Introduction
Inflammatory myofibroblastic tumor (IMT) is a
mesenchymal neoplasm composed of myofibroblastic spindle cells in a
myxoid to collagenous stroma with an inflammatory infiltrate
chiefly comprised of plasma cells and lymphocytes, occasionally
admixed with eosinophils and neutrophils (1). IMT usually affects children and
adolescents, although a broad age range has been documented. The
most common anatomical locations are the abdominopelvic region,
lung, mediastinum and retroperitoneum (1).
In a mesenteric inflammatory myofibroblastic tumor,
T1 weighted sequence four minutes after Gadolinium injection
demonstrated intense enhancement of the peripheral inflammatory
component, whilst the central fibrotic component is hypovascular
(2). IMT is considered to be a soft
tissue tumor with an intermediate biological behavior. However, a
small percentage of cases behave aggressively (3).
Epithelioid inflammatory myofibroblastic sarcoma
(EIMS) is considered to be a variant of inflammatory
myofibroblastic tumor (IMT), as it has similar malignant
characteristics and mainly consists of round-to-epithelioid cells
(4) with a pattern of nuclear
membrane or perinuclear immunostaining for ALK receptor tyrosine
kinase (hereafter ALK). ALK is a receptor tyrosine kinase gene
located on chromosome 2p23. Rearrangements involving this gene
generally result in hyperactivity of ALK protein and correlate well
with ALK protein expression by immunohistochemistry (5). Subsequent studies further demonstrated
that this distinctive nuclear membrane staining pattern of ALK
corresponded to ALK-RANBP2 fusion (6–8). The
nuclear membrane staining pattern of ALK seems to be suggestive of
RANBP2-ALK fusion in IMT because it has not been observed in other
ALK rearrangements to date. The RANBP2 gene, located on chromosome
2q12, encodes a 358-kd nuclear pore protein and, therefore, has
been attributed to the nuclear membranous localization of ALK
expression by immunostaining (6,7).
Clinically, EIMS is more clinically aggressive than IMT and
patients exhibit reduced disease-free survival rates (4). Recognizing EIMS as a distinct variant of
IMT is very important as patients with ALK-rearrangement EIMS may
benefit from targeted therapy. EIMS closely resembles malignant
mesothelioma (MM), anaplastic large cell lymphoma (ALCL),
gastrointestinal stromal tumor (GIST) and other cancer types,
meaning it is difficult to diagnose. Magnetic resonance imaging
(MRI) enhanced scanning is a MRI scan following an intravenous
injection of a contrast agent. This is a useful clinical technique
for evaluating the severity, location and extent of the tumor
(9). As EIMS has not been widely
recognized, we analyzed its imaging, clinicopathological,
immunohistochemical, molecular cytogenetics and treatment. The aim
of the present study was to improve understanding of the
disease.
Materials and methods
Patient information and selection
A 26-year-old male patient diagnosed with EIMS, who
had been in abdominal pain for eighteen days and abdominal
distention for 1 week, presented to the Capital Medical University
Affiliated Beijing Shijitan Hospital (Beijing, China) in November
2015. This patient was selected for the present study as they
presented with inflammatory myofibroblastic tumor cells with a
round or epithelial morphology. The present study was approved by
ethics committee of the Capital Medical University Affiliated
Beijing Shijitan Hospital (Beijing, China) and the patient provided
written informed consent for inclusion.
Imaging examination
The lesion was observed with magnetic resonance
imaging enhanced scanning at the Capital Medical University
Affiliated Beijing Shijitan Hospital (Beijing, China). A MRI scan
was carried out following intravenous injection of some kind of
contrast agent Gd-DTPA (0.2 ml/kg, Consun Pharmaceutical Group
Limited, Guangzhou, China). Dynamic contrast-enhanced MRI has
become an important component of the multiparametric strategy and
is emerging as a useful clinical technique for evaluating the
severity, location and extent of the tumor (9).
Histopathological analysis
Removed surgical specimens were fixed in 10%
phosphate-buffered, neutral formaldehyde solution at room
temperature for 24 h and dehydrated in an ascending series of
ethanol. Samples were routinely embedded in paraffin, washed with
xylene, then rehydrated in a descending series of alcohol, washed
with distilled water, and stained with hematoxylin and eosin for 30
min at room temperature. Sections (4-µm thick) were observed under
a light microscope with the magnifications of ×40, ×100, ×200 and
×400.
Immunohistochemical study
Removed surgical specimens were fixed in 10%
phosphate-buffered, neutral formaldehyde solution at room
temperature for 24 h. Tissue sections (4-µm thick) were
deparaffinized, rehydrated and antigen retrieval with working
solution of EnVision, FLEX Target Retrieval solution High Ph (50×)
according to the manufacturer's protocol [EnVision
FLEX+, Mouse, high Ph (Link) HRP; cat. no. K8002; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA] in PT Link (cat.
no. PT100; Dako; Agilent Technologies, Inc.) at 95°C for 20 min,
and washed in distilled water (10).
Endogenous peroxidase was blocked by DAKO Envision flex
peroxidase-blocking reagent for 10 min, then washed again three
times in the EnVision™ FLEX Wash buffer (Dako; Agilent
Technologies, Inc.). The slides were incubated for 20–30 mins at
room temperature in humidity chamber with appropriate dilutions of
primary antibodies (primary antibodies are detailed in Table I) along with their positive and
negative controls. Immunohistochemistry was done manually according
to DAKO EnVision method (10), The
sections (4-µm thick) were then incubated with secondary antibody
(EnVision FLEX/HRP, cat. no. K8002; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) for coupling reaction for 20–30 min at
room temperature. The substrate (EnVision FLEX DAB+ Chromogen) was
used to produce crisp brown color at the site of target antigen.
The hematoxylin (1–2 dips) was used as a counter stain. Sections
were observed under a light microscope with the magnifications of
40, 100, 200 and ×400.
 | Table I.Primary antibodies used for
immunohistochemistry. |
Table I.
