Introduction
Endometrial cancer (EC) is the fourth most common
cancer in females, behind breast, colorectal and lung cancer
(1). EC can be classified into type I
and type II based on etiology, clinical behavior and pathological
characteristics (2). Type I is
estrogen-dependent endometrioid endometrial carcinoma (EEC), while
type II is non-EEC (2). EECs account
for 70–80% of ECs, and are associated with unopposed estrogen
stimulation (2). EC is often
diagnosed in its early stages due to early clinical signs,
including abnormal uterine bleeding and vaginal discharge (3). For patients in early stage EC, surgery
is the best choice of treatment and the 5-year survival rate is
~96% (3). However, a number of
patients with advanced stage EC develop abdominal or pelvic pain,
abdominal distension, early satiety or change in bowel or bladder
function, and the survival rate markedly decreases (3). Although these patients in advanced
stages are traditionally treated using chemotherapy, radiotherapy
and hormonal therapy, there are still numerous patients who are
less sensitive to present therapy. Therefore, the identification of
novel molecular biomarkers may be helpful for the early diagnosis
and treatment of patients with EC.
Programmed cell death 4 (PDCD4) was first identified
as a gene upregulated following initiation of apoptosis (4). Subsequently, it has been identified as a
novel tumor suppressor gene that performs an important role in
inhibiting tumorigenesis and tumor progression at the
transcriptional and translational levels (5,6). Several
studies have demonstrated that PDCD4 affects the transcription of
specific genes by modulating the activities of certain
transcription factors, including c-Jun (7), Sp1 and p53 (8,9). PDCD4
suppresses protein translation in two different ways: The
eIF4A-dependent mechanism (PDCD4 interacts with the eukaryotic
translation initiation factor eIF4A to inhibit the RNA helicase
activity of eIF4A) (10), and the
eIF4A-independent mechanism [PDCD4 directly interacts with poly
(A)-binding protein through the RNA binding domains and regulates
protein translation] (11). PDCD4 is
expressed ubiquitously in normal tissues, including liver (12), kidney and brain (13,14), but
downregulated or lost in various human tumors, including lung
(15), colorectal (16), breast and ovarian cancers (17,18).
However, the expression of PDCD4 in patients with EEC and the
clinicopathological significance remain unclear.
The present study aimed to detect the expression
status of PDCD4 in EEC, the most common type of EC (2), and analyze the association between PDCD4
expression and clinicopathological parameters of patients with
EEC.
Materials and methods
Samples collection
A total of 15 frozen, fresh EEC tissues and 52
formalin-fixed, paraffin-embedded EEC specimens, as well as 18
frozen, fresh control endometrium and 70 formalin-fixed,
paraffin-embedded control specimens (including in the 36
proliferative phase and 34 from the secretory phase) were obtained
from The Department of Gynecology and Obstetrics, Jinan Central
Hospital affiliated to Shandong University (Shandong, China)
between February 2012 and December 2015. A total of 67 patients
with EEC, with mean age of 56.2, were confirmed by histological
examination of biopsies obtained during surgery. None of the
patients had received neoadjuvant chemotherapy or hormone treatment
prior to the operation. Endometrial tissues were categorized as
proliferative or secretory according to the patient's menstrual
history, which was confirmed by histopathological examination. All
slides were evaluated by two gynecological pathologists to confirm
the histological type, International Federation of Gynecology and
Obstetrics (FIGO) stage, the histological grade, the depth of
myometrial invasion and the estrogen receptor (ER) and progesterone
receptor (PR) expression status. FIGO stage and histological grade
were evaluated as described in previous reports (19,20). The
ER and PR staining was performed and evaluated as described
previously (21). The above
clinicopathological parameters of patients with EEC were obtained
from Jinan Central Hospital affiliated to Shandong University and
outlined in Table I. The present
study was approved by the Institutional Ethics Committee of
Shandong University and all patients provided written, informed
consent.
 | Table I.Associations of PDCD4 expression with
clinicopathological parameters in endometrioid endometrial
carcinoma. |
Table I.
Associations of PDCD4 expression with
clinicopathological parameters in endometrioid endometrial
carcinoma.
|
| PDCD4
expression |
|
|---|
|
|
|
|
|---|
| Parameters | Total patients,
n | Negative-weak, n
(%) | Moderate-strong, n
(%) | P-value
(χ2 test) |
|---|
| Age |
|
|
|
|
| ≤55
years | 20 | 9 (45) | 11 (55) | 0.0637 |
| >55
years | 31 | 22 (71) | 9 (29) |
|
| Myometrial
invasion |
|
|
|
|
|
≤50% | 32 | 17 (53) | 15 (47) | 0.146 |
|
>50% | 19 | 14 (74) | 5 (26) |
|
| FIGO stage |
|
|
|
|
| IA | 33 | 19 (59) | 14 (41) | 0.5251 |
|
IB-III | 18 | 12 (67) | 6 (33) |
|
| Histological
grade |
|
|
|
|
| Grade
1 | 14 | 3 (21) | 11 (79) | 0.0013 |
| Grade
2/3 | 38 | 27 (71) | 11 (29) |
|
| Estrogen
receptor |
|
|
|
|
|
Negative-weak | 12 | 5 (42) | 7 (58) | 0.1167 |
|
Moderate-strong | 31 | 21 (68) | 10 (32) |
|
| Progesterone
receptor |
|
|
|
|
|
Negative-weak | 12 | 7 (58) | 5 (42) | 0.7957 |
|
Moderate-strong | 37 | 20 (54) | 17 (46) |
|
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Tiangen Biotech Co., Ltd., Beijing,
China) was used to isolate total RNA of frozen fresh EEC tissues
and control endometrium. RNA was reverse transcribed to cDNA with
the FastQuant RT kit (Tiangen Biotech Co., Ltd.) according to the
manufacturer's protocol. RT-qPCR was used to detect the mRNA
expression of PDCD4 in reaction mixture containing cDNA, UltraSYBR
Mixture (CWBIO, Beijing, China) and specific primers using a
Bio-Rad CFX96 real-time PCR instrument (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The PDCD4 primers were: forward,
5′-ACAGGTGTATGATGTGGAGGA-3′ and reverse,
5′-TTCTCAAATGCCAGTCTTTCATCCAA-3′. GAPDH was used as an internal
control. The specific primers for GAPDH were: forward,
5′-AACGGATTTGGTCGTATTGGG-3′ and reverse,
5′-CCTGGAAGATGGTGATGGGAT-3′. The reaction mixture was denatured at
95°C for 10 min, followed by 39 cycles of 95°C for 15 sec, 60°C for
1 min and 65°C for 5 sec to stop the reaction. Each sample was
performed in triplicate and the 2−ΔΔCq method (22) was used to analyze the results.
