Introduction
Esophageal cancer is a common type of malignancy
worldwide with an estimated 482,300 new cases and 406,800
mortalities globally in 2008 (1). In
China, esophageal cancer is also recognized as a high-incidence
tumor and greatly contributes to global esophageal cancer incidence
(2). Histologically, the majority of
patients with esophageal cancer have esophageal squamous cell
carcinoma (ESCC) worldwide and in China, whereas esophageal
adenocarcinoma may occur more frequently in developed countries,
including the USA (3). The primary
worldwide risk factors of ESCC include tobacco smoking and alcohol
consumption; in China, the consumption of salted vegetables or
other nutritional factors are also associated with a high incidence
of ESCC (3). To date, the exact ESCC
pathogenesis and mechanisms remain unclear; a deeper understanding
of ESCC development and progression may lead to the identification
of novel therapeutic strategies for the regulation of this fatal
disease.
5-lipoxygenase (5-LO) is an important enzyme that
catalyzes arachidonic acid biosynthesis to hydroxyeicosatetraenoic
acids or leukotrienes (LTs). Previous studies demonstrated that
5-LO may act through a number of signaling pathways to promote
tumorigenesis, and 5-LO expression was identified to be upregulated
in several human cancer types, including breast (4), lung (5),
pancreatic (6), colon (7), prostate (8,9) and
esophageal cancer (10–12). Two previous studies on esophageal
cancer also indicated that the inhibition of 5-LO enzymatic
activity was able to induce apoptosis in tumor cells (13,14).
However, data is currently lacking regarding whether upregulated
5-LO expression is associated with the clinical importance and
prognosis of ESCC. Thus, the aims of the present study were to
investigate 5-LO expression in a large ESCC cohort for its
association with clinicopathological and survival data, and to then
explore 5-LO expression or knockdown in the regulation of ESCC
proliferation and migration capacity in vitro. The results
of the present study may provide further insights into the role of
5-LO in ESCC progression and may support the future targeting of
5-LO in the management or treatment of patients with ESCC.
Materials and methods
Patients
A total of 297 tissue samples were collected from
patients with ESCC who underwent surgical treatment at Shantou City
Center Hospital (Shantou, China) between January 2007 and December
2011. All patients were pathologically diagnosed and confirmed with
ESCC in the Anatomic Pathology Department of the Hospital according
to the 8th edition of the tumor-node-metastasis (TNM)
classification released by the International Union against Cancer
and guidelines of the World Health Organization Pathological
Classification of Tumors (15). Among
the patients, there were 232 males and 65 females with a medium age
of 59 years (range, 39–88 years). Patients underwent surgical tumor
resection and received radiochemotherapy thereafter. All patients
were followed up regularly until they succumbed. The maximum length
of follow-up was 60 months with a medium of 26.77 months.
Clinicopathological data was also collected from the medical
records, including gender, age, stage of disease and tumor
differentiation. The study was performed in accordance with the
Declaration of Helsinki and Good Clinical Practice guidelines. The
present study was approved by the Ethics Committee of the Shantou
City Center Hospital and all enrolled patients provided written
informed consent.
Tissue samples and construction of
tissue microarrays
Paraffin blocks from the 297 tissue specimens were
retrieved from the Pathology Department, and the tissue sections
were prepared for hematoxylin and eosin (HE) staining to confirm
the diagnosis and to select areas for the construction of tissue
microarrays as previously described (16). Briefly, representative areas of tumor
tissues were selected from paraffin blocks after reviewing the
HE-stained tissue sections. From each tissue specimen, two tissue
cores were selected with a diameter of 1.8 mm and length of 1.0–3.0
mm, which depended on the depth of the tissue specimens in the
donor blocks. A new paraffin block was then precisely generated
with the arrayed cores from the original paraffin blocks. The
quality of tissue samplings was verified via paraffin-embedded
tissue sectioning and HE staining. The tissue microarray sections
were then baked at 56°C overnight and subjected to
immunohistochemistry.
Immunohistochemistry
For immunohistochemistry, 10% neutral buffered
formalin-fixed, paraffin-embedded specimens were cut into
4-µm-thick sections. The sections were dewaxed in xylene and
rehydrated in a series of graded alcohols. Subsequently, slides
were submerged in a peroxidase quenching solution containing 30%
hydrogen peroxide and absolute methanol at a ratio of 1:9,
respectively, for 10 min. Subsequent to rinsing in
phosphate-buffered saline, antigen retrieval from the tissue was
performed by autoclaving in 0.01 mol/l sodium citrate buffer (pH
6.0) at 120°C for 3 min. Next, sections were blocked in 10% normal
goat serum (ZSGB-BIO; OriGene Technologies, Beijing, China) for 10
min at room temperature, and then incubated overnight at 4°C with
rabbit monoclonal antibody against 5-LO (1:100 dilution; AP7856C,
San Diego, CA, USA). Then, the sections were subjected to
immunostaining using the SuperPicture Polymer Detection kit
(Product code 879363, Thermo Fisher Scientific, Inc., Waltham,
USA), which contained a ready-to-use secondary antibody. The slides
were examined with an Olympus IX51 light microscope (Olympus,
Tokyo, Japan) at magnification, ×100. The immunostained tissue
microarray section was then reviewed and independently evaluated by
two histopathologists who did not know the clinicopathological data
of the patients. Immunoreactivity was evident according to brown
color on the cell membrane or in the cytoplasm. In the study, the
5-LO expression level and the subcellular 5-LO distribution were
respectively recorded. Regarding the 5-LO expression level, the
scores that reflected the staining intensity were as follows: 3,
strong; 2, moderate; 1, weak; and 0, negative, and the staining
percentages were ranked as 4, >75; 3, 51–75; 2, 26–50; 1, 5–25;
and 0, <5%. Final scores were recorded by multiplying the
intensity and percentage scores to produce a 0 to 12 digit scoring.