Primary antibodies used for
immunohistochemistry.
| Target | Supplier | Catalog number | Dilution | Staining |
|---|
| ALK | Origene Technologies,
Inc., Beijing, China | TA801287 | Ready to use | + |
| PD-L1 | Origene
Technologies, Inc. | TA507087 | Ready to use | + |
| PD-1 | Origene
Technologies, Inc. | TA314461 | Ready to use | Lymphocyte + |
| Ki-67 | Origene
Technologies, Inc. | UM870033 | 1:100 | Index 40% |
| P53 | Gene Tech Co.,
Ltd., Shanghai, China | GM700101 | Ready to use | + |
| DES | Origene
Technologies, Inc. | TA502328 | 1:60 | Focally + |
| CK | Origene
Technologies, Inc. | ZM-0069 | 1:80 | Focal + |
| EMA | Gene Tech Co.,
Ltd. | GM061329 | 1:200 | − |
| CAM5.2 | Origene
Technologies, Inc. | ZM-0316 | Ready to use | Focal + |
| Myoglobin | Origene
Technologies, Inc. | ZA-0192 | Ready to use | − |
| CALDES | Gene Tech Co.,
Ltd. | GM355701 | Ready to use | − |
| Vimentin | Gene Tech Co.,
Ltd. | GM072529 | 1:120 | + |
| CD30 | Gene Tech Co.,
Ltd. | GM075129 | 1:35 | − |
| CD3 | Origene
Technologies, Inc. | ZM-0417 | 1:120 | Lymphocyte + |
| CD20 | Gene Tech Co.,
Ltd. | GM075529 | 1:200 | Little lymphocyte
+ |
| CD4 | Origene
Technologies, Inc. | ZM-0418 | Ready to use | Lymphocyte + |
| CD8 | Gene Tech Co.,
Ltd. | GT211202 | Ready to use | Lymphocyte + |
| MC | Gene Tech Co.,
Ltd. | ZM-0386 | Ready to use | − |
| Calretinin | Origene
Technologies, Inc. | TA353630 | 1:60 | Focal + |
| WT-1 | Gene Tech Co.,
Ltd. | GM356102 | Ready to use | + |
| D2-40 | Gene Tech Co.,
Ltd. | GM361929 | 1:60 | − |
| HMB45 | Origene
Technologies, Inc. | ZM-0187 | Ready to use | − |
| CD117 | Origene
Technologies, Inc. | ZA-0523 | Ready to use | − |
| DOG1 | Origene
Technologies, Inc. | ZM-0371 | Ready to use | − |
| SMA | Origene
Technologies, Inc. | ZM-0003 | Ready to use | − |
Fluorescence in situ hybridization
(FISH)
FISH was performed on 4µm-thick paraffin sections
with Vysis ALK Break Apart FISH Probe kit, according to the
manufacturer's protocol (Abbott Laboratories, Chicago, IL, USA).
The probe of ALK dual color separation of Abbott Company are
composed of 3 ′one ALK orange fluorescent probe ~300 KB and 5 ′one
ALK green fluorescent probe ~442 KB. The hybridization results were
viewed by an epi-illumination fluorescence microscope
(maginification, ×1,000). For no ALK rearrangement, these specimens
contain fused orange and green signals or a single green signal
without a corresponding orange signal; whilst for ALK
rearrangement, these nuclei contained rearranged or ‘broken apart’
signals, 2 or more signal diameters apart.
Reverse transcription-polymerase chain
reaction (RT-PCR)
RT-PCR analysis was performed to investigate the
fusion location of the RAN binding protein 2 (RANBP2) and ALK gene.
Total RNA was extracted from 20 µm-thick paraffin sections
(AmoyDx® FFPE DNA and RNA Extraction kits; Amoy
Diagnostics Co., Ltd., Xiamen, China), and reverse transcription
(cat. no. ADx-AE01; Amoy Diagnostics Co., Ltd.) was conducted by
random hexamer primers method to cDNA. The primer sequences of
RANBP2-ALK were as follows: Forward, 5′-CAGACTCAGTGCCTGATGGATA-3′
and reverse, 5′-CGGAGCTTGCTCAGCTTGTA-3′. The PCR (2X Taq Master
mix, Takara Bio, Inc., Otsu, Japan) reaction ran for 35 cycles
under the following conditions: 95°C for 30 sec, 50°C for 30 sec
and 72°C for 60 sec. An expected 139-bp amplified product was
detected in the present case. β-actin was included as a reference
gene, the primer sequences of β-actin were as follows: Forward,
5′-CACAGTAGGTCTGAACAGACTC-3′, and reverse,
5′-AGTGATCTCCTTCTGCATCCTG-3′. RANBP2-ALK fusion point was confirmed
by direct sequencing (YMFX Biotech Co., Ltd.) of the chimeric cDNA
product. The 2−∆∆Cq method (11) was used to determine the relative
quantification of miR expression in the tissue samples.
Results
Magnetic resonance imaging
examination
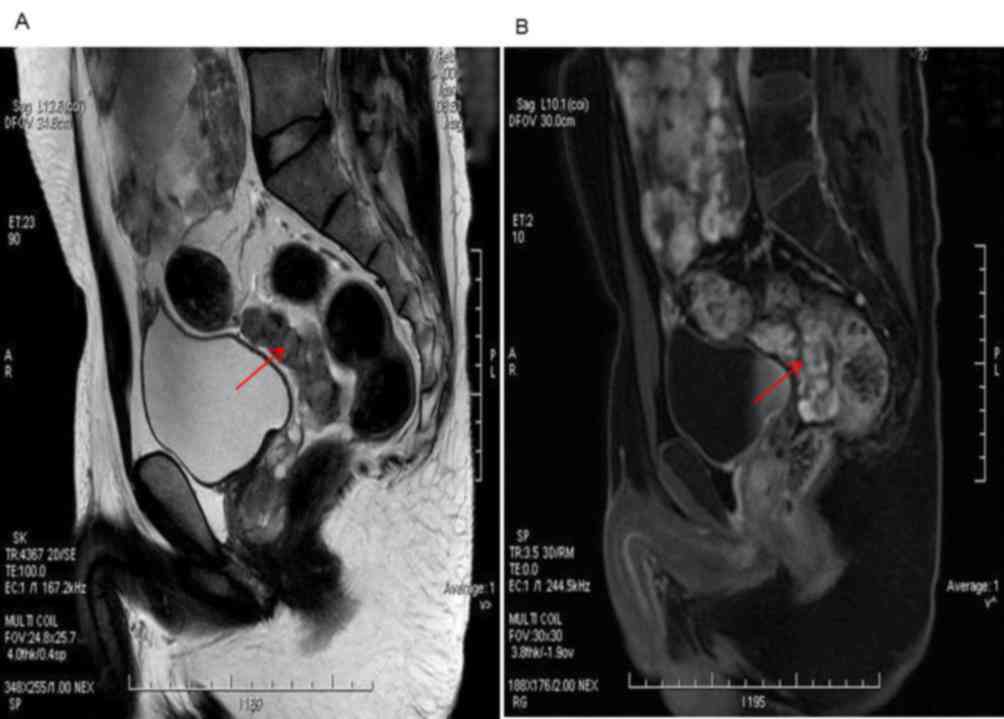
Images from magnetic resonance imaging enhanced
scanning demonstrated that the morphology of the tumor was
irregular, and signal was medley consisting of high and low hybrid
reinforcement. Tumors located in the bladder and rectal pit in the
lower part of the lower abdomen indicated the malignancy and
involvement of the small intestine and rectum (Fig. 1A). Inhomogeneity was observed
following enhanced scanning imaging (Fig.
