Introduction
Gastric cancer is one of the most common types of
malignancy worldwide and was the second leading cause of
cancer-associated morbidity and mortality in China in 2015
(1). The main treatment for gastric
cancer is surgery; however, due to the absence of symptoms in early
gastric cancer, as well as the lack of simple and sensitive
screening methods, over half of patients are diagnosed at advanced
stages and have missed the opportunity for radical surgery.
Therefore, it is important to identify and validate biomarkers for
the early diagnosis of gastric cancer.
Annexin is a member of the calcium ion-dependent
phospholipid-binding protein superfamily that contains A, B, C, D
and E families, which all have similar chemical structures
(2). Annexin has N-terminal calcium-
and phospholipid-binding domains, and a conserved C-terminal
membrane phospholipid-binding domain. The differences among the
family members are the various lengths and sequences of the
N-terminal structural domains (3).
The Annexins expressed in vertebrate cells are in the Annexin A
family, which contains 12 members (Annexin A1 to A11, and A13).
Annexin A family members participate in various biological
processes, including cytoskeletal structure and activity,
pinocytosis, exocytosis, conformation and activation of cell
membrane receptors, function of cell transmembrane ion channels,
cell signaling, as well as cell division, proliferation,
differentiation and apoptosis (3–8). The human
Annexin A7 gene was first identified in the 1970s by Creutz et
al (9) and is expressed in
various tissues (10,11). Annexin A7 regulates cell growth,
differentiation, proliferation and apoptosis by inhibiting
coagulation and phospholipase A2 activity, promoting cell
secretion, accelerating chromaffin particle gathering and
modulating cell signal transduction (4,12–14). Annexin A7-knockout mice develop
spontaneous tumors (15).
Furthermore, a number of studies have demonstrated that Annexin A7
serves an important role in tumorigenesis and that it is
dysregulated in multiple types of tumor tissue (16). However, the roles of Annexin A7 in
tumorigenesis remain inconclusive. Annexin A7 is downregulated in
malignant glioma (17), glioblastoma
multiform (18), melanoma (19) and prostate cancer (20), but it is upregulated in liver cancer
(16), gastric cancer (21), nasopharyngeal carcinoma (22), colorectal cancer (23), cervical squamous cell carcinoma
(24) and breast cancer (25). These observations indicated that
Annexin A7 may either suppress or promote tumorigenesis, depending
on the type of tumor. Therefore, it is important to further
elucidate the molecular mechanisms of Annexin A7 in the
tumorigenesis of different types of cancer.
Previously, we reported that the expression of
Annexin A7 is upregulated in gastric cancer tissues, compared with
para-carcinoma tissues, and that a high expression of Annexin A7 is
a predictive factor for lymphatic metastasis of gastric cancer
(26). However, the expression status
of Annexin A7 in different stages of gastric cancer remains
unknown. In addition, evasion of apoptosis serves a key role in
gastric cancer progression, but the association between Annexin A7
expression and gastric cancer cell apoptosis remains unclear.
In order to assess the associations between Annexin
A7 expression, and tumor differentiation and apoptosis, the present
study analyzed the expression of Annexin A7 and detected apoptosis
in gastric cancer clinical tissues and cell lines with various
differentiation grades through reverse transcription-quantitative
polymerase chain reaction (RT-qPCR), western blot analysis,
terminal deoxynucleotidyl transferase-mediated dUTP nick end
labeling (TUNEL) and flow cytometry experiments.
Materials and methods
Cell lines and reagents
The highly differentiated human gastric
adenocarcinoma MKN74 cell line, the moderately differentiated human
gastric adenocarcinoma SGC7901 cell line and the poorly
differentiated human gastric adenocarcinoma BGC823 cell line were
provided by the Fourth Hospital of Hebei Medical University (Hebei,
China). All cell lines were maintained in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd., Hangzhou, China), 100 U/ml
penicillin and 100 mg/ml streptomycin in a saturated humidity
incubator with 5% CO2 at 37°C.
Collection and preparation of gastric
cancer specimens of various differentiation grades
A total of 85 fresh gastric cancer specimens were
collected from patients who had been diagnosed with gastric cancer
and had undergone either radical distal gastrectomy, proximal
subtotal gastrectomy or total gastrectomy in the Department of
General Surgery of the Fourth Hospital of Hebei Medical University
between January 2013 and March 2014. Of the patients, 49 were male
and 36 were female, with a mean age of 55±6.2 years (range: 39–79
years). Patients who also had other malignancies and had received
preoperative radiotherapy, chemotherapy or biotherapy were
excluded. The patients were confirmed as having gastric
adenocarcinoma through postoperative pathology, and the numbers of
highly, moderately and poorly differentiated gastric adenocarcinoma
cases were 23, 30 and 32, respectively. Each specimen was divided
into three sections following surgical removal, and those used for
RT-qPCR and western blot analysis were immediately placed into
liquid nitrogen for quick freezing, and were subsequently
transferred and stored at −80°C. The tissues used for TUNEL
staining were fixed with 4% paraformaldehyde at room temperature
for 48 h, embedded in paraffin and routinely sliced (5-µm thick).
The aforementioned study protocol was approved by the Ethics
Committee of the Fourth Hospital of Hebei Medical University and
all patients provided preoperative written informed consent.
RT-qPCR
Total RNA was extracted from tissues and cells using
the TRIzol one-step method (Invitrogen; Thermo Fisher Scientific,
Inc.), and the integrity, content and purity of the RNA was
detected through agarose gel electrophoresis and ultraviolet
spectroscopy, respectively. Total RNA (5 µg) was reverse
transcribed to cDNA using an M-MLV reverse transcription kit
(Promega Corporation, Madison, WI, USA), and qPCR was conducted
using SYBR-Green (Promega, Corporation) on a fluorescence
quantitative PCR machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction conditions were as follows:
Pre-denaturation at 95°C for 5 min; 40 cycles of denaturation at
95°C for 15 sec, annealing at 60°C for 34 sec, and extension at
72°C for 25 sec; followed by denaturation at 95°C for 15 sec,
annealing at 57°C for 1 min, and termination with extension at 95°C
for 30 sec. The primer sequences were as follows: Annexin A7
forward, 5′-GTATCCACAGCCACCTTCACAGTC-3′ and reverse,
5′-CGCTGAGTACGTCGTGGAGTC-3′; and GAPDH forward,
5′-CGCTGAGTACGTCGTGGAGTC-3′ and reverse,
5′-GCTGATGATCTTGAGGCTGTTGTC-3′. The PCR primers were synthesized by
Beijing Dingguo Changsheng Biotechnology Co., Ltd. (Beijing,
China). GAPDH was used as the internal reference gene, and the
expression level of mRNA of the target gene was measured by the
quantitative cycle (Cq) method (27),
the formulas of which were as follows: ∆Cq (test)=Cq (target,
test)-Cq (ref, test); and ∆Cq (calibrator)=Cq (target,
calibrator)-Cq (ref, calibrator). The ∆Cq value of the calibrator
was used to normalize that of the test, which was ∆∆Cq=∆Cq
(test)-∆Cq (calibrator). Finally, the relative expression (RQ) of
the target gene was calculated by RQ=2−∆∆Cq. The value
of 2−ΔΔCq in the measurement group was set as 1;
therefore, the multiple proportion relations of other experimental
groups relative to the measurement group were obtained (27,28). The
mean of three experiments was taken as the actual value of each
sample.
