Introduction
Prostate cancer (PCa) is the most frequently
diagnosed cancer in males. Furthermore, PCa is the second leading
cause of cancer-associated mortality in males in the USA and there
were 220,800 reported cases of PCa in the USA in 2015 (1). Additionally, the mortality and incidence
rates of PCa have increased rapidly in China (2). Surgery and radiotherapy are successful
treatments for early and localized tumors (3,4), but the
preferred therapy for advanced PCa is androgen-deprivation therapy.
The majority of patients eventually develop androgen-independent or
hormone-refractory PCa (5), and
chemotherapy is the only remaining option for advanced
hormone-refractory PCa (6). Further
investigation into the molecular mechanisms underlying the
tumorigenesis and development of PCa, and the consequential
development of novel targeted therapeutics, is required.
Numerous studies have demonstrated that microRNAs
(miRNAs/miRs) negatively regulate the expression of a number of
genes that are important for tumorigenesis and development, which
highlights a novel mechanism underlying PCa pathogenesis (7–12). miRNAs
are endogenously expressed small, non-coding, single-stranded RNAs.
miRNAs are usually 21–23 nucleotides in length and they negatively
regulate gene expression by binding to target gene 3′ untranslated
regions (3′-UTRs). This miRNA binding activity induces mRNA
degradation or inhibition of translation (13–15). miRNA
activity may therefore directly contribute to numerous fundamental
biological and cellular processes, including stem cell
differentiation, cell differentiation, and proliferation and
apoptosis (16). Despite the
increasing volume of evidence for the identification of miRNAs in
PCa carcinogenesis, limited information is available regarding
their specific roles and underlying mechanisms in PCa
development.
Numerous F-box proteins are involved in
tumorigenesis via their important roles in cell differentiation,
cell cycle regulation and proliferation. As an F-box protein, only
F-box and WD repeat domain containing 8 (Fbxw8) interact with
S-phase kinase associated protein 1, ring-box 1 and cullin 7 to
form an E3 ligase (17,18). Growth and cellular senescence is
regulated by Fbxw8 targeting insulin receptor substrate 1 (IRS-1).
IRS-1 is a critical mediator in insulin and insulin-like growth
factor-1 signaling (19).
Downregulation of Fbxw8 arrests cell cycle progression at the
G2/M phase in JEG3 choriocarcinoma cells (20). miRNA-218, which targets Fbxw8,
inhibits the proliferation of the human choriocarcinoma JEG-3 cell
line (21).
The present study revealed that miR-3160-5p was
highly expressed in PCa cells and that Fbxw8 is a target of
miR-3160-5p. Overexpression of miR-3160-5p repressed DU145 cell
proliferation and arrested the cell cycle in the G2/M
phase by targeting Fbxw8. The results of the present study
suggested that miR-3160-5p may serve as a novel potential
therapeutic target for PCa.
Materials and methods
Cells and culture
Human PCa DU145, LNCaP and 22Rv1 cells were obtained
from the American Type Culture Collection (Manassas, VA, USA) and
were cultured in RPMI-1640 medium, supplemented with 10% fetal calf
serum (FCS) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) 100 mg/ml streptomycin and 100 U/ml penicillin in 5%
CO2 at 37°C.
RNA oligonucleotide and cell
transfection
TargetScan (http://www.targetscan.org/vert_71/) was used to
predict the microRNA which could bind to the 3′-UTR of the Fbxw8
transcript. Guangzhou RiboBio Co., Ltd. (Guangzhou, China)
chemically synthesized miR-3160-5p mimics (cat no. miR10019212-1-5)
and their scramble microRNA (miR-SCR) (cat no. miR01201-1-5). Cells
were seeded onto 6-well plates and, once the cell density had
reached 60%, 50 nm oligonucleotides were transfected into DU145
cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
treated cells were harvested at 24 and 48 h for RNA or protein
extraction, respectively.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from DU145
cells. For clearance of DNA contamination in the synthesis of RNA
and cDNA, a PrimeScript® RT reagent kit with gDNA Eraser
was used (Life Technologies; Thermo Fisher Scientific, Inc.).
RT-qPCR was subsequently performed using the ABI-7500 system
employing the SYBR® Select Master mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). A total of 2 µg RNA
was used for reverse transcription. The mixture was incubated for
10 min at 25°C, then 37°C for 120 min, at last 5 min at 85°C. The
protocol of qPCR as follows: Denaturation at 95°C for 10 min, then
40 amplification cycles of 95°C for 5 sec and 60°C for 60 sec.
miR-3160-5P, miR-548a-3p and miR-4426 expression levels in PCa
LNCaP, 22Rv1 and DU145 cells, were assessed using RT-qPCR and were
normalized to U6 small nuclear RNA. Fbxw8 mRNA expression levels in
PCa cells were determined by qPCR and were normalized to β-actin.
