Introduction
Colorectal cancer (CRC) is the third most common
type of cancer and the second leading cause of cancer-associated
mortality globally (1,2). The major CRC prognostic factor is the
stage at diagnosis. Early diagnosis of patients with CRC allows a
5-year survival rate of 90%, but <10% when advanced metastases
occur (3). Between 20 and 25% of
patients diagnosed with the disease already have metastases to
other organs, and between 50 and 60% of the remaining patients will
develop metastases (4,5).
The main treatment for CRC is surgery. However, in
patients with advanced CRC, surgery is not always able to prevent
progression of the disease. Therefore, chemotherapy is used
complementarily to decrease the risk of local recurrence (5,6). In total,
~50% of patients with CRC are candidates to receive chemotherapy
(5,6).
5-Fluorouracil (5-FU) has been the first-line and gold standard of
chemotherapy for the treatment of advanced CRC. 5-FU, a pyrimidine
antagonist, is converted intracellularly into active metabolites
that exert antitumor effects through the inhibition of thymidylate
synthase and disruption of RNA and DNA synthesis (7).
It is clear that 5-FU-based chemotherapy decreases
tumor recurrence and improves the overall survival rates of
patients with advanced CRC. However, only between 10 and 15% of
patients respond to 5-FU as a single first-line treatment as drug
resistance limits the effectiveness of monotherapy (8). To enhance the antitumor activity of 5-FU
and overcome its clinical resistance, this drug has been used in
combination with other cytotoxic agents. Different combinations of
these agents with 5-FU as the principal drug have been used to
develop a variety of chemotherapy protocols to treat patients with
advanced CRC. These modern chemotherapy regimens, including 5-FU +
lucoverin + irinotecan, 5-FU + oxaliplatin and capecetabine (a 5-FU
prodrug) + oxaliplatin with or without monoclonal antibodies, have
improved the response rate and outcome in patients with advanced
CRC (6,9–11). Despite
these substantial advances, the long-term survival of patients with
metastatic CRC has not been achieved (6). Therefore, the design of novel
chemotherapy protocols using more active drugs with fewer side
effects is urgently required.
Acriflavine (ACF), a naturally occurring compound,
is a mixture of 3,6-diamino-10-methylacridinium chloride
(trypaflavin) and 3,6-diaminoacridine (proflavine) and has a
history of clinical use (12). ACF is
a US Food and Drug Administration-approved drug that has been
administered topically or systemically for the treatment of
microbial infections (12). The
median lethal dose (LD50) of ACF in humans is unclear,
but the LD50 of ACF in mice is 30 mg/kg (13). It has been demonstrated that ACF
exhibits antitumor activity in several types of cancer, including
breast cancer, osteosarcoma and hepatocellular carcinoma (14–16). It
has also been demonstrated previously that ACF limits tumor growth
and progression in mouse models of colorectal cancer through
inhibition of hypoxia-inducible factor (HIF) (17). Importantly, long-term administration
of ACF to patients as an antiviral agent has not revealed any major
side effects (18).
Several molecular mechanisms have been proposed for
the anticancer property of ACF. Studies by Shay et al
(17) and Hassan et al
(19) proposed that cytotoxic
property of ACF in CRC cells may be associated with inhibition of
topoisomerase 2 and HIF-1α activity. However, the exact molecular
mechanism of action of ACF against CRC remains to be determined
(19). To the best of our knowledge,
it has not been investigated previously whether ACF is able to act
through the alteration of mRNA expression of these two important
proteins in CRC cells.
The aim of the present study was to investigate
whether it was possible to potentiate the anticancer property of
5-FU when combined with ACF in CRC cells. If this combination
protocol significantly enhanced the efficacy of 5-FU based
chemotherapy, it may be a basis for the development of other
preclinical and clinical studies to design new chemotherapy
regimens using ACF for those patients with advanced CRC who are
5-FU-resistant. In addition, the effect of ACF on the mRNA
expression level of topoisomerase 2 and HIF-1α was evaluated as a
potential molecular mechanism underlying the cytotoxic effect of
this drug on CRC cells.
Materials and methods
Chemicals and reagents
ACF, 5-FU, irinotecan and MTT were purchased from
Sigma; Merck KGaA (Darmstadt, Germany). Dimethylsulfoxide (DMSO)
was from Merck KGaA. Other reagents used for cell culture were
obtained from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Reagents were prepared and stored according to the
manufacturers' protocol.
Cell lines and cell culture
The human colon cancer cell lines SW480, HCT116 and
LS174T were obtained from the National Cell Bank of Iran (Pasteur
Institute, Tehran, Iran). Cells were cultured in either RPMI-1640
medium (SW480) or Dulbecco's modified Eagle's medium (DMEM; HCT116
and LS174T) supplemented with 10% fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in a humidified
atmosphere containing 5% CO2.