Western blot analysis
Total protein was extracted from the gastric cancer
tissues and cell lines using radioimmunoprecipitation assay lysis
buffer (1% Nonidet P-40, 0.5% sodium deoxycholate and 0.1% SDS in
1× phosphate buffer solution) containing protease inhibitors (2
µg/ml aprotinin, 2 µg/ml leupeptin and 1 M phenylmethane sulfonyl
fluoride) for 30 min on ice. The protein concentration was
determined using a bicinchoninic acid assay. Equal amounts of
proteins (10 µg) were separated by 10% SDS-PAGE, prior to being
transferred onto nitrocellulose membranes. The membranes were
blocked in 2% nonfat milk in Tris-buffered saline (TBS) for 1 h at
room temperature. The membranes were incubated with a rabbit
anti-human Annexin A7 primary antibody (dilution, 1:1,000;
10154-2-AP; ProteinTech Group, Inc., Chicago, IL, USA) or a rabbit
anti-human GAPDH primary antibody (dilution, 1:3,000; YT5052;
ImmunoWay Biotechnology, Plano, TX, USA) overnight at 4°C, followed
by washing with TBS containing 0.1% Tween-20 and incubation with an
IRDye 680LT-conjugated secondary antibody (dilution, 1:1,000; Goat
anti-Rabbit, A11369; Invitrogen; Thermo Fisher Scientific, Inc.) at
room temperature in the dark for 2 h. An Odyssey double color
infrared laser imaging system (Odyssey Sa Software CD Version 2.0;
LI-COR Biosciences, Lincoln, NE, USA) was adopted for scanning and
calculating the integral absorbance values of the bands, and the
ratio of the gray values of the target band and the corresponding
GAPDH band was used to determine the target protein expression.
Detection of apoptosis using a TUNEL
assay
Cell apoptosis was detected using a TUNEL apoptosis
detection kit (Roche Diagnostics, Basel, Switzerland), according to
the manufacturer's protocol. In brief, the paraffin-embedded
sections of gastric cancer tissues were dewaxed at 60°C, washed
twice with xylene for 5 min each time, rehydrated in a descending
alcohol series (100, 95, 90, 80 and 70% for 5 min of each alcohol
concentration) and processed with proteinase K at 37°C for 20 min,
followed by processing with 0.1% Triton X-100 for 8 min and rinsing
with phosphate-buffered saline (PBS) twice, for 5 min each time.
After incubation with TUNEL reaction mixture at 37°C in the dark
for 60 min, the sections were washed with PBS 3 times (5 min/wash).
The sections were further counterstained with 5 µg/ml propidium
iodide (PI) dye at room temperature for 5 min and mounted with gum.
The number of apoptotic cells per 100 cells in each field of view
of five random fields was counted under an inverted fluorescence
microscope (magnification, ×400; Nikon Corporation, Japan). The
apoptosis index (AI) was calculated as follows: AI=(the number of
apoptotic cells/total cell count) ×100%.
Detection of apoptosis through flow
cytometry
Cells (1×105) were centrifuged at 111.8 ×
g for 5 min at room temperature and were washed twice with
precooled PBS. The cell pellets were resuspended in 100 µl of
binding buffer, followed by the addition of 5 µl of Annexin
V-fluorescein isothiocyanate (FITC) and incubation in the dark at
room temperature for 15 min. Subsequently, the samples were mixed
with 10 µl PI and were incubated in the dark at room temperature
for 5 min. The samples were analyzed using a flow cytometer
(FACSCalibur; BD Biosciences, San Diego, CA, USA) with an
excitation wavelength of 488 nm within 1 h. A band-pass filter with
a wavelength of 515 nm was applied to detect FITC fluorescence,
while another filter with a wavelength of 560 nm was used to detect
PI. The apoptosis rate=(the number of early apoptotic cells + the
number of late apoptotic cells)/total cell count ×100%. The Annexin
V-FITC/PI apoptosis detection kit was purchased from BD
Biosciences.
Statistical analysis
All statistical analyses were processed using SPSS
19.0 software (IBM Corp., Armonk, NY, USA) and the enumeration data
are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
One-way analysis of variance was applied for comparisons among
groups. With regard to pairwise comparison, the least significant
difference test was applied for homogeneity of variance, while
Dunnett's T3 test was utilized for heterogeneity of variance.
Spearman's rank correlation was used for correlation analysis. The
level of significance used was α=0.05.
