the
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol.
Western blot analysis
Total protein extraction and bicinchoninic acid
protein assay kits were purchased from Nanjing KeyGen Biotech Co.,
Ltd. (Nanjing, China). Polyvinylidene difluoride membranes were
purchased from Merck KGaA. Antibodies against PRL-3 (ab50276;
1:400), AKT (ab8805; 1:500), p-AKT S473 (ab81283; 1:1,000), MMP-2
(ab97779; 1:1,000) and MMP-9 (ab137867; 1:1,000) were purchased
from Abcam (Cambridge, UK). The antibody for β-actin (60008-1-Ig;
1:5,000) was purchased from Wuhan Sanying Biotechnology (Wuhan,
China). These antibodies were used in the present study was
described previously (23–26). Membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (ab6721; 1:3,000) or goat anti-mouse secondary antibody
(Wuhan Sanying Biotechnology; SA00012-6; 1:2,000) and the western
blotting protocol were performed as previously described (21). Reactive protein was detected by an
enhanced chemiluminescence kit (EMD Millipore, Billerica, MA, USA).
Band intensity was quantified by using the Quantity One image
analysis software version 4.62 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Cell migration and invasion
assays
The migratory and invasive abilities of SGC7901
cells transfected with PRL-3/LY (LY294002) or empty control
vector/LY were determined using a Transwell assay. A total of
1.0×105 cells in serum-free DMEM medium were seeded in
the upper chamber of an 8-µm pore, 6.5-mm polycarbonate filter
(Corning Incorporated, Corning, NY, USA) for cell migration. For
cell invasion, the upper chamber was coated with 20 µg Matrigel and
the following protocols were the same as those for cell migration.
DMEM containing 10% FBS was added to the lower chamber. The
following steps were performed as previously described (21). All assays were independently repeated
≥3 times. Cells in five microscopic fields (magnification, ×200)
were counted and photographed. Each assay was done at least in
triplicate. Images were captured using a fluorescence microscope
(Olympus Corporation, Tokyo, Japan).
Nude mouse peritoneal metastasis
model
Specific pathogen-free grade male BALB/c nude mice
(n=24, 5 weeks old, weighing 16–17 g) were housed in the Laboratory
Animal Center of the First Affiliated Hospital of Nanchang
University (Nanchang, Jiangxi, China). The 5-week old nude mice
were randomly separated into 4 groups of 6. SGC7901/PRL-3/dimethyl
sulfoxide (DMSO, SGC7901/PRL-3/LY294002 and SGC7901/vector/DMSO
cells were injected into the abdominal cavity at 2.0×107
cells/mouse. The control group contained nude mice injected with an
equivalent volume of phosphate buffered saline. After 1 week,
twice-weekly intraperitoneal injections of 50 mg/kg LY294002 were
performed, as described previously (27), for 4 weeks. The mice were then
sacrificed by cervical dislocation and the peritoneal cavity was
opened for visual inspection and photography.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6
(GraphPad Software, Inc., La Jolla, CA, USA). All data are
presented as the mean ± standard deviation. The χ2 test
was used to analyze the relationship between the expression levels
of PRL-3. Differences between control and experiment groups were
analyzed using a one-way analysis of variance test followed by
least significance difference post-hoc test. Each experiment was
repeated ≥3 times. P<0.05 was considered to indicate a
statistically significant difference.