1B).
Gross examination
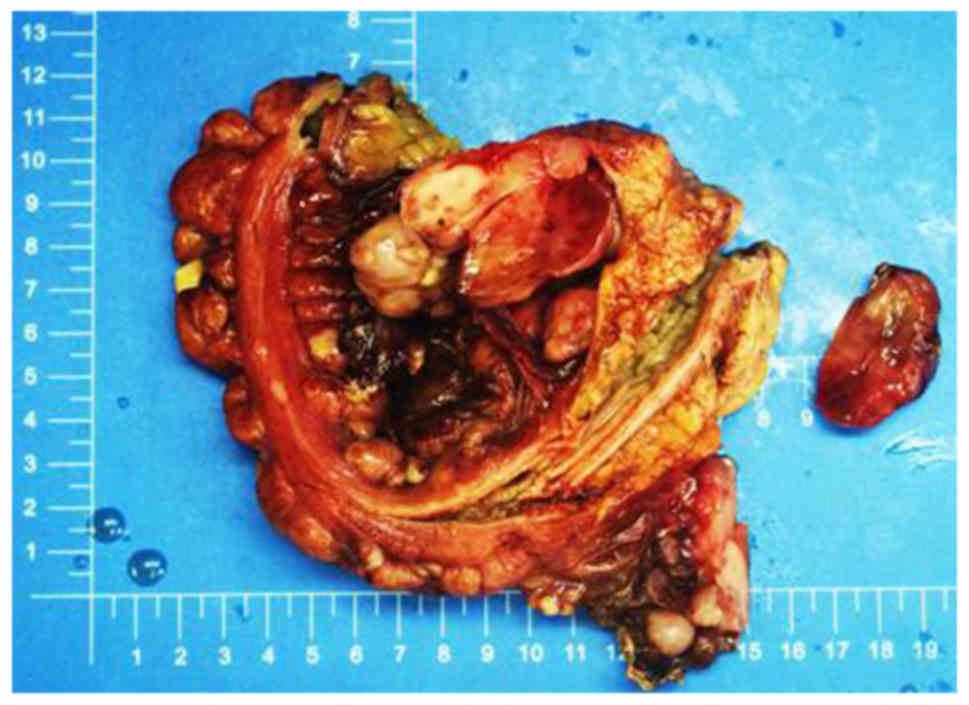
Gross examination revealed that the tumor was
located in the right abdominal wall (5.0×3.2×0.5 cm), left iliac
fossa (2.5×2.0×1.0 cm), the greater omentum (17×12×4.5cm), part of
the right hemi-colon (1.8×1.5×1.5–0.3×0.3×0.2 cm), the rectum
(5.6×3.5×4.0 cm) and the abdominal cavity (25×19×10 cm). The tumor
was solid and had a variegated appearance, with alternating fleshy
and mucoid areas at the cut surface (Fig.
2).
Histopathological examination
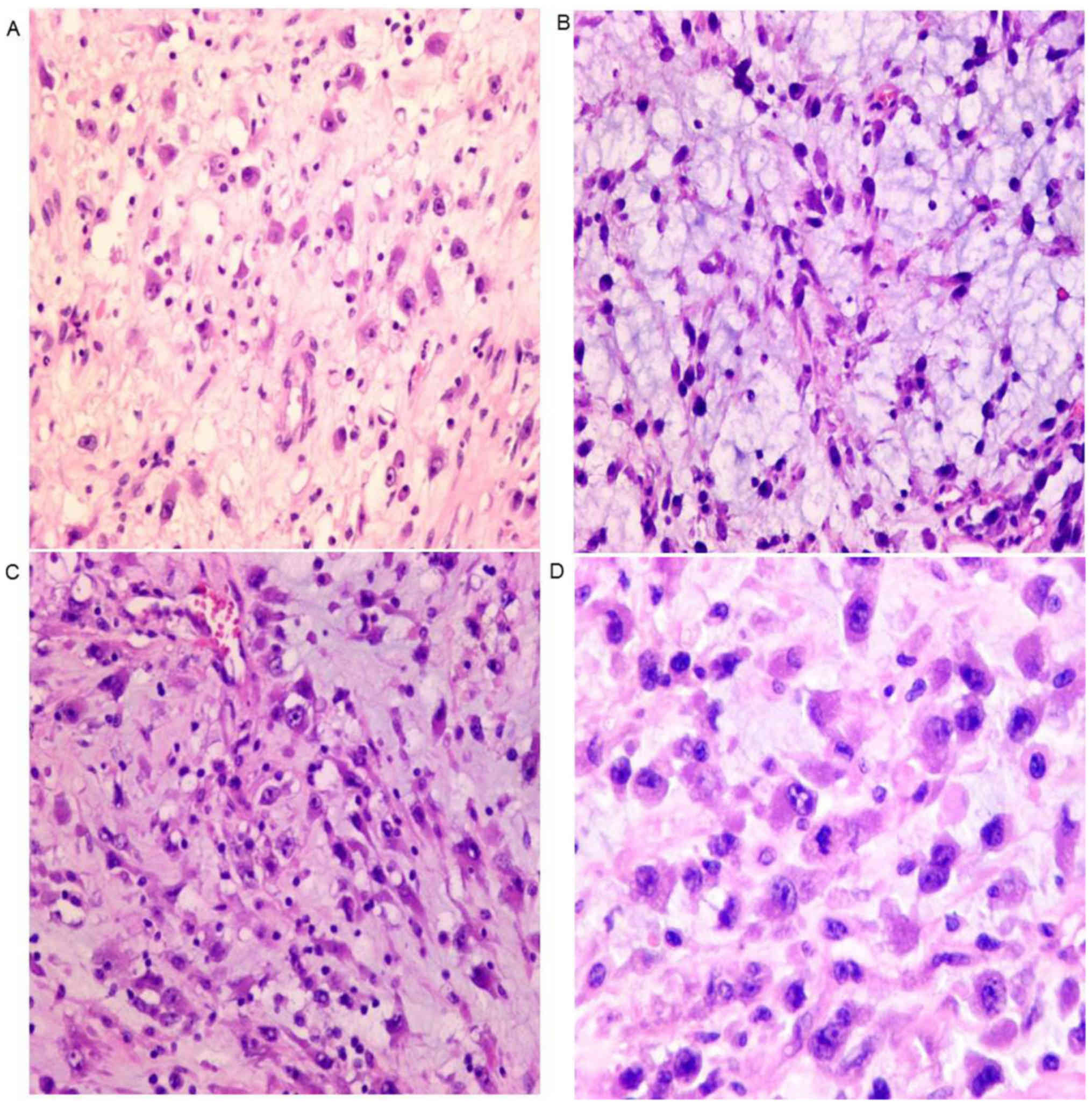
Microscopically, tumor cells were not densely
arranged. (Fig. 3A), and stroma is
myxoid (Fig. 3B). Lymphocytes
infiltrated into the stroma (Fig.