Drug cytotoxicity assay
An MTT assay was used to determine the cytotoxic
effect of ACF, 5-FU and irinotecan, a standard chemotherapy drug
routinely used with 5-FU, on CRC cell lines, as described
previously (20). The optimum number
of cells/well for 72 h of incubation was first determined. CRC
cells were seeded in 96-well plates at density of 8×103
cells/well in 100 µl DMEM or RPMI medium. At 1 day after seeding,
ACF (0.07–5 µM), 5-FU (0.125–128 µM) and irinotecan (2.5–80 µM)
were added at the specified concentration and incubated at 37°C for
24, 48 or 72 h. The medium of untreated control cells was
replenished with medium without drugs. Following drug treatment, 20
µl MTT reagent was added to each well at a final concentration of
0.5 mg/ml and incubated for 4 h at 37°C in a humidified atmosphere
containing 5% CO2. The medium was then aspirated and
crystals were dissolved in 150 µl DMSO/well. The optical density at
570 nm (OD570) was determined using a microtiter plate
reader. After 72 h, the confluency of untreated control was between
80 and 90%. Cell viability was calculated using the following
formula: Cell viability=OD570 (sample)/OD570
(control) ×100.
Determination of half-maximal
inhibitory concentration (IC50) and 30% maximal
inhibitory concentration (IC30)
The IC50 and IC30 values
associated with the cytotoxic effects of the drugs were calculated
using GraphPad Prism software (version 5.00; GraphPad Software, San
Diego, CA, USA) using non-linear regression model and dose-response
equations.
Drug co-treatment protocol
The stock solutions of ACF and 5-FU were prepared
and diluted in cell culture medium. Cells were treated with
different concentrations of 5-FU (0.5, 1, 2, 4, 8, 16, 32 and 64
µM) or ACF (0.07, 015, 0.31, 0.62, 1.25, 2.5 and 5 µM) for 72 h.
The IC50 values for the cytotoxic effects of either 5-FU
or ACF were subsequently calculated. To evaluate the effect of ACF
on the sensitivity of cells to 5-FU, cells were simultaneously
treated with a low cytotoxic concentration (IC30) of ACF
and different concentrations of 5-FU for 72 h. The cell viability
and the IC50 value of 5-FU in the co-treatment protocol
was compared with the IC50 value of 5-FU when used for
72 h as a single drug.
Drug pretreatment protocol
CRC cells were treated with different concentrations
of ACF (0.15, 0.31, 0.62, 1.25, 2.5 and 5 µM) or 5-FU (0.125, 0.25,
0.5, 1, 2, 4, 8, 16, 32, 64 and 128 µM) for 24 and 48 h,
respectively. IC50 values for the cytotoxic effects of
5-FU and IC30 values of ACF were calculated. To evaluate
the effect of ACF on the sensitivity of cells to 5-FU, cells were
pretreated with the IC30 of ACF for 24 h, then the
medium was aspirated and replenished with a medium containing 5-FU
(at concentrations between 0.125 and 128 µM) for another 48 h. In
the same protocol, the effect of irinotecan (IC30
concentration table III), a
standard chemotherapy drug, on the sensitivity of CRC cells to 5-FU
was also assessed and compared with the results of the ACF
pretreatment experiment. The overall time for drug treatment in
each protocol was 72 h.
 | Table III.IC30 values of ACF and
irinotecan for CRC cell lines. |
Table III.
IC30 values of ACF and
irinotecan for CRC cell lines.
|
| IC30
value, µM |
|---|
|
|
|
|---|
| CRC cell line | ACF | Irinotecan |
|---|
| SW480 | 4.85±1.03 | 56.97±4.52 |
| HCT116 | 4.41±0.57 | 80.01±1.91 |
| LS174T | 3.10±0.24 | 33.12±3.06 |
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
CRC cells (250,000 cells/well) were cultured in
6-well plates and treated with the IC30 of ACF for 24 h.
Following treatment, total RNA was extracted using an miRNeasy Mini
kit (Qiagen, Inc., Valencia, CA, USA), according to the
manufacturer's protocol. The quality and quantity of RNA were
determined using agarose gel electrophoresis and a NanoDrop 1000
instrument (NanoDrop Technologies; Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA), respectively. A 1 µg amount of total RNA was
used for cDNA synthesis using a PrimeScript™ First-Strand cDNA
Synthesis kit (Takara Bio, Inc., Otsu, Japan). qPCR assays for the
quantitative determination of HIF-1α, topoisomerase 2 and β-actin
(internal control) were performed in duplicate using a Corbett
RotorGene RG-6000 instrument (Qiagen, Inc.). Primer sequences are
presented in Table I. Amplifications
were performed in 25 µl mixtures containing 2 µl cDNA, 1 µl 10 µM
solutions of each of the forward and reverse primers, along with
12.5 µl SYBR Green PCR master mix (SYBR Premix Ex Taq™, Takara
Bio). The thermocycling conditions consisted of initial
denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec, annealing at 59°C (β-actin), 52°C (HIF-1α) and 56°C
(Topoisomerase 2) for 30 sec and extension at 60°C for 30 sec. The
relative amount of mRNA was calculated using the 2−ΔΔCq
method (21) and normalized to the
level of β-actin.
 | Table I.Primer sequences used for
quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences used for
quantitative polymerase chain reaction analysis.
| Gene | Forward primer | Reverse primer |
|---|
| β-actin |
5′-GCCTTTGCCGATCCGC-3′ |
5′-GCCGTAGCCGTTGTCG-3′ |
| HIF-1α |
5′-AGGAAATGAGAGAAATGCTTA-3′ |
5′-GGTTGGTTACTGTTGGTAT-3′ |
| Topoisomerase
2 |
5′-ATGTATCACCTTTCAGCCT-3′ |
5′-TTCATCCAACTTGTCCTTC-3′ |
Statistical analysis
Results are expressed as the mean ± standard
deviation. Differences between IC50 values of three or
more groups were determined using a Kruskal-Wallis test and Dunn's
post hoc test. A Mann-Whitney U test was performed on experiments
with two groups. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using SPSS software (version 12.0; SPSS, Inc., Chicago,
IL, USA).