The relative expression was calculated using the 2−∆∆Cq
method (22). The specific primers
for the aforementioned miRNAs, U6 and Fbxw8, were synthesized by
Guangzhou RiboBio Co., Ltd. The sequences of primers were as
follows: Fbxw8, forward: 5′-TCAGGGGATGTGAGAGTGTGG-3′, reverse:
5′-TCAGGGGATGTGAGAGTGTGG-3′, β-actin, forward:
5′-CATGTACGTTGCTATCCAGGC-3′, reverse:
5′-CTCCTTAATGTCACGCACGAT-3′.
Western blot analysis
Radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, P.R. China) was used to extract total
cellular proteins of DU145, PC-3 and 22RV1 cells, then the protein
concentration was detected using a bicinchoninic acid assay
according to the manufacturer's protocol (Pierce™ BCA Protein Assay
Kit; cat no. 23227; Life Technologies; Thermo Fisher Scientific,
Inc.). A total of 50 µg proteins/well were separated using a
denatured 10% polyacrylamide gel and were subsequently transferred
to a nitrocellulose membrane. The membranes were blocked with 5%
non-fat milk for 1 h at room temperature, then incubated with
primary antibodies at 4°C overnight. Antibodies against Fbxw8
(ab85647; 1:500) were obtained from Abcam (Cambridge, UK).
Antibodies against β-actin (60008-1-Ig; 1:1,000) were obtained from
ProteinTech Group, Inc. (Chicago, IL, USA). p-CDC2 (BM3428; 1:200),
CDC-2 (BM0027; 1:200) and CDC-25C (BM1580; 1:200) were purchased
from Boster Biological Technology Co., Ltd. (Wuhan, China).
Subsequently, the membranes were rinsed three times with
Tris-buffered saline with Tween-20 for 10 min at room temperature
and incubated with horseradish peroxidase-conjugated secondary
antibodies (A16104; 1:2,000) for 1 h. Enhanced chemiluminesence was
performed using Western blot detection reagents according to the
manufacturer's protocol (Pierce™ ECL Plus Western
Blotting Substrate; cat no. 32132; Life Technologies; Thermo Fisher
Scientific, Inc.) in order to visualize the signals.
MTT assay
Cells were seeded at 3×103 onto 96-well
plates and were stained once per day for 5 consecutive days with
100 µl sterile MTT dye (0.5 mg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C for 4 h. This was followed by the
removal of the culture medium and the addition of 150 µl of DMSO
(Sigma-Aldrich; Merck KGaA) to dissolve the purple formazan. The
absorbance was detected at 570 nm using 655 nm as the reference
wavelength in order to account for background interference. The
experiments were performed in triplicate.
Luciferase reporter assay
In a 12-well plate, PCa DU145 cells were seeded at
1.5×105 cells/well. A psiChECK-2 Fbxw8 3′-UTR WT vector
(500 ng) or miR-3160-5P site mutation vector (500 ng) were
co-transfected with miRNA-3160-5P mimic using Lipofectamine 2000.
After 48 h, luciferase activities were detected using a Dual
Luciferase Reporter Assay kit (Promega Corporation, Madison, WI,
USA). The results are expressed as ratio of Firefly luciferase to
Renilla luciferase. Each experiment was performed three times and
the results are presented as the mean ± standard deviation.
Cell cycle assay
DU145 cells were transfected with miR-3160-5P for 48
h, then harvested. Cold phosphate-buffered saline (PBS) was used to
wash the cells following harvesting, then cells were fixed in 70%
ethanol overnight at 4°C. The cells were washed again in cold PBS
three times and incubated with 0.5 µg/ml RNase A (Sigma-Aldrich;
Merck KGaA) for 30 min at 37°C. Subsequently, the cells were
stained with 50 mg/ml propidium iodide (Sigma-Aldrich; Merck KGaA)
at room temperature for 20 min. Fluorescence-activated cell sorter
(FACS) analysis was performed using a flow cytometer (BD
Biosciences, San Jose, CA, USA). ModFit LT software (version 3.2.1;
BD Biosciences) was used for analysis.