3C). The tumor cells were epithelioid, with eccentric nuclei,
prominent nucleoli and abundant eosinophilic cytoplasm (Fig. 3D).
Immunohistochemical examination
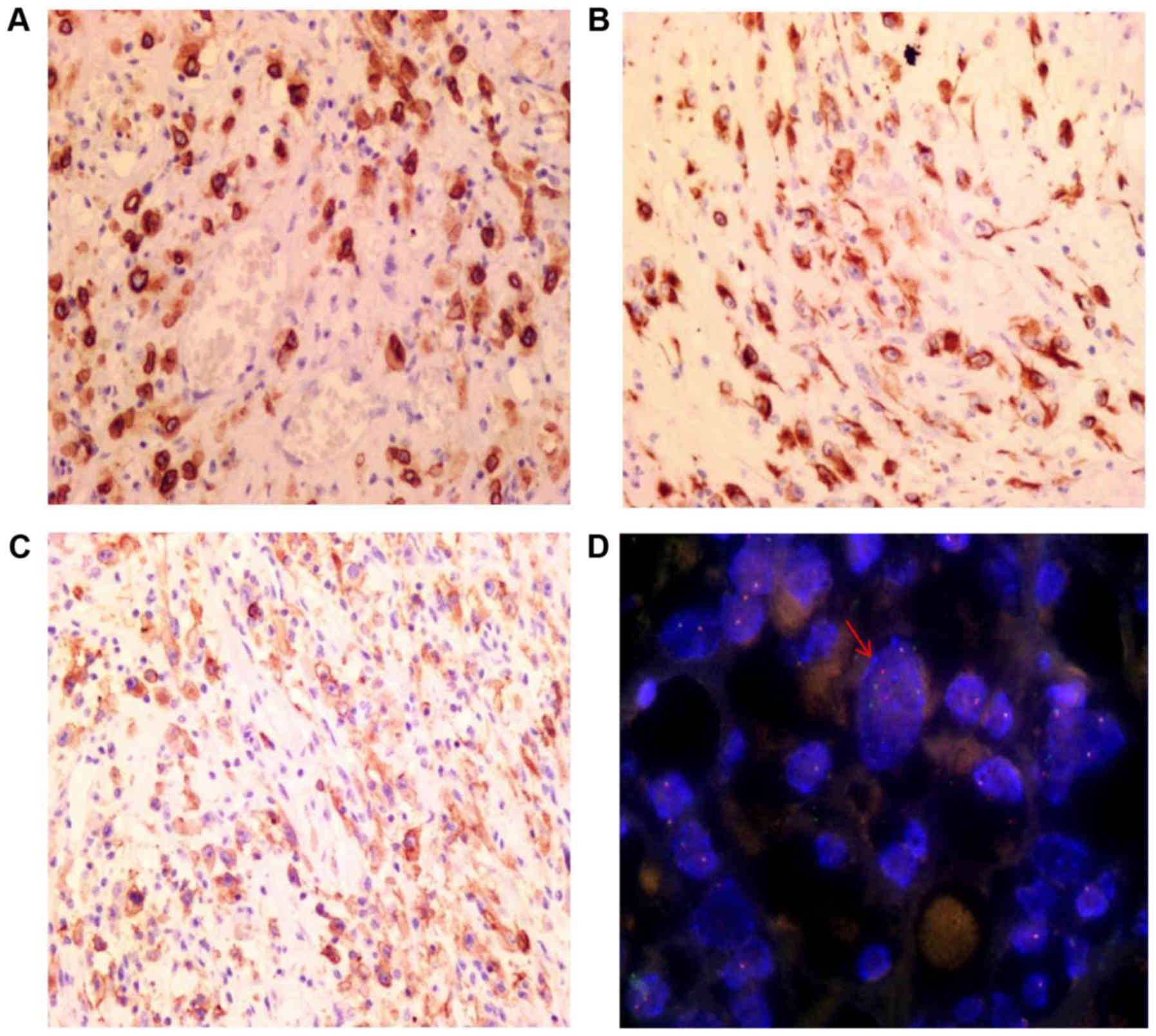
Immunohistochemical staining results are depicted in
Table I. The tumor cells exhibited
diffuse strong staining for ALK (Fig.
4A), DES (Fig. 4B), PDL1
(Fig. 4C) and vimentin. Similarly,
there was positive staining for Wilms' tumor 1 and tumor protein
P53. The Ki-67 index was ~40%. The tumor cells also exhibited
positive staining for the cytokeratins (CK) CAM5.2 and calretinin,
although this staining was weaker. There was no reactivity to
epithelial membrane antigen (EMA), smooth muscle actin (SMA),
anoctamin-1 (DOG1), CD117, antibody HMB-45, antibody D2-40,
mesothelial cells (MC), CD30, caldesmon or myoglobin. Infiltrated
lymphocytes were mainly T cells, with a small number of B cells
also present.
FISH analysis
FISH analysis revealed the presence of ALK
rearrangements through the identification of a set of separate
green and orange signals, a fused signal in tumor cell nuclei or
through only one orange signal and a fused signal (Fig. 4D).