Results
Effect of ACF and 5-FU co-treatment on
the sensitivity of CRC cells to 5-FU
To assess the cytotoxic effects of ACF or 5-FU, CRC
cells (SW480, HCT116 and LS174T) were treated with a graded
concentration of drugs for 72 h and cell viability was determined
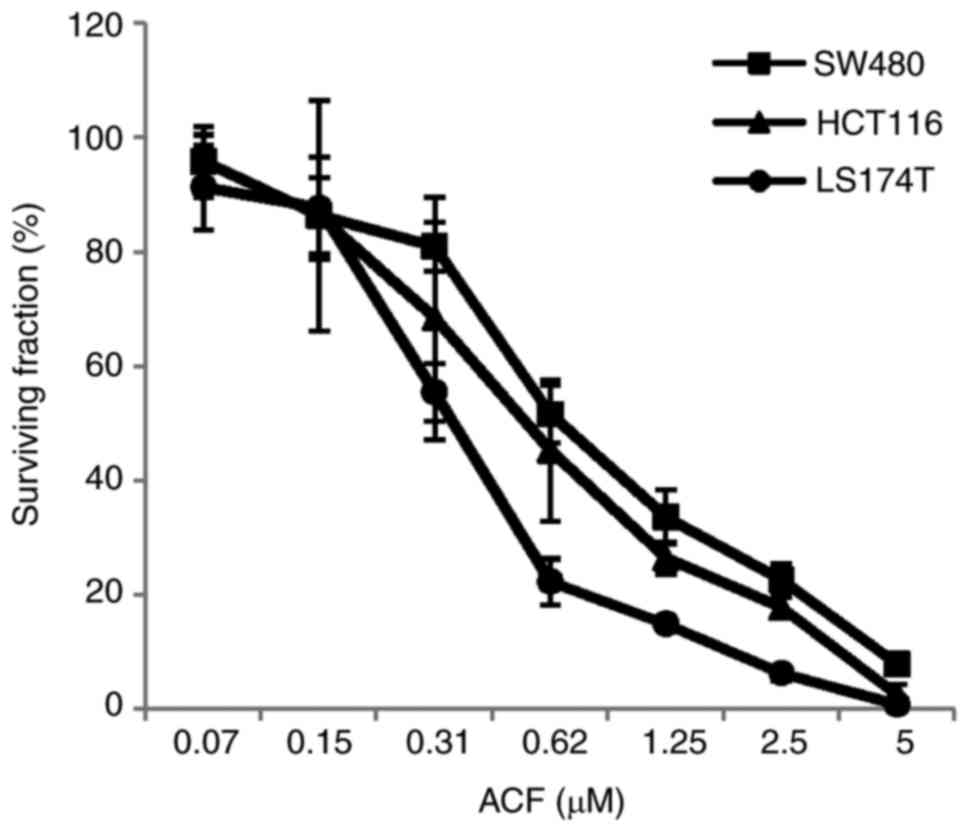
using an MTT assay. The IC50 values of ACF for SW480,
HCT116 and LS174T cells were 0.75±0.10, 0.57±0.22 and 0.36±0.05 µM,
respectively. ACF caused inhibitory effects on the cell growth in a
dose-dependent manner (Fig. 1). The
same pattern was also obtained for 5-FU (data not shown). The
IC30 values of ACF for SW480, HCT116 and LS174T cells
were 0.36±0.07, 0.29±0.14 and 0.20±0.04 µM, respectively. The
sensitivity of CRC cells against ACF and 5-FU was determined by
calculation of the IC50 values as presented in Table II. SW480 cells exhibited the highest
resistance to ACF and 5-FU in comparison with the other two cell
lines. To determine the effect of ACF on the sensitivity of cells
against 5-FU, cells were simultaneously treated with the
IC30, the low cytotoxic concentration, of ACF and
different concentrations of 5-FU for 72 h. As indicated in Table II, the results indicated that the
co-treatment protocol was not able to significantly alter the
IC50 value of 5-FU on CRC cells. Therefore, an
alternative treatment protocol was designed (pretreatment
protocol).
 | Table II.Sensitivity of CRC cell lines to 5-FU
when used as single agents or in combination (5-FU + ACF). |
Table II.