Results
LY294002-mediated PI3K/AKT inhibition
decreases SGC7901 cell proliferation
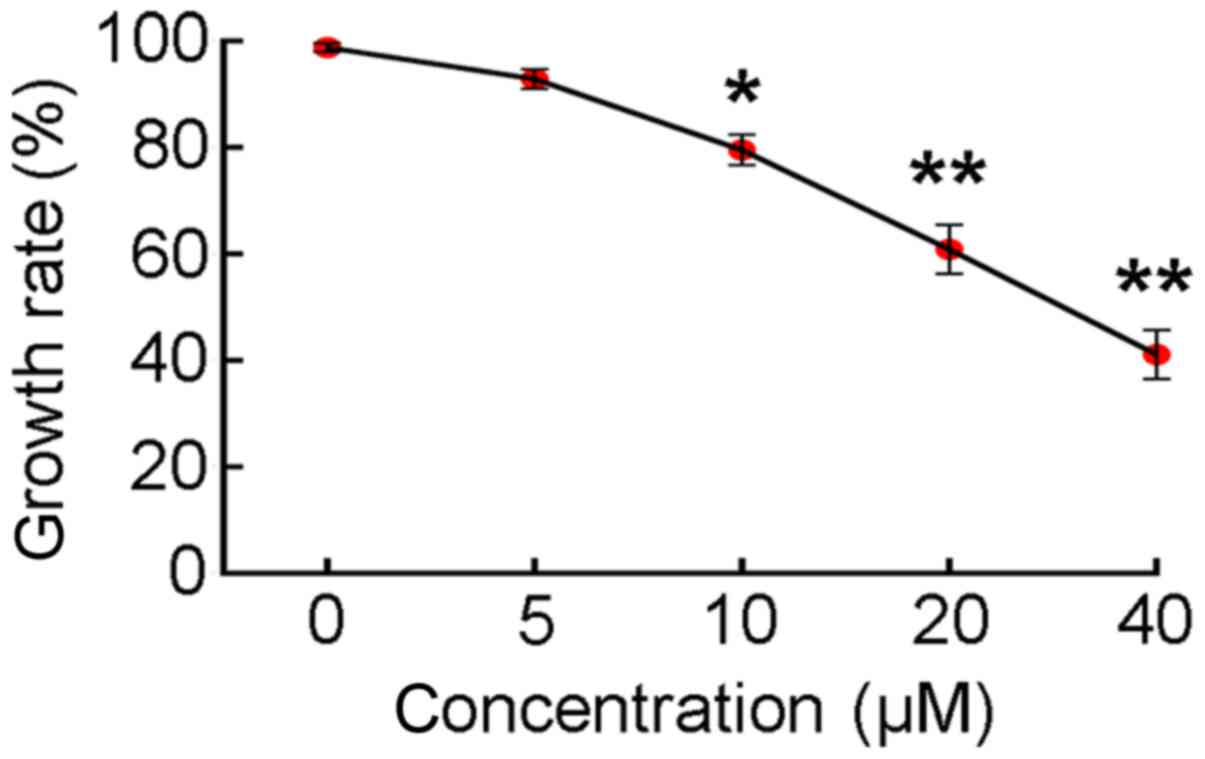
A CCK-8 assay was used to assess effect of
LY294002-mediated inhibition of PI3K/AKT activity on SGC7901 cell
proliferation. When SGC7901 cells were cultured in medium
containing 0, 5, 10, 20 or 40 µM LY294002 for 24 h, the rate of
cell proliferation decreased in a dose-dependent manner (Fig. 1). Treatment with ≥10 µM LY294002
reduced the rate of cell proliferation to ≤80%. Therefore, a dosage
of 10 µM LY294002 was selected for use in subsequent
experiments.
LY294002 inhibits the expression of
p-AKT (Ser473), MMP-2 and MMP-9 proteins in pEGFP-PRL-3-SGC7901
cells
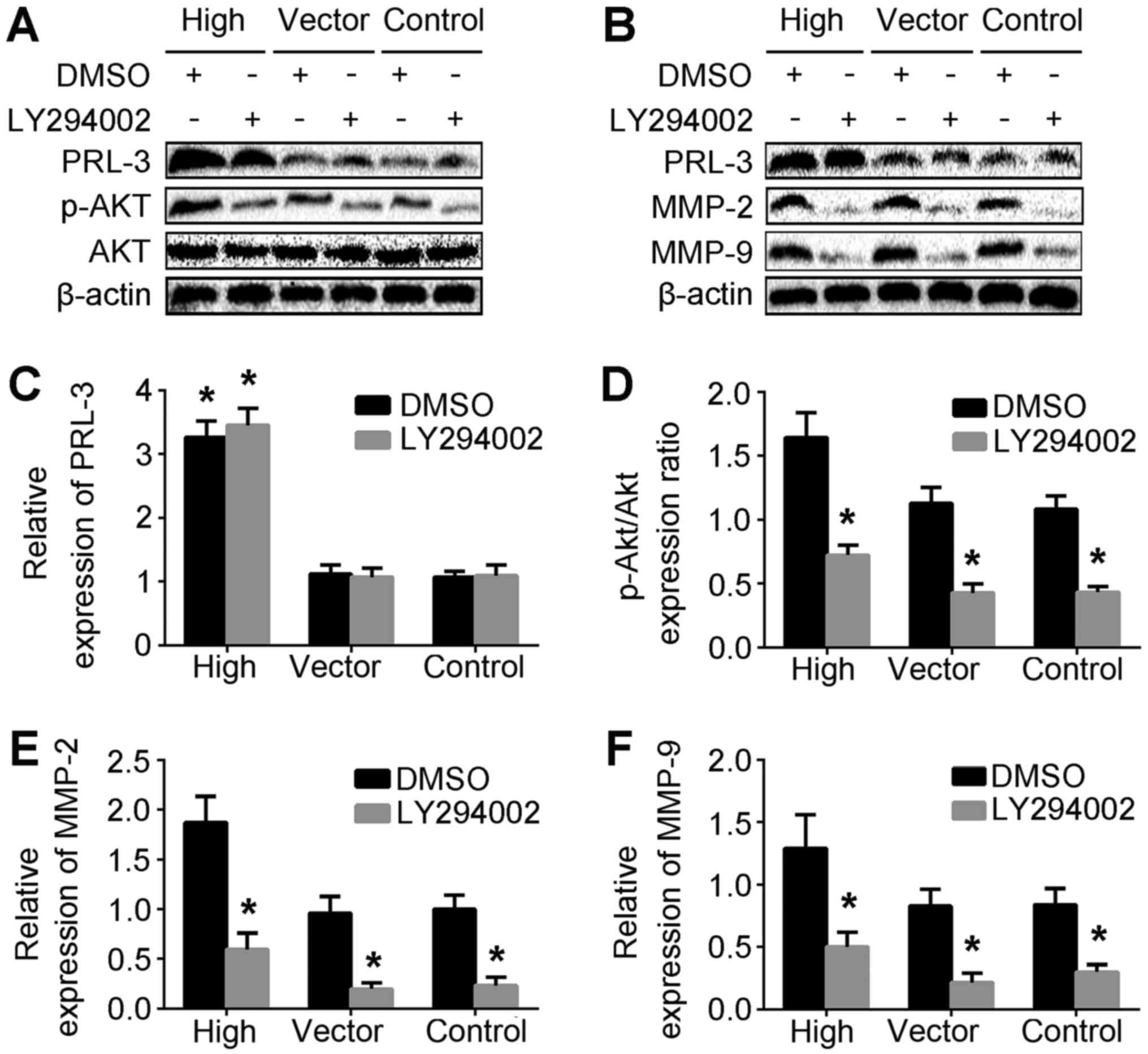
To investigate the association between PRL-3 and
p-AKT levels in the SGC7901/PRL-3 and LY/SGC7901/PRL-3 treatment
groups, western blotting was performed to determine the protein
levels of PRL-3, AKT and p-AKT (Fig.
2A). MMPs, including MMP-2 and MMP-9, can promote cells to
migrate through the basement membrane (28). To dissect the function and
gelatinolytic activities of MMP-2 and MMP-9 in PRL-3-promoted cell
invasion, the protein expression of MMP-2 and MMP-9 in the
LY/SGC7901/PRL-3 and DMSO/SGC7901/PRL-3 treatment groups was
analyzed by western blotting (Fig.
2B). The levels of p-AKT, MMP-2 and MMP-9 protein in the
LY/SGC7901/PRL-3 treatment group were significantly lower than
those in control groups (Fig. 2D-F).
Taken together, these results indicated that PRL-3 may upregulate
MMP-2 and MMP-9 expression via the PI3K/AKT pathway.