Genetic features
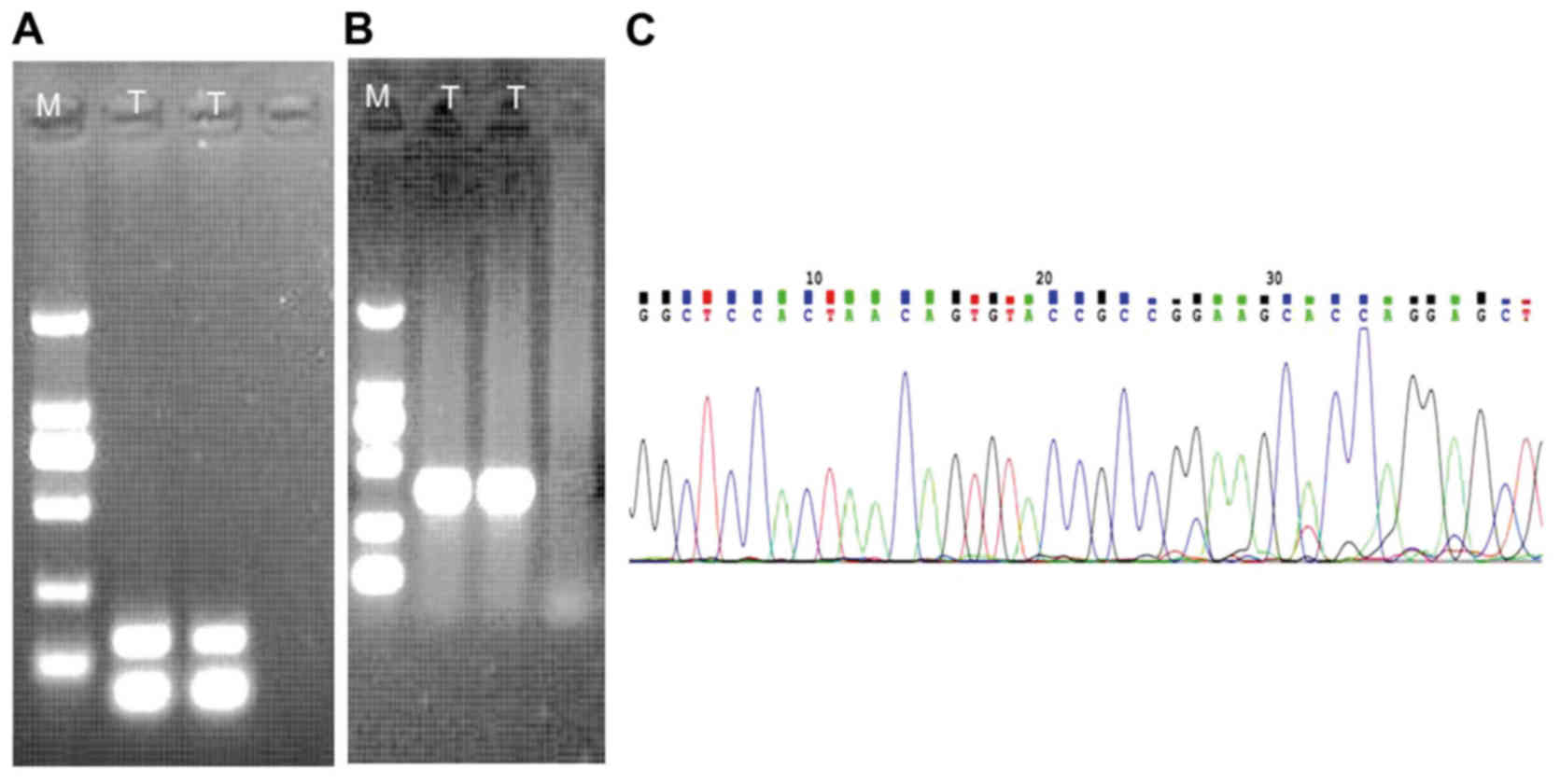
An expected 139-bp amplified product was detected in
the sample (Fig. 5A). Positively
amplified results (268-bp) of β-actin as a house-holding gene were
also present (Fig. 5B). The
RANBP2-ALK fusion point was at exon 18 of RANBP2 and exon 20 of ALK
(Fig. 5C), which was confirmed by
direct sequencing of the chimeric cDNA product.
Discussion
Clinically, EIMS occurs mainly in children and
adolescents, although the overall range in age varies widely, with
previous studies reporting on patients aged between 7 months and 65
years, with a median age of 33.4 years (Table II) (4,12–20).
 | Table II.Literature review of published EIMS
cases. |
Table II.
Literature review of published EIMS
cases.
| Author, year | Case | Age/Sex | Anatomic site | Treatment | Follow up,
months | (Ref.) |
|---|
| Marino-Enriquez
et al, 2011 | 1 | 59 years/M | Mesentery of the
small bowel | SE+CT | 12 (STD) | (4) |
|
| 2 | 41 years/M | Omentum | SE+CT+ALKi | 40 (ANED) |
|
|
| 3 | 6 years/M | Omentum | SE+CT | 13 (AWD) |
|
|
| 4 | 28 years/M | Mesentery of the
small bowel | NA | NA |
|
|
| 5 | 63 years/M | Mesentery of the
small bowel | SE+CT | 3 (DOD) |
|
|
| 6 | 42 years/M |
Intra-abdominal | SE+CT | 13 (AWD) |
|
|
| 7 | 7 months/M | Peritoneum | SE+CT+RT | 36 (STD) |
|
|
| 8 | 40 years/M | Peritoneum | SE+CT+RT | 28 (STD) |
|
|
| 9 | 31 years/F | Mesentery of the
small bowel | SE+CT | 11 (STD) |
|
|
| 10 | 6 years/M | Omentum and
mesentery | SE | NO |
|
|
| 11 | 39 years/M | Mesentery of the
small bowel | SE | NO |
|
| Li et al,
2013 | 12 | 19 years/F | Pelvic cavity | SE | 3 (STD) | (12) |
|
| 13 | 39 years/M | Pelvic cavity | SE+CT | Recurrent |
|
| Liu et al,
2015 | 14 | 22 years/M | Pelvic cavity | SE+ALKi | No recurrence | (13) |
| Kimbara et
al, 2014 | 15 | 22 years/M | Pelvic cavity | SE+CT+ALKi | TS | (14) |
| Kurihara-Hosokawa
et al, 2014 | 16 | 22 years/M | Pelvic cavity | SE+CT+ALKi | TS | (15) |
| Zhou et al,
2015 | 17 | 8 years/M | Pelvic cavity | SE | 8 (STD) | (16) |
| Wu et al,
2015 | 18 | 47 years/F | Pelvic cavity | SE+CT+RT | 8 (STD) | (17) |
| Bai et al,
2015 | 19 | 65 years/M | Colon | Resection SE+
Chinese medicine | Metastases | (18) |
| Yu et al,
2016 | 20 | 37 years/F | Rectum | SE | 8 (ANED) | (19) |
|
| 21 | 55 years/M | Mesentery of
ileum | SE+CT | 10 (ANED) |
|
|
| 22 | 22 years/M | Mesentery of
colon | SE+ALKi | 14 (AWD) |
|
|
| 23 | 58 years/F | Omentum | SE+CT | 8 (STD) |
|
|
| 24 | 15 years/F | Transverse
colon | SE | 7 (ANED) |
|
| Jiang et al,
2017 | 25 | 45 years/M | Abdominal
cavity | SE+crizotinib | 2 (DOD) | (20) |
| Kozu et al,
2014 | 26 | 57 years/M | Pleural cavity | ALKi | NO | (21) |
| Fu et al,
2015 | 27 | 21 years/M | Lung with multiple
bone metastases | SE+ALKi | 4 (STD) | (22) |
| Current case | 28 | 26 years/M | Pelvic cavity | SE+CT | 8 (STD) | − |
The majority of patients with EIMS are male. In
Table II, the male:female ratio is
21:7. EIMS is mainly located in abdominal cavity (4). In Table
II, 26 cases occurred in the abdominopelvic area (4,12–20), with two cases located in the pleural
cavity (21,22). The most common symptom of EIMS is
abdominal pain prior to surgery (19).