Statistical analysis
The results are expressed as the mean ± SD. One-way
analysis of variance was performed using SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA). Multiple comparison between the groups was
performed using Fisher's least significant difference test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-3160-5P is expressed in PCa
cells
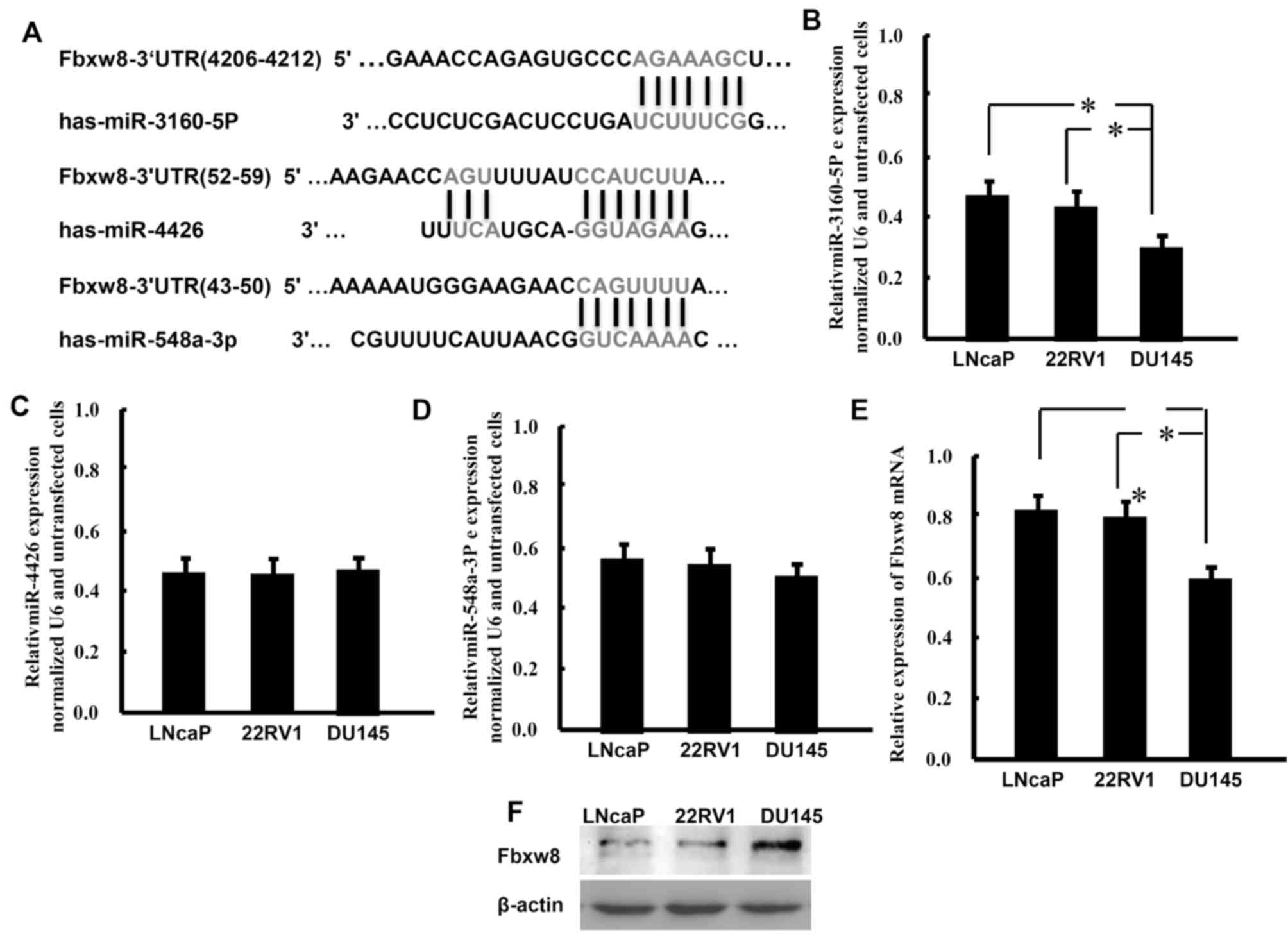
Targetscan predicted that miR-3160-5P, miR-548a-3p
and miR-4426 may target the 3′-UTR of the Fbxw8 transcript
(Fig. 1A). The present study detected
the expression patterns of these miRNAs in the PCa LNCaP, 22Rv1 and
DU145 cell lines using RT-qPCR analysis (Fig. 1B-D). The results demonstrated that
these miRNAs were expressed in PCa cells. Compared with the other
two cell lines and miRNAs, DU145 cells exhibit significantly lower
expression levels of miR-3160-5P. Conversely, DU145 cells expressed
markedly higher mRNA and protein levels of Fbxw8 compared with
LNCaP and 22Rv1 cells (Fig. 1E and
F). These results demonstrated that lower miR-3160-5P
expression levels were associated with higher expression levels of
Fbxw8 protein in DU145 cells.
miR-3160-5P targets Fbxw8 mRNA
It was predicted that miR-3160-5p negatively
regulated the expression of Fbxw8. There is a negative association
between the expression of miR-3160-5p and Fbxw8 in DU145, therefore
DU145 cells were selected for the present study. DU145 cells were
transfected with miR-3160-5P in order to investigate the
association between miR-3160-5P and Fbxw8 in DU145 cells. RT-qPCR
revealed that the expression of miR-3160-5P was significantly
upregulated 48 h after transfection compared with expression in the
control group (Fig. 2A). In addition,
RT-qPCR and western blot analysis demonstrated that enhanced
miR-3160-5P expression in DU145 cells significantly repressed Fbxw8
mRNA and protein expression (Fig. 2B and
C). The present study also constructed a reporter vector
consisting of the luciferase coding sequence followed by the 3′-UTR
of Fbxw8 (WT-Fbxw8) in order to determine whether the 3′-UTR of
Fbxw8 mRNA is a functional target of miR0316-5P in DU145 cells, as
well as a mutant-Fbxw8 3′-UTR luciferase reporter vector
(Mut-Fbxw8) by deleting the predicted 7-base pair miR-3160-5P
binding site in the 3′-UTR of the Fbxw8 transcript (Fig. 2D). The WT-Fbxw8 or Mut-Fbxw8 vector
and miR-3160-5P mimics or control RNA were co-transfected into
DU145 cells, respectively. miR-3160-5P-transfected cell luciferase
activity was significantly reduced compared with that in the
control cells (Fig. 2E). In addition,
the mutant putative binding site abolished miR-3160-5P-mediated
repression of luciferase activity.