Migration and invasion abilities
conferred by PRL-3 are reduced by LY249002
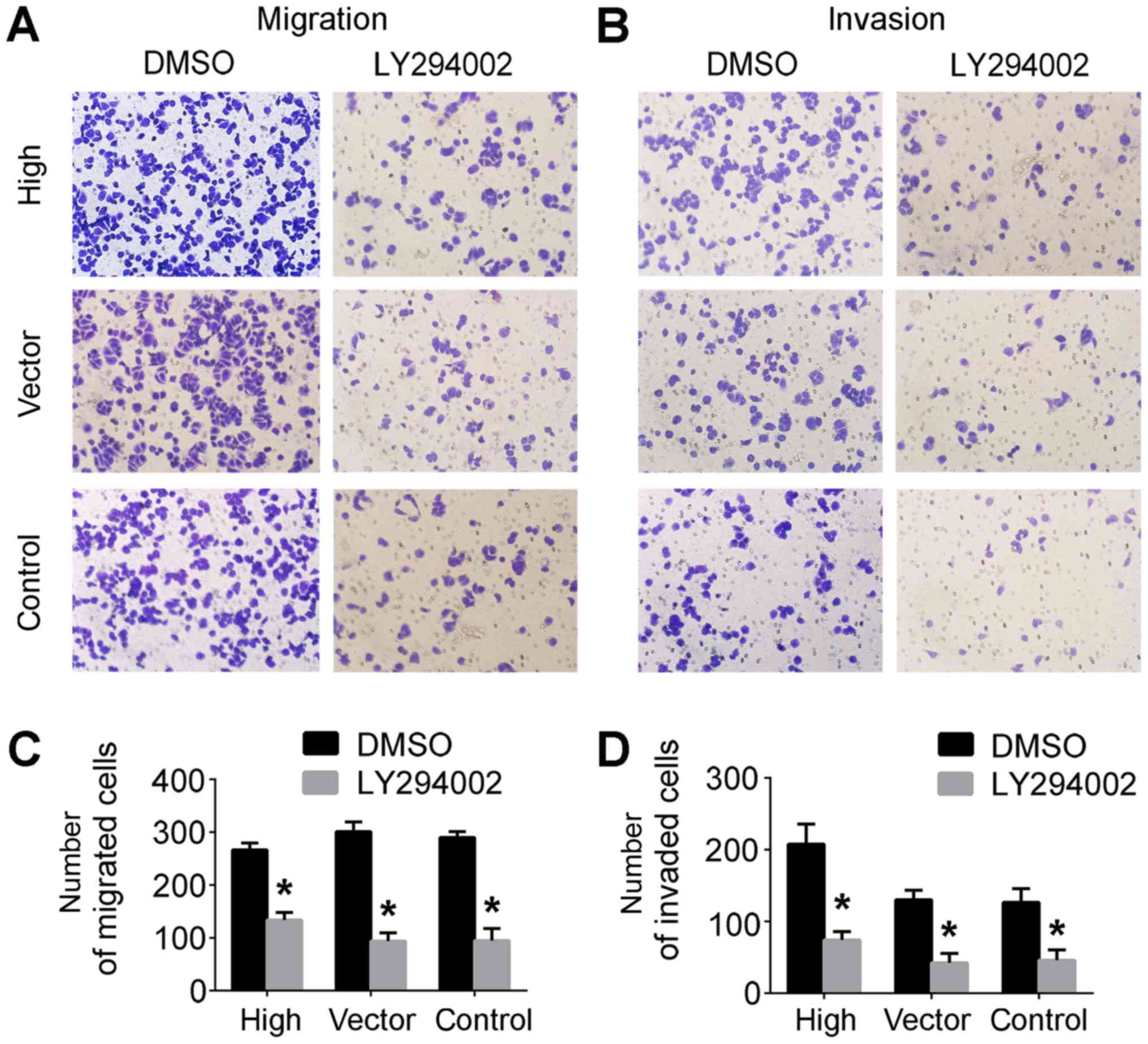
Transwell assays were performed to assess cell
migratory and invasive capabilities. Compared with the control
groups, the cells in the lower Transwell chamber were significantly
decreased in the SGC7901/LY249002 group compared with controls
(Fig. 3A and B). Taken together,
these results demonstrated that LY294002 inhibited PRL-3-conferred
invasive and migratory abilities of SGC7901 cells (Fig. 3C and D), indicating that PRL-3 is
involved in cell migration and invasion via the PI3K/AKT signaling
pathway in vitro.