In soft tissue sarcomas, the tumors are often
heterogeneous in composition, including areas of fibrosis, mucous
degeneration, necrosis, and hemorrhage (23). Magnetic resonance imaging (MRI)
enhanced scanning shows that high signal is a rich blood supply
area (usually a tumor area), no enhancement area is a blood free
region (equivalent to a liquefied necrotic area). From high signal
to no signal, there also are different levels of reinforcement,
which are related to tumor tissue structure, tumor angiogenesis and
so on. A tumor can also appear high and low hybrid reinforcement.
In the present case, the tumor comprises mucous degeneration and
necrosis. The signal was medley consisting of high and low hybrid
reinforcement.
Common characteristics of EIMS include: i)
Round-to-epithelioid tumor cells; ii) abundant myxoid stroma with
inflammatory infiltrates; iii) immunopositivity for ALK with a
nuclear membrane or perinuclear staining pattern; iv) Desmin ES
positive in the cytoplasm of tumor cells; and v) the frequent
presence of the RANBP2-ALK fusion gene. All of these features were
identified in the present case, meaning a diagnosis of EIMS was
reached.
Immunohistochemically, EIMS exhibited nuclear
membrane or perinuclear accentuation staining pattern of ALK, which
was observed in 100% (25/25) of cases from a selection of previous
studies (Table III) (4,13–22). Another diagnostic immunophenotype is
the diffuse strong expression of DES in almost all cases (92%;
23/25). In addition, the tumors displayed variable expression of
SMA (45.5%, 10/22), CD30 (57.9%, 11/19) and cytokeratin (20%, 4/20)
(Table III) (4,13–22).
 | Table III.Immunohistochemical, cytogenetic and
molecular features of 28 cases of epithelioid inflammatory
myofibroblastic sarcoma. |
Table III.
Immunohistochemical, cytogenetic and
molecular features of 28 cases of epithelioid inflammatory
myofibroblastic sarcoma.
|
|
|
| Case | IHC | ALK |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Author, year |
| DES | SMA | CALDES | CD30 | CK | EMA | MYF4 | S100 | IHC | FISH | RT-PCR | (Refs.) |
|---|
| Marino-Enriquez
et al, 2011 | 1 | + + + | + | NA | NA | − | NA | − | − | +NM | + | NA | (4) |
|
| 2 | + + + | − | − | + + + | − | − | − | − | +NM | + | RANBP2-ALK |
|
|
| 3 | + + + | − | NA | NA | − | NA | − | − | +NM | + | NA |
|
|
| 4 | + + + | + + | NA | + + | − | − | NA | − | +NM | NA | NA |
|
|
| 5 | + + + | − | − | + + + | − | − | − | − | +PN | + | NA |
|
|
| 6 | + + + | NA | NA | NA | NA | NA | NA | NA | +NM | NA | NA |
|
|
| 7 | + + + | MA | − | + + + | NA | NA | − | NA | +PN | + | NA |
|
|
| 8 | − | + + | − | + + | − | − | − | − | +NM | + | NA |
|
|
| 9 | + + + | − | − | + + | − | NA | − | − | +NM | + | NA |
|
|
| 10 | + + + | NA | − | + + + | NA | NA | NA | NA | +NM | + | RANBP2-ALK |
|
|
| 11 | + + + | + + | − | + + + | − | NA | − | − | +NM | + | RANBP2-ALK |
|
| Liu et al,
2015 | 12 | + + | − | NA | + + | − | NA | NA | − | +PN | + | RANBP2-ALK | (13) |
| Kimbara et
al, 2014 | 13 | + + | + + | NA | NA | NA | NA | − | − | +NM | NA | RANBP2-ALK | (14) |
| Kurihara-Hosokawa
et al, 2014 | 14 | NA | NA | NA | NA | NA | NA | NA | NA | +NM | NA | RANBP2-ALK | (15) |
| Zhou et al,
2015 | 15 | + + + | + + + | NA | + + + | − | − | − | − | +NM | + | NA | (16) |
| Wu et al,
2015 | 16 | + + + | − | NA | + + + | − | − | NA | − | +PN | + | RANBP2-ALK | (17) |
| Bai et al,
2015 | 17 | + + + | + + + | NA | NA | + + | NA | NA | − | NA | NA | NA | (18) |
| Yu et al,
2016 | 18 | + + + | − | − | − | − | NA | − | − | +NM | + | NA | (19) |
|
| 19 | + + + | − | NA | − | − | − | − | − | +NM | + | NA |
|
|
| 20 | + + + | − | − | − | − | − | − | − | +PN | + | NA |
|
|
| 21 | + + + | + + | − | − | + + | NA | − | − | +NM | + | NA |
|
|
| 22 | − | + + + | NA | − | − | − | − | − | +NM | + | NA |
|
| Jiang et al,
2017 | 23 | + + + | + + + | NA | NA | NA | NA | NA | NA | +NM | + | RANBP2-ALK | (20) |
| Kozu et al,
2014 | 24 | + + + | − | − | − | + + | − | − | − | +NM and +PN | + | RANBP2-ALK | (21) |
| Fu et al,
2015 | 25 | + + + | − | − | − | − | − | − | − | +NM | + | NA | (22) |
| Current case | 26 | + + + | − | − | − | + + | − | − | − | +NM | + | RANBP2-ALK | − |
| Total |
| 92% | 45.5% | 0% | 57.9% | 20% | 0% | 0% | 0% | 100% | 100% | 100% |
|
|
|
| (23/25) | (10/22) | (0/13) | (11/19) | (4/20) | (0/12) | (0/18) | (0/21) | (25/25) | (21/21) | (10/10) |
|
Of the patients with IMT examined by Gleason and
Hornick (3), ~50% aberrantly
expressed the ALK protein, triggered by clonal rearrangements of
the ALK gene located on chromosome 2p23. EIMS tumors harbor a
specific RANBP2-ALK fusion gene resulting from t(2;2)(2q12; 2p23).
All 21 cases tested by FISH exhibited an ALK translocation signal.
Notably, all 10 cases in which the cDNA fusions were examined by
RT-PCR exhibited identical fusion points, between exon 18 of RANBP2
and exon 20 of ALK (Table III).
RANBP2 encodes a nuclear pore protein, which is likely to lead to
the nuclear membrane or perinuclear staining pattern in EIMS.
