Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
prevalent malignancies in southern China and Southeast Asia
(1). Radiotherapy (RT) is one of the
most powerful, highly effective treatments for NPC (1,2). However,
resistance to radiation is the leading cause of treatment failure
(2). Therefore, increasing the
radiosensitivity of NPC is important. The potential mechanism
underlying the radiosensitivity of NPC remains unclear. Therefore,
markers associated with radiosensitivity need to be examined, and
the molecular mechanisms of these markers need to be further
investigated.
The migration and invasion inhibitory protein (MIIP)
gene, also termed IIp45 gene, has a key role in tumorigenesis
(3–5).
MIIP gene, which is located in the chromosome 1p36 region and spans
12.6 kb of genomic DNA, inhibits the migration and invasion of
cells (3–6). The chromosome 1p36 region containing
MIIP is absent in a wide range of human cancer cases, including NPC
(3–13), but the role of MIIP in
radiosensitivity of NPC has not been studied.
DNA double-stranded breaks (DSBs) are the most
dangerous lesions caused by ionizing radiation (IR) as they
seriously threaten cell viability and genome stability. The
phosphorylation of H2AX is one of the earliest events that occur in
the chromatin surrounding DNA DSBs (14,15).
Through phosphorylation-dependent protein-protein interactions,
phosphorylated H2AX (γ-H2AX) recruits abundant DNA damage-response
(DDR) proteins to areas of damaged chromatin and initiates the DDR,
which includes DNA repair and cell cycle checkpoint (16). Apart from activating the checkpoint,
γ-H2AX may also be involved in the repair of damaged DNA directly
by stabilizing the broken ends (17).
When DNA has been repaired, the γ-H2AX foci disappear and the
checkpoint is closed, allowing re-entry into the cell cycle
(18). Thus, timely dephosphorylation
of γ-H2AX is critical to the dissociation of repair proteins and to
the release of the cells from cell cycle checkpoints. As a sensor
of DNA damage signaling, γ-H2AX is widely thought to be a molecular
marker for IR-induced DSBs, and it is one of the popular topics in
the research on mechanisms of DDR.
In the current study, the effects of MIIP gene on
radiosensitivity in NPC cells and the possible molecular mechanism
were investigated. It was indicated that the overexpression of MIIP
may enhance the radiosensitivity of NPC cells. MIIP gene induces
the expression and persistence of γ-H2AX, which stands for the
earliest occurrence in the IR-induced DNA DSBs.
Materials and methods
Cell lines and cell culture
The human NPC 5-8F and CNE2 cell lines were provided
by the Research Center of Clinical Oncology of the Affiliated
Jiangsu Cancer Hospital (Nanjing Medical University, Nanjing,
China). Although it was reported that the CNE2 cell line was
potentially contaminated on September 2014 (19), several studies based on this cell line
have been published afterwards (20–23), which
seem to support the authors' view that the misidentification issue
was unlikely to affect the outcomes of the present study.
MIIP was overexpressed in 5-8F and CNE2 cell lines
by lentivirus-mediated transduction. All cells were cultured in
Roswell Park Memorial Institute-1640 medium (Corning Incorporated,
Corning, NY, USA) containing fetal bovine serum (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at a final concentration of 10%
and grown in a humidified incubator with 5% CO2 at
37°C.
Colony-forming assay
Cell growth following treatment with IR was analyzed
by colony-formation assay. The cells were seeded in six-well plates
at different cell densities (2×102-8×102
cells/well) for 12 h and exposed to IR at 0, 2, 4, 6 and 8 Gy.
Then, the cells were cultured at 37°C for 10 days, and colonies
were stained with Giemsa at 25°C for 2 h. The surviving colonies
with >50 cells were counted using a light microscope (Olympus
Corp., Tokyo, Japan). The experiments were performed three
times.
Flow cytometric analysis
For apoptosis analysis, negative control (5–8F NC
and CNE2 NC) and MIIP-transfected (5–8F OE and CNE2 OE) cells were
seeded in six-well plates for 12 h (10×104 cells/well).
Two parallel holes for each cell line were exposed to 6 Gy IR.
Then, the cells were incubated at 37°C for 72 h and washed twice
with ice-cold PBS. The apoptotic cells were detected by Annexin
V-fluorescein isothiocyanate/propidium iodide (PI) staining.
For cell cycle analysis, negative control and
MIIP-transfected cells were plated in 60 mm2 culture
dishes for 12 h (10×104 cells/well). Two parallel holes
for each cell line were exposed to 6 Gy IR. Then, the cell cultures
were terminated after 24 h. The cells were collected and fixed with
70% ice-cold ethanol and stained with PI to detect cell cycle
distribution.
The percentage of apoptotic cells and the
distribution of cell cycle were detected by flow cytometry (FCM),
and the data were analyzed by flow cytometry analysis software
(Kaluza 1.6; Beckman Coulter, Inc., Brea, CA, USA). The
aforementioned procedures were conducted in three replicates.
Immunofluorescence
Negative control and MIIP-transfected cells were
seeded on cover glasses and placed in six-well plates for 12 h
(10×104 cells/well). The cells were exposed to 6 Gy IR,
and then the cell cultures were terminated after 0, 1 and 24 h. The
cells were fixed with 4% paraformaldehyde for 30 min at room
temperature and permeabilized with 0.5% Triton X-100 solution.
Then, the samples were incubated with the primary antibody against
γ-H2AX (1:100; catalog no. 2577; Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C overnight. This was followed by incubation
with secondary antibody Cy3-conjugated goat anti-rabbit IgG (1:100;
red; catalog no. GB21303; Servicebio, Wuhan, China) at room
temperature for 1 h. The DNA was stained using DAPI. Finally, DSBs
were detected by an immunofluorescence microscopy (Olympus Corp.,
Tokyo, Japan) and ZNE Lite (version 2.3; ZEISS Corp., Jena,
Germany).