PRL-3 promotes peritoneal spreading
via PI3K/AKT signaling in vivo
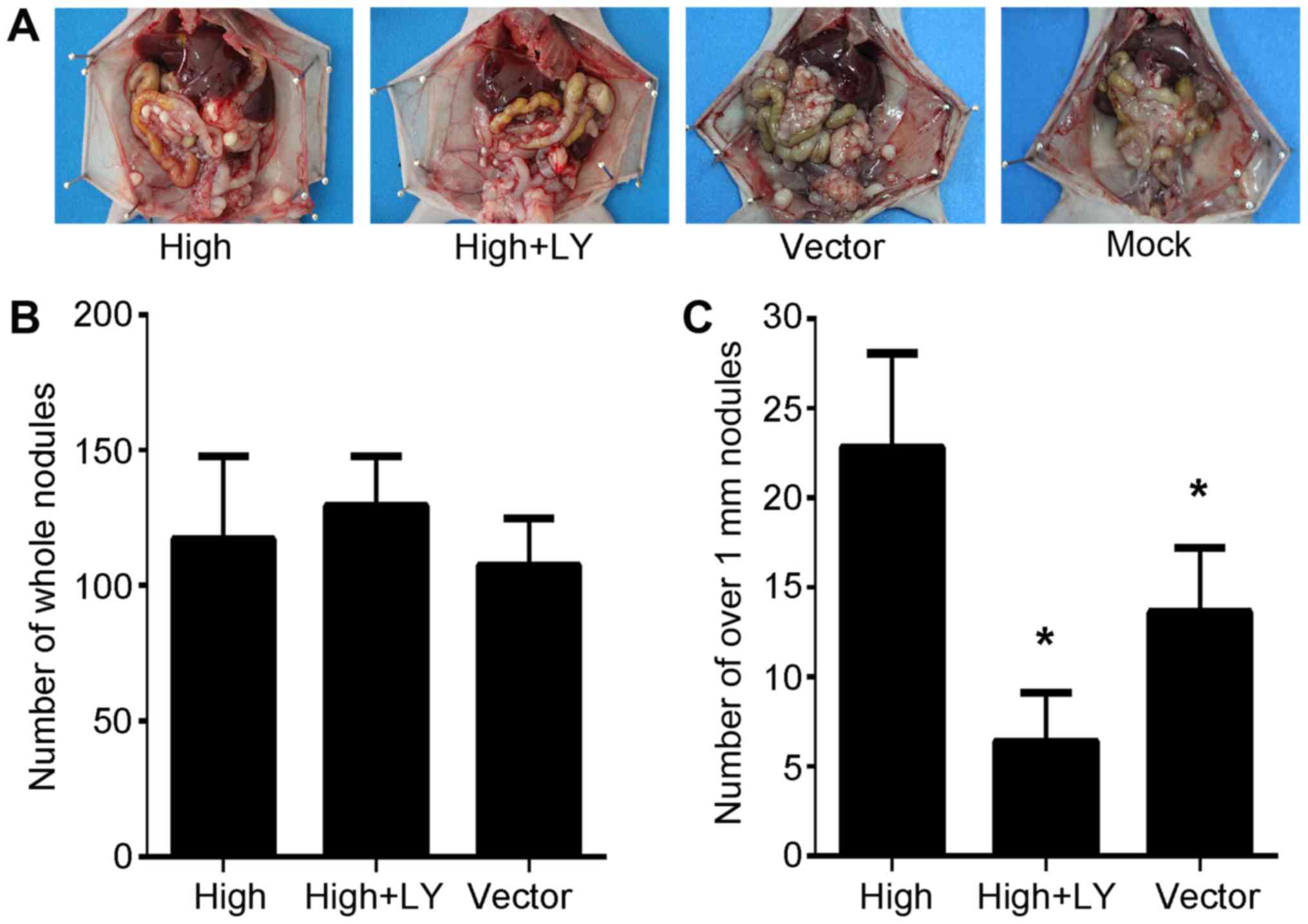
The in vivo effects of PRL-3 overexpression
on peritoneal spreading and metastasis via the PI3K/AKT pathway
were investigated in the present study. SGC7901/PRL-3,
SGC7901/PRL-3/LY294002 and SGC7901/Vector cells were injected into
the abdomens of nude mice. Extensive peritoneal spread was observed
in all three groups (Fig. 4A).
Differences in the total number of metastatic nodules on the
peritoneal surface were not statistically significant (Fig. 4B). However, the number of metastatic
nodules exceeding 1.0 mm3 in size significantly differed
between treatment groups (P<0.05; Fig.
4C). These results are consistent with those previously
reported (27,29), and indicate that the PI3K inhibitor
effectively suppresses PRL-3-mediated invasion in this peritoneal
metastasis model.
Discussion
The current gastric cancer drugs are designed to
inhibit or alter PI3K/AKT signaling to prevent metastasis (30–32). PRL-3
is a regulator of the PI3K/AKT signaling pathway (20). To a certain extent, blocking the
PI3K/AKT pathway influences the tumorigenic effects of PRL-3
(21). The present study aimed to
confirm this at a whole-animal level. LY294002 effectively inhibits
the growth of a variety of tumor cells in vitro and in
vivo through the inhibition of the PI3K/AKT pathway (33–35).
Therefore, in the case of peritoneal metastasis, it is possible
that LY294002 treatment had a negative effect on PRL-3-mediated
tumorigenesis.