Western blotting
Frozen, fresh EEC and control endometrium specimens
were ground mechanically, and total protein was extracted using
radioimmunoprecipitation assay buffer containing 1%
phenylmethanesulfonyl fluoride and 0.5% phosphatase inhibitor
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Following
centrifugation at 12,000 × g for 30 min at 4°C, the protein
concentration of supernatant was measured with a bicinchoninic acid
kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Protein (30 µg per sample) was separated
with 10% SDS-PAGE and transferred to a polyvinylidene fluoride
membrane. The membrane was blocked with 5% bovine serum albumin
(Gibco; Thermo Fisher Scientific, Inc.) and incubated with a rabbit
anti-human PDCD4 monoclonal antibody (dilution, 1:1,000; cat. no.,
9535; Cell Signaling Technology, Inc. Danvers, MA, USA) and a
rabbit anti-human β-actin polyclonal antibody (dilution, 1:1,000;
cat. no., 20536-1-AP; Proteintech, Wuhan, China) at 4°C overnight.
Following 3 washes with phosphate-buffered saline, the membrane was
incubated with goat anti-rabbit IgG conjugated with horseradish
peroxidase (HRP; dilution, 1:2,000; cat. no., ZB-2301, ZSJQB Co.
Ltd., Beijing, China) for 1 h at room temperature. The protein
bands were detected using the enhanced chemiluminescence method
with the reagents immobilon western chemilum HRP substrate solution
(cat. nos., 16267A4; EMD Millipore, Billerica, MA, USA) and
immobilon western chemilum HRP substrate Luminol Peroxide solution
(cat. nos., 16267B4; EMD Millipore), according to the
manufacturer's protocol.
Immunohistochemistry (IHC)
The 4-µm sections from 4% formalin-fixed,
paraffin-embedded EEC specimens and control endometrium were
deparaffinized and rehydrated with an ethanol gradient (100, 100,
95, 85, 70 and 60%), and then antigen retrieval was performed by
microwave heating. The slides were blocked using 3%
H2O2, and 10% normal goat serum (Beijing
Dingguo Changsheng Biotechology Co., Ltd., Beijing, China) for 15
min at 37°C was used to block non-specific binding. The sections
were then incubated with a rabbit anti-human PDCD4 monoclonal
antibody (dilution, 1:100; cat. no., 9535; Cell Signaling
Technology, Inc.) overnight in a wet chamber at 4°C. The slides
were incubated for 30 min at 37°C with HRP-conjugated goat
anti-rabbit IgG (dilution, 1:100; cat. no., ZB-2301; ZSJQB Co.,
Ltd.), and visualized using a diaminobenzidine (DAB) kit (ZSJQB
Co., Ltd.) according to the manufacturer's protocol, followed by
counterstaining with hematoxylin.
The results of IHC staining were scored by
evaluating the extent and intensity of staining in 5 fields of view
using light microscopy (Olympus Co. Tokyo, Japan) at ×100, ×200 and
×400 magnification, using a previously described immunoreactive
scoring method (23). The staining
intensity was divided into four grades: -, score 0; +, score 1; ++,
score 2; and +++, score 3. The positive expression area was also
classified into four categories: -, <1%, score 0; +, 1–33%,
score 1; ++, 34–66%, score 2; and +++, 67–100%, score 3. The sum of
intensity and area scores was used as the final PDCD4 staining
score. The expression of PDCD4 was defined as follows: No
expression, total score 0; weak expression, total score 1 and 2;
moderate expression, total score 3 and 4; strong expression, total
score 5 and 6. All slides were scored by two independent
pathologists who were blind to the clinical data of patients. When
there were discrepancies between two pathologists, the mean score
was used.
Isolation and culture of endometrial
glandular epithelial and stromal cells
Methods for the isolation and culture of endometrial
cells were based on methods in previously published reports
(24). Briefly, secretory phase
control endometrium samples were extracted from the previously
described patients. The samples were placed immediately in a
mixture of Dulbecco's modified Eagle's medium/F12 (1:1)
supplemented with 100 U/ml of penicillin and 100 U/ml of
streptomycin (all HyClone; GE Healthcare Life Sciences, Logan, UT,
USA). The tissues were then minced into small pieces and digested
with 0.25% collagenase type IA (Sigma-Aldrich; Merck KgaA,
Darmstadt, Germany) in an agitating water bath for 50 min at 37°C.
The pre-warmed DMEM/F12 medium containing 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) was used to stop the
collagenase activity. The cell suspension was firstly filtered
through a 154-mm monofilament nylon mesh and then a 38.5-mm
monofilament nylon mesh. To obtain stromal cells, the cell
suspension was collected and centrifuged at 800 × g for 10 min at
room temperature, the pellet was resuspended in medium and
incubated in 24-well plates with coverslips for 2 h at 37°C in 95%
air and 5% CO2. The medium was then replaced with fresh
medium in order to purify the stromal cells. To obtain glandular
epithelial cells, the 38.5-mm monofilament was washed thoroughly
upside down with medium. The medium was collected and centrifuged
at 800 × g for 10 min at room temperature, the pellet was
resuspended in medium and incubated in 24-well plates with
coverslips at 37°C in 5% CO2. The cultured endometrial
glandular epithelial cells and stromal cells were characterized by
immunocytochemistry (ICC) with mouse anti-human vimentin and
cytokeratin antibodies (ZSJQB Co., Ltd. Beijing, China).