Sensitivity of CRC cell lines to 5-FU
when used as single agents or in combination (5-FU + ACF).
|
| IC50
value, µM |
|
|---|
|
|
|
|
|---|
| CRC cell line | 5-FU | 5-FU + ACF | P-value |
|---|
| SW480 | 41.85±16.44 | 64.66±8.22 | 0.275 |
| HCT116 | 7.36±4.14 | 11.59±1.80 | 0.465 |
| LS174T | 2.35±1.10 | 3.07±2.27 | 0.564 |
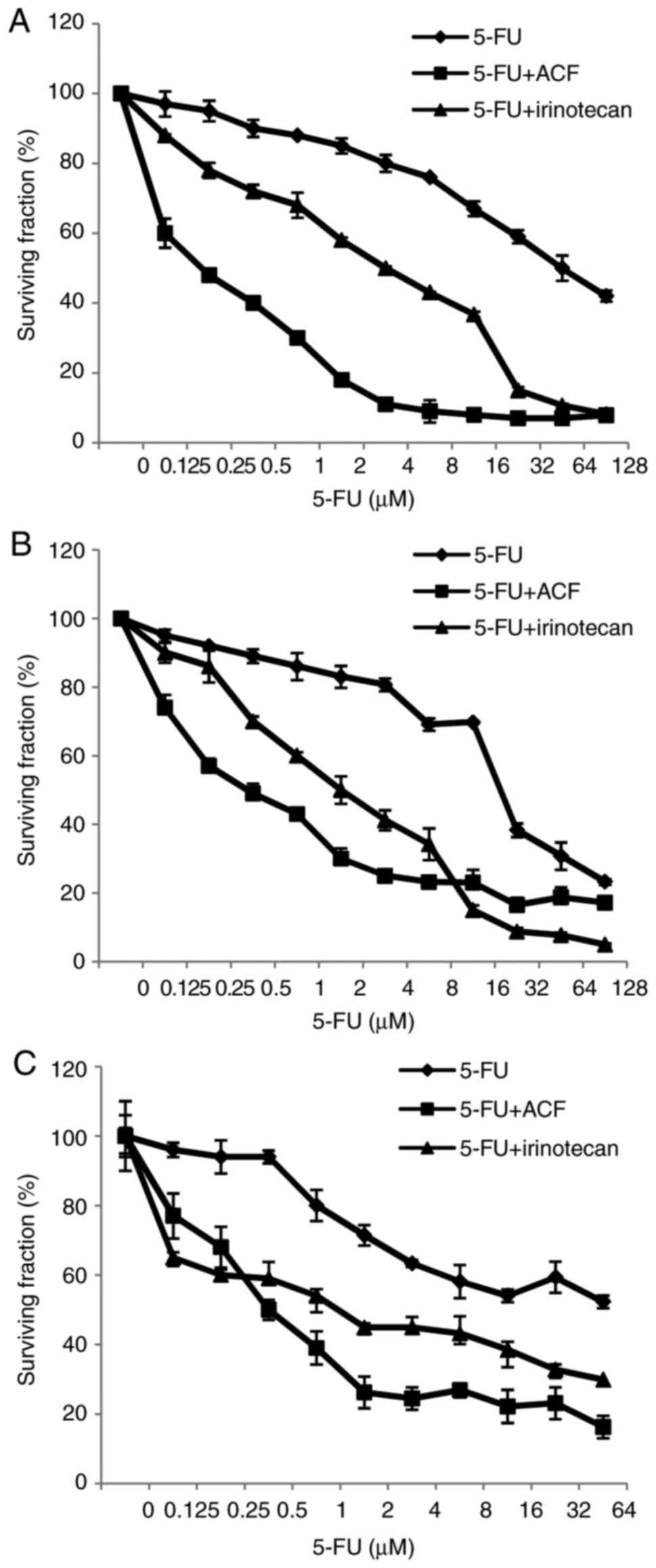
Effects of ACF pretreatment on the
sensitivity of CRC cells to 5-FU
To investigate the effects of ACF pretreatment on
5-FU cytotoxicity, CRC cells were pretreated with the
IC30 of ACF for 24 h, and the cells were incubated with
various concentrations of 5-FU and the viability of cells was
assessed. Table III presents the
IC30 values of ACF for 24 h of treatment. In Fig. 2, the pattern of CRC cell responses to
the cytotoxic effect of ACF + 5-FU is presented. In all
ACF-pretreated cell lines, at the low concentration of 5-FU, an
increased amount of cell death occurred. Furthermore, the
IC50 value of 5-FU in the pretreatment protocol was
significantly lower compared with the IC50 value of 5-FU
when used as a single drug (Table
IV). In fact, ACF pretreatment was able to sensitize CRC cells
to the low concentration of 5-FU.
 | Table IV.Sensitivity of colorectal cancer cell
lines to 5-FU when used as a single agent or when pretreated with
ACF (5-FU + ACF) and/or irinotecan (5-FU + irinotecan). |
Table IV.
Sensitivity of colorectal cancer cell
lines to 5-FU when used as a single agent or when pretreated with
ACF (5-FU + ACF) and/or irinotecan (5-FU + irinotecan).