For statistical analyses, scores of 0 to 4 were regarded as low
expression, whereas 5–12 indicated high expression.
Cell lines and culture
The human ESCC cell lines SHEEC, KYSE510, KYSE150,
KYSE180, and KYSE450 were obtained from the Key Laboratory of
Molecular Biology for High Cancer Incidence Coastal Chaoshan Area,
Shantou University Medical College (Shantou, China) and cultured in
RPMI-1640 or Dulbecco's modified Eagle's medium/F-12 medium
containing 10% fetal calf serum (Thermo Fisher Scientific, Inc.) at
37°C in a humidified 5% CO2 atmosphere. It is necessary
to emphasize that the SHEEC cell line was developed and provided by
the Department of Tumor Pathology, Medical College of Shantou
University (17).
Protein extraction and western
blotting
Total cellular protein was extracted from esophageal
cancer cell lines using RIPA buffer (Maygene, Guangzhou, China).
Protein determination was performed using the BCA method, and 80 µg
total protein samples were separated using 10% SDS-PAGE and
transferred on to a polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). The membrane was then blocked in 5%
skim milk at the room temperature for 1 h and then incubated at 4°C
overnight with an anti-5-LO antibody (cat. no., 3289, Cell
Signaling Technology, Inc., Danvers, MA, USA) at a dilution of
1:500 or with an anti-β-actin antibody (cat. no., A2066;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at a dilution of
1:1,000. On the next day, the membrane was washed with PBS-Tween 20
thrice and further incubated with horseradish peroxidase-conjugated
secondary antibody (cat. no., sc-2004; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at a dilution of 1:5,000 at room temperature
for 2 h. The protein bands were subsequently visualized with ECL
reagent (Thermo Fisher Scientific, Inc.) and the FluorChem 8900
image analysis system (ProteinSimple, San Jose, CA, USA) was used
to capture images and quantitatively analyze 5-LO levels following
normalization to the level of β-actin.
Vector construction and gene
transfection
Human recombinant 5-LO eukaryotic expression plasmid
vector pcDNA3.1 (+)/5-LO was gifted by Dr Eiko Matsui from Gifu
University (Gifu, Japan). A total of 50 µg 5-LO cDNA-containing
plasmid was transfected into KYSE 150 cells, and an empty plasmid
was used as a negative control using Lipofectamine® 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Furthermore, two
different 5-LO small interfering (si)RNA (150 pmol) sequences
(18,19) were also designed to knock down 5-LO
expression in ESCC cells. These sequences were synthesized by
GenePharma (Shanghai, China): siRNA-1, 5′-AUUGCCCUGAAAAACUGUG-3′
and siRNA-2, 5′-AGAAAUCCCAAGAUCAGUG-3′. These oligonucleotides were
transiently transfected into ESCC SHEEC cells using Lipofectamine
2000 transfection reagent according to the manufacturer's protocol
and the nonsense siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′) was
transfected into the cells as a negative control.
Tumor cell proliferation and migration
assays
A real-time cell electronic sensing RTCA DP analyzer
with Galaxy 48R (Real-Time Cell analyzer, Roche Applied Science,
Penzberg, Germany) was used to assess the effects of 5-LO
manipulation on the regulation of ESCC cell viability and migration
capacity. Briefly, human ESCC cells transfected with 5-LO cDNA or
siRNA were seeded in E-plate to assess changes in cell
proliferation or the cell invasion and migration (CIM)-plate to
assess changes in cell migration followed by culturing for up to 2
days. Using the cell index (CI), data was obtained on changes
regarding the cell proliferation and the migration rates. A total
of 10,000 cells in RPMI-1640 medium containing 10% bovine serum
albumin (BSA; Thermo Fisher Scientific, Inc.) were added to each
well of the E-plate. In addition, a total of 50,000 cells in
DMEM/F-12 medium containing 10% BSA were added to each well of the
CIM-plate.
Statistical analysis
Associations between 5-LO expression and
clinicopathological characteristics, including gender, age, tumor
TNM classification, histology, size, lymph node metastasis and
distant metastasis, were evaluated using Pearson's chi-square test.