ICC
KLE cells (G3 endometrioid endometrial carcinoma
cells) were purchased from China Center for Type Culture Collection
(Wuhan, China). The isolated glandular epithelial, stromal and KLE
cells were cultured at 37°C overnight in 24-well plates with
coverslips and used for the following ICC analysis. The cells on
coverslips were fixed with 4% paraformaldehyde for 30 min at room
temperature, and then blocked with 0.3% H2O2
for 10 min at room temperature. The coverslips were incubated with
rabbit anti-human PDCD4 (dilution, 1:100; cat. no., 9535; Cell
Signaling Technology, Inc.), mouse anti-human vimentin (dilution,
1:100; cat. no., ZM-0260) and anti-cytokeratin (dilution, 1:100;
cat. no., ZM-0071; both ZSJQB Co., Ltd.) antibodies for 2 h at
37°C. The coverslips were then incubated with a HRP-conjugated goat
anti-rabbit secondary antibody (dilution, 1:100; cat. no., ZB-2301;
ZSJQB Co., Ltd.) and HRP-conjugated goat anti-mouse secondary
antibody (dilution, 1:100; cat. no., ZB-2305; ZSJQB Co., Ltd.), and
then visualized using the aforementioned DAB kit according to the
manufacturer's protocol, followed by counterstaining with
hematoxylin. The slides were observed under a light microscope
(Olympus Corporation, Tokyo, Japan) with ×400 magnification.
Statistical analysis
Unpaired t-tests were performed to analyze the
results of RT-qPCR, and the data are presented as the mean ±
standard error of mean. The comparison of PDCD4 protein expression
between the EEC group and the control group was assessed using a
Mann-Whitney test; the result was reported as the median. The
χ2 test was used to analyze the data of
immunohistochemical staining and the association of PDCD4 protein
expression with clinicopathological parameters. GraphPadPrism 5