Results
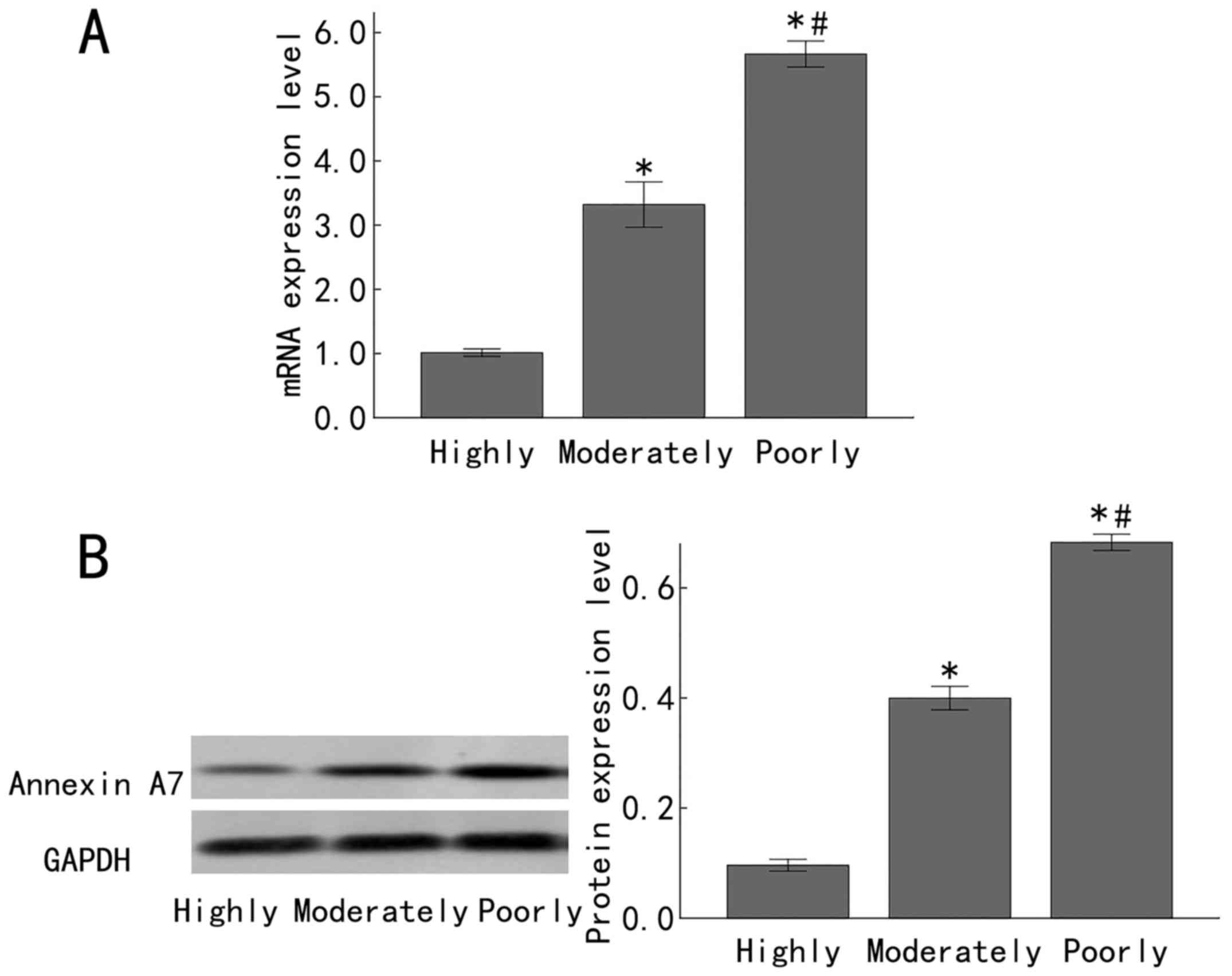
Annexin A7 expression is downregulated
in late-stage gastric adenocarcinoma tissues
In order to investigate the expression status of
Annexin A7 in differently differentiated gastric adenocarcinomas,
the Annexin A7 mRNA levels in 23, 30 and 32 cases of highly,
moderately and poorly differentiated gastric adenocarcinomas,
respectively, were determined by RT-qPCR, with GAPDH as the
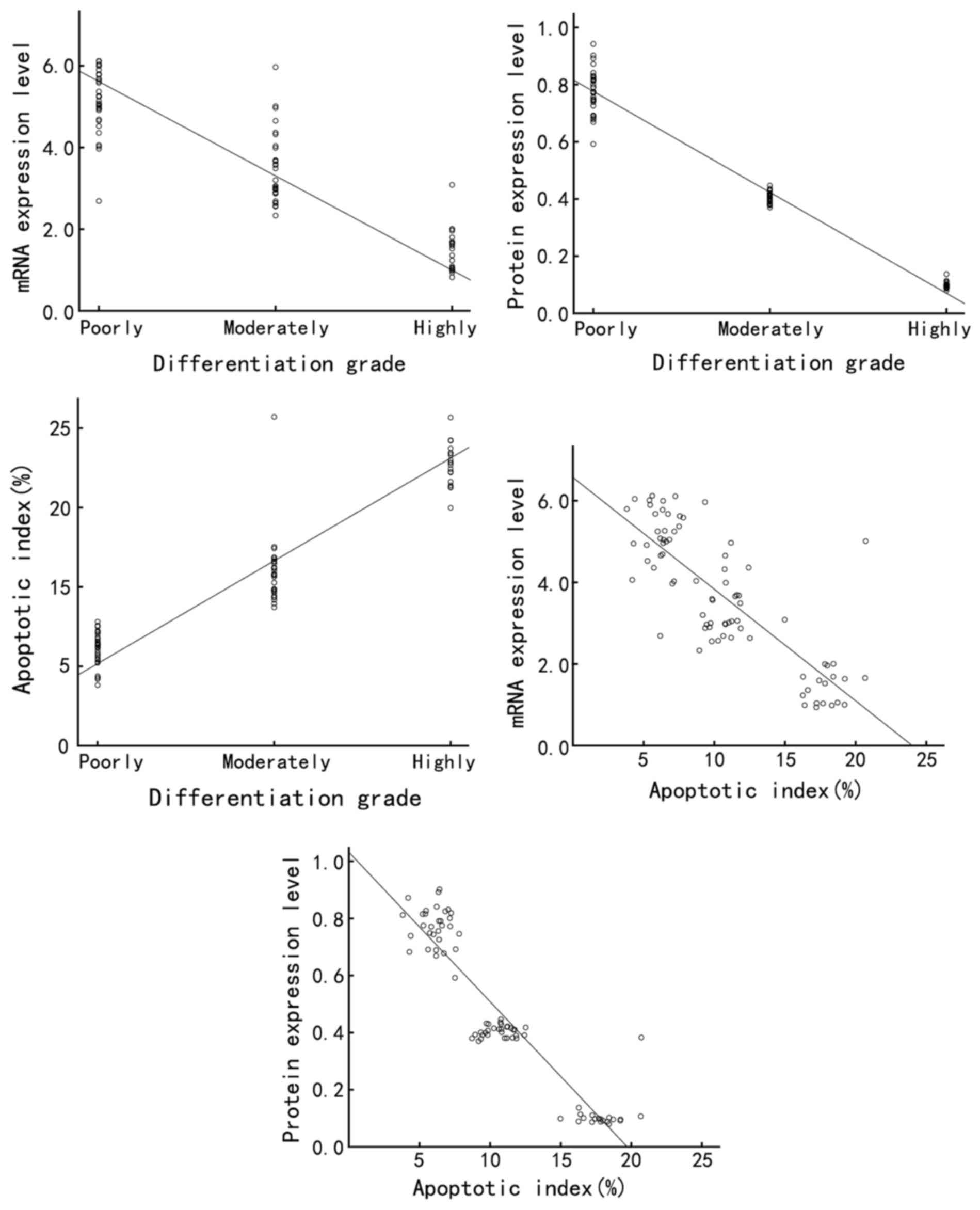
internal control. There was a significantly increased Annexin A7
mRNA expression in the moderately differentiated gastric
adenocarcinoma samples, compared with that in the highly
differentiated gastric adenocarcinoma samples (P<0.05).