A log-rank test was applied to analyze the Kaplan-Meier curves for
associations between 5-LO expression and overall survival (OS). OS
was defined as the duration between surgery and mortality
associated with esophageal cancer. Associations between the
clinicopathological characteristics and 5-LO expression with
overall survival were assessed using the multivariate Cox
proportional hazards regression model. Data are represented as the
mean ± standard deviation. All analyses were performed with SPSS
for Windows 13.0 software (SPSS, Inc., Chicago, IL, USA). All
P-values were two-tailed and P≤0.05 was considered to indicate a
statistically significant difference.
Results
Differential 5-LO expression in ESCC
and pericarcinoma esophageal tissues
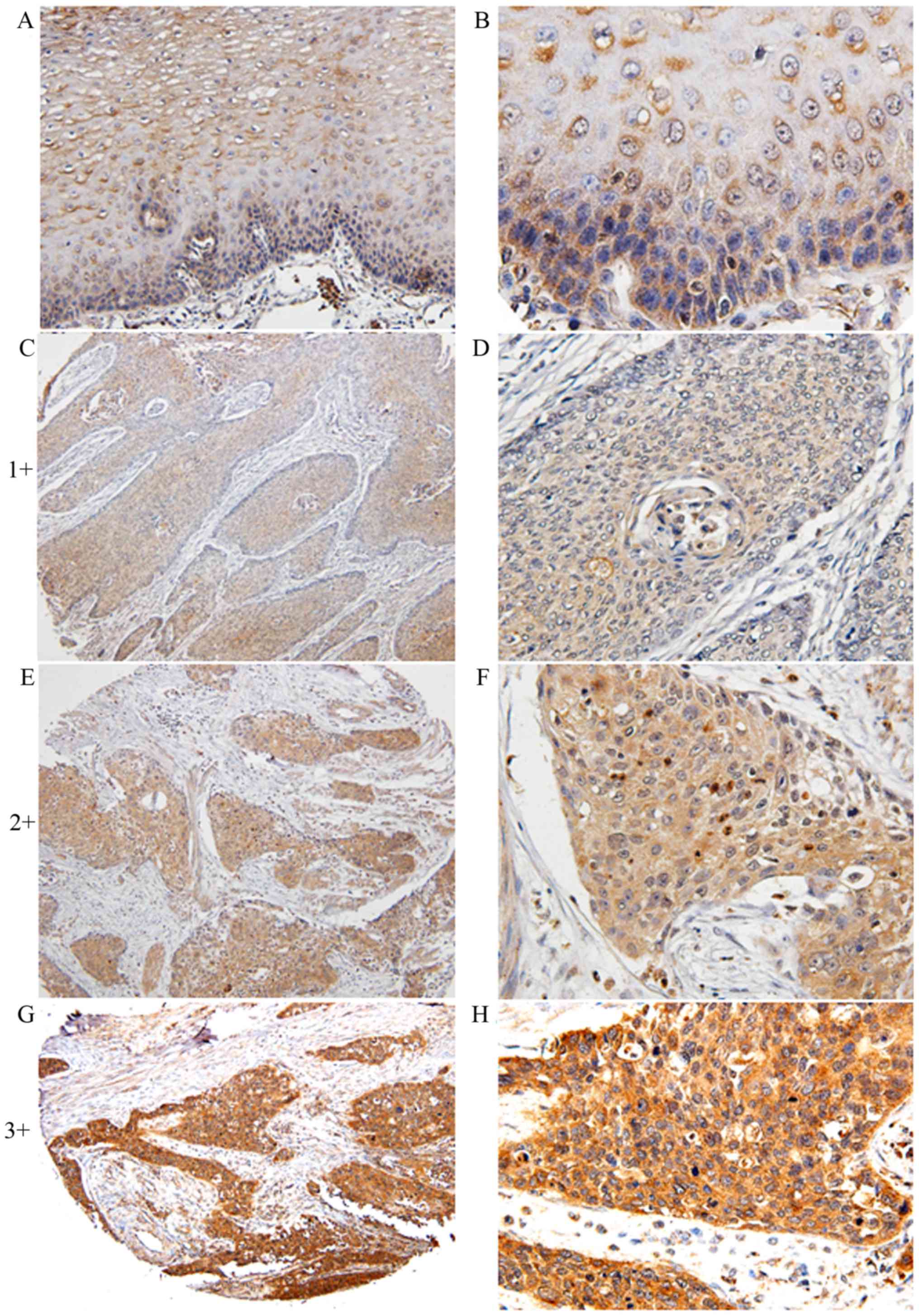
5-LO expression was detected in 297 ESCC samples and
66 pericarcinoma esophageal tissues using immunohistochemical
staining (Fig. 1). Specifically, in
pericarcinoma esophageal tissues, 5-LO was primarily expressed in
the cell cytoplasm or membrane (Fig. 1A
and B). In ESCC, 5-LO expression was localized in the cytoplasm
and/or nucleus of the tumor cells, but the expression intensity and
the percentage of positive cells were upregulated (Fig. 1C-G). 5-LO staining patterns in cancer
cells were heterogeneous and high 5-LO expression (sum index score
≥5) was detected in 122/297 (41.1%) tumor samples, but only 6/66
(11%) pericarcinoma esophageal tissues (P<0.05).