Western blot analysis
The cells were extracted and prepared in modified
RIPA buffer (Beyotime Institute of Biotechnology, Haimen, China).
Total protein was extracted, and protein concentration was
quantified using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Equivalent quantities of protein (20 mg) were run
on 10% SDS-PAGE gels. Then, the proteins were transferred onto
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were blocked with 5% non-fat milk
at room temperature for 2 h. The membranes were incubated with the
relevant primary antibodies against γ-H2AX (1:1,000; catalog no.
2577; Cell Signaling Technology, Inc.) or B-cell lymphoma 2 (Bcl-2;
1:500; catalog no. sc-7382; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) or BCL2 associated X, apoptosis regulator (Bax 1:1,000;
catalog no. 2772; Cell Signaling Technology, USA) in TBS-Tween-20
(TBST) containing 5% non-fat milk (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 4°C overnight, followed by three washes in
TBST for 10 min per wash. Subsequently, the membranes were
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit (catalog no. 7074) and HRP-conjugated goat anti-mouse
(catalog no. 7076) IgG (1:1,000; Cell Signaling Technology, USA)
for 1 h at room temperature. β-actin (1:500; catalog no. BM0627;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) was used as
a loading control. Protein bands were visualized using the ECL
detection reagent (EMD Millipore, Billerica, MA, USA) and analyzed
using Bio-Rad Laboratories Quantity One software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). All data analyses were
repeated three times independently.
Statistical analysis
The data are expressed as the mean ± standard
deviation (SD). Statistical analysis was performed using unpaired
Student's t-test and one-way analysis of variance (ANOVA) test with
Student-Newman-Keuls test using Graphpad (version 5.0; GraphPad
Software, Inc., La Jolla, CA, USA) and SPSS (version 19.0; IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of MIIP affects the
sensitivity of 5-8F and CNE2 cells to IR
The radiosensitizing effects of MIIP were initially
measured using clonogenic assay. The colony formation assay of 5-8F
and CNE2 cells that were treated with or without 4 Gy IR are
indicated in Fig. 1A and D. The
survival fractions of cells following exposure to 4 Gy IR are shown
in Fig. 1B and E. Notably, the
survival fraction of cells in the 5-8F OE and CNE2 OE groups
significantly decreased following irradiation compared with the
negative control and untreated groups. As shown in Fig. 1C and F, a dose-dependent decrease in
survival occurred in 5-8F and CNE2 cells following irradiation (0,
2, 4, 6 and 8 Gy). These results confirmed that the overexpression
of MIIP was able to suppress the growth of NPC cells following
irradiation.
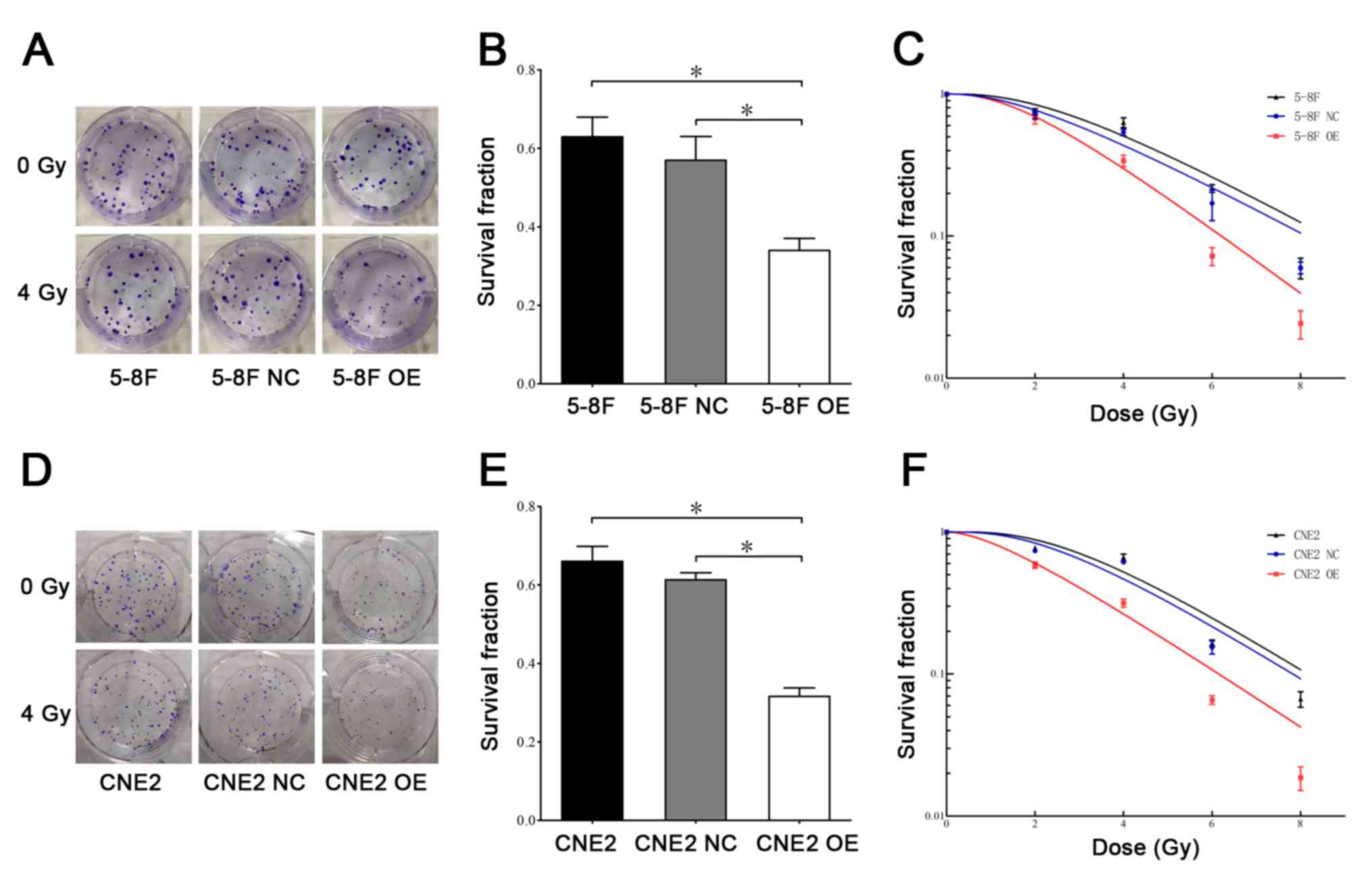 | Figure 1.Overexpression of MIIP gene affects
the radiosensitivity of 5-8F cells. (A) The colony formation assay
in 5-8F, 5-8F NC and 5-8F OE cells that were treated with or
without IR. (B) The survival fractions of 5-8F, 5-8F NC and 5-8F OE
cells following exposure to 4 Gy IR. (C) The survival fractions of
5-8F, 5-8F NC and 5-8F OE cells at different doses of IR (0, 2, 4,
6 and 8 Gy). (D) The colony formation assay in CNE2, CNE2 NC and
CNE2 OE cells that were treated with or without IR. (E) The
survival fractions of CNE2, CNE2 NC and CNE2 OE cells following
exposure to 4 Gy IR. (F) The survival fractions of CNE2, CNE2 NC,
and CNE2 OE cells at different doses of IR (0, 2, 4, 6 and 8 Gy).