Peritoneal metastasis accounts for the majority of
incidences of gastric cancer-associated mortality (9). Previous in vitro results have
demonstrated that PRL-3 is involved in peritoneal metastasis via
the PI3K/AKT signaling pathway (21).
The growth of malignant tumors is the result of interactions
between tumor cells and the local microenvironment, which is
affected by a variety of cytokines and other complex factors that
influence in vivo growth (36). Therefore, in vitro experiments
do not reliably reflect the in vivo environmental context.
To determine whether PRL-3 can influence tumor growth via the
PI3K/AKT pathway in vivo, a mouse model of gastric cancer
peritoneal metastasis was established. Significant differences
between the number of metastatic nodules on the peritoneal surface
>1 mm3 were observed between the PRL-3/DMSO and
PRL-3/LY groups. This is likely to be due to the fact that the
growth of solid tumors >1 mm3 is dependent on
specific oncogenes (37).
Furthermore, it was indicated that PI3K inhibition might prevent
PRL-3-mediated peritoneal metastasis. However, treatment with
LY294002 did not completely inhibit tumor growth. Therefore, it is
possible that the PI3K/AKT signaling pathway was only partially
inhibited by LY294002 treatment, which represent a limitation of
the present study. Other pathways may also be involved in this
process, which may function similarly to the PI3K/AKT pathway. This
speculation requires further investigation.
Invasion and metastasis are key steps in tumor
development. MMPs serve notable roles in tumor cell migration,
metastasis and invasion (38,39). Additionally, the expression of MMPs
increase when the PI3K/AKT pathway is activated (40–42). MMP-2
and MMP-9 are closely associated with the lymphatic metastasis of
gastric cancer (43). In the present
study, a significant difference in the level of MMP-2 and MMP-9
expression was identified between transfected gastric tumor cells
treated with LY249002 and control (P<0.05). This result
indicates that the LY249002-mediated inhibition of MMP-2, MMP-9 and
p-AKT expression occurred via PRL-3. These results indicated that
LY249002 has the potential to inhibit PRL-3-mediated invasion and
metastasis in vitro and in vivo.
Targeted therapies provide the most efficacious and
selective treatment to suppress tumor development (44). The present study demonstrated that
PRL-3 promoted the peritoneal metastasis of gastric cancer via the
PI3K/AKT pathway in vivo and in vitro. These results
may provide a novel candidate for gene-targeted therapy, and are
likely to translate into improved strategies for the treatment of
gastric cancer.
Acknowledgements
The authors thank the Key Laboratory of Basic
Pharmacology of Nanchang University and the Department of Histology
and Embryology of Medical College of Nanchang University (Nanching,
China) for technical assistance in the experiments.
Funding
The present study was supported by the National
Science Foundation of China (grant no. 81360362/81160304), the
Education Department of Jiangxi Province Science and Technology
Research Projects (grant no. GJJ13126), the Training Program for
Young Scientists of Jiangxi Province (grant no. 20133BCB23020) and
the Graduate Student Innovation Special Fund Project of Nanchang
University (grant no. cx2016383).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC, ZL, YZ, XF and ZJ conceived and designed the
experiment. YZ and XF performed the experiments. HC, YZ and XF
analyzed the data. JX, GZ, XL, KL, YC, ZH, FW, LX and GD
contributed reagents, materials and analysis tools. HC, ZL, YZ, XF
and ZJ contributed to the writing of the manuscript. The final
version of the manuscript has been read and approved by all
authors.
Ethics approval and consent to
participate
Animal experiments were performed in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals, and the present study was approved by the
Ethics Committee of Nanchang University (Nanchang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Best LM, Mughal M and Gurusamy KS:
Laparoscopic versus open gastrectomy for gastric cancer. Cochrane
Database Syst Rev. 3:Cd0113892016.PubMed/NCBI
|
|
2
|
Correa P: Gastric cancer: Overview.
Gastroenterol Clin North Am. 42:211–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee KS, Oh DK, Han MA, Lee HY, Jun JK,
Choi KS and Park EC: Gastric cancer screening in Korea: Report on
the national cancer screening program in 2008. Cancer Res Treat.
43:83–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamashima C, Shibuya D, Yamazaki H, Inoue
K, Fukao A, Saito H and Sobue T: The Japanese guidelines for
gastric cancer screening. Jpn J Clin Oncol. 38:259–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung KW, Park S, Kong HJ, Won YJ, Lee JY,
Seo HG and Lee JS: Cancer statistics in Korea: Incidence,
mortality, survival, and prevalence in 2009. Cancer Res Treat.