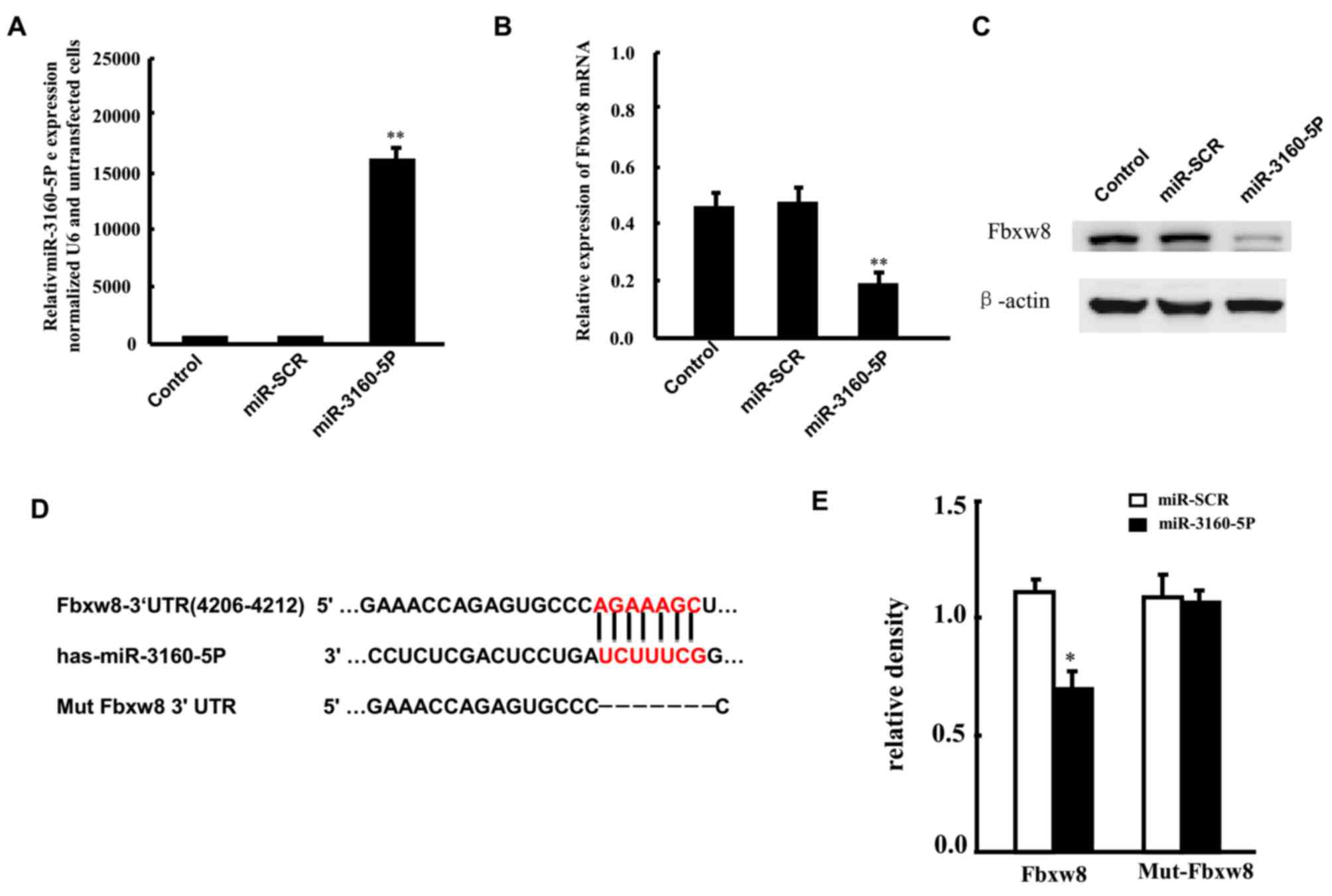 | Figure 2.Fbxw8 is targeted by miR-3160-5P
following binding to the 3′-UTR. (A) RT-qPCR was used to assess the
upregulated expression of miR-3160-5P in DU145 cells transfected
with miR-3160-5P mimics, compared with controls or
miR-SCR-transfected cells. Fbxw8 (B) mRNA and (C) protein
expression levels in DU145 cells that were transfected with
miR-3160-5P or miR-SCR were assessed by RT-qPCR and western blot
analysis. (D) The predicted miR-3160-5P binding site in the Fbxw8
3′-UTR and its mutated version due to site mutagenesis. (E) The
relative luciferase activities of the WT and Mut Fbxw8 3′-UTR
regions (mean ± SD of three independent experiments, performed in
triplicate). **P<0.01. The mean ± SD of three representative
experiments are presented. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; miR,
microRNA; miR-SCR, scramble microRNA; Fbxw8, F-box and WD repeat