n=3 for each group. *P<0.05. IR, ionizing radiation; MIIP,
migration and invasion inhibitory protein. |
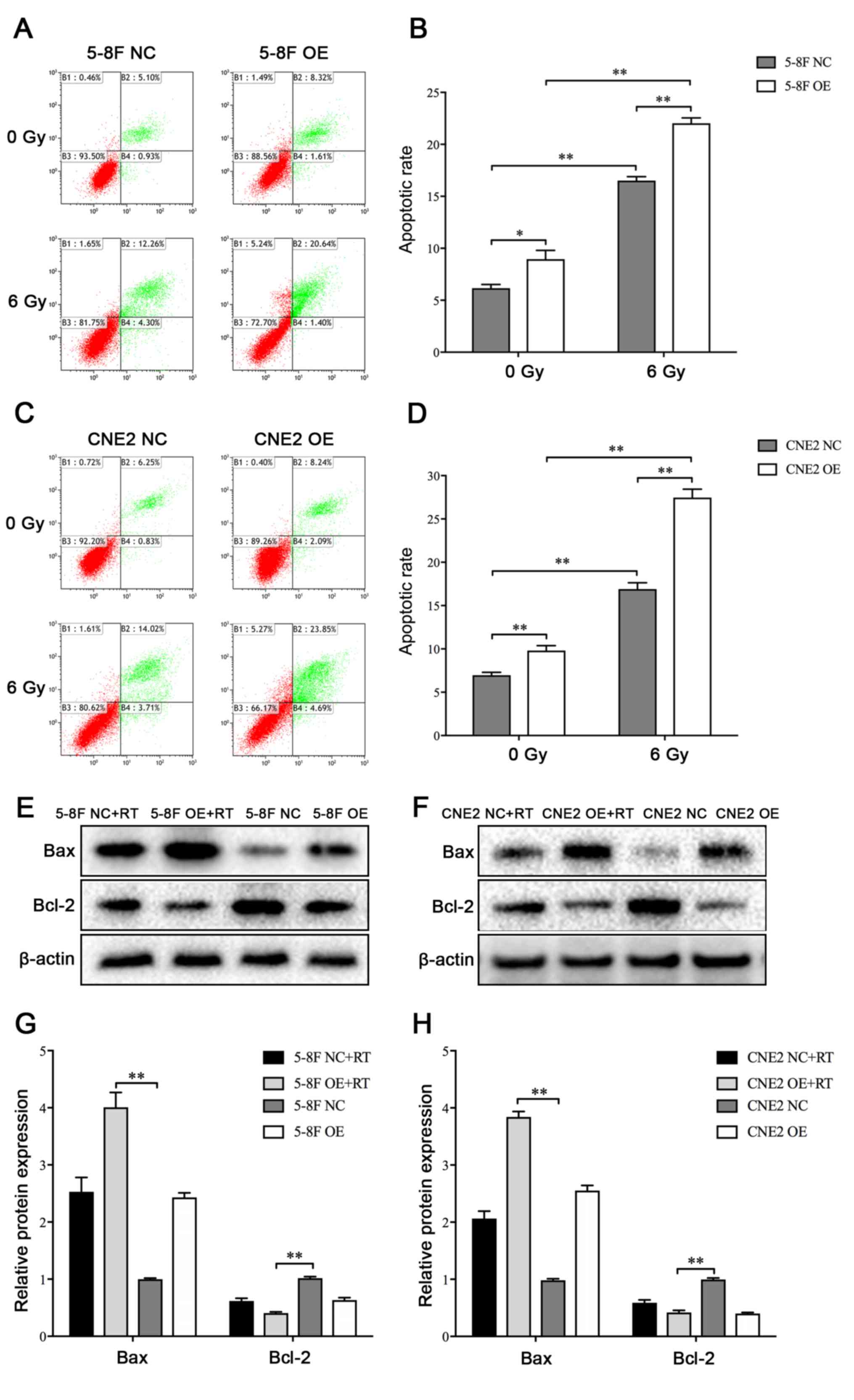
Effect of the MIIP gene on cell
apoptosis
Apoptotic rates were analyzed 72 h following
irradiation treatments, and untreated cells were used as controls.
Exposure of the NPC cells to irradiation could significantly
increase the apoptosis of NPC cells (Fig.