(GraphPad Software, Inc., La Jolla, CA, USA) was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of PDCD4 mRNA and protein
in the EEC samples and control endometrium detected by RT-qPCR and
western blotting
To evaluate the expression levels of PDCD4 in EEC
tissues, frozen fresh EEC samples and control endometrium were
firstly collected, and PDCD4 mRNA expression was detected by
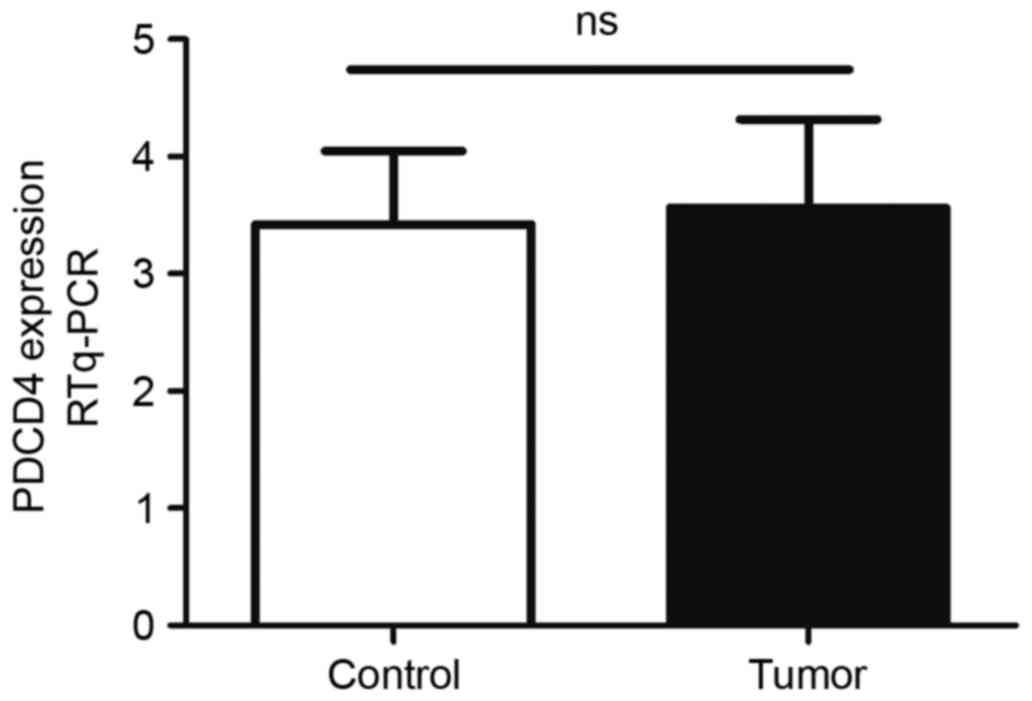
RT-qPCR. As shown in Fig. 1, there
was no significant difference in PDCD4 mRNA between the control and
EEC group (P=0.88). The expression of PDCD4 protein in these
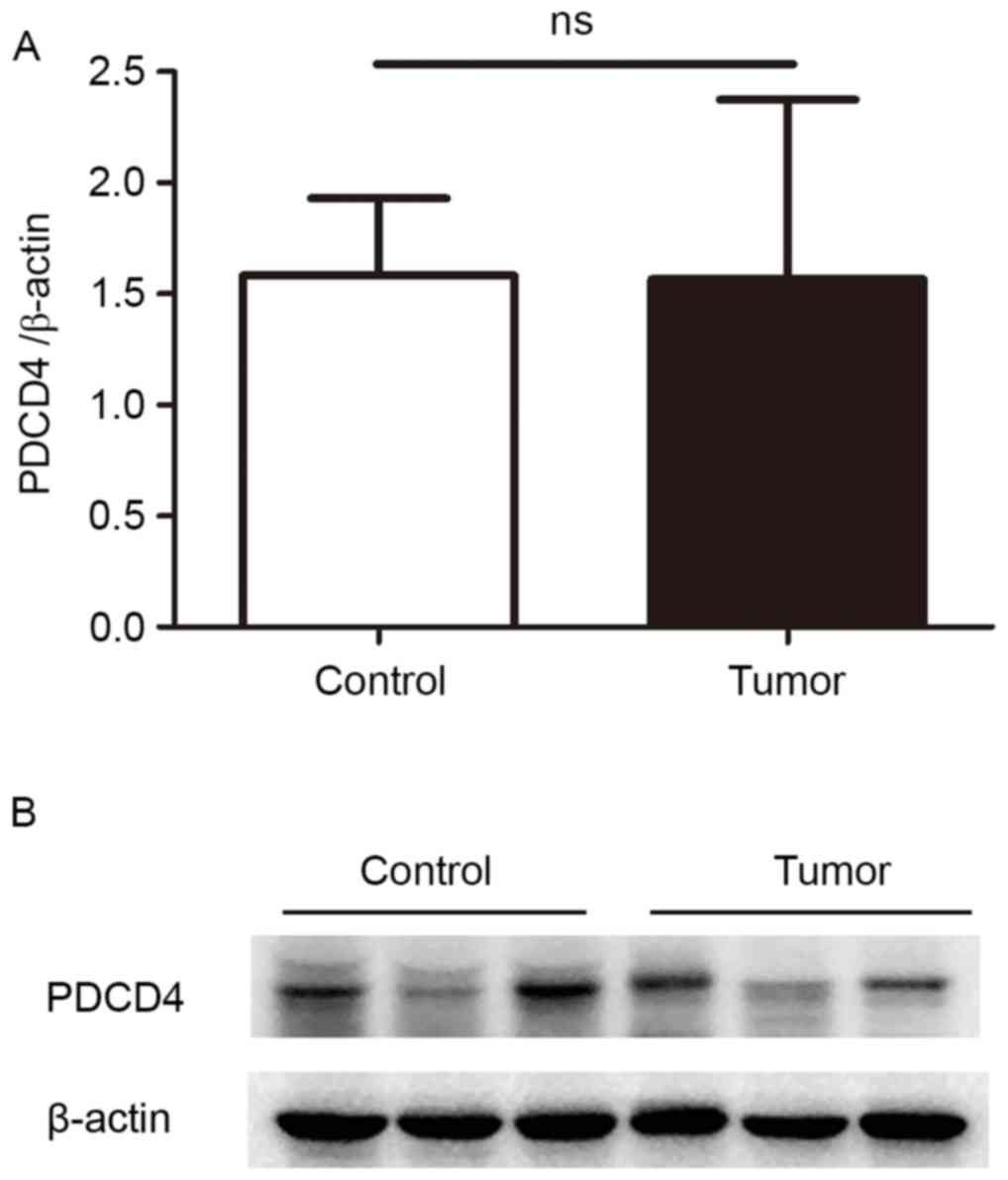
samples was then detected by western blotting. The data
demonstrated that, in the patients with EEC, PDCD4 protein
expression remained the same as the control (P=0.10; Fig. 2), which is consistent with the
findings of RT-qPCR.
Expression of PDCD4 protein in the EEC
samples and control endometrium detected by IHC staining
To confirm the location and levels of PDCD4 protein
in the EEC tissues and control endometrium, formalin-fixed,
paraffin-embedded EEC samples and control endometrium were
recruited, and the expression of PDCD4 was detected by IHC.
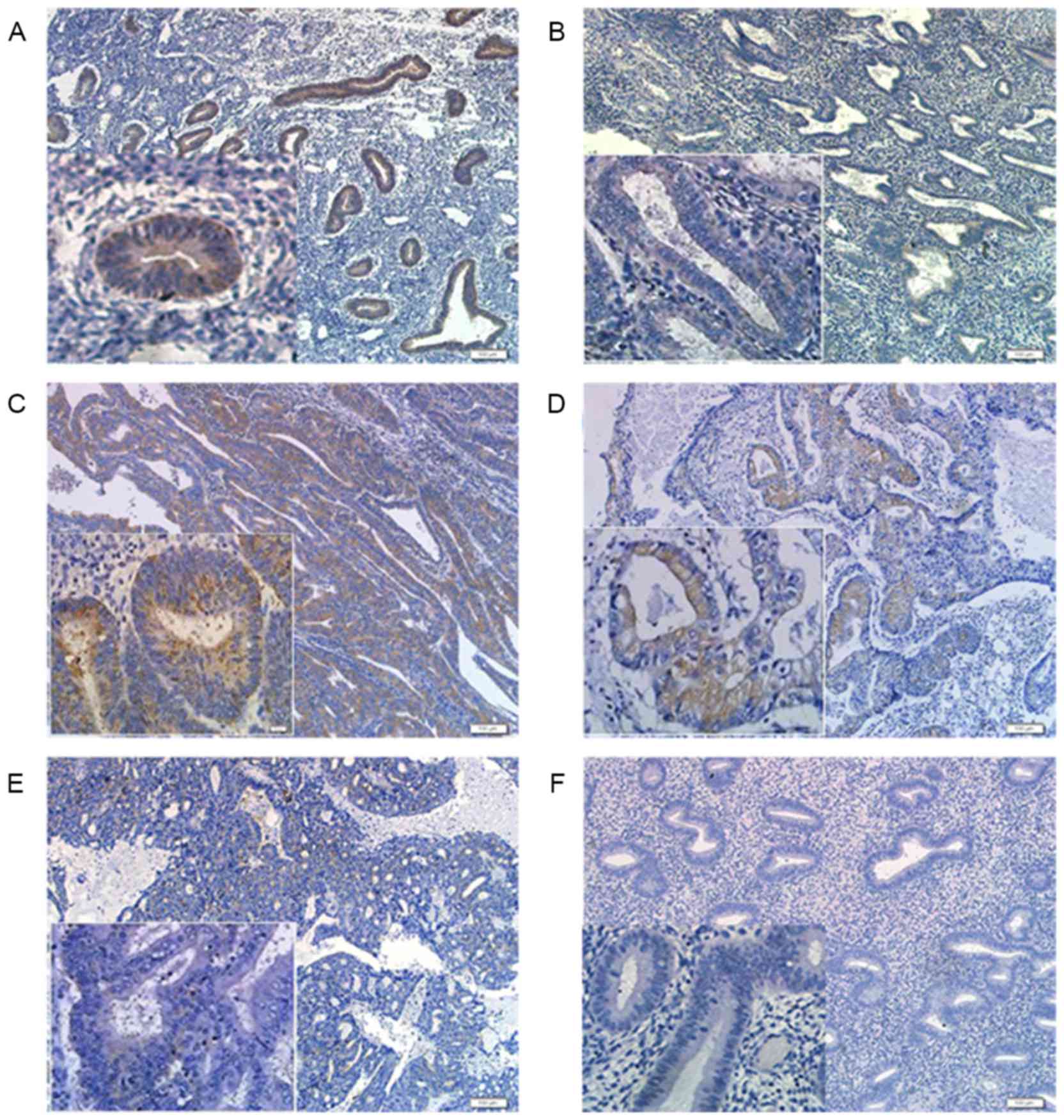
The results showed that PDCD4 positive staining was
mainly located in the cytoplasm of endometrial glandular epithelial
cells, but no immunoreactivity of PDCD4 was observed in endometrial
stromal cells (Fig. 3A and B). To
explore whether the expression of PDCD4 is affected by ovarian
steroid, differences in PDCD4 expression were compared between
proliferative and secretory phase control endometrium, and it was
found that the staining index of PDCD4 in the proliferative phase
(n=36) was significantly increased compared with that in the
secretory phase (n=34) of control endometrium (P<0.001; Fig. 3A and B).