domain containing 8; 3′-UTR, 3′ untranslated region; WT, wild type;
Mut, mutant; SD, standard deviation. |
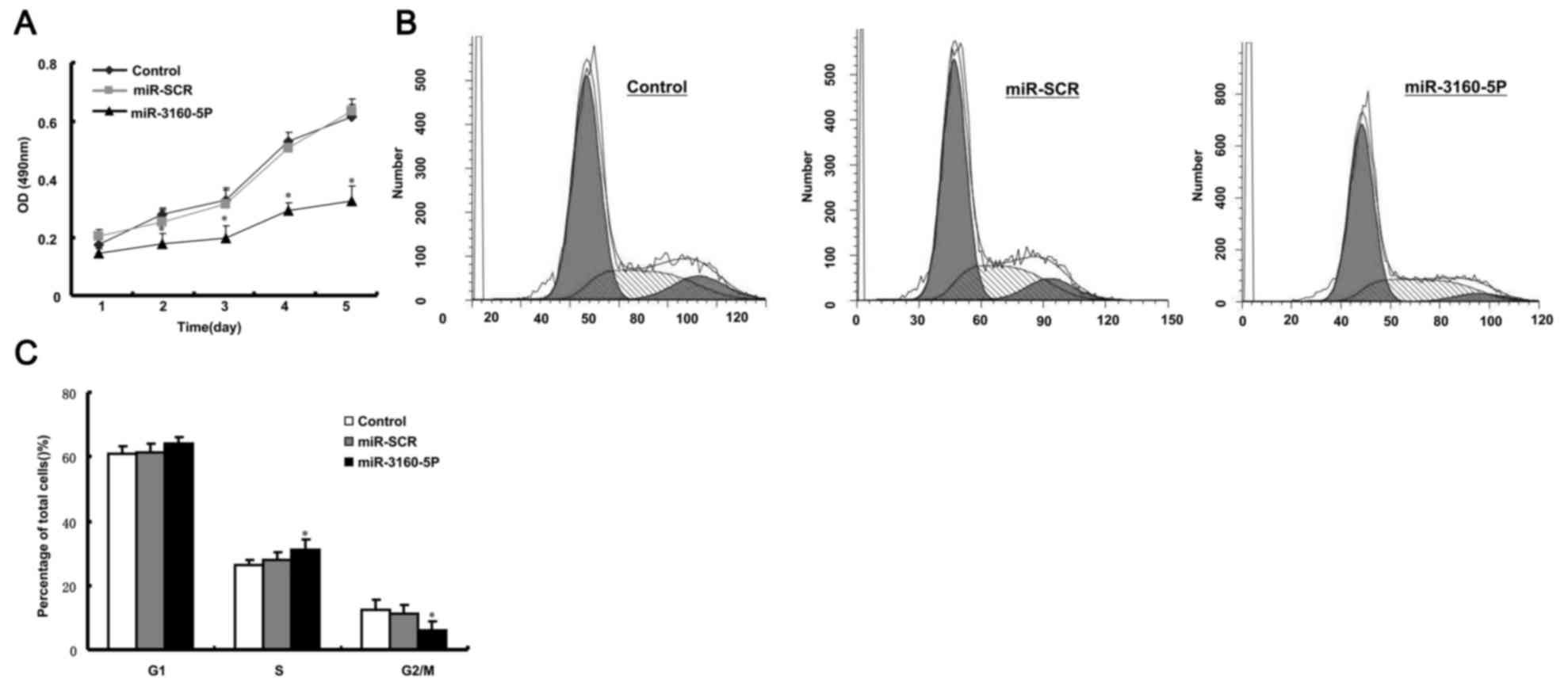
miR-3160-5P inhibits cell growth in
PCa DU145 cells
We previously reported that Fbxw8 has important
effects on cell proliferation as a potential oncogene in human
cancer (13). Therefore, the present
study hypothesized that miR-3160-5P may repress DU145 cell
proliferation via Fbxw8 downregulation. To elucidate the functional
significance of miR-3160-5P in DU145 cells, the present study
examined its effect on proliferation using MTT and cell cycle
analysis. It was revealed that miR-3160-5P significantly decreased
the proliferation of DU145 cells (Fig.
3A). Furthermore, FACS revealed that the percentage of cells in
the G2/M phase was increased following miR-3160-5P
transfection (Fig. 3B and C). These
results demonstrated that miR-3160-5P inhibited DU145 cell
proliferation.