Furthermore, in comparison to expression in the moderately
differentiated gastric adenocarcinoma samples, there was a
significantly increased expression of Annexin A7 mRNA in the poorly
differentiated gastric adenocarcinoma samples (P<0.05; Fig. 1A). Similarly, western blot analysis
demonstrated that the Annexin A7 protein levels were downregulated
in late-stage gastric adenocarcinoma. There was significantly
increased Annexin A7 protein expression in moderately
differentiated gastric adenocarcinoma, compared with that in the
highly differentiated gastric adenocarcinoma samples (P<0.05).
In comparison to expression in the moderately differentiated
gastric adenocarcinoma samples, the Annexin A7 protein level in the
poorly differentiated gastric adenocarcinoma samples was
significantly increased (P<0.05; Fig.
1B).
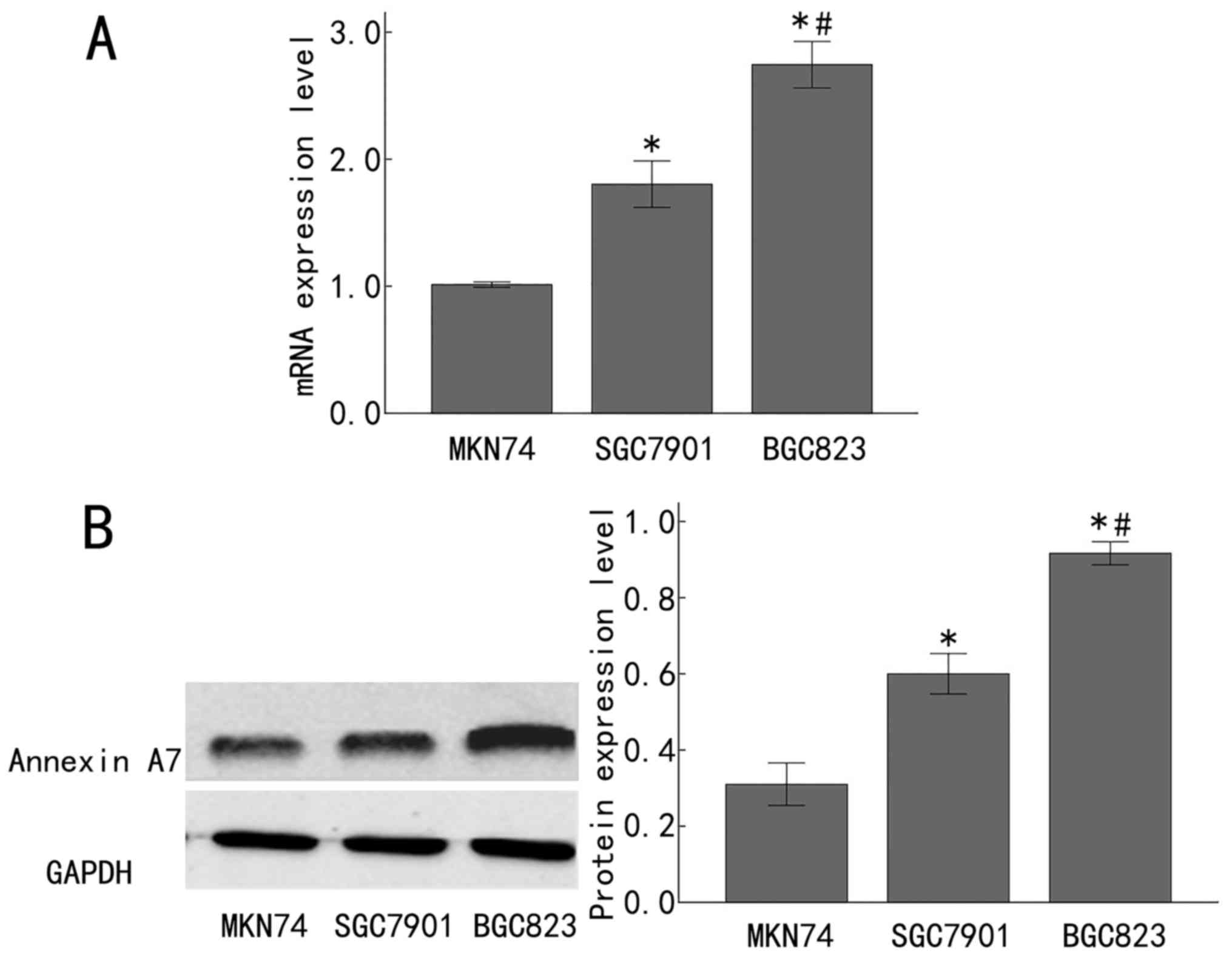
Expression of Annexin A7 is
downregulated in late-stage gastric adenocarcinoma cell lines
To expand on the aforementioned observations in
clinical samples, the Annexin A7 mRNA and protein expression was
measured in the poorly differentiated human gastric adenocarcinoma