In the EEC samples, PDCD4 staining was also mainly
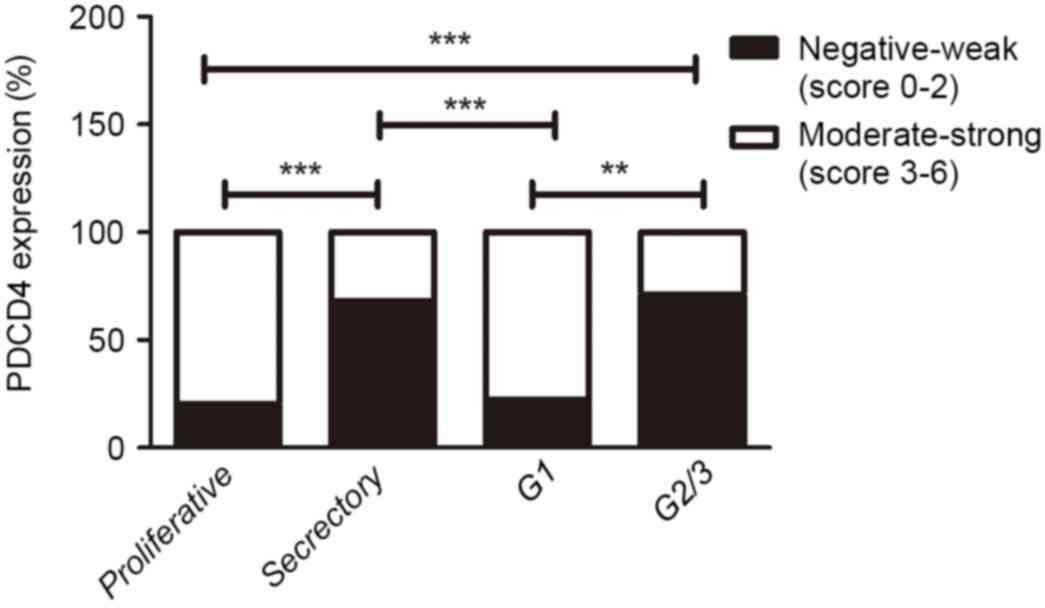
present in the cytoplasm of EEC cells (Fig. 3C-E). Furthermore, there was
significantly decreased PDCD4 expression in grade (G) 2/3 EEC
tissues compared with the proliferative phase of control
endometrium (P<0.001; Fig. 4).
Additionally, the PDCD4 expression in G1 EEC samples was increased
compared with the secretory phase of control endometrium. However,
no statistically significant difference in PDCD4 staining was
observed between the proliferative phase of control endometrium and
G1 EEC tissues, as well as between the secretory phase of control
endometrium and G2/3 EEC tissues (Fig.
4). These results suggested that the expression of PDCD4 could
vary in a cyclic fashion in control endometrium and PDCD4 protein
was downregulated in the G2/3 EEC tissues.
Expression of PDCD4 protein in control
glandular epithelial cells and KLE cells detected by ICC
staining
To verify the location of the PDCD4 protein and
differences in PDCD4 expression between the control glandular
epithelial cells and endometrial carcinomas cells, endometrial
glandular epithelial cells and stromal cells were isolated and
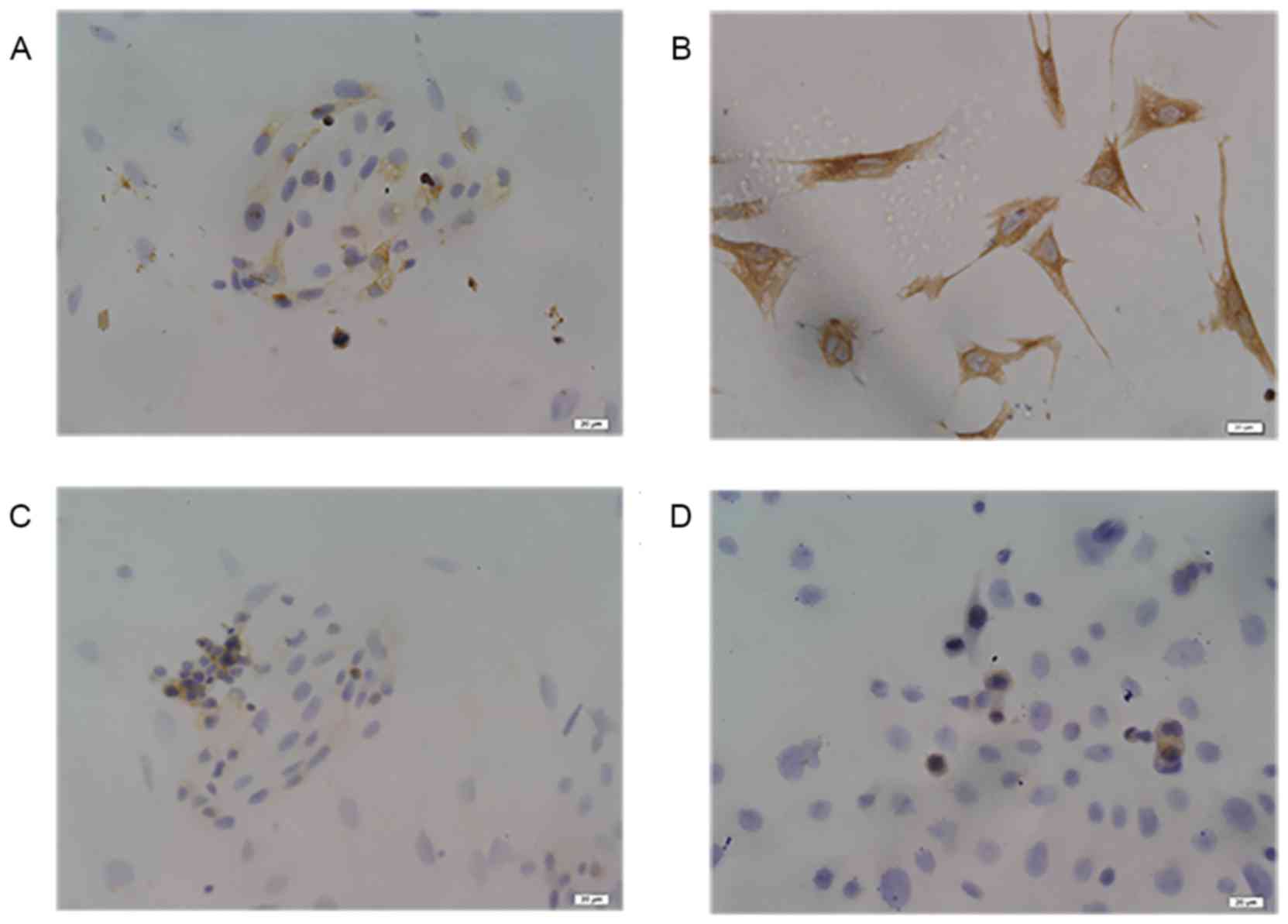
cultured. As shown in Fig. 5A and B,
glandular epithelial cells and stromal cells were respectively
characterized by ICC with mouse anti-human cytokeratin and vimentin
antibodies. In addition, the results from PDCD4 staining showed
that PDCD4 immunostaining in control glandular epithelial and KLE
cells was mostly localized in the cytoplasm (Fig. 5C and D). KLE cells exhibited weak
PDCD4 staining, which was similar to the secretory phase of control
endometrial glandular epithelial cells.
Association analysis between PDCD4
expression in EEC samples and clinicopathological parameters of
patients
The association between PDCD4 protein expression
detected by IHC and clinicopathological parameters was analyzed. No
significant associations were observed between PDCD4 expression and
age, the depth of myometrial invasion, FIGO stage, ER or PR.
However, PDCD4 expression was significantly associated with the
tumor grade; the PDCD4 level in G1 EEC tissues was higher than that
in the G2/3 EEC group (P<0.01; Fig.
4; Table I), which suggests that
PDCD4 expression may be associated with the malignant progression
of patients with EEC.
Discussion
In the majority of human cancers, including
colorectal (25), gastric (26), glioma and ovarian cancers (18,27), PDCD4
mRNA and protein expression is lost or reduced compared with normal
tissues. However, in lung squamous cell carcinoma samples, PDCD4
protein and mRNA level alterations do not correlate; the mRNA is
low but the protein is unchanged or even upregulated (28). In addition, PDCD4 protein was also
found to be highly expressed in bladder and breast carcinoma
compared with the corresponding normal tissues (17,29). These
data demonstrated that PDCD4 expression levels are varied in
different tissues, which indicates that PDCD4 has tissue-specific
roles. In the present study, it was observed that PDCD4 protein
expression in EEC tissues remain the same as the control. The