Association between 5-LO expression
and clinicopathological data
Statistical analysis revealed an association between
5-LO expression and regional lymph node metastasis (48.3 vs. 34%;
Χ2=0.145; P=0.013) and advanced pathological (p)TNM
stage (50.4 vs. 33.5%; X2=0.17; P=0.004). However, no
significant associations were identified between 5-LO expression
and other clinicopathological factors, including gender, age, tumor
location or distant metastasis (Table
I).
 | Table I.Association between 5-LO expression
and parameters in with esophageal squamous cell carcinoma. |
Table I.
Association between 5-LO expression
and parameters in with esophageal squamous cell carcinoma.
| Clinical
parameters | Low 5-LO
expression | High 5-LO
expression | Χ2 | P-value |
|---|
| Age (years) |
|
| 0.008 | 0.907 |
|
<59 | 89 | 63 |
|
|
| ≥59 | 86 | 56 |
|
|
| Sex |
|
| 0.055 | 0.393 |
| Male | 140 | 92 |
|
|
|
Female | 35 | 30 |
|
|
| Tumor size (cm) |
|
| 0.073 | 0.216 |
| ≤3 | 37 | 28 |
|
|
|
3–5 | 77 | 63 |
|
|
|
>5 | 59 | 31 |
|
|
| Tumor location |
|
| 0.021 | 0.735 |
|
Upper | 11 | 8 |
|
|
|
Middle | 77 | 56 |
|
|
|
Lower | 87 | 58 |
|
|
| Differentiation
grade |
|
| 0.088 | 0.119 |
| G1 | 32 | 13 |
|
|
| G2 | 129 | 98 |
|
|
| G3 | 14 | 11 |
|
|
| Invasive depth |
|
| 0.094 | 0.134 |
|
T1+T2 | 39 | 18 |
|
|
|
T3+T4 | 136 | 104 |
|
|
| Regional lymph
nodes |
|
| 0.145 | 0.013a |
| N0 | 99 | 51 |
|
|
|
N1+N2+N3 | 76 | 71 |
|
|
| pTNM stage |
|
| 0.17 | 0.004a |
|
I+II | 109 | 55 |
|
|
|
III+IV | 66 | 67 |
|
|
Association between 5-LO expression
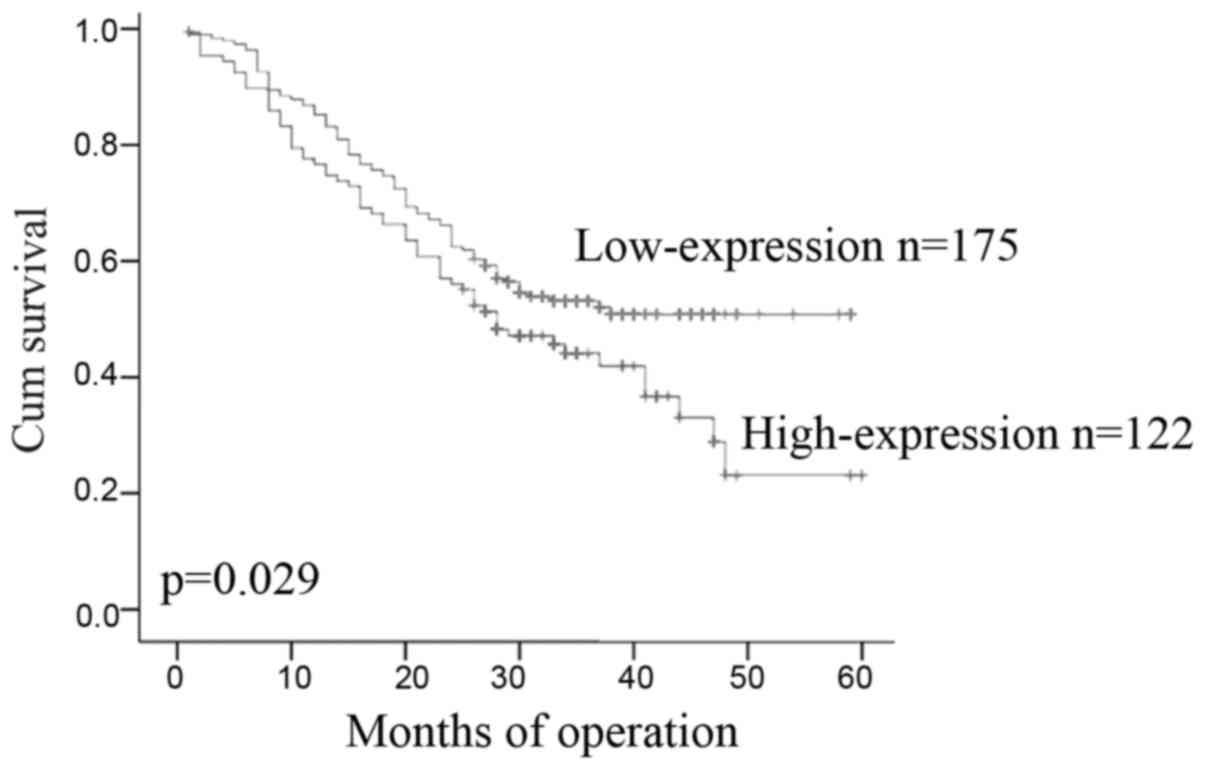
and overall survival in ESCC patients
The median post-operative survival time was 26.77
months. A total of 122 (41.1%) patients had ESCC expressing high
5-LO and 175 (58.9%) patients had ESCC expressing low 5-LO. The
Kaplan-Meier curves showed that stronger 5-LO immunoreactivity was
significantly associated with poorer overall survival in patients
with ESCC (P=0.029; Fig. 2).