2A-D). Moreover, the MIIP gene overexpression groups exhibited
markedly higher apoptotic rate compared with the negative control
groups in the absence of IR. Following radiation with 6 Gy, the
apoptotic rates of the MIIP gene overexpression groups were also
significantly higher compared with that of the negative control
groups (Fig. 2A-D). To further
uncover the underlying mechanism by which MIIP gene regulates
radiosensitivity, the expression levels of Bax and Bcl-2 proteins,
which were related to cell apoptosis, were analyzed by western
blotting. The 5-8F OE and CNE2 OE cells exhibited notably higher
Bax expression and considerably lower Bcl-2 expression compared
with 5-8F NC and CNE2 NC cells (Fig.
2E-H). Therefore, the overexpression of the MIIP gene may
enhance the apoptosis of NPC cells following irradiation.
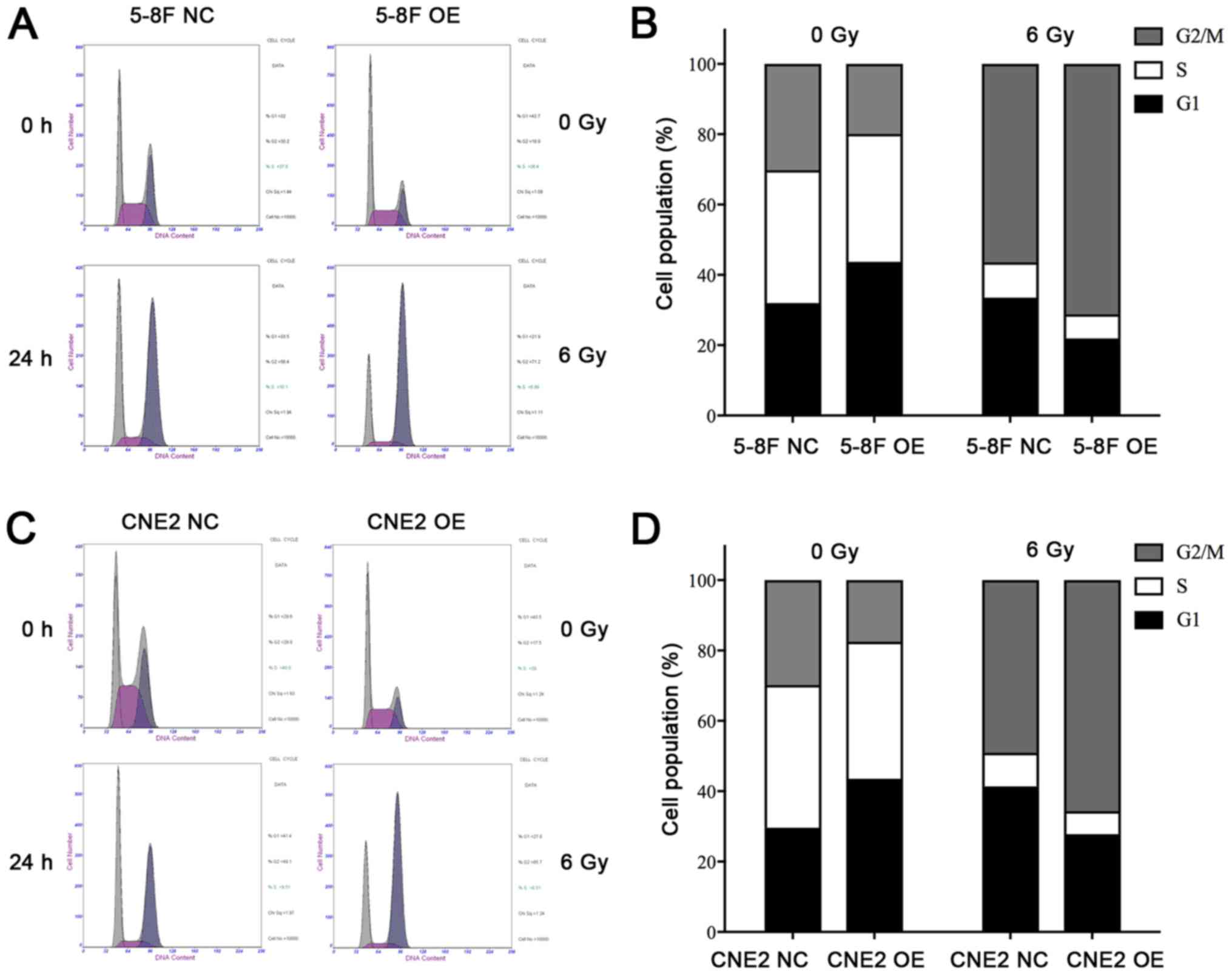
Effect of the MIIP gene on cell cycle
distribution
To further assess the causes of radiation
sensitivity, FCM analysis was employed to confirm the effect of
MIIP on the distribution of cell cycle following irradiation. The
cells were exposed to 6 Gy irradiation, and analyses were conducted
at 0 and 24 h following treatment. As shown in Fig. 3A-D, irradiation was able to
significantly disrupt cell cycle progression and cause a sharp
increase in the proportion of cells in the G2/M phase in 5-8F OE
and CNE2 OE cells compared with 5-8F NC and CNE2 NC cells. In the
absence of IR, no significant difference was observed between the
cell cycle profiles of cells in the MIIP gene overexpression and
control groups (Fig. 3A-D). By
contrast, the percentage of cells in the MIIP gene overexpression
group was markedly higher in the G2/M phase compared with the
control cells at 24 h following 6 Gy irradiation (Fig. 3B and D). The overexpression of MIIP
gene enhanced the G2/M cell cycle arrest that was induced by
IR.