|
| IC50
value, µM |
|
|
|
|---|
|
|
|
|
|
|
|---|
| CRC cell line | 5-FU | 5-FU + ACF | 5-FU +
irinotecan |
P-valuea |
P-valueb |
P-valuec |
|---|
| SW480 | 107.93±5.13 | 0.22±0.03 | 9.96±0.55 | 0.001 | 0.004 | 0.003 |
| HCT116 | 35.44±1.04 | 0.28±0.06 | 2.20±0.38 | 0.007 | 0.009 | 0.006 |
| LS174T | 53.35±10.73 | 0.32±0.04 | 1.16±0.17 | 0.006 | 0.009 | 0.008 |
Irinotecan is one of the standard drugs routinely
used in combination with 5-FU for the treatment of patients with
CRC. CRC cells were pretreated with irinotecan using the same
protocol as for ACF, and the IC50 value of 5-FU was
determined and compared with the IC50 value of 5-FU when
used as a single agent or when pretreated with ACF. Irinotecan has
an inhibitory effect on cell viability in a dose-dependent manner
(data not shown). The IC30 values of irinotecan for 24 h
of treatment are presented in Table
III. Pretreatment with IC30 of irinotecan
significantly increased the antitumor activity of 5-FU in CRC cells
(Table IV). In comparison with
irinotecan, ACF was identified to be a more potent agent for
enhancing the antitumor activity of 5-FU (Table IV). CRC cells that were pretreated
with ACF were significantly more sensitive to 5-FU compared with
the cells pretreated with irinotecan (Table IV).
It is worthy of mention that the pretreatment
protocol is independent of the co-treatment protocol and the two
protocols were not compared.
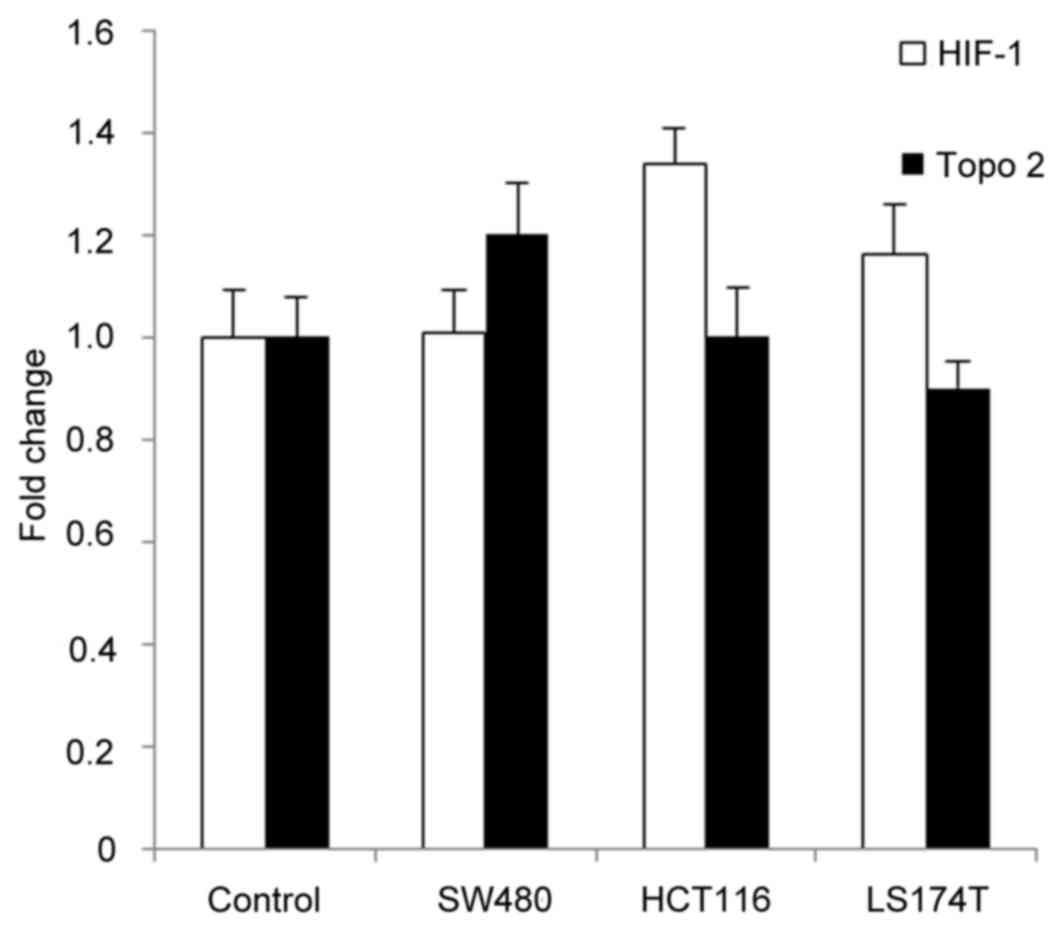
Effects of ACF treatment on mRNA
expression levels of HIF-1α and topoisomerase 2 in CRC cells
To assess the possible cytotoxic mechanism of ACF
action, cells were treated with the IC30 of ACF for 24
h. qPCR was subsequently used to determine mRNA levels of HIF-1α
and topoisomerase 2. The results identified that ACF did not
significantly alter the mRNA levels of either HIF-1α or
topoisomerase 2 (Fig. 3).
Discussion
Conventional chemotherapy regimens have exhibited
limited curative effects in CRC and a significant proportion of
patients with advanced CRC exhibit resistance to chemotherapy
(22,23). 5-FU is a first-line treatment in
patients with advanced CRC. However, only a small proportion of
patients respond to 5-FU when used as a single agent.
Administration of other chemotherapy drugs in combination with 5-FU
is reported to improve the response rate of patients (9,11).
In the present study, the effect of ACF on the
anticancer activity of 5-FU in CRC cells was investigated. The
results indicated that ACF inhibits the growth of three CRC cell
lines (SW480, LS174T and HCT116) in a dose-dependent manner.