Using a Cox proportional hazards model, a
multivariate analysis was performed to assess predictors for
overall survival of this cohort of patients. It was demonstrated
that pTNM stage (P<0.001), depth of tumor invasion (P=0.031),
Histology (P=0.012), age (P=0.035), regional lymph node metastasis
(P<0.001) and 5-LO expression (P=0.041) were independent
prognostic factors for survival in patients with ESCC (Table II).
 | Table II.Multivariate Cox regression analysis
of clinicopathological factors for risk prediction in 297 patients
with esophageal squamous cell carcinoma. |
Table II.
Multivariate Cox regression analysis
of clinicopathological factors for risk prediction in 297 patients
with esophageal squamous cell carcinoma.
| Factors | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| Age | 1.419 | 1.026–1.964 | 0.035 |
| Gender | 0.912 | 0.618–1.346 | 1.346 |
| Tumor size | 0.844 | 0.583–1.222 | 0.369 |
| Tumor location | 1.311 | 0.713–2.41 | 0.383 |
| Invasive depth | 0.482 | 0.248–0.934 | 0.031 |
| Histology | 0.433 | 0.226–0.831 | 0.012 |
| Lymph node
metastasis | 1.992 | 1.429–2.779 | <0.001 |
| pTNM stage | 2 | 1.44–2.778 | <0.001 |
| 5-LO high
expression | 1.412 | 1.014–1.967 | 0.041 |
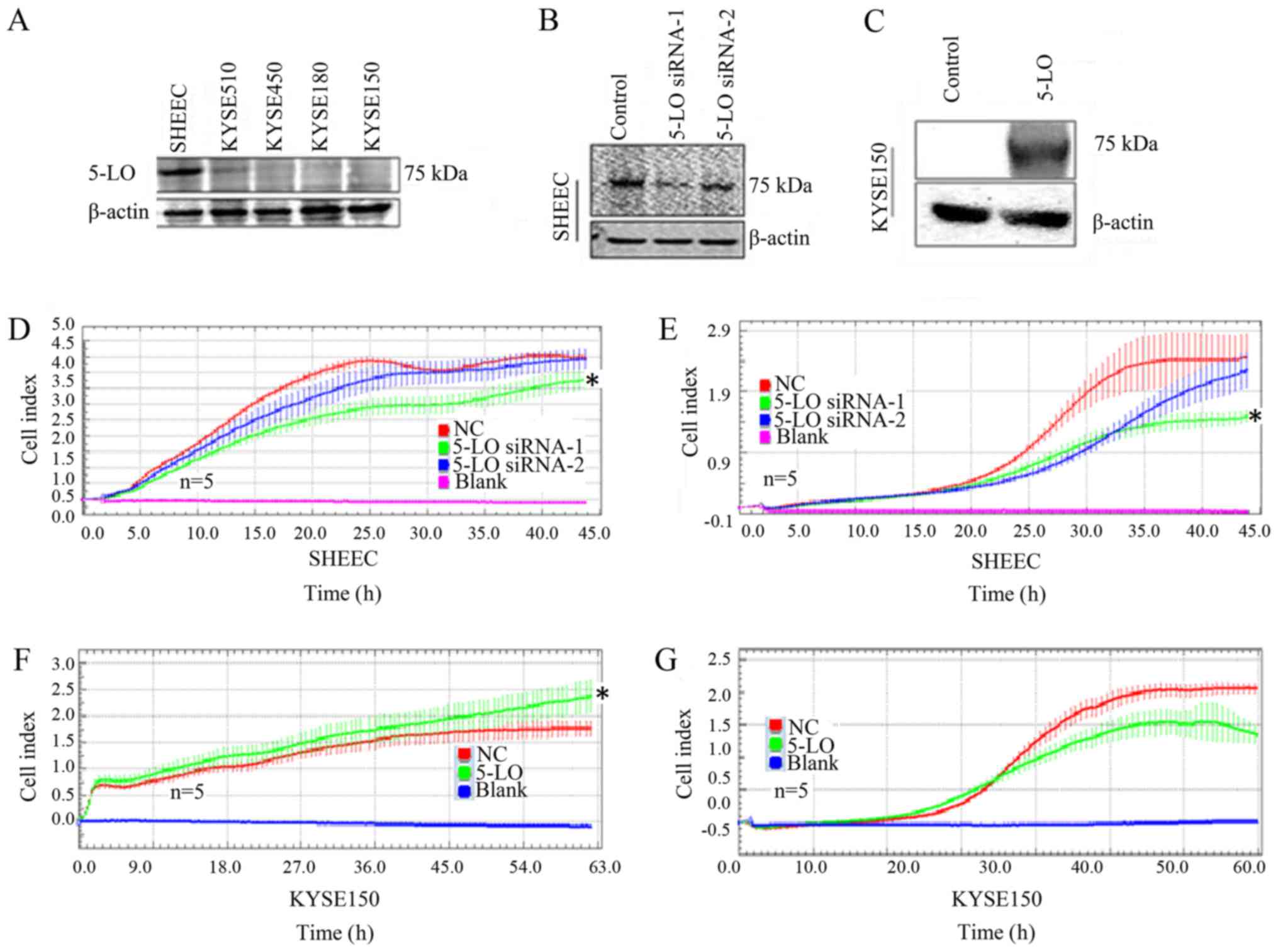
5-LO expression regulates ESCC cell
viability and migration in vitro
The expression of 5-LO in ESCC cell lines was
analyzed and manipulated to determine the effects on cell
proliferation, and migration (Fig.