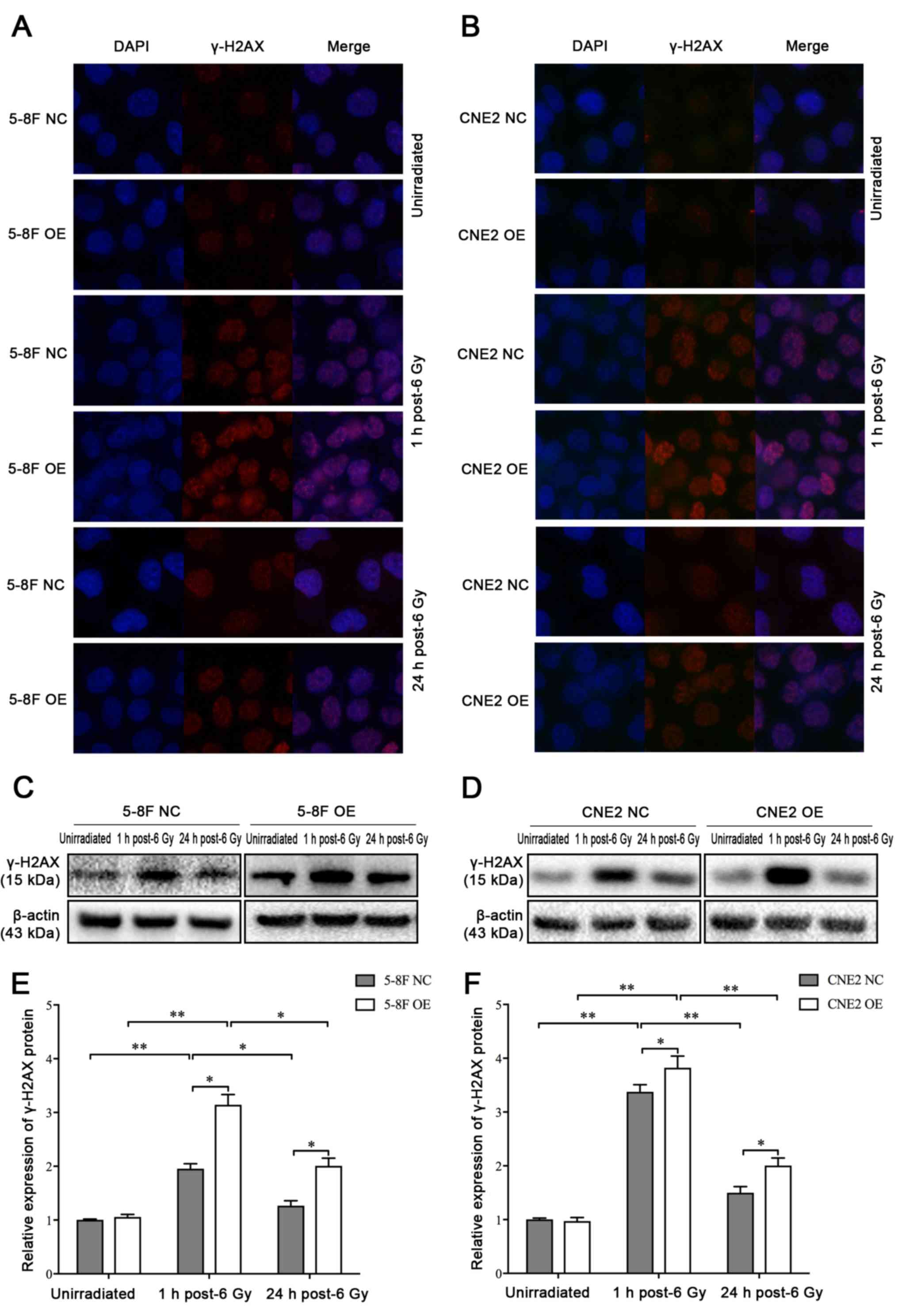
MIIP participates in IR-induced γ-H2AX
foci formation
IR inflicts various types of damage to the genome to
kill cells (24). It was speculated
that the radiosensitizing effect of MIIP gene on NPC cells may
originate from the impairment in the repair of DSBs. Therefore, the
levels of DSBs in 5-8F and CNE2 cells following exposure to IR at
different time points were determined by immunofluorescence
staining of γ-H2AX foci. Following irradiation at 6 Gy, the number
of nuclear foci containing γ-H2AX at 1 h was markedly higher in
5-8F OE and CNE2 OE cells compared with 5-8F NC and CNE2 NC cells.
As a result of DBS repair, the number of foci decreased from 1 to
24 h. Meanwhile, the 5-8F OE and CNE2 OE cells exhibited slower
decay of γ-H2AX foci following irradiation compared with 5-8F NC
and CNE2 NC cells. In addition, 5-8F OE and CNE2 OE groups
exhibited higher levels of γ-H2AX compared with 5-8F NC and CNE2
NC, respectively (Fig. 4A and B). The
same results were observed by western blot analysis (Fig. 4C-F). Therefore, the MIIP gene was able
to increase the induction and persistence of IR-induced γ-H2AX
foci.
Discussion
Clinically, radiosensitivity and radioresistance
have important roles in treatment of NPC (25,26).
However, the accurate molecular mechanisms underlying their roles
remain unclear. Several reports demonstrated that numerous tumor
suppressor genes and oncogenes are associated with radiosensitivity
(27–33). MIIP was first identified in a yeast
two-hybrid screen for proteins that interact and inhibit
insulin-like growth factor binding protein 2 (6). Further studies on insulin-like growth
factor binding protein 2 indicated that MIIP regulates cell
migration and mitosis (34). MIIP is
underexpressed in a wide range of types of human cancer, including
glioma, endometrial cancer, breast cancer, lung cancer, esophageal
cancer, prostate cancer, neuroblastoma and pheochromocytoma
(3–10). A decreased MIIP expression is
associated with tumorigenesis and progression of endometrial cancer
as MIIP inhibits the migration and invasion of endometrial cancer
cells (4). Moreover, MIIP inhibits
the migration and invasion of glioma cells (6). Wen et al (5) found that MIIP accelerates epidermal
growth factor receptor protein turnover and attenuates the
proliferation of non-small cell lung cancer cells. Additionally, a
previous study conducted by our team indicated that the expression
of MIIP mRNA was reduced in human NPC cell lines (5–8F and CNE2)
compared with normal nasopharyngeal epithelial cell line (NP69),
and the MIIP gene played a notable role in the pathogenesis of NPC
(unpublished). Therefore, the current study was designed to
investigate the association between MIIP and radiosensitivity of
NPC cells.