expression of PDCD4 mRNA in EEC samples was slightly increased
compared with the control group, however no significant difference
was observed. Torres et al (30) evaluated the expression of PDCD4 in 20
endometrial cancer and 10 normal endometrium samples by qPCR, and
the results showed that there were no significant differences in
the mRNA expression level of PDCD4 between endometrial cancer and
control groups. However, due to the small number of fresh frozen
samples, whether the expression of PDCD4 mRNA and protein in EEC
tissues was similar to that in the control endometrium remains
unclear.
In the present study, 70 formalin-fixed,
paraffin-embedded control specimens (including 36 proliferative and
34 secretary phase of endometrium) and 52 EEC samples were
collected. The expression of PDCD4 in normal endometrium was
detected by IHC, and the staining index of PDCD4 in the
proliferative phase was found to be significantly higher than that
in the secretory phase, which suggests the expression of PDCD4
could vary in a cyclic fashion. It has been reported that the
expression of some certain proteins is different in the
proliferative phase or the secretory phase of normal endometrium
(31,32). Chen et al (31) found that the staining index of
epithelial Musashi-1 in the proliferative phase endometrium was
significantly increased compared with the secretory phase
endometrium. In addition, COX-2 expression suffers variations
during the menstrual cycle in response to the fluctuating levels of
estrogen and progesterone in the normal cycling endometrium
(32). Estrogen was found to be a
potent stimuli of COX-2 expression, whereas progesterone may have
the opposite effect, diminishing the expression of COX-2 in the
glandular epithelium during the secretory phase (32,33). These
data suggested that the expression of PDCD4 may also be affected by
the fluctuating levels of estrogen and progesterone.
The expression status of PDCD4 in EEC tissues was
investigated by IHC, and the association between PDCD4 expression
and clinicopathological parameters was analyzed. The present
results indicated PDCD4 expression in G2/3 EEC was decreased
compared with the proliferative phase of control endometrium.
However, no marked difference was observed between G2/3 EEC and the
secretory phase control endometrium. ICC results also confirmed
that PDCD4 staining in the KLE cells (high grade endometrial
adenocarcinoma cells) expressed similar weak PDCD4 protein compared
with the secretory phase of control endometrial glandular
epithelial cells. A χ2 test showed that the level of
PDCD4 had no significant association with age, the depth of
myometrial invasion, tumor stage, ER and PR, while PDCD4 expression
was associated with the histological grade of tumor. The level of
PDCD4 in G1 EEC group was higher compared with G2/3 EEC group.
Similarly, in ovarian cancer (34),
renal cell cancer (13) and
pancreatic cancer (35), PDCD4
expression was significantly decreased in G2/3 cancers compared
with the G1 cancers. However, Gao et al found that the loss
of PDCD4 in gliomas had no significant association with tumor grade
or histological type (27).