Treatment of cells with different concentrations of ACF for 24 or
72 h indicated that SW480 cells have the most and LS174T cells have
the least sensitivity to ACF. Anticancer effects of ACF on
different cancer cell lines and in mouse models of cancer have been
demonstrated previously (14–16). Hassan et al (19) demonstrated that ACF has a cytotoxic
effect in monolayer and multicellular spheroid culture of CRC
cells, and their results demonstrated that ACF has a good cellular
penetration and cytotoxic activity against hypoxic CRC cells.
For advanced CRC, different combinations of drugs
were used as chemotherapy regimens in which 5-FU was considered as
the main drug. Because of the powerful selection that results in
the eventual emergence of cellular resistance to chemotherapy
drugs, the combinatory use of different agents is critical for the
successful treatment of CRC. However, in the majority of previous
studies, the anticancer activity of ACF against CRC was
investigated as a single agent. In the present study, the effects
of ACF on the sensitivity of CRC cells to 5-FU were investigated
using two separate protocols. In the first protocol, CRC cells were
co-treated with a low concentration (IC30) of ACF and
different concentrations of 5-FU for 72 h. The results revealed
that ACF co-treatment was not able to improve the sensitivity of
cells to 5-FU. In the second protocol, CRC cells were pretreated
with the IC30 of ACF for 24 h, then the drug was omitted
and various concentrations of 5-FU were added. Pretreatment with
ACF significantly increased the antiproliferative effect of 5-FU in
comparison with 5-FU alone. The IC50 value of SW480, the
most resistant cell, for 5-FU was decreased from 107 to 0.22 µM
following pretreatment with ACF. Additionally, ACF-pretreated CRC
cells were significantly more sensitive to 5-FU than the cells
pretreated with irinotecan, a standard chemotherapy drug that is
routinely used along with 5-FU. These results imply that ACF is a
more suitable agent compared with irinotecan for enhancing the
efficacy of 5-FU-based chemotherapy. Weijer et al (24) demonstrated that pretreatment with ACF
for 24 h improves the response of human perihilar
cholangiocarcinomas cells to photodynamic therapy and decreases
tumor cell survival. In addition, pretreatment of the HCT116 cell
line with ACF has been demonstrated to potentiate radiation-induced
cell death (25).
It has been identified that the loss of p53 function
is associated with tumor resistance to 5-FU (26–28). The
responses of cells and patients to 5-FU chemotherapy are dependent
on p53 status, with cells and patients with a mutated form of p53
having a higher resistance to 5-FU chemotherapy (29,30).
Disrupting both alleles of TP53 in a colon cancer cell line made
the cells highly resistant to apoptosis induced by 5-FU (26). Seth et al (31) demonstrated that restoration of
wild-type p53 may overcome the drug resistance of human cancer
associated with p53 dysfunction. In the present study, SW480 cells
were used that express a mutated form of p53 (32) and two other cell lines, HCT116 and
LS174T, with wild-type p53 (33). In
the present study, SW480 cells exhibited an increased
IC50 value and were more resistant to the cytotoxic
effect of 5-FU compared with the other two cell lines. However,
pretreatment of cells with ACF markedly sensitized all three cell
lines to the anticancer effects of 5-FU, regardless of the p53
status of cells. Therefore, the combination of 5-FU and ACF, the
naturally occurring product with low side effects, may be a
promising strategy to increase 5-FU-mediated cytotoxicity even in
patients with p53-mutated CRC.
It appears that the underlying molecular mechanism
of the antitumor property of ACF varies depending on the type and
origin of cancer. ACF may intercalate DNA and RNA, and inhibit
nucleolar RNA synthesis and topoisomerase 2 activity (13). It has been identified that certain
anticancer activities of ACF are associated with disruption of the
cell-surface membrane that leads to protein kinase C inhibition
(34). ACF may sensitize cells to
chemotherapeutic agents through the suppression of the expression
of xenobiotic-metabolizing genes (35). Furthermore, in previous studies, the
effect of ACF on cell cycle (24),
caspase activity (14,24,25) and
expression of angiogenic genes (16)
were investigated. In hepatocellular carcinoma cells, ACF induced
apoptosis through the suppression of B-cell lymphoma 2 expression
(14). ACF was also identified to
bind HIF-1α and inhibit its transcriptional activity, which was
associated with an inhibitory effect on tumor growth and
vascularization in prostate cancer xenografts (36). In the present study, other effects of
ACF that, to the best of our knowledge, have not been determined
previously and were more associated with CRC were investigated.
As aforementioned, certain anticancer effects of ACF
were associated with the inhibition of HIF-1α and topoisomerase 2
activity. Overexpression of HIF-1α has been identified to be
involved in the pathogenesis of CRC (37,38). In
previous studies of CRC, the effects of ACF on the expression of
genes for HIF-1α and topoisomerase 2 by cells were not assessed. In
the present study, the effects of ACF on the cellular expression of
genes encoding HIF-1α and topoisomerase 2 were investigated using
RT-qPCR. The results identified that pretreatment of cells with ACF
was not able to significantly alter the expression of HIF-1α and
topoisomerase 2. Therefore, it appears that the cytotoxic effect of
ACF is not exerted through suppression of transcription of HIF-1α
and topoisomerase 2 genes in CRC cells.