One of the most reliable methods to evaluate cell
survival is the colony formation assay, which is the gold standard
for detecting radiosensitivity (35).
In the present study, all radiosensitization parameters were
calculated using the linear-quadratic model (25). Consequently, it was demonstrated that
the survival fraction significantly decreased in the MIIP gene
overexpression groups at a given dose of irradiation in comparison
with the negative control and untreated groups. Moreover, a
dose-dependent decrease in survival was observed in 5-8F and CNE2
cells following irradiation. Therefore, the MIIP gene may exert a
radiosensitization effect on NPC cells.
In previous studies, tumor radiosensitivity is
associated with numerous factors, including tumor microenvironment,
apoptosis, cell cycle regulation and DNA repair dysfunction
(36). Apoptosis is one of the most
important mechanisms of cell death following IR, and the apoptosis
index is positively correlated with tumor radiosensitivity
(37). Moreover, several studies
indicated that Bcl-2 and Bax have a significant role in cell
apoptosis (38,39). Following irradiation, the apoptotic
rate in the 5-8F OE and CNE2 OE groups increased along with
increased Bax expression and decreased Bcl-2 protein expression. In
theory, the inhibition of MIIP would lead to the suppression of the
radiation-induced apoptosis of NPC cells. However, in a previous
study by the present authors, it was indicated that the expression
of MIIP gene is very low in NPC cell lines (unpublished).
Therefore, in the present study, the overexpression of MIIP was
carried out instead of knockdown. It was demonstrated that the
overexpression of MIIP and irradiation increased cell apoptosis by
activating the Bax/Bcl-2 signaling pathway in NPC cells, which may
be one of the potential underlying mechanisms of
radiosensitization.
Apart from stimulating apoptosis, DNA damage
maintains genomic integrity by causing responses to conserved DNA
damage, activating cell cycle checkpoints, and allowing DNA repair
(40,41). Cells in the G2/M phase are the most
sensitive to IR, whereas those in the S phase are resistant
(42). Radiosensitization had been
achieved in previous studies by inducing cell cycle arrest at G2/M
using gene therapy or taxanes (43,44). The
present study analyzed the changes in cell cycle by flow cytometry.
The overexpression of MIIP increased the proportion of 5-8F and
CNE2 cells in the G2/M phase following exposure to IR, thereby
indicating that G2 phase delay may result in the sensitization of
irradiated cells.
The activation of checkpoint mechanisms following
exposure to DNA damage is critical to the maintenance of genomic
integrity and prevention of cancer development (45). DNA DSBs induce a checkpoint response
that inhibits further progression of cell cycle and promotes repair
of damaged DNA in response to genotoxic stress (46).
In IR-induced DSBs, γ-H2AX occurs immediately
following the appearance of DSBs and is crucial to the formation of
foci at the chromatin surrounding the DSB. Then, numerous other
substrates are modified, which leads to checkpoint activation, DNA
repair and/or apoptosis. After finishing the repair of damaged DNA,
γ-H2AX foci disappear, and the checkpoint is closed, which allows
re-entry into the cell cycle. In mammalian cells, γ-H2AX could
accumulate around damaged chromatin (14,47–49). In
the present study, γ-H2AX appeared in nuclear foci within 1 h
following exposure to IR. The overexpression of MIIP markedly
increased the number of γ-H2AX foci in 5-8F and CNE2 cells. The
same results were observed in the western blot analysis. These
results indicated that the overexpression of MIIP may enhance the
radiosensitivity of NPC cells, and then promote the cascade of DNA
damage signal induced by IR, accumulating and retaining DDR
proteins at the DNA damage sites. However, further studies are
needed to examine other mechanisms of radiosensitization.
In conclusion, MIIP improved the radiosensitivity of
NPC cells via promoting cell apoptosis by regulating the expression
of bax and bcl-2, and inducing cell cycle arrest at the G2/M phase,
as well as inhibiting the repair of DBS. MIIP appears to be a
potential radiotherapy sensitization agent for the treatment of
NPC.
Acknowledgements
The authors would like to thank Dr. Wenjie Xia
(Department of Thoracic surgery, Jiangsu Cancer Hospital) for
technical assistance and helpful discussion.
Funding
The present study was supported by the Jiangsu
Provincial Commission of Health and Family Planning Young Scholars
Award (grant no. Q201501), the Jiangsu Clinical Medicine Science
and Technology Special Fund (grant no. BL2014091), the National
Natural Science Foundation of China (grant no. 81672989) and the
Medical Young Talent Foundation of Jiangsu Provincial Health
Department (grant no. QNRC2016648).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH, LY and HPZ conceived and designed the
experiments. HPZ, LXQ and NZ performed the experiments. NZ, JJG and
KD coordinated the research and analyzed the data. HPZ and LXQ
wrote the manuscript. JW, MYD, ZWL and HMZ supported the
experiments and helped to draft the manuscript. JZW, XH and LY
supervised laboratorial experimentation. JZW also provided
technical assistance in the experiment. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang L, Huang Y, Hong S, Yang Y, Yu G,
Jia J, Peng P, Wu X, Lin Q, Xi X, et al: Gemcitabine plus cisplatin
versus fluorouracil plus cisplatin in recurrent or metastatic
nasopharyngeal carcinoma: A multicentre, randomised, open-label,
phase 3 trial. Lancet. 388:1883–1892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang S, Pan Y, Zhang R, Xu T, Wu W, Zhang
R, Wang C, Huang H, Calin CA, Yang H and Claret FX: Hsa-miR-24-3p
increases nasopharyngeal carcinoma radiosensitivity by targeting
both the 3′UTR and 5′UTR of Jab1/CSN5. Oncogene. 35:6096–6108.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song F, Zhang L, Ji P, Zheng H, Zhao Y,
Zhang W and Chen K: Altered expression and loss of heterozygosity
of the migration and invasion inhibitory protein (MIIP) gene in
breast cancer. Oncol Rep. 33:2771–2778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Hu L, Ji P, Teng F, Tian W, Liu Y,
Cogdell D, Liu J, Sood AK, Broaddus R, et al: MIIP remodels
Rac1-mediated cytoskeleton structure in suppression of endometrial
cancer metastasis. J Hematol Oncol. 9:1122016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen J, Fu J, Ling Y and Zhang W: MIIP
accelerates epidermal growth factor receptor protein turnover and
attenuates proliferation in non-small cell lung cancer. Oncotarget.