3). It was revealed that SHEEC cells had a high level of 5-LO
protein expression, KYSE510 exhibited intermediate levels of 5-LO
expression, and KYSE150, KYSE180 and KYSE450 had low expression
levels (Fig. 3A). Next, 5-LO
expression was manipulated in the selected ESCC cell lines via
transfection with 5-LO cDNA or siRNA. Two constructs of 5-LO siRNAs
were transfected into SHEEC cells that expressed high levels of
5-LO protein, and these two siRNAs were able to knock down 5-LO
expression (Fig. 3B). Real-time
analysis of cell proliferation and migration revealed that 5-LO
knockdown reduced tumor cell proliferation and migration capacities
compared with the control (Fig. 3D and
E). Regardless of cell proliferation or mobile ability, for
0–45 h after siRNA interference, the level of 5-LO expression
relative to the control group was significantly decreased,
particularly in the 5-LO siRNA-1-transfeced cells.
Furthermore, plasmids carrying 5-LO cDNA were
transfected into KYSE150 cells, which increased the protein
expression of 5-LO (Fig. 3C).
Real-time analysis of cell proliferation demonstrated that 5-LO
significantly increased cell proliferation for 0–63 h after 5-LO
transfection compared with the vector control-transfected cells
(t-test, P<0.05, Fig. 3F, the
statistical significance on the relevant graphs using *,); however,
the migration of ESCC cells was not significantly affected by 5-LO
expression for 0–70 h (P>0.05; Fig.
3G).
Discussion
5-LO was originally reported to be primarily
expressed in leukocytes, including B-lymphocytes, polymorphonuclear
leukocytes (neutrophils and eosinophils), mast cells,
monocytes/macrophages and foam cells or dendritic cells of human
atherosclerotic tissue (20). The
function of 5-LO is to convert arachidonic acid to
hydroxyeicosatetraenoic acids (5-HETE) or LTs. However, previous
studies revealed that an increased expression of 5-LO was
associated with human carcinogenesis (4–12). In
esophageal cancer, a previous cDNA microarray study of 14,803 genes
identified nine genes that were upregulated and 36 genes that were
downregulated in ESCC tissue specimens (11). In addition, nine of the altered gene
expressions were associated with arachidonic acid metabolism and
5-LO was upregulated in esophageal cancer (11). The present study, together with other
studies (10,12), indicated that 5-LO upregulation may
play a role in ESCC development; thus, the current study aimed to
further investigate 5-LO expression in ESCC tissue specimens, and
its association with clinicopathological and survival data from
patients with ESCC. The data of the current study revealed that
5-LO was highly expressed in ESCC tissue specimens, expression of
which was significantly associated with advanced stage of disease
and tumor lymph node metastasis, and was identified as an
independent predictor for overall survival of patients.
Additionally, the limited in vitro data indicated that 5-LO
expression induced tumor cell proliferation, whereas the knockdown
of 5-LO expression suppressed tumor cell proliferation and
migration. The current data indicated that 5-LO expression may
serve as a prognostic marker and that targeting 5-LO may represent
a viable therapeutic strategy in patients with ESCC.
Numerous studies have verified that 5-LO is
overexpressed in a variety of tumors. Increased 5-LO expression was
observed in glioblastoma cell lines and astrocytoma tissue
specimens (21). The current study
demonstrated that 5-LO expression was upregulated in ESCC tissues
compared with pericarcinoma tissue, and that high 5-LO expression
was associated with ESCC metastasis to the regional lymph nodes and
advanced pTNM stage. The results of the present study are
consistent with previous studieson other cancer types (4–9); for
example, 5-LO expression was associated with lymph node metastasis
in breast cancer (4). To the best of
our knowledge, the current study was the first to associate 5-LO
expression with poor overall survival in patients with ESCC. This
is consistent with previous studies on other cancer types
demonstrating an association between 5-LO expression and poor
survival in patients with high-grade astrocytoma, and breast cancer
(4,20).