In the present study, the co-treatment protocol was
not able to enhance the cytotoxicity of 5-FU, but pretreatment with
ACF was able to significantly increase 5-FU cytotoxicity. The
reasons for this are unclear. It appears that pretreatment with ACF
predisposes CRC cells to the cytotoxic effect of the main drug,
i.e. 5-FU, through the inhibition of HIF-1α and topoisomerase 2
activity as suggested previously (17,19).
However, the exact molecular mechanism of ACF cytotoxicity against
CRC cells remains to be elucidated (19). Additionally, it has been identified
that 5-FU exerts its anticancer property primarily through the
inhibition of thymidylate synthase (7). Therefore, investigating the effect of
ACF on thymidylate synthase, which is a target of 5-FU, is a focus
of future research. A limitation of the present study is that the
cytotoxic effect of ACF and 5-FU on normal colon epithelial cell as
a control was not determined.
Taken together, the results of the present study
reveal for the first time that the pretreatment with a low
concentration of a naturally occurring product, ACF, markedly
increases the cytotoxic effects of 5-FU in CRC cells. This effect
is independent of the p53 status of cells and is not exerted
through the suppression of the expression of mRNAs for HIF-1α and
topoisomerase 2 in CRC cells. The combination of ACF and 5-FU may
be considered as a potential new chemotherapy regimen to overcome
5-FU resistance and improve the survival of patients with advanced
CRC. However, for optimizing the ACF dose for the treatment of
human CRC, other in vivo studies are required.
Acknowledgements
This study was a part of the dissertation of Parisa
Zargar (The effect of acriflavine on HIF-1α expression and
5-fluorouracil chemosensitivity in colorectal cancer cells),
submitted to Hormozgan University of Medical Sciences in partial
fulfillment of the requirements for the MSc in Physiology.
Funding
The present study was supported by the Deputy of
Research, Hormozgan University of Medical Sciences, Bandar Abbas,
Iran (grant number 91-F-4).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
PZ performed experiments and collected data. EG
analyzed and interpreted data. AR analyzed data and performed
experiments. FJM analyzed and interpreted data, prepared figures
and wrote the introduction section. EE developed the concept and
designed the study, and wrote the manuscript. All authors read and
approved the final article.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coppedè F, Lopomo A, Spisni R and Migliore
L: Genetic and epigenetic biomarkers for diagnosis, prognosis and
treatment of colorectal cancer. World J Gastroenterol. 20:943–956.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Cutsem E and Oliveira J: ESMO
Guidelines Working Group: Advanced colorectal cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20:61–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aschele C, Bergamo F and Lonardi S:
Chemotherapy for operable and advanced colorectal cancer. Cancer
Treat Rev. 35:509–516. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prenen H, Vecchione L and Van Cutsem E:
Role of targeted agents in metastatic colorectal cancer. Target
Oncol. 8:83–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
9
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peeters M, Price TJ, Cervantes A, Sobrero
AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ, et al:
Randomized phase III study of panitumumab with fluorouracil,
leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as
second-line treatment in patients with metastatic colorectal
cancer. J Clin Oncol. 28:4706–4713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wainwright M: Acridine-a neglected
antibacterial chromophore. J Antimicrob Chemother. 47:1–13. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SG, Kim CW, Ahn ET, Lee KY, Hong EK,
Yoo BI and Han YB: Enhanced anti-tumour effects of acriflavine in
combination with guanosine in mice. J Pharm Pharmacol. 49:216–222.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee CJ, Yue CH, Lin YY, Wu JC and Liu JY:
Antitumor activity of acriflavine in human hepatocellular carcinoma
cells. Anticancer Res. 34:3549–3556. 2014.PubMed/NCBI
|
|
15
|
Fan J, Yang X and Bi Z: Acriflavine
suppresses the growth of human osteosarcoma cells through apoptosis
and autophagy. Tumor Biol. 35:9571–9576. 2014. View Article : Google Scholar
|
|
16
|
Yin T, He S, Shen G and Wang Y: HIF-1
Dimerization inhibitor acriflavine enhances antitumor activity of
sunitinib in breast cancer model. Oncol Res. 22:139–145. 2015.
View Article : Google Scholar
|
|
17
|
Shay JE, Imtiyaz HZ, Sivanand S, Durham
AC, Skuli N, Hsu S, Mucaj V, Eisinger-Mathason TS, Krock BL,
Giannoukos DN and Simon MC: Inhibition of hypoxia-inducible factors
limits tumor progression in a mouse model of colorectal cancer.
Carcinogenesis. 35:1067–1077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mathé G, Pontiggia P, Orbach-Arbouys S,
Triana K, Ambetima N, Morette C, Hallard M and Blanquet D: AIDS
therapy with two, three or four agent combinations, applied in
short sequences, differing from each other by drug rotation. I.