7:9118–9134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song SW, Fuller GN, Khan A, Kong S, Shen
W, Taylor E, Ramdas L, Lang FF and Zhang W: IIp45, an insulin-like
growth factor binding protein 2 (IGFBP-2) binding protein,
antagonizes IGFBP-2 stimulation of glioma cell invasion. Proc Natl
Acad Sci USA. 100:13970–13975. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song F, Ji P, Zheng H, Song F, Wang Y, Hao
X, Wei Q, Zhang W and Chen K: Definition of a functional single
nucleotide polymorphism in the cell migration inhibitory gene MIIP
that affects the risk of breast cancer. Cancer Res. 70:1024–1032.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gibbs M, Stanford JL, McIndoe RA, Jarvik
GP, Kolb S, Goode EL, Chakrabarti L, Schuster EF, Buckley VA,
Miller EL, et al: Evidence for a rare prostate
cancer-susceptibility locus at chromosome 1p36. Am J Hum Genet.
64:776–787. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choma MK, Lumb J, Kozik P and Robinson MS:
A genome-wide screen for machinery involved in downregulation of
MHC class I by HIV-1 Nef. PLoS One. 10:e01404042015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujita T, Igarashi J, Okawa ER, Gotoh T,
Manne J, Kolla V, Kim J, Zhao H, Pawel BR, London WB, et al: CHD5,
a tumor suppressor gene deleted from 1p36.31 in neuroblastomas. J
Natl Cancer Institute. 100:940–949. 2008. View Article : Google Scholar
|
|
11
|
Shao JY, Wang HY, Huang XM, Feng QS, Huang
P, Feng BJ, Huang LX, Yu XJ, Li JT, Hu LF, et al: Genome-wide
allelotype analysis of sporadic primary nasopharyngeal carcinoma
from southern China. Int J Oncol. 17:1267–1275. 2000.PubMed/NCBI
|
|
12
|
Huang B, Zhang L, Yan J, Liang Q and Fang
Y: Fine mapping of the loss of heterozygosity for chromosome
1pter-p36.11 in nasopharyngeal carcinoma. Zhonghua Bing Li Xue Za
Zhi. 30:110–113. 2001.(In Chinese). PubMed/NCBI
|
|
13
|
Shao JY, Huang XM, Yu XJ, Huang LX, Wu QL,
Xia JC, Wang HY, Feng QS, Ren ZF, Ernberg I, et al: Loss of
heterozygosity and its correlation with clinical outcome and
Epstein-Barr virus infection in nasopharyngeal carcinoma.
Anticancer Res. 21:3021–3029. 2001.PubMed/NCBI
|
|
14
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Downs JA, Lowndes NF and Jackson SP: A
role for Saccharomyces cerevisiae histone H2A in DNA repair.
Nature. 408:1001–1004. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Foster ER and Downs JA: Histone H2A
phosphorylation in DNA double-strand break repair. FEBS J.
272:3231–3240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Celeste A, Difilippantonio S,
Difilippantonio MJ, Fernandez-Capetillo O, Pilch DR, Sedelnikova
OA, Eckhaus M, Ried T, Bonner WM and Nussenzweig A: H2AX
haploinsufficiency modifies genomic stability and tumor
susceptibility. Cell. 114:371–383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berkovich E, Monnat RJ Jr and Kastan MB:
Roles of ATM and NBS1 in chromatin structure modulation and DNA
double-strand break repair. Nat Cell Biol. 9:683–690. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strong MJ, Baddoo M, Nanbo A, Xu M,
Puetter A and Lin Z: Comprehensive high-throughput RNA sequencing
analysis reveals contamination of multiple nasopharyngeal carcinoma
cell lines with HeLa cell genomes. J Virol. 88:10696–10704. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan Y, Guo Q, Xie Q, Wang K, Yuan B, Zhou
Y, Liu J, Huang J, He X, Yang X, et al: Single-walled carbon
nanotubes (SWCNTs)-assisted cell-systematic evolution of ligands by
exponential enrichment (cell-SELEX) for improving screening
efficiency. Anal Chem. 86:9466–9472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu T, Sun Q, Li Q, Yang H, Zhang Y, Wang
R, Lin X, Xiao D, Yuan Y, Chen L and Wang W: Dual PI3K/mTOR
inhibitors, GSK2126458 and PKI-587, suppress tumor progression and
increase radiosensitivity in nasopharyngeal carcinoma. Mol Cancer
Ther. 14:429–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Z, Zhang L, Xie B, Wang X, Yang X,
Ding N, Zhang J, Liu Q, Tan G, Feng D and Sun LQ: FOXC2 promotes
chemoresistance in nasopharyngeal carcinomas via induction of
epithelial mesenchymal transition. Cancer Lett. 363:137–145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuang CM, Fu X, Hua YJ, Shuai WD, Ye ZH,
Li Y, Peng QH, Li YZ, Chen S, Qian CN, et al: BST2 confers
cisplatin resistance via NF-kappaB signaling in nasopharyngeal
cancer. Cell Death Dis. 8:e28742017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawamura K, Qi F and Kobayashi J:
Potential relationship between the biological effects of low-dose
irradiation and mitochondrial ROS production. J Radiat Res. Feb
3–2018.Doi: 10.1093/jrr/rrx091. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan L, Yi HM, Yi H, Qu JQ, Zhu JF, Li LN,
Xiao T, Zheng Z, Lu SS and Xiao ZQ: Reduced RKIP enhances
nasopharyngeal carcinoma radioresistance by increasing ERK and AKT
activity. Oncotarget. 7:11463–11477. 2016.PubMed/NCBI
|
|
26
|
Chistiakov DA, Voronova NV and Chistiakov
PA: Genetic variations in DNA repair genes, radiosensitivity to
cancer and susceptibility to acute tissue reactions in
radiotherapy-treated cancer patients. Acta Oncol. 47:809–824. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Lang J, Cao Z, Li R, Wang X and
Wang W: Radiation-induced SOD2 overexpression sensitizes colorectal
cancer to radiation while protecting normal tissue. Oncotarget.