Diagnosing EMIS can be challenging owing to the
unusual epithelioid-to-round cell morphology and atypical nuclear
features. EMIS should be differentially diagnosed with diseases as
follows: i) Distinguishing EIMS from ALCL can be difficult, as the
rare sarcomatoid variant of ALCL can exhibit spindle cell
morphology and an overlapping immunophenotype, including reactivity
for CD30, ALK and SMA and non-reactivity for EMA. Strong expression
of DES and the distinctive nuclear membrane pattern of ALK staining
are not observed in ALCL. The RANBP2-ALK has never been reported in
ALCL either. ii) Malignant mesothelioma (MM), MC, CK5 and
calretinin are present in MM, but ALK is absent. iii) Epithelioid
GIST is positive for CD117, DOG1 and CD34. Mutations to c-Kit and
platelet derived growth factor-α are also present in GIST. GIST
exhibits negative ALK staining. iv) The solid variant of alveolar
rhabdomyosarcoma is frequently ALK-positive, lacks fibrovascular
stroma and forms sheets of round cells with variable
rhabdomyoblastic differentiation. Antibodies against MyoD and
myogenin are highly specific and sensitive for its diagnosis; and
v) epithelioid leiomyosarcoma (ELS) ELS commonly displays greater
cellular atypia and pleomorphism, and higher cellular density. ELS
generally lacks an extensive myxoid background and inflammatory
infiltrates (12) and lacks ALK
nuclear membrane expression (Table
IV).
 | Table IV.Differential diagnosis of EMIS. |
Table IV.
Differential diagnosis of EMIS.
| Disease | IHC | Molecular
pathology |
|---|
| EMIS | CD30 (+), ALK (+),
SMA (+), EMA (+) and DES (+) | RANBP2-ALK |
| ALCL | CD30 (+), ALK (+),
SMA (+), EMA (+) and DES (−) | T cell gene
rearrangement |
| MM | MC (+), CK5 (+),
Calretinin (+) and DES (−) | P16/CDKN2A |
| GIST | CD117 (+), DOG1 (+)
and CD34 (+) | c-Kit and
PDGFα |
| Alveolar
Rhabdomyosarcoma | Myogenin (+) | PAX3-FOXO1 and
PAX7-FOXO1 fusion gene |
| Epithelioid
leiomyosarcoma | ALK (−) | HMGA and MED12 |
Currently, the majority of cases of EMIS are treated
by surgical resection combined with chemotherapy (4,12). Several
reports indicated that patients with an ALK gene rearrangement had
a notable response to ALK targeted therapy (13–15);
however, disease recurrence is common following resection (24). In the present case, the patient was
treated with chemotherapy prior to and following surgical
resection, but succumbed as a result of cachexia due to tumor
metastasis to the thoracic cavity.
Staining for programmed death-ligand 1 (PD-L1) was
diffusely positive in the present case. The expression of PD-L1 in
this patient was comparable to that in other genitourinary cancer
types, such as bladder cancer (20%) (25), and non-genitourinary cancer types,
such as breast cancer (23.4%) (26),
colorectal cancer (36%) (27),
testicular seminomas (73%) (28) and
cancer of the oral cavity (73%) (29). The PD-1/PD-L1 axis has a notable role
in the immune antitumor response (30,31). PD-L1
expression in tumor cells is considered to be predictive of the
tumor response to immunomodulatory therapies targeting the
PD-1/PD-L1 pathway (32). Unlike
chemotherapy and molecularly targeted therapy, the checkpoint
blockade immunotherapies result in durable clinical responses
through the induction, activation, and expansion of tumor-specific
cytotoxic T cells. Immune checkpoints serve an essential role in
maintaining self-tolerance and regulating the amplitude and
duration of T cell responses (33).
Immunotherapies with checkpoint blockade antibodies that block PD-1
(or its ligand PD-L1) can restore and augment cytotoxic T cell
responses against chemotherapy-refractory tumors, leading to
durable responses and prolonged overall survival with tolerable
toxicity (33).
In conclusion, EIMS is a highly aggressive IMT
variant with epithelioid-to-round cell morphology, myxoid stroma
and nuclear membrane or perinuclear ALK staining. Detection of ALK
rearrangement in the present study provides further evidence for
the diagnosis of the tumor and a reliable reference for
ALK-targeted therapy. The expression of PD-L1 in EIMS indicated the
immune checkpoint blockade, which could represent a novel anti-EIMS
therapy.
Acknowledgements
The authors would like to thank to Mr. Yongqi Chen
(Department of Pathology, Beijing Aerospace General Hospital,
Beijing, China) for editing the language of this paper.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
2016 Basic clinical cooperation project of China Capital Medical
University (grant no. 3500-11722913).
Availability of data and materials
The datasets generated in the present study are
available on reasonable request from the corresponding author.
Authors' contributions
XD was responsible for consulting literature,
reviewing slices and drafting the manuscript. YG conducted the
molecular genetic studies and immunohistochemistry experiments. HZ
made was responsible for the paraffin sections. BL obtained and
analyzed the MRI report. WX conducted the FISH experiment. DW took
part in analysis and interpretation of data, edited the language of
this paper and provided funding support.
Ethics approval and consent to
participate
The study protocol was approved by the Medical
Ethics Committee of Beijing Shijitan Hospital, Capital Medical
University (Beijing, China).
Consent for publication
Written informed consent was obtained from patient's
father.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fletcher CDM, Bridge JA, Hogendoorn PCW
and Mertens F: World health organization classification of tumors
of soft tissue and bone. 4th edition. Lyon, France: IARC Press; pp.
83–84. 2013
|
|
2
|
Kirchgesner T, Danse E, Sempoux CH, Annet
L, Dragean ChA, Trefois P, Orabi Abbes N and Kartheuser A:
Mesenteric inflammatory myofibroblastic tumor: MRI and CT imaging
correlated to anatomical pathology. JBR-BTR. 97:301–302.
2014.PubMed/NCBI
|
|
3
|
Gleason BC and Hornick JL: Inflammatory
myofibroblastic tumours: Where are we now? J Clin Pathol.
61:428–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mariño-Enríquez A, Wang WL, Roy A,
Lopez-Terrada D, Lazar AJ, Fletcher CD, Coffin CM and Hornick JL:
Epithelioid inflammatory myofibroblastic sarcoma: An aggressive
intra-abdominal variant of inflammatory myofibroblastic tumor with
nuclear membrane or perinuclear ALK. Am J Surg Pathol. 35:135–144.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cook JR, Dehner LP, Collins MH, Ma Z,
Morris SW, Coffin CM and Hill DA: Anaplastic lymphoma kinase (ALK)
expression in the inflammatory myofibroblastic tumor: A comparative
immunohistochemical study. Am J Surg Pathol. 25:1364–1371. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patel AS, Murphy KM, Hawkins AL, Cohen JS,
Long PP, Perlman EJ and Griffin CA: RANBP2 and CLTC are involved in
ALK rearrangements in inflammatory myofibroblastic tumors. Cancer
Genet Cytogenet. 176:107–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma Z, Hill DA, Collins MH, Morris SW,
Sumegi J, Zhou M, Zuppan C and Bridge JA: Fusion of ALK to the
Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic
tumor. Genes Chromosomes Cancer. 37:98–105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen ST and Lee JC: An inflammatory
myofibroblastic tumor in liver with ALK and RANBP2 gene
rearrangement: combination of distinct morphologic,
immunohistochemical, and genetic features. Hum Pathol.