BGC823 cell line, the moderately differentiated human gastric
adenocarcinoma SGC7901 cell line, and the highly differentiated
human gastric adenocarcinoma MKN74 cell line. The mRNA level of
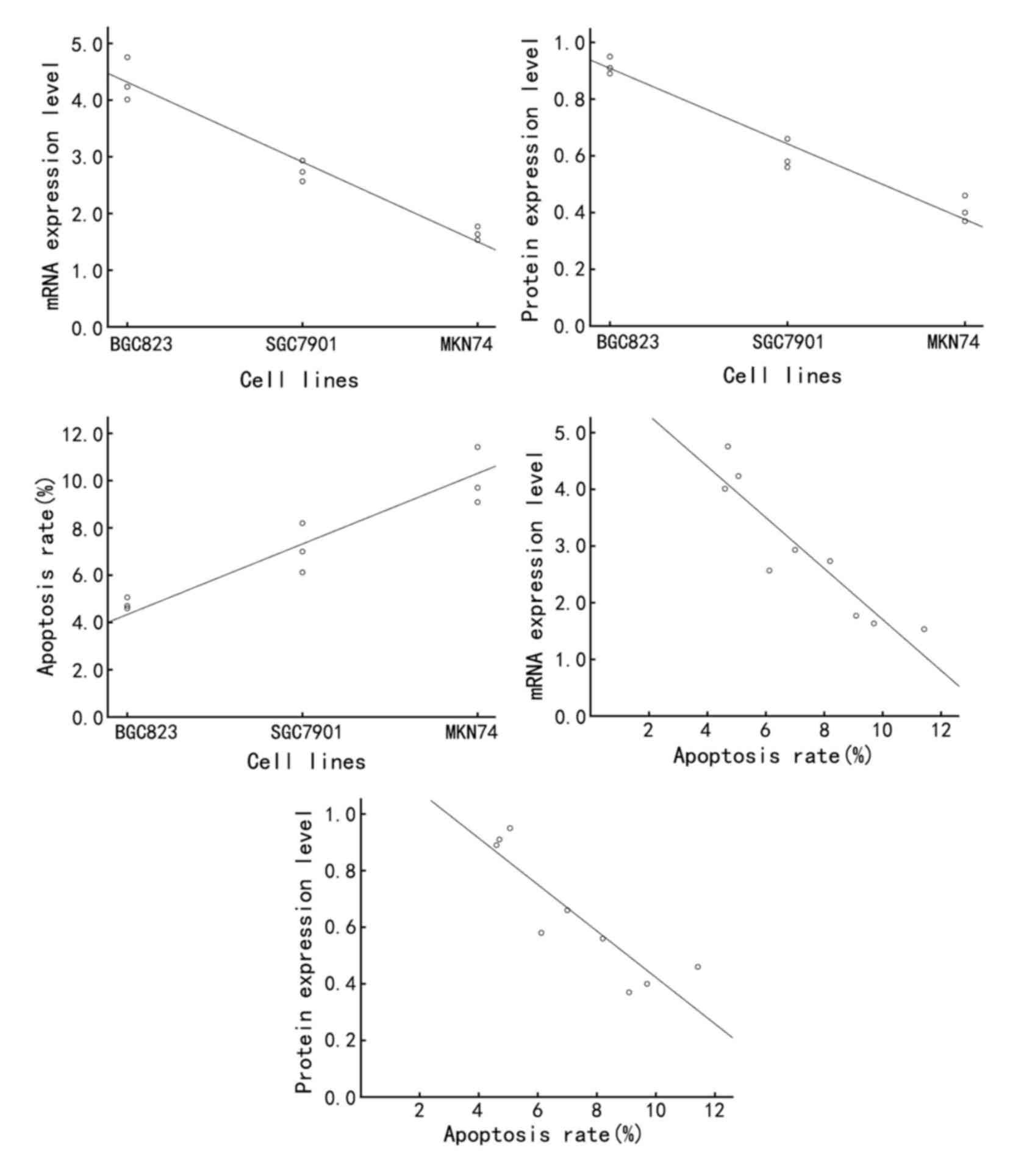
Annexin A7 was significantly increased in the SGC7901 cells,
compared with that in the MKN74 cells (P<0.05), and was
significantly increased in the BGC823 cells, compared with that in
the SGC7901 cells (P<0.05; Fig.
2A). In line with this, Annexin A7 protein expression was
significantly upregulated in the SGC7901 cells, compared with that
in the MKN74 cells (P<0.05), and was significantly increased in
the BGC823 cells, compared with that in the SGC7901 cells
(P<0.05; Fig. 2B). Taken together,
these results demonstrated that Annexin A7 was significantly
downregulated in late-stage gastric adenocarcinoma tissues and cell
lines, implying that Annexin A7 expression is gradually
downregulated with the progression of gastric adenocarcinoma.
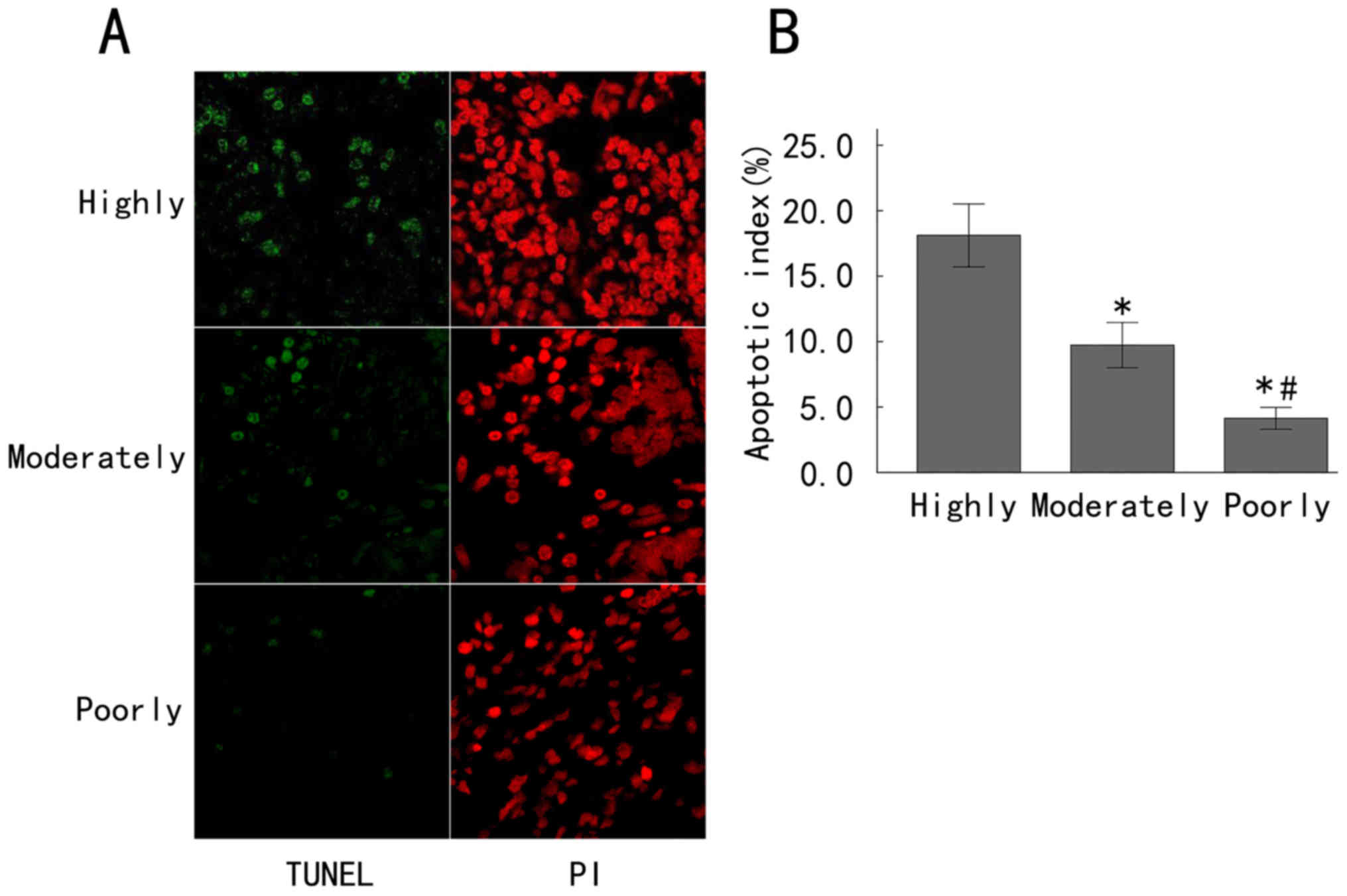
Enhanced apoptosis in late-stage
gastric adenocarcinoma tissues
Evasion of apoptosis is a hallmark of cancer cells.