8:7791–7800. 2017.PubMed/NCBI
|
|
28
|
Liu ZG, Jiang G, Tang J, Wang H, Feng G,
Chen F, Tu Z, Liu G, Zhao Y, Peng MJ, et al: c-Fos over-expression
promotes radioresistance and predicts poor prognosis in malignant
glioma. Oncotarget. 7:65946–65956. 2016.PubMed/NCBI
|
|
29
|
Tewari D, Monk BJ, Al-Ghazi MS, Parker R,
Heck JD, Burger RA and Fruehauf JP: Gene expression profiling of in
vitro radiation resistance in cervical carcinoma: A feasibility
study. Gynecol Oncol. 99:84–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harima Y, Togashi A, Horikoshi K, Imamura
M, Sougawa M, Sawada S, Tsunoda T, Nakamura Y and Katagiri T:
Prediction of outcome of advanced cervical cancer to
thermoradiotherapy according to expression profiles of 35 genes
selected by cDNA microarray analysis. Int J Radiat Oncol Biol Phys.
60:237–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cengel KA, Voong KR, Chandrasekaran S,
Maggiorella L, Brunner TB, Stanbridge E, Kao GD, McKenna WG and
Bernhard EJ: Oncogenic K-Ras signals through epidermal growth
factor receptor and wild-type H-Ras to promote radiation survival
in pancreatic and colorectal carcinoma cells. Neoplasia. 9:341–348.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langland GT, Yannone SM, Langland RA,
Nakao A, Guan Y, Long SB, Vonguyen L, Chen DJ, Gray JW and Chen F:
Radiosensitivity profiles from a panel of ovarian cancer cell lines
exhibiting genetic alterations in p53 and disparate DNA-dependent
protein kinase activities. Oncol Rep. 23:1021–1026. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang EY, Chen YF, Chen YM, Lin IH, Wang
CC, Su WH, Chuang PC and Yang KD: A novel radioresistant mechanism
of galectin-1 mediated by H-Ras-dependent pathways in cervical
cancer cells. Cell Death Dis. 3:e2512012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Wen J and Zhang W: MIIP, a
cytoskeleton regulator that blocks cell migration and invasion,
delays mitosis, and suppresses tumorogenesis. Curr Protein Pept
Sci. 12:68–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nahas SA and Gatti RA: DNA double strand
break repair defects, primary immunodeficiency disorders, and
‘radiosensitivity’. Curr Opin Allergy Clin Immunol. 9:510–516.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Selzer E and Hebar A: Basic principles of
molecular effects of irradiation. Wien Med Wochenschr. 162:47–54.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Williams JR, Zhang Y, Zhou H, Russell J,
Gridley DS, Koch CJ and Little JB: Genotype-dependent
radiosensitivity: Clonogenic survival, apoptosis and cell-cycle
redistribution. Int J Radiat Biol. 84:151–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Selvarajah J, Elia A, Carroll VA and
Moumen A: DNA damage-induced S and G2/M cell cycle arrest requires
mTORC2-dependent regulation of Chk1. Oncotarget. 6:427–440. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Toyoshima M: Analysis of p53 dependent
damage response in sperm-irradiated mouse embryos. J Radiat Res.
50:11–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hiro J, Inoue Y, Toiyama Y, Yoshiyama S,
Tanaka K, Mohri Y, Miki C and Kusunoki M: Possibility of paclitaxel
as an alternative radiosensitizer to 5-fluorouracil for colon
cancer. Oncol Rep. 24:1029–1034. 2010.PubMed/NCBI
|
|
44
|
Zheng L, Tang W, Wei F, Wang H, Liu J, Lu
Y, Cheng Y, Bai X, Yu X and Zhao W: Radiation-inducible protein
RbAp48 contributes to radiosensitivity of cervical cancer cells.
Gynecol Oncol. 130:601–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bartek J, Bartkova J and Lukas J: DNA
damage signalling guards against activated oncogenes and tumour
progression. Oncogene. 26:7773–7779. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bartek J and Lukas J: DNA damage
checkpoints: From initiation to recovery or adaptation. Curr Opin
Cell Biol. 19:238–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
van Attikum H, Fritsch O, Hohn B and
Gasser SM: Recruitment of the INO80 complex by H2A phosphorylation
links ATP-dependent chromatin remodeling with DNA double-strand
break repair. Cell. 119:777–788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Park JH, Park EJ, Lee HS, Kim SJ, Hur SK,
Imbalzano AN and Kwon J: Mammalian SWI/SNF complexes facilitate DNA
double-strand break repair by promoting gamma-H2AX induction. EMBO
J. 25:3986–3997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kobayashi J, Fujimoto H, Sato J, Hayashi
I, Burma S, Matsuura S, Chen DJ and Komatsu K: Nucleolin
participates in DNA double-strand break-induced damage response
through MDC1-dependent pathway. PLoS One. 7:e492452012. View Article : Google Scholar : PubMed/NCBI
|