39:1854–1858. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verma S, Turkbey B, Muradyan N, Rajesh A,
Cornud F, Haider MA, Choyke PL and Harisinghani M: Overview of
dynamic contrast-enhanced MRI in prostate cancer diagnosis and
management. AJR Am J Roentgenol. 198:1277–1288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashmi AA, Hussain ZF, Faridi N and
Khurshid A: Distribution of Ki67 proliferative indices among WHO
subtypes of non-Hodgkin'slymphoma: Association with other clinical
parameters. Asian Pac J Cancer Prev. 15:8759–8763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Yin WH, Takeuchi K, Guan H, Huang YH
and Chan JK: Inflammatory myofibroblastic tumor with RANBP2 and ALK
gene rearrangement: A report of two cases and literature review.
Diagn Pathol. 8:1472013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Q, Kan Y, Zhao Y, He H and Kong L:
Epithelioid inflammatory myofibroblastic sarcoma treated with ALK
inhibitor: A case report and review of literature. Int J Clin Exp
Pathol. 8:15328–15332. 2015.PubMed/NCBI
|
|
14
|
Kimbara S, Takeda K, Fukushima H, Inoue T,
Okada H, Shibata Y, Katsushima U, Tsuya A, Tokunaga S, Daga H, et
al: A case report of epithelioid inflammatory myofibroblastic
sarcoma with RANBP2-ALK fusion gene treated with the ALK inhibitor,
crizotinib. Jpn J Clin Oncol. 44:868–871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kurihara-Hosokawa K, Kawasaki I, Tamai A,
Yoshida Y, Yakushiji Y, Ueno H, Fukumoto M, Fukushima H, Inoue T
and Hosoi M: Epithelioid inflammatory myofibroblastic sarcoma
responsive to surgery and an ALK inhibitor in a patient with
panhypopituitarism. Intern Med. 53:2211–2214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Jiang G, Zhang D, Zhang L, Xu J,
Li S, Li W, Ma Y, Zhao A and Zhao Z: Epithelioid inflammatory
myofibroblastic sarcoma with recurrence after extensive resection:
Significant clinicopathologic characteristics of a rare aggressive
soft tissue neoplasm. Int J Clin Exp Pathol. 8:5803–5807.
2015.PubMed/NCBI
|
|
17
|
Wu H, Meng YH, Lu P, Ning HY, Hong L, Kang
XL and Duan MG: Epithelioid inflammatory myofibroblastic sarcoma in
abdominal cavity: A case report and review of literature. Int J
Clin Exp Pathol. 8:4213–4219. 2015.PubMed/NCBI
|
|
18
|
Bai Y, Jiang M, Liang W and Chen F:
Incomplete intestinal obstruction caused by a rare epithelioid
inflammatory myofibroblastic sarcoma of the colon. Medicine
(Baltimore). 94:e23422015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu L, Liu J, Lao IW, Luo Z and Wang J:
Epithelioid inflammatory myofibroblastic sarcoma: A
clinicopathological, immunohistochemical and molecular cytogenetic
analysis of five additional cases and review of the literature.
Diagn Pathol. 11:672016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Q, Tong HX, Hou YY, Zhang Y, Li JL,
Zhou YH, Xu J, Wang JY and Lu WQ: Identification of EML4-ALK as an
alternative fusion gene in epithelioid inflammatory myofibroblastic
sarcoma. Orphanet J Rare Dis. 12:972017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozu Y, Isaka M, Ohde Y, Takeuchi K and
Nakajima T: Epithelioid inflammatory myofibroblastic sarcoma
arising in the pleural cavity. Gen Thorac Cardiovasc Surg.
62:191–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu X, Jiang J, Tian XY and Li Z: Pulmonary
epithelioid inflammatory myofibroblastic sarcoma with multiple bone
metastases: Case report and review of literature. Diagn Pathol.
10:1062015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang W, Beckett BR, Tudorica A, Meyer JM,
Afzal A, Chen Y, Mansoor A, Hayden JB, Doung YC, Hung AY, et al:
Evaluation of soft tissue sarcoma response to preoperative
chemoradiotherapy using dynamic contrast-enhanced magnetic
resonance imaging. Tomography. 2:308–316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujiya M and Kohgo Y: ALK inhibition for
the treatment of refractory epithelioid inflammatory
myofibroblastic sarcoma. Intern Med. 53:2177–2178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Faraj SF, Munari E, Guner G, Taube J,
Anders R, Hicks J, Meeker A, Schoenberg M, Bivalacqua T, Drake C
and Netto GJ: Assessment of tumoral PD-L1 expression and
intratumoral CD8+ T cells in urothelial carcinoma. Urology.
85:703.e1–e6. 2015. View Article : Google Scholar
|
|
26
|
Muenst S, Schaerli AR, Gao F, Däster S,
Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE,
et al: Expression of programmed death ligand 1 (PD-L1) is
associated with poor prognosis in human breast cancer. Breast
Cancer Res Treat. 146:15–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Droeser RA, Hirt C, Viehl CT, Frey DM,
Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A,
Rosso R, et al: Clinical impact of programmed cell death ligand 1
expression in colorectal cancer. Eur J Cancer. 49:2233–2242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fankhauser CD, Curioni-Fontecedro A,
Allmann V, Beyer J, Tischler V, Sulser T, Moch H and Bode PK:
Frequent PD-L1 expression in testicular germ cell tumors. Br J
Cancer. 113:411–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Straub M, Drecoll E, Pfarr N, Weichert W,
Langer R, Hapfelmeier A, Götz C, Wolff KD, Kolk A and Specht K:
CD274/PD-L1 gene amplification and PD-L1 protein expression are
common events in squamous cell carcinoma of the oral cavity.
Oncotarget. 7:12024–12034. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zandberg DP and Strome SE: The role of the
PD-L1: PD-1 pathway in squamous cell carcinoma of the head and
neck. Oral Oncol. 50:627–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berger R, Rotem-Yehudar R, Slama G, Landes
S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A and Nagler A:
Phase I safety and pharmacokinetic study of CT-011, a humanized
antibody interacting with PD-1, in patients with advanced
hematologic malignancies. Clin Cancer Res. 14:3044–3051. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma W, Gilligan BM, Yuan J and Li T:
Current status and perspectives in translational biomarker research
for PD-1/PD-L1 immunecheckpoint blockade therapy. J Hematol Oncol.
9:472016. View Article : Google Scholar : PubMed/NCBI
|