Furthermore, a larger panel of data supported that
the 5-LO signaling pathway exerts profound effects on human
tumorigenesis and progression, and that targeting 5-LO may lead to
cancer treatment or prevention (20–25).
However, the mechanism underlying how 5-LO promotes malignant
transformation and malignant tumor behavior in vivo remains
unclear. The present study revealed that the knockdown of 5-LO
expression inhibited ESCC proliferation and migration capacity,
whereas 5-LO overexpression promoted ESCC cell proliferation, but
did not promote tumor cell migration. The current in vitro
data are preliminary, and further studies are required to
investigate the effects of 5-LO knockdown and expression in the
regulation of ESCC viability, apoptosis, migration, invasion, and
independent growth in soft agar, as well as the underlying
molecular mechanism. In other cancer types, 5-LO was revealed to
enhance cell proliferation and inhibit apoptosis (22). 5-LO was also reported to selectively
inhibit tumor suppressor gene p53 transcription (23). Furthermore, 5-LO expression promoted
tumor angiogenesis by inducing the expression of vascular
endothelial growth factor (VEGF) (24), which is currently one of the strongest
targets in promoting or generating tumor blood vessel formation
(20). 5-LO was reported to induce
VEGF transcription through 5-HETE, a product of 5-LO (20), whereas 5-LO inhibitors were able to
decrease VEGF expression in colon cancer cells (25). These data clearly demonstrated that
targeting 5-LO expression or activity may modulate certain human
cancer activity; however, the arachidonic acid metabolism pathway
is complicated and difficult to regulate. Thus, further studies are
warranted to determine whether targeting 5-LO is sufficient for
regulating ESCC.
In conclusion, the results of the current study
revealed that increased 5-LO expression in ESCC tissue specimens is
associated with ESCC metastasis to the lymph node and advanced
stages of disease. Increased 5-LO expression was also identified as
an independent predictor for ESCC survival. Furthermore, the
inhibition of 5-LO expression suppressed the proliferation and
migration of ESCC cells in vitro. These results supported
that 5-LO is a potential target for the prevention and treatment of
esophageal cancer in clinical.
Acknowledgements
The authors would like to thank the Inner Mongolia
Key Laboratory of Human Genetic Diseases (Medical College of
Chifeng University, Chifeng, Inner Mongolia, China), for provide
cell culture instruments for this study.
Funding
The present study was supported in part by the
Natural Science Foundation of China (grant no. 81360331 to CB,
grant no. 81201844 to JZ and grant no. 81502138 to ZD), the
National Basic Research Program (grant no. 2012CB526600 to LX), the
National High Technology Research and Development Program of China
(grant no. 2012AA02A503 to EL) and the Natural Science Foundation
of China-Guangdong Joint Fund (grant no. U1301227 to LX).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
EML and LYX designed the study. CYB and BLW were
responsible for statistical analysis. TWS and CYB were responsible
for cell culture, protein extraction and western blotting. ZPD, ZYW
and JYW collected tissue samples and clinical data of patients. YQB
and JuYZ were responsible for tumor cell proliferation and
migration assays. SHW and RYT were responsible for collection of
follow-up data for ESCC patients after surgery. XEX was responsible
for construction of tissue microarrays. The immunostained tissue
microarray section was reviewed and evaluated by YQB and JiYZ. CYB
was a major contributor in writing the manuscript. EML, LYX and
JiYZ revised the article. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Shantou University Medical College. Written informed consents were
obtained from all the study participants.
Consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W: Cancer statistics: Updated cancer
burden in China. Chinese J Cancer Res. 27:12015. View Article : Google Scholar
|
|
3
|
Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey
SM, Dong ZW, Mark SD, Qiao YL and Taylor PR: Prospective study of
risk factors for esophageal and gastric cancers in the Linxian
general population trial cohort in China. Int J Cancer.
113:456–463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang WG, Douglas-Jones AG and Mansel RE:
Aberrant expression of 5-lipoxygenase-activating protein (5-LOXAP)
has prognostic and survival significance in patients with breast
cancer. Prostaglandins Leukot Essent Fatty Acids. 74:125–134. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ou D, Bonomi P, Jao W, Jadko S, Harris JE
and Anderson KM: The mode of cell death in H-358 lung cancer cells
cultured with inhibitors of 5-lipoxygenase or the free radical spin
trap, NTBN. Cancer Lett. 166:223–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hennig R, Ding XZ, Tong WG, Schneider MB,
Standop J, Friess H, Büchler MW, Pour PM and Adrian TE:
5-Lipoxygenase and leukotriene B(4) receptor are expressed in human
pancreatic cancers but not in pancreatic ducts in normal tissue. Am
J Pathol. 161:421–428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ihara A, Wada K, Yoneda M, Fujisawa N,
Takahashi H and Nakajima A: Blockade of leukotriene B4 signaling
pathway induces apoptosis and suppresses cell proliferation in
colon cancer. J Pharmacol Sci. 103:24–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuyama M, Yoshimura R, Mitsuhashi M,
Hase T, Tsuchida K, Takemoto Y, Kawahito Y, Sano H and Nakatani T:
Expression of lipoxygenase in human prostate cancer and growth
reduction by its inhibitors. Int J Oncol. 24:821–827.