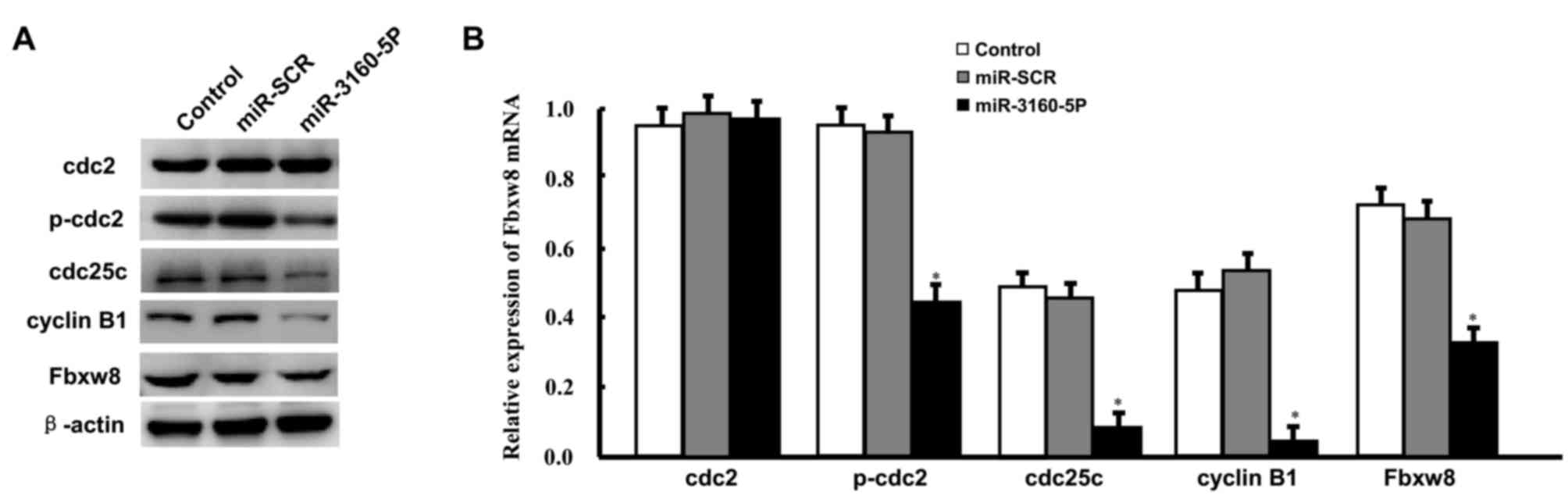
Induced expression of miR-3160-5P
inhibited p-CDC2, CDC25C, and cyclin B1 expression
Subsequently, the present study evaluated the
expression levels of proteins that were responsible for the
G2/M cell cycle phase transition by western blot
analysis, since downregulation of Fbxw8 induced G2/M
arrest in DU145 cells. These results were consistent with the cell
cycle assay results. miR-3160-5P was decreased in p-CDC2, CDC25C
and cyclin B1 in DU145 cells following a 48-h treatment (Fig. 4A and B). Treatment with miR-3160-5P
did not affect the expression level of total CDC2.
Discussion
The second leading cause of cancer-associated
mortality in males is PCa. The mechanisms underlying PCa
development and progression remain unknown despite the fact that
certain mechanisms that serve a significant role in PCa
pathogenesis have been elucidated.
The inability of a cell to modulate its
proliferation is a distinctive feature of cancer. The present study
identified a novel miRNA that regulates Fbxw8 expression and
elucidated the role of this novel miRNA on the proliferation of PCa
DU145 cells. Using Targetscan, it was initially hypothesized that
miR-3160-5P may bind to the 3′-UTR of the Fbxw8 transcript. The
present study evaluated luciferase activity using a vector
containing the two alleles to demonstrate that miR-3160-5P directly
targets the 3′-UTR. There was significantly decreased activity with
the luciferase vector that exhibited the mutant-type allele
compared with the wild-type vector. Overexpression of miR-3160-5P
reduced the luciferase activity, indicating that miR-3160-5P
directly inhibited Fbxw8 expression. Fbxw8 mRNA and protein
expression levels were decreased following transfection with
miR-3160-5P mimics, which occurred in a precursor miRNA
concentration-dependent manner. It has been suggested that
miR-3160-5P suppressed endogenous Fbxw8 protein expression by
regulating the stability of Fbxw8 mRNA transcripts. Considering the
results of the present study, it was suggested that miR-3160-5P
negatively regulates Fbxw8.
miRNAs serve an important role in the progression of
the majority of types of human cancer. They participate in numerous
cellular processes, including proliferation, differentiation,
apoptosis, metastasis, stem cell maintenance and metabolism
(13,23–27).
miRNAs are involved in PCa pathogenesis via the regulation of
oncogene upregulation or tumor suppressor downregulation in PCa
(13). The results from RT-qPCR in
the present study demonstrated that miR-3160-5P, which is a
potential miRNA that targets the Fbxw8 3′-UTR, was downregulated in
DU145 cells. The results of the present study revealed that
miR-3160-5P participated in the proliferation of DU145 cells. The
present study indicated that miRNA may act as a potential tumor
suppressor in solid cancer.
An increasing volume of data has revealed the role
of Fbxw8 E3 ligase in cancer (20,21,28,29).