Therefore, in certain types of cancer, including EEC, PDCD4
expression decreases with the decline of differentiation degree,
which suggests downregulation of PDCD4 may serve an important role
in de-differentiation and progression of certain cancers, and PDCD4
may be an indicator of tumor grade in specific cancers. In
addition, PDCD4 protein expression in the proliferative phase of
control endometrium and G1 EEC tissues was increased compared with
the secretory phase of control endometrium and G2/3 EEC samples,
which may be why no significant difference was observed between
control endometrium and EEC tissues detected by RT-qPCR and western
blotting.
To confirm the subcellular localization of PDCD4,
PDCD4 expression sites were observed in control endometrial
glandular epithelial and EEC cells detected by IHC and ICC.
Notably, PDCD4 positive staining mainly existed in the cytoplasm of
these cells, while no evident staining was observed in the nucleus.
The present results are consistent with the study by Zhang et
al (36), which identified that
PDCD4 protein was localized in the cytoplasm of adjacent
non-cancerous hepatocytes and hepatocellular carcinoma cells.
However, Chen et al (15)
reported that PDCD4 staining was localized in the nuclei and
cytoplasm of lung cancer cells. These discrepancies may be due to
the fact that PDCD4 could shuttle between nucleus and cytoplasm
(37). It has been reported that a
nuclear translocation of PDCD4 is controlled by protein kinase
B/Akt-mediated PDCD4 phosphorylation at Ser67 and Ser457 (38). Another explanation for different
subcellular localization may be a cell-type specific action of
PDCD4.
In conclusion, the present study demonstrated that
PDCD4 protein expression was downregulated in G2/3 EEC compared
with the proliferative phase of normal endometrium and G1 of EEC.
These results indicated that PDCD4 serves an important role in
determining the tumor grade of EEC. With respect to these findings,
PDCD4 may be a valuable indicator of the degree of tumor malignancy
in patients with EEC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National 973
Basic Research Program of China (grant no. 2011CB503906), the
National Natural Science Foundation of China (grant nos. 81471437
and 31470856) and the Natural Science Foundation of Shandong (grant
nos. ZR2012HM091 and ZR2013HM105).
Availability of data and materials
The datasets generated during the present study are
available upon reasonable request from the corresponding
author.
Author's contributions
ZW designed the project and conducted the
experimental study. XW made substantial contributions to
experimental design and implementation, drafted the manuscript and
revised it critically for intellectual content. YanL collected the
samples, performed the experiments and wrote the manuscript. HS
participated in designing the experiments, drafting and revising
the manuscript. HM collected the samples. MG and XT were involved
in performing experiments. YuL and YaL were involved in data
collection and statistical analysis. GMM and LZ were involved in
made substantial contributions to analysis and interpretation of
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All the subjects included in the study signed
informed consents. The Institutional Ethics Committee of Shandong
University approved the present study.
Consent for publication
The patients provided written informed consent for
the publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shevra CR, Ghosh A and Kumar M: Cyclin D1
and Ki-67 expression in normal, hyperplastic and neoplastic
endometrium. J Postgrad Med. 61:15–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llauradó M, Ruiz A, Majem B, Ertekin T,
Colás E, Pedrola N, Devis L, Rigau M, Sequeiros T, Montes M, et al:
Molecular bases of endometrial cancer: New roles for new actors in
the diagnosis and the therapy of the disease. Mol Cell Endocrinol.
358:244–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shibahara K, Asano M, Ishida Y, Aoki T,
Koike T and Honjo T: Isolation of a novel mouse gene MA-3 that is
induced upon programmed cell death. Gene. 166:297–301. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jansen AP, Camalier CE and Colburn NH:
Epidermal expression of the translation inhibitor programmed cell
death 4 suppresses tumorigenesis. Cancer Res. 65:6034–6041. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allgayer H: Pdcd4, a colon cancer
prognostic that is regulated by a microRNA. Crit Rev Oncol Hematol.
73:185–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bitomsky N, Böhm M and Klempnauer KH:
Transformation suppressor protein Pdcd4 interferes with
JNK-mediated phosphorylation of c-Jun and recruitment of the
coactivator p300 by c-Jun. Oncogene. 23:7484–7493. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leupold JH, Yang HS, Colburn NH, Asangani
I, Post S and Allgayer H: Tumor suppressor Pdcd4 inhibits
invasion/intravasation and regulates urokinase receptor (u-PAR)
gene expression via Sp-transcription factors. Oncogene.
26:4550–4562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wedeken L, Singh P and Klempnauer KH:
Tumor suppressor protein Pdcd4 inhibits translation of p53 mRNA. J
Biol Chem. 286:42855–42862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang HS, Jansen AP, Komar AA, Zheng X,
Merrick WC, Costes S, Lockett SJ, Sonenberg N and Colburn NH: The
transformation suppressor Pdcd4 is a novel eukaryotic translation
initiation factor 4A binding protein that inhibits translation. Mol
Cell Biol. 23:26–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fehler O, Singh P, Haas A, Ulrich D,
Müller JP, Ohnheiser J and Klempnauer KH: An evolutionarily
conserved interaction of tumor suppressor protein Pdcd4 with the
poly(A)-binding protein contributes to translation suppression by
Pdcd4. Nucleic Acids Res. 42:11107–11118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Ozaki I, Mizuta T, Hamajima H,
Yasutake T, Eguchi Y, Ideguchi H, Yamamoto K and Matsuhashi S:
Involvement of programmed cell death 4 in transforming growth
factor-beta1-induced apoptosis in human hepatocellular carcinoma.
Oncogene. 25:6101–6112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Xin S, Yang D, Li X, He Z, Che X,
Wang J, Chen F, Wang X and Song X: Down-regulation of PDCD4
expression is an independent predictor of poor prognosis in human
renal cell carcinoma patients. J Cancer Res Clin Oncol.