44:11–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishikawa K, Iwase K, Aono T, Yoshida H,
Nomura M, Tamagawa H, Matsuda C, Deguchi T, Kawada J, Higashi S, et
al: Efficacy of capecitabine/cisplatin chemotherapy after failure
of all conventional therapies in patients with advanced gastric
cancer. Gan To Kagaku Ryoho. 40:57–60. 2013.(In Japanese).
PubMed/NCBI
|
|
9
|
Turaga KK, Gamblin TC and Pappas S:
Surgical treatment of peritoneal carcinomatosis from gastric
cancer. Int J Surg Oncol. 2012:4056522012.PubMed/NCBI
|
|
10
|
Bessette DC, Qiu D and Pallen CJ: PRL
PTPs: Mediators and markers of cancer progression. Cancer
Metastasis Rev. 27:231–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slordahl TS, Abdollahi P, Vandsemb EN,
Rampa C, Misund K, Baranowska KA, Westhrin M, Waage A, Rø TB and
Børset M: The phosphatase of regenerating liver-3 (PRL-3) is
important for IL-6-mediated survival of myeloma cells. Oncotarget.
7:27295–27306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miskad UA, Semba S, Kato H and Yokozaki H:
Expression of PRL-3 phosphatase in human gastric carcinomas: Close
correlation with invasion and metastasis. Pathobiology. 71:176–184.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li ZR, Wang Z, Zhu BH, He YL, Peng JS, Cai
SR, Ma JP and Zhan WH: Association of tyrosine PRL-3 phosphatase
protein expression with peritoneal metastasis of gastric carcinoma
and prognosis. Surg Today. 37:646–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Q, Ding X, Chang B, Wang H and Wang
A: PRL-3 promotes migration and invasion and is associated with
poor prognosis in salivary adenoid cystic carcinoma. J Oral Pathol
Med. 45:111–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kato H, Semba S, Miskad UA, Seo Y, Kasuga
M and Yokozaki H: High expression of PRL-3 promotes cancer cell
motility and liver metastasis in human colorectal cancer: A
predictive molecular marker of metachronous liver and lung
metastases. Clin Cancer Res. 10:7318–7328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai N, Lu AP, Shou CC and Li JY:
Expression of phosphatase regenerating liver 3 is an independent
prognostic indicator for gastric cancer. World J Gastroenterol.
15:1499–1505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fruman DA and Rommel C: PI3K and cancer:
Lessons, challenges and opportunities. Nat Rev Drug Discov.
13:140–156. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vara Fresno JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and Gonzalez-Baron M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tapia O, Riquelme I, Leal P, Sandoval A,
Aedo S, Weber H, Letelier P, Bellolio E, Villaseca M, Garcia P and
Roa JC: The PI3K/AKT/mTOR pathway is activated in gastric cancer
with potential prognostic and predictive significance. Virchows
Arch. 465:25–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Quah SY, Dong JM, Manser E, Tang
JP and Zeng Q: PRL-3 down-regulates PTEN expression and signals
through PI3K to promote epithelial-mesenchymal transition. Cancer
Res. 67:2922–2926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiong J, Li Z, Zhang Y, Li D, Zhang G, Luo
X, Jie Z, Liu Y, Cao Y, Le Z, et al: PRL-3 promotes the peritoneal
metastasis of gastric cancer through the PI3K/Akt signaling pathway
by regulating PTEN. Oncol Rep. 36:1819–1828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vlahos CJ, Matter WF, Hui KY and Brown RF:
A specific inhibitor of phosphatidylinositol 3-kinase,
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol
Chem. 269:5241–5248. 1994.PubMed/NCBI
|
|
23
|
Liu Y, Zheng P, Liu Y, Ji T, Liu X, Yao S,
Cheng X, Li Y, Chen L, Xiao Z, et al: An epigenetic role for PRL-3
as a regulator of H3K9 methylation in colorectal cancer. Gut.