Impaired proliferation kinetics was identified in the
Fbxw8−/− mouse embryonic fibroblasts (30). The degradation of cyclin D1 by Fbxw8
in HCT116 cells was revealed to be required for cancer cell
proliferation (28). Endogenous
homeodomain-interacting protein kinase 1 expression was restored by
downregulation of Fbxw8 and it also suppresses cell proliferation
in pancreatic ductal adenocarcinoma (29).
The present study investigated the hypothesis that
miR-3160-5P-mediated inhibition of Fbxw8 regulated the
proliferation of DU145 cells. Notably, miR-3160-5P and Fbxw8
protein expression levels were negatively associated with each
other in DU145 cells lines.
Upregulated miR-3160-5P expression suppressed the
proliferation of DU145 cells and increased the number of cells
arrested in the G2/M phase of the cell cycle. The
present study confirmed that miR-3160-5P and Fbxw8 are associated
with each other. It was also revealed that miR-3160-5P inhibited
DU145 cell proliferation by targeting Fbxw8. Target-inhibition of
Fbxw8 arrested cell cycle progression at the G2/M phase
in choriocarcinoma JEG3 cells by decreasing the expression level of
G2/M-associated cell cycle regulators and increasing the
expression level of p27 (20,21). The in-depth molecular mechanisms
underlying cell cycle arrest induced by miR-3160-5P were
investigated further. A number of cyclin-dependent kinases (CDKs)
were involved in the regulation of the cell cycle (31,32).
Activation of CDK1 and CDK2 is primarily associated with cyclin A
and B1 in the progression of the G2/M cell cycle phase.
Cyclin B1 and CDC2 kinase serve important roles in the initiation
stage of the M phase (33). Previous
studies demonstrated that the activation of CDC2 kinase depends on
the accumulation of cyclin B and dephosphorylation of CDC2
(34). The present study confirmed
that there is a vital molecular association between Fbxw8,
miR-3160-5P, cdc2 kinase, cyclin B1 and cdc25c protein expression.
At the protein level, induced expression of miR-3160-5P in DU145
cells was revealed to effectively inhibit Fbxw8, cyclin B1, CDC2
kinase and total CDC25C expression. This suggested a potential
inverse association between miR-3160-5P and Fbxw8 in DU145 cells.
The main effect of Fbxw8 is an autocrine effect as miR-3160-5P
downregulates Fbxw8 mRNA and protein levels in the cells. In
addition, the present study suggested that miR-3160-5P inhibited
cyclin B1, CDC2 kinase and CDC25C expression levels via Fbxw8
signaling. The results of the present study sufficiently
demonstrated that miR-3160-5P, at least in part, serves a role in
the suppression of cellular proliferation due to the direct
inhibition of Fbxw8 expression.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that miR-3160-5P is a
tumor suppressor in PCa. miR-3160-5P inhibited cell proliferation
by directly inhibiting the expression of Fbxw8 and indirectly
suppressing the expression of cyclin B1, CDC2 kinase and CDC25C.
miR-3160-5P is a potential therapeutic target for PCa. miR-3160-5P
effects on PCa proliferation in vivo, as well as the
underlying mechanisms, remain unknown. Future studies are required
to elucidate the effect of anti-miR-3160-5P therapy in the
clinic.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172417) to
Xiaoguang Yu.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL, LZ and XY conception and design, provision of
study material, data analysis and interpretation, collection and
assembly of data. PL, LZ, WS, ZY, HS, DL, RC, XZ: experiment
performing. PL and XY: manuscript writing.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:25–29. 2015. View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moore TH, King AJ, Evans M, Sharp D,
Persad R and Huntley AL: Supportive care for men with prostate
cancer: Why are the trials not working? A systematic review and
recommendations for future trials. Cancer Med. 4:1240–1251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hudson SV, O'Malley DM and Miller SM:
Achieving optimal delivery of follow-up care for prostate cancer
survivors: Improving patient outcomes. Patient Relat Outcome Meas.
6:75–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyamoto H, Messing EM and Chang C:
Androgen deprivation therapy for prostate cancer: Current status
and future prospects. Prostate. 61:332–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Helsen C, Van den Broeck T, Voet A,
Prekovic S, Van Poppel H, Joniau S and Claessens F: Androgen
receptor antagonists for prostate cancer therapy. Endocr Relat
Cancer. 21:T105–T118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu B, Huang Y, Niu X, Tao T, Jiang L, Tong
N, Chen S, Liu N, Zhu W and Chen M: Hsa-miR-146a-5p modulates
androgen-independent prostate cancer cells apoptosis by targeting
ROCK1. Prostate. 75:1896–1903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khanmi K, Ignacimuthu S and Paulraj MG:
MicroRNA in prostate cancer. Clin Chim Acta. 451:154–160. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang YX and Gao WQ: Roles of microRNAs
during prostatic tumorigenesis and tumor progression. Oncogene.