138:529–535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao F, Wang X, Zhu F, Wang Q, Zhang X, Guo
C, Zhou C, Ma C, Sun W, Zhang Y, et al: PDCD4 gene silencing in
gliomas is associated with 5′CpG island methylation and
unfavourable prognosis. J Cell Mol Med. 13:4257–4267. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Knösel T, Kristiansen G, Pietas A,
Garber ME, Matsuhashi S, Ozaki I and Petersen I: Loss of PDCD4
expression in human lung cancer correlates with tumour progression
and prognosis. J Pathol. 200:640–646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mudduluru G, Medved F, Grobholz R, Jost C,
Gruber A, Leupold JH, Post S, Jansen A, Colburn NH and Allgayer H:
Loss of programmed cell death 4 expression marks adenoma-carcinoma
transition, correlates inversely with phosphorylated protein kinase
B, and is an independent prognostic factor in resected colorectal
cancer. Cancer. 110:1697–1707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Afonja O, Juste D, Das S, Matsuhashi S and
Samuels HH: Induction of PDCD4 tumor suppressor gene expression by
RAR agonists, antiestrogen and HER-2/neu antagonist in breast
cancer cells. Evidence for a role in apoptosis. Oncogene.
23:8135–8145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei ZT, Zhang X, Wang XY, Gao F, Zhou CJ,
Zhu FL, Wang Q, Gao Q, Ma CH, Sun WS, et al: PDCD4 inhibits the
malignant phenotype of ovarian cancer cells. Cancer Sci.
100:1408–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang T, Qiu H, Bao W, Li B, Lu C, Du G,
Luo X, Wang L and Wan X: Epigenetic inactivation of EFEMP1 is
associated with tumor suppressive function in endometrial
carcinoma. PLoS One. 8:e674582013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walentowicz-Sadlecka M, Sadlecki P, Bodnar
M, Marszalek A, Walentowicz P, Sokup A, Wilińska-Jankowska A and
Grabiec M: Stromal derived factor-1 (SDF-1) and its receptors CXCR4
and CXCR7 in endometrial cancer patients. PLoS One. 9:e846292014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeon YT, Park IA, Kim YB, Kim JW, Park NH,
Kang SB, Lee HP and Song YS: Steroid receptor expressions in
endometrial cancer: Clinical significance and epidemiological
implication. Cancer Lett. 239:198–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
24
|
Zhang H, Li M, Zheng X, Sun Y, Wen Z and
Zhao X: Endometriotic stromal cells lose the ability to regulate
cell-survival signaling in endometrial epithelial cells in vitro.
Mol Hum Reprod. 15:653–663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang KH, Miller N, Kheirelseid EA,
Ingoldsby H, Hennessy E, Curran CE, Curran S, Smith MJ, Regan M,
McAnena OJ and Kerin MJ: MicroRNA-21 and PDCD4 expression in
colorectal cancer. Eur J Surg Oncol. 37:597–603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu W, Gao T, Shen J, Sun Y, Zheng X, Wang
J, Ma J, Hu XY, Li J and Hu MJ: MicroRNA-183 inhibits apoptosis and
promotes proliferation and invasion of gastric cancer cells by
targeting PDCD4. Int J Clin Exp Med. 7:2519–2529. 2014.PubMed/NCBI
|
|
27
|
Gao F, Zhang P, Zhou C, Li J, Wang Q, Zhu
F, Ma C, Sun W and Zhang L: Frequent loss of PDCD4 expression in
human glioma: Possible role in the tumorigenesis of glioma. Oncol
Rep. 17:123–128. 2007.PubMed/NCBI
|
|
28
|
Kalinichenko SV, Kopantzev EP, Korobko EV,
Palgova IV, Zavalishina LE, Bateva MV, Petrov AN, Frank GA,
Sverdlov ED and Korobko IV: Pdcd4 protein and mRNA level
alterations do not correlate in human lung tumors. Lung Cancer.
62:173–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lei Y, Hu X, Li B, Peng M, Tong S, Zu X,
Wang Z, Qi L and Chen M: miR-150 modulates cisplatin
chemosensitivity and invasiveness of muscle-invasive bladder cancer
cells via targeting PDCD4 in vitro. Med Sci Monit. 20:1850–1857.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Torres A, Torres K, Paszkowski T, Radej S,
Staśkiewicz GJ, Ceccaroni M, Pesci A and Maciejewski R: Highly
increased maspin expression corresponds with up-regulation of
miR-21 in endometrial cancer: A preliminary report. Int J Gynecol
Cancer. 21:8–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen YZ, Wang JH, Yan J, Liang Y, Zhang XF
and Zhou F: Increased expression of the adult stem cell marker
Musashi-1 in the ectopic endometrium of adenomyosis does not
correlate with serum estradiol and progesterone levels. Eur J
Obstet Gynecol Reprod Biol. 173:88–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maia H Jr, Maltez A, Studard E, Zausner B,
Athayde C and Coutinho E: Effect of the menstrual cycle and oral
contraceptives on cyclooxygenase-2 expression in the endometrium.
Gynecol Endocrinol. 21:57–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bulun SE, Zeitoun KM, Takayama K and
Sasano H: Molecular basis for treating endometriosis with aromatase
inhibitors. Hum Reprod Update. 6:413–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Wei Z, Gao F, Zhang X, Zhou C, Zhu
F, Wang Q, Gao Q, Ma C, Sun W, et al: Expression and prognostic
significance of PDCD4 in human epithelial ovarian carcinoma.
Anticancer Res. 28:2991–2996. 2008.PubMed/NCBI
|
|
35
|
Ma G, Zhang H, Dong M, Zheng X, Ozaki I,
Matsuhashi S and Guo K: Downregulation of programmed cell death 4
(PDCD4) in tumorigenesis and progression of human digestive tract
cancers. Tumour Biol. 34:3879–3885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang S, Li J, Jiang Y, Xu Y and Qin C:
Programmed cell death 4 (PDCD4) suppresses metastastic potential of
human hepatocellular carcinoma cells. J Exp Clin Cancer Res.
28:712009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Böhm M, Sawicka K, Siebrasse JP,
Brehmer-Fastnacht A, Peters R and Klempnauer KH: The transformation
suppressor protein Pdcd4 shuttles between nucleus and cytoplasm and
binds RNA. Oncogene. 22:4905–4910. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Palamarchuk A, Efanov A, Maximov V,
Aqeilan RI, Croce CM and Pekarsky Y: Akt phosphorylates and
regulates Pdcd4 tumor suppressor protein. Cancer Res.
65:11282–11286. 2005. View Article : Google Scholar : PubMed/NCBI
|