62:571–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mollevi DG, Aytes A, Padulles L,
Martínez-Iniesta M, Baixeras N, Salazar R, Ramos E, Figueras J,
Capella G, Villanueva A, et al: PRL-3 is essentially overexpressed
in primary colorectal tumours and associates with tumour
aggressiveness. Br J Cancer. 99:1718–1725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mayinuer A, Yasen M, Mogushi K, Obulhasim
G, Xieraili M, Aihara A, Tanaka S, Mizushima H, Tanaka H and Arii
S: Upregulation of protein tyrosine phosphatase type IVA member 3
(PTP4A3/PRL-3) is associated with tumor differentiation and a poor
prognosis in human hepatocellular carcinoma. Ann Surg Oncol.
20:305–317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khapare N, Kundu ST, Sehgal L, Sawant M,
Priya R, Gosavi P, Gupta N, Alam H, Karkhanis M, Naik N, et al:
Plakophilin3 loss leads to an increase in PRL3 levels promoting K8
dephosphorylation, which is required for transformation and
metastasis. PLoS One. 7:e385612012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang H, Fan D, Zhou G, Li X and Deng H:
Phosphatidylinositol 3-kinase inhibitor (LY294002) induces
apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J
Exp Clin Cancer Res. 29:342010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo Y, Liang F and Zhang ZY: PRL1 promotes
cell migration and invasion by increasing MMP2 and MMP9 expression
through Src and ERK1/2 pathways. Biochemistry. 48:1838–1846. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsuoka T, Yashiro M, Nishioka N,
Hirakawa K, Olden K and Roberts JD: PI3K/Akt signalling is required
for the attachment and spreading, and growth in vivo of metastatic
scirrhous gastric carcinoma. Br J Cancer. 106:1535–1542. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun L, Liu L, Liu X, Wang Y, Li M, Yao L,
Yang J, Ji G, Guo C, Pan Y, et al: MGr1-Ag/37LRP induces cell
adhesion-mediated drug resistance through FAK/PI3K and MAPK pathway
in gastric cancer. Cancer Sci. 105:651–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Almhanna K, Strosberg J and Malafa M:
Targeting AKT protein kinase in gastric cancer. Anticancer Res.
31:4387–4392. 2011.PubMed/NCBI
|
|
32
|
Jia Y, Sun H, Wu H, Zhang H, Zhang X, Xiao
D, Ma X and Wang Y: Nicotine Inhibits Cisplatin-Induced Apoptosis
via regulating α5-nAChR/AKT signaling in human gastric cancer
cells. PLoS One. 11:e01491202016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Semba S, Itoh N, Ito M, Harada M and
Yamakawa M: The in vitro and in vivo effects of
2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific
inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer
cells. Clin Cancer Res. 8:1957–1963. 2002.PubMed/NCBI
|
|
34
|
Hu L, Zaloudek C, Mills GB, Gray J and
Jaffe RB: In vivo and in vitro ovarian carcinoma growth inhibition
by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin
Cancer Res. 6:880–886. 2000.PubMed/NCBI
|
|
35
|
Zhao K, Zhu BS, Gong W, Zhu ML, Gao ZT, Wu
YY, Chen Q, Yang XD and Xing CG: SN50 enhances the effects of
LY294002 on cell death induction in gastric cancer cell line
SGC7901. Arch Med Sci. 9:990–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Polacheck WJ, Zervantonakis IK and Kamm
RD: Tumor cell migration in complex microenvironments. Cell Mol
Life Sci. 70:1335–1356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Foda HD and Zucker S: Matrix
metalloproteinases in cancer invasion, metastasis and angiogenesis.
Drug Discov Today. 6:478–482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Furuya F, Hanover JA and Cheng SY:
Activation of phosphatidylinositol 3-kinase signaling by a mutant
thyroid hormone beta receptor. Proc Natl Acad Sci USA.
103:1780–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL,
Kim JS and Yoo YA: Metastatic function of BMP-2 in gastric cancer
cells: the role of PI3K/AKT, MAPK, the NF-κB pathway, and MMP-9
expression. Exp Cell Res. 317:1746–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chien CS, Shen KH, Huang JS, Ko SC and
Shih YW: Antimetastatic potential of fisetin involves inactivation
of the PI3K/Akt and JNK signaling pathways with downregulation of
MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell
Biochem. 333:169–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu JEC, Yao Y, Ren S, Wang G and Jin H:
Matrix metalloproteinase expression and molecular interaction
network analysis in gastric cancer. Oncol Lett. 12:2403–2408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brannon-Peppas L and Blanchette JO:
Nanoparticle and targeted systems for cancer therapy. Adv Drug
Deliv Rev. 64:206–212. 2012. View Article : Google Scholar
|