In order to assess the potential associations between Annexin A7
expression and apoptosis in gastric cancer and different degrees of
differentiation, the apoptosis of gastric cancer tissues at various
differentiation grades was detected by a TUNEL assay and
fluorescence microscopy (Fig. 3A).
The apoptotic cells exhibited green fluorescence, while the PI
staining exhibited red fluorescence, representing the total number
of cells. A large amount of green fluorescence could be seen in the
highly differentiated gastric cancer tissues, indicating a large
number of apoptotic cells, while the moderately differentiated
gastric cancer tissues exhibited less green fluorescence and poorly
differentiated gastric cancer tissues exhibited the least green
fluorescence (Fig. 3A). The apoptosis
indices in the highly, moderately and poorly differentiated groups
were 18.12±2.40, 9.73±1.73 and 4.13±0.83%, respectively. The
apoptosis index in the poorly differentiated group was
significantly decreased compared with that in the moderately
differentiated group, and the apoptosis index in the moderately
differentiated group was also significantly decreased compared with
that in the highly differentiated group (all P<0.05; Fig. 3B). These results demonstrated that the
apoptosis index increased with an increase of the differentiation
grade of gastric cancer.
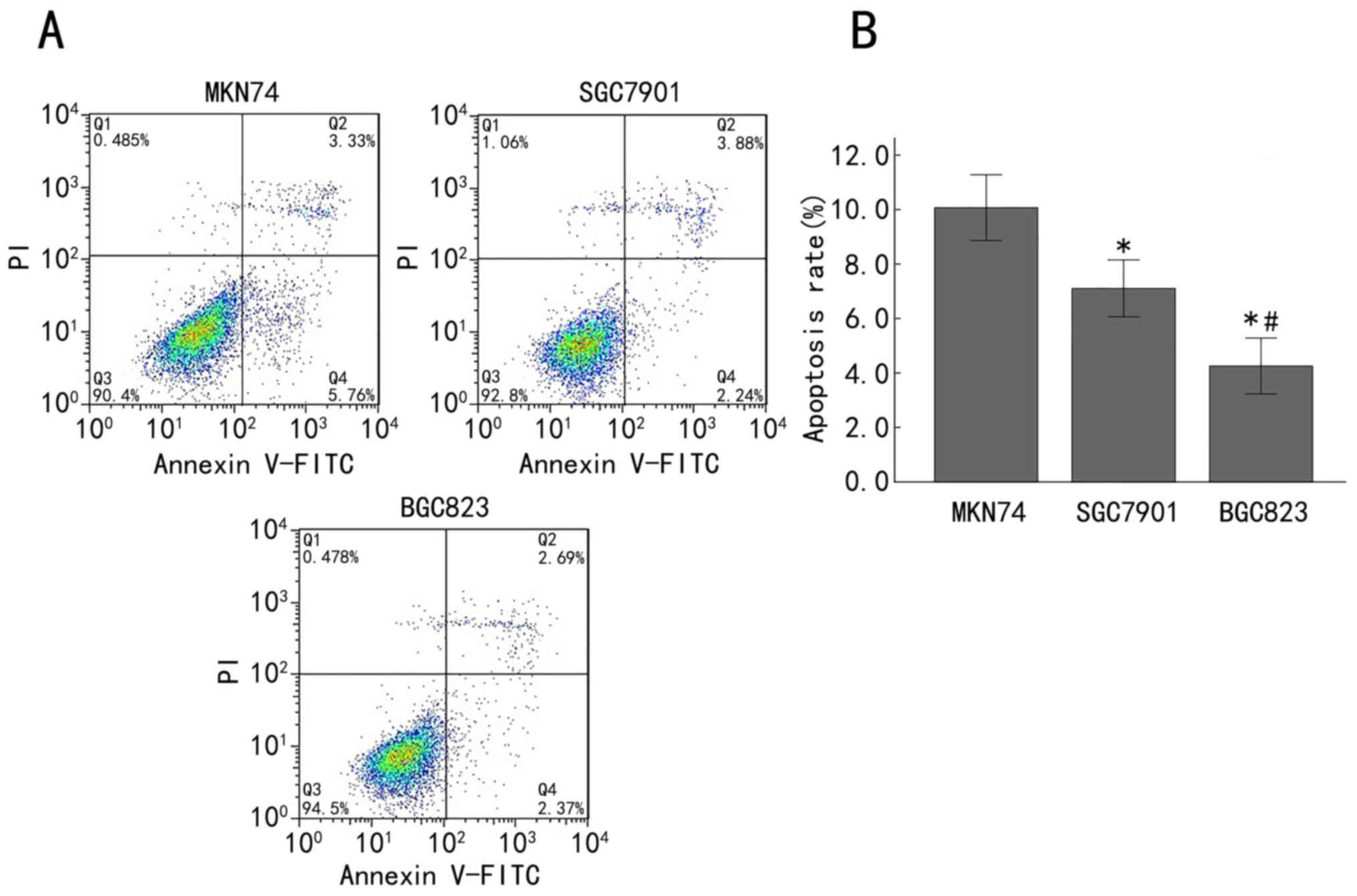
Enhanced apoptosis in late-stage
gastric adenocarcinoma cell lines
Subsequently, the apoptosis rate in MKN74, SGC7901
and BGC823 cells was analyzed by Annexin V-FITC/PI staining and
flow cytometry. The double-variable flow scatter diagram indicates
that the number of BGC823 cells in the right lower quadrant (early
apoptotic cells) and the right upper quadrant (advanced apoptotic
cells) was markedly decreased, while the SGC7901 group exhibited
more apoptotic cells and the MKN74 group exhibited the most
apoptotic cells (Fig. 4A). The
apoptosis rates of the MKN74, SGC7901 and BGC823 cells were
10.07±1.21, 7.11±1.04 and 4.25±1.02%, respectively. The apoptosis
rate in the BGC823 cells was significantly decreased compared with
that in the SGC7901 cells (P<0.05), and the apoptosis rate in
the SGC7901 cells was significantly lower than that in the MKN74
cells (P<0.05; Fig. 4B). These
results demonstrated that the apoptosis rate was enhanced with the
advancement of differentiation of gastric cancer cells.