33:135–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hassan O, Ahmad A, Sethi S and Sarkar FH:
Recent updates on the role of microRNAs in prostate cancer. J
Hematol Oncol. 5:92012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu TI, Hsu CH, Lee KH, Lin JT, Chen CS,
Chang KC, Su CY, Hsiao M and Lu PJ: MicroRNA-18a is elevated in
prostate cancer and promotes tumorigenesis through suppressing STK4
in vitro and in vivo. Oncogenesis. 3:e992014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu
M, Tepper CG, Evans CP, Kung HJ and deVere White RW: An
androgen-regulated miRNA suppresses Bak1 expression and induces
androgen-independent growth of prostate cancer cells. Proc Natl
Acad Sci USA. 104:19983–19988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Li B, Li L and Wang T:
MicroRNA-497 suppresses proliferation and induces apoptosis in
prostate cancer cells. Asian Pac J Cancer Prev. 14:3499–3502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nazarov PV, Reinsbach SE, Muller A, Nicot
N, Philippidou D, Vallar L and Kreis S: Interplay of microRNAs,
transcription factors and target genes: Linking dynamic expression
changes to function. Nucleic Acids Res. 41:2817–2831. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cellini F, Morganti AG, Genovesi D,
Silvestris N and Valentini V: Role of microRNA in response to
ionizing radiations: Evidences and potential impact on clinical
practice for radiotherapy. Molecules. 19:5379–5401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dias DC, Dolios G, Wang R and Pan ZQ:
CUL7: A DOC domain-containing cullin selectively binds Skp1. Fbx29
to form an SCF-like complex. Proc Natl Acad Sci USA.
99:16601–16606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huber C, Dias-Santagata D, Glaser A,
O'Sullivan J, Brauner R, Wu K, Xu X, Pearce K, Wang R, Uzielli ML,
et al: Identification of mutations in CUL7 in 3-M syndrome. Nat
Genet. 37:1119–1124. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu X, Sarikas A, Dias-Santagata DC, Dolios
G, Lafontant PJ, Tsai SC, Zhu W, Nakajima H, Nakajima HO, Field LJ,
et al: The CUL7 E3 ubiquitin ligase targets insulin receptor
substrate 1 for ubiquitin-dependent degradation. Mol Cell.
30:403–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin P, Fu J, Zhao B, Lin F, Zou H, Liu L,
Zhu C, Wang H and Yu X: Fbxw8 is involved in the proliferation of
human choriocarcinoma JEG-3 cells. Mol Biol Rep. 38:1741–1747.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi D, Tan Z, Lu R, Yang W and Zhang Y:
MicroRNA-218 inhibits the proliferation of human choriocarcinoma
JEG-3 cell line by targeting Fbxw8. Biochem Biophys Res Commun.
450:1241–1246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ,
Lee KH, Yeh SD, Hong TM and Chen YL: MicroRNA-320 suppresses the
stem cell-like characteristics of prostate cancer cells by
downregulating the Wnt/beta-catenin signaling pathway.
Carcinogenesis. 34:530–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu C, Kelnar K, Vlassov AV, Brown D, Wang
J and Tang DG: Distinct microRNA expression profiles in prostate
cancer stem/progenitor cells and tumor-suppressive functions of
let-7. Cancer Res. 72:3393–3404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin M, Zhang T, Liu C, Badeaux MA, Liu B,
Liu R, Jeter C, Chen X, Vlassov AV and Tang DG: miRNA-128
suppresses prostate cancer by inhibiting BMI-1 to inhibit
tumor-initiating cells. Cancer Res. 74:4183–4195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurisetty VV, Lakshmanaswamy R and
Damodaran C: Pathogenic and therapeutic role of miRNAs in breast
cancer. Front Biosci (Landmark Ed). 19:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okabe H, Lee SH, Phuchareon J, Albertson
DG, McCormick F and Tetsu O: A critical role for FBXW8 and MAPK in
cyclin D1 degradation and cancer cell proliferation. PLoS One.
1:e1282006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Chen Y, Lin P, Li L, Zhou G, Liu
G, Logsdon C, Jin J, Abbruzzese JL, Tan TH and Wang H: The
CUL7/F-box and WD repeat domain containing 8 (CUL7/Fbxw8) ubiquitin
ligase promotes degradation of hematopoietic progenitor kinase 1. J
Biol Chem. 289:4009–4017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsunematsu R, Nishiyama M, Kotoshiba S,
Saiga T, Kamura T and Nakayama KI: Fbxw8 is essential for Cul1-Cul7
complex formation and for placental development. Mol Cell Biol.
26:6157–6169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Molinari M: Cell cycle checkpoints and
their inactivation in human cancer. Cell Prolif. 33:261–274. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hartwell LH and Weinert TA: Checkpoints:
Controls that ensure the order of cell cycle events. Science.
246:629–634. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiang NJ, Lin CI, Liou JP, Kuo CC, Chang
CY, Chen LT and Chang JY: A novel synthetic microtubule inhibitor,
MPT0B214 exhibits antitumor activity in human tumor cells through
mitochondria-dependent intrinsic pathway. PLoS One. 8:e589532013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
King KL and Cidlowski JA: Cell cycle and
apoptosis: Common pathways to life and death. J Cell Biochem.
58:175–180. 1995. View Article : Google Scholar : PubMed/NCBI
|