2004.PubMed/NCBI
|
|
9
|
Gupta S, Srivastava M, Ahmad N, Sakamoto
K, Bostwick DG and Mukhtar H: Lipoxygenase-5 is overexpressed in
prostate adenocarcinoma. Cancer. 91:737–743. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoque A, Lippman SM, Wu TT, Xu Y, Liang
ZD, Swisher S, Zhang H, Cao L, Ajani JA and Xu XC: Increased
5-lipoxygenase expression and induction of apoptosis by its
inhibitors in esophageal cancer: A potential target for prevention.
Carcinogenesis. 26:785–791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhi H, Zhang J, Hu G, Lu J, Wang X, Zhou
C, Wu M and Liu Z: The deregulation of arachidonic acid
metabolism-related genes in human esophageal squamous cell
carcinoma. Int J Cancer. 106:327–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boger PC, Shutt JD, Neale JR, Wilson SJ,
Bateman AC, Holloway JW, Patel P and Sampson AP: Increased
expression of the 5-lipoxygenase pathway and its cellular
localization in Barrett's adenocarcinoma. Histopathology.
61:509–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang H, Jia X, Chen X, Yang CS and Li N:
Time-selective chemoprevention of vitamin E and selenium on
esophageal carcinogenesis in rats: the possible role of nuclear
factor kappaB signaling pathway. Int J Cancer. 131:1517–1527. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi HY, Lv FJ, Zhu ST, Wang QG and Zhang
ST: Dual inhibition of 5-LOX and COX-2 suppresses esophageal
squamous cell carcinoma. Cancer Lett. 309:19–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amin MB, Edge S, Greene FL, et al: AJCC
Cancer Staging Manual (M). 8th edition. New York: Springer; pp.
185–202. 2017
|
|
16
|
Fang WK, Gu W, Li EM, Wu ZY, Shen ZY, Shen
JH, Wu JY, Pan F, Lv Z, Xu XE, et al: Reduced membranous and
ectopic cytoplasmic expression of DSC2 in esophageal squamous cell
carcinoma: An independent prognostic factor. Hum Pathol.
41:1456–1465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen Z, Cen S, Shen J, Cai W, Xu J, Teng
Z, Hu Z and Zeng Y: Study of immortalization and malignant
transformation of human embryonic esophageal epithelial cells
induced by HPV18 E6E7. J Cancer Res Clin Onco. 126:589–594. 2000.
View Article : Google Scholar
|
|
18
|
Lisovyy OO, Dosenko VE, Nagibin VS,
Tumanovska LV, Korol MO, Surova OV and Moibenko OO:
Cardioprotective effect of 5-lipoxygenase gene (ALOX5) silencing in
ischemia-reperfusion. Acta Biochima Pol. 56:687–694. 2009.
|
|
19
|
Ding X, Zhou X, Zhang H, Qing J, Qiang H
and Zhou G: Triptolide augments the effects of 5-lipoxygenase RNA
interference in suppressing pancreatic tumor growth in a xenograft
mouse model. Cancer Chemother Pharmacol. 69:253–261. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Radmark O and Samuelsson B:
5-Lipoxygenase: Mechanisms of regulation. J Lipid Res. 50
Suppl:S40–S45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nathoo N, Prayson RA, Bondar J, Vargo L,
Arrigain S, Mascha EJ, Suh JH, Barnett GH and Golubic M: Increased
expression of 5-lipoxygenase in high-grade astrocytomas.
Neurosurgery. 58:347–354; discussion 347–354S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tong WG, Ding XZ, Witt RC and Adrian TE:
Lipoxygenase inhibitors attenuate growth of human pancreatic cancer
xenografts and induce apoptosis through the mitochondrial pathway.
Mol Cancer Ther. 1:929–935. 2002.PubMed/NCBI
|
|
23
|
Gilbert B, Ahmad K, Roos J, Lehmann C,
Chiba T, Ulrich-Rückert S, Smeenk L, van Heeringen S, Maier TJ,
Groner B and Steinhilber D: 5-Lipoxygenase is a direct p53 target
gene in humans. Biochim Biophys Acta. 1849:1003–1016. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Romano M, Catalano A, Nutini M, D'Urbano
E, Crescenzi C, Claria J, Libner R, Davi G and Procopio A:
5-lipoxygenase regulates malignant mesothelial cell survival:
Involvement of vascular endothelial growth factor. FASEB J.
15:2326–2336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye YN, Wu WK, Shin VY and Cho CH: A
mechanistic study of colon cancer growth promoted by cigarette
smoke extract. Eur J Pharmacol. 519:52–57. 2005. View Article : Google Scholar : PubMed/NCBI
|