Association among Annexin A7
expression, differentiation grade and apoptosis rate in gastric
cancer tissues and cell lines
Finally, the association among Annexin A7
expression, differentiation grade and apoptosis rate in gastric
cancer tissues and cell lines was assessed by Spearman's rank
correlation analysis. In gastric cancer clinical samples, the
expression of Annexin A7 mRNA and protein was negatively correlated
with the differentiation grade of gastric cancer (r=−0.926,
P<0.01 and r=−0.950, P<0.01, respectively), while the
apoptosis index was positively correlated with the differentiation
grade (r=0.949, P<0.01) and the apoptosis index of gastric
cancer was negatively correlated with the Annexin A7 mRNA and
protein expression (r=−0.978, P<0.01 and r=−0.973, P<0.01,
respectively) (Table I; Fig. 5). In the gastric cancer cell lines,
the Annexin A7 mRNA and protein expression was negatively
correlated with the differentiation grade (r=−0.934, P<0.01 and
r=−0.938, P<0.01, respectively), while the apoptosis rate was
positively correlated with the differentiation grade (r=0.936,
P<0.01). In addition, the apoptosis rate was negatively
correlated with the expression of Annexin A7 mRNA and protein
(r=−0.917, P<0.01 and r=−0.933, P<0.01, respectively)
(Table I; Fig. 6).
 | Table I.Correlation among Annexin A7
expression, differentiation grade and apoptosis rate in gastric
cancer tissues and cell lines was assessed by Spearman's rank
correlation analysis. |
Table I.
Correlation among Annexin A7
expression, differentiation grade and apoptosis rate in gastric
cancer tissues and cell lines was assessed by Spearman's rank
correlation analysis.
| Variable | r-value | P-value |
|---|
| Gastric cancer
tissues |
|
|
| Annexin
A7 mRNA expression vs. differentiation grade | −0.926 | <0.01 |
| Annexin
A7 protein expression vs. differentiation grade | −0.950 | <0.01 |
| Apoptosis
index vs. differentiation grade | 0.949 | <0.01 |
| Apoptosis
index vs. Annexin A7 mRNA expression | −0.978 | <0.01 |
| Apoptosis
index vs. Annexin A7 protein expression | −0.973 | <0.01 |
| Gastric cancer cell
lines |
|
|
| Annexin
A7 mRNA expression vs. differentiation grade | −0.934 | <0.01 |
| Annexin
A7 protein expression vs. differentiation grade | −0.938 | <0.01 |
| Apoptosis
index vs. differentiation grade | 0.936 | <0.01 |
| Apoptosis
index vs. Annexin A7 mRNA expression | −0.917 | <0.01 |
| Apoptosis
index vs. Annexin A7 protein expression | −0.933 | <0.01 |
Discussion
Gastric cancer is an aggressive malignancy with a
poor prognosis, and the majority of patients are not suitable for
surgery due to the late stage of disease at diagnosis (29). The results of the present study
demonstrated that Annexin A7 was significantly downregulated while
the rate of apoptosis was increased in late-stage gastric
adenocarcinoma tissues and cell lines. Furthermore, Annexin A7
expression was negatively correlated with the differentiation grade
and apoptosis rate. These results indicated that Annexin A7
expression is gradually downregulated while apoptosis is gradually
upregulated with the progression of gastric adenocarcinoma.
The differentiation grade is a critical factor for
tumor prognosis. Highly differentiated tumors have a low malignancy
degree and a good prognosis, while poorly differentiated tumors
have a high malignancy degree and a poor prognosis. The
differentiation degree is also associated with the proliferation,
invasion and metastasis of cancer cells; therefore, elucidating the
mechanism of tumor differentiation is important in individualizing
treatment and prognosis. In the present study, the expression
levels of Annexin A7 mRNA and protein in gastric cancer tissues and
cell lines with different grades of differentiation were
determined. The expression levels of Annexin A7 mRNA and protein
were decreased and were negatively correlated with the
differentiation grade of gastric cancer, suggesting that Annexin A7
expression increases with the decrease in differentiation grade,
further supporting the important role for Annexin A7 in the
development of gastric cancer. The results of the present study
were consistent with the previous observation that the expression
of Annexin A7 may be used as an important predictive indicator for
the survival rate of patients with gastric cancer (26). By contrast, Hsu et al (21) reported that the expression of Annexin
A7 is associated with the differentiation grade and that positive
expression rates exhibited a gradual decrease in highly
differentiated tubular and papillary adenocarcinoma, moderately
differentiated tubular adenocarcinoma, and poorly differentiated
signet ring cell carcinoma and mucinous adenocarcinoma. In
addition, Xi and Zhao (30) have
demonstrated that the lower the gastric cancer differentiation
grade, the lower the expression of Annexin A7. However, Gong et
al (31) reported that
differences in the expression of Annexin A7 in highly, moderately
and poorly differentiated gastric tubular adenocarcinoma are not
statistically significant. This discrepancy may be due to the
different methods used to detect Annexin A7 expression and the
different sample sizes. A multi-center study with a large sample
size and unified methods is required in order to confirm the
expression of Annexin A7 in distinctly differentiated gastric
cancer.