First of two parts: A phase I trial equivalent, concerning five
virostatics: AZT, ddI, ddC, acriflavine and an ellipticine
analogue. Biomed Pharmacother. 50:220–227. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hassan S, Laryea D, Mahteme H, Felth J,
Fryknäs M, Fayad W, Linder S, Rickardson L, Gullbo J, Graf W, et
al: Novel activity of acriflavine against colorectal cancer tumor
cells. Cancer Sci. 102:2206–2213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eftekhar E, Jaberie H and Naghibalhossaini
F: Carcinoembryonic antigen expression and resistance to radiation
and 5-fluorouracil-induced apoptosis and autophagy. Int J Mol Cell
Med. 5:80–89. 2016.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao C, Yan TD, Black D and Morris DL: A
systematic review and meta-analysis of cytoreductive surgery with
perioperative intraperitoneal chemotherapy for peritoneal
carcinomatosis of colorectal origin. Ann Surg Oncol. 16:2152–2165.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma J, Zhang Y, Shen H, Kapesa L, Liu W,
Zeng M and Zeng S: Association between mismatch repair gene and
irinotecan-based chemotherapy in metastatic colon cancer. Tumor
Biol. 36:9599–9609. 2015. View Article : Google Scholar
|
|
24
|
Weijer R, Broekgaarden M, Krekorian M,
Alles LK, van Wijk AC, Mackaaij C, Verheij J, van der Wal AC, van
Gulik TM, Storm G and Heger M: Inhibition of hypoxia inducible
factor 1 and topoisomerase with acriflavine sensitizes perihilar
cholangiocarcinomas to photodynamic therapy. Oncotarget.
7:3341–3356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim MJ, Ahn JY, Han Y, Yu CH, Kim MH, Lee
SL, Lim DS and Song JY: Acriflavine enhances radiosensitivity of
colon cancer cells through endoplasmic reticulum stress-mediated
apoptosis. Int J Biochem Cell Biol. 44:1214–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bunz F, Hwang PM, Torrance C, Waldman T,
Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW and
Vogelstein B: Disruption of p53 in human cancer cells alters the
responses to therapeutic agents. J Clin Invest. 104:263–269. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Longley DB, Boyer J, Allen WL, Latif T,
Ferguson PR, Maxwell PJ, McDermott U, Lynch M, Harkin DP and
Johnston PG: The role of thymidylate synthase induction in
modulating p53-regulated gene expression in response to
5-fluorouracil and antifolates. Cancer Res. 62:2644–2649.
2002.PubMed/NCBI
|
|
28
|
Pereira DM, Simões A, Gomes SE, Castro RE,
Carvalho T, Rodrigues CM and Borralho PM: MEK5/ERK5 signaling
inhibition increases colon cancer cell sensitivity to
5-fluorouracil through a p53-dependent mechanism. Oncotarget.
7:34322–34340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sui X, Kong N, Wang X, Fang Y, Hu X, Xu Y,
Chen W, Wang K, Li D, Jin W, et al: JNK confers 5-fluorouracil
resistance in p53-deficient and mutant p53-expressing colon cancer
cells by inducing survival autophagy. Sci Rep. 4:46942014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Akasaka T, Tsujii M, Kondo J, Hayashi Y,
Ying J, Lu Y, Kato M, Yamada T, Yamamoto S, Inoue T, et al: 5-FU
resistance abrogates the amplified cytotoxic effects induced by
inhibiting checkpoint kinase 1 in p53-mutated colon cancer cells.
Int J Oncol. 46:63–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seth P, Katayose D, Li Z, Kim M, Wersto R,
Craig C, Shanmugam N, Ohri E, Mudahar B, Rakkar AN, et al: A
recombinant adenovirus expressing wild type p53 induces apoptosis
in drug-resistant human breast cancer cells: A gene therapy
approach for drug-resistant cancers. Cancer Gene Ther. 4:383–390.
1997.PubMed/NCBI
|
|
32
|
Rodrigues NR, Rowan A, Smith M, Kerr IB,
Bodmer WF, Gannon JV and Lane DP: p53 mutations in colorectal
cancer. Proc Natl Acad Sci USA. 87:7555–7559. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Nan L, Yu D, Lindsey JR, Agrawal S
and Zhang R: Anti-tumor efficacy of a novel antisense anti-MDM2
mixed-backbone oligonucleotide in human colon cancer models:
p53-dependent and p53-independent mechanisms. Mol Med. 8:185–199.
2002.PubMed/NCBI
|
|
34
|
Hannun Y and Bell RM: Aminoacridines,
potent inhibitors of protein kinase C. J Biol Chem. 263:5124–5131.
1988.PubMed/NCBI
|
|
35
|
Kim SG, Cho JY, Chung YS, Ahn ET, Lee KY
and Han YB: Suppression of xenobiotic-metabolizing enzyme
expression in rats by acriflavine, a protein kinase C inhibitor.
Effects on epoxide hydrolase, glutathione s-transferases, and
cytochromes P450. Drug Met Dispos. 26:66–72. 1998.
|
|
36
|
Lee K, Zhang H, Qian DZ, Rey S, Liu JO and
Semenza GL: Acriflavine inhibits HIF-1 dimerization, tumor growth,
and vascularization. Proc Natl Acad Sci USA. 106:17910–17915. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Waldner MJ and Neurath MF: The molecular
therapy of colorectal cancer. Mol Aspect Med. 31:171–178. 2010.
View Article : Google Scholar
|
|
38
|
Kuwai T, Kitadai Y, Tanaka S, Onogawa S,
Matsutani N, Kaio E, Ito M and Chayama K: Expression of
hypoxia-inducible factor-1alpha is associated with tumor
vascularization in human colorectal carcinoma. Int J Cancer.
105:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|