Apoptosis serves an important role in the
physiological and pathological processes of the body, particularly
in the development and progression of cancer. Apoptosis is a
programmed cell death that involves extracellular and intracellular
signals (32). A TUNEL assay,
currently the most specific, fastest and most sensitive technique
to detect single-cell apoptosis through in situ staining,
revealed that highly differentiated gastric cancer exhibited
significantly more apoptotic cells, while poorly differentiated
gastric cancer exhibited significantly fewer apoptotic cells in
clinical gastric tissues. Furthermore, the use of Annexin V-FITC/PI
staining with flow cytometry in order to detect gastric cancer cell
lines at various differentiation grades revealed that the apoptosis
rate of poorly differentiated BGC823 cells was markedly decreased,
compared with the rates in highly differentiated MKN74 cells and
moderately differentiated SGC7901 cells. These observations in cell
lines were consistent with those of TUNEL staining in clinical
samples, demonstrating a gradual increase of apoptosis with the
advancement of gastric cancer differentiation.
In order to further understand the mechanism of
gastric cancer progression and to identify a novel target for
treating gastric cancer, the associations among the expression of
Annexin A7, the differentiation grade and the apoptosis rate of
gastric cancer cells in gastric cancer tissues and cell lines at
various differentiation grades were analyzed. It was revealed that
the expression of Annexin A7 was negatively correlated with the
differentiation of gastric cancer, while the apoptosis rate was
positively correlated with the differentiation of gastric cancer.
In addition, Annexin A7 expression was negatively correlated with
apoptosis. These results indicated that the expression of Annexin
A7 was high and the apoptosis rate was low in poorly differentiated
gastric cancer, suggesting that Annexin A7 may inhibit apoptosis
during the development and progression of gastric cancer. However,
this mechanism requires confirmation by genetic modulation of
Annexin A7 expression in vitro and in animal models.
In summary, by analyzing the Annexin A7 expression
and the apoptosis rate in gastric cancer tissues and cell lines at
various differentiation grades, it was revealed that Annexin A7
expression was gradually downregulated while apoptosis was
gradually upregulated with the progression of gastric
adenocarcinoma. The results of the present study suggested that
Annexin A7 is a potential biomarker for the diagnosis, prognosis
and treatment of gastric adenocarcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL designed the research. WY, LF and QZ collected
clinical data and performed the clinical studies. WY, HY and BT
performed the experiments. WY, HY and ZZ analyzed the data. YL, WY
and BT wrote the manuscript.
Ethics approval and consent to
participate
This research was performed in accordance with the
Declaration of Helsinki and approved by the Ethics Committee of The
Fourth Hospital of Hebei Medical University.
Consent for publication
Patients, parents or guardians provided written
informed consent for the publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monastyrskaya K, Babiychuk EB and Draeger
A: The Annexins: Spatial and temporal coordination of signaling
events during cellular stress. Cell Mol Life Sci. 66:2623–2642.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerke V, Creutz CE and Moss SE: Annexins:
Linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell
Biol. 6:449–461. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang KL, Wu TT, Resetkova E, Wang H,
Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton
SR and Albarracin CT: Expression of Annexin A1 in esophageal and
esophagogastric junction adenocarcinomas: Association with poor
outcome. Clin Cancer Res. 12:4598–4604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan GR, Ding W, Xu SH, Xu Z, Xiao CL, Yin
XF and He QY: Characterization of phosphoproteins in gastric cancer
secretome. OMICS. 15:83–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lauritzen SP, Boye TL and Nylandsted J:
Annexins are instrumental for efficient plasma membrane repair in
cancer cells. Semin Cell Dev Biol. 45:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Ye J, Dong Y, Xu Z and Du Q:
Expression and significance of Annexin A2 in patients with gastric
adenocarcinoma and the association with E-cadherin. Exp Ther Med.
10:549–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Creutz CE, Pazoles CJ and Pollard HB:
Identification and purification of an adrenal medullary protein
(synexin) that causes calcium-dependent aggregation of isolated
chromaffin granules. J Biol Chem. 253:2858–2866. 1978.PubMed/NCBI
|
|
10
|
Selbert S, Fischer P, Pongratz D, Stewart
M and Noegel AA: Expression and localization of Annexin VII
(synexin) in muscle cells. J Cell Sci. 108:85–95. 1995.PubMed/NCBI
|
|
11
|
Clemen CS, Hofmann A, Zamparelli C and
Noegel AA: Expression and localisation of Annexin VII (synexin)
isoforms in differentiating myoblasts. J Muscle Res Cell Motil.
20:669–679. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fatimathas L and Moss SE: Annexins as
disease modifiers. Histol Histopathol. 25:527–532. 2010.PubMed/NCBI
|
|
13
|
Huang Y, Du Y, Zhang X, Bai L, Mibrahim M,
Zhang J, Wei Y, Li C, Fan S, Wang H, et al: Down-regulated
expression of Annexin A7 induces apoptosis in mouse hepatocarcinoma
cell line by the intrinsic mitochondrial pathway. Biomed
Pharmacother. 70:146–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song MY, Tang JW, Sun MZ, Liu SQ and Wang
B: Localization and expression of CLIC1 in hepatocarcinoma ascites
cell lines with high or low potentials of lymphatic spread.
Zhonghua Bing Li Xue Za Zhi. 39:463–466. 2010.(In Chinese).
PubMed/NCBI
|
|
15
|
Srivastava M, Montagna C, Leighton X,
Glasman M, Naga S, Eidelman O, Ried T and Pollard HB:
Haploinsufficiency of Anx7 tumor suppressor gene and consequent
genomic instability promotes tumorigenesis in the Anx7(+/-) mouse.
Proc Natl Acad Sci USA. 100:14287–14292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srivastava M, Torosyan Y, Raffeld M,
Eidelman O, Pollard HB and Bubendorf L: ANXA7 expression represents
hormone-relevant tumor suppression in different cancers. Int J
Cancer. 121:2628–2636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yadav AK, Renfrow JJ, Scholtens DM, Xie H,
Duran GE, Bredel C, Vogel H, Chandler JP, Chakravarti A, Robe PA,
et al: Monosomy of chromosome 10 associated with dysregulation of
epidermal growth factor signaling in glioblastomas. JAMA.
302:276–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hung KS and Howng SL: Prognostic
significance of Annexin VII expression in glioblastomas multiforme
in humans. J Neurosurg. 99:886–892. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kataoka TR, Ito A, Asada H, Watabe K,
Nishiyama K, Nakamoto K, Itami S, Yoshikawa K, Ito M, Nojima H and
Kitamura Y: Annexin VII as a novel marker for invasive phenotype of
malignant melanoma. Jpn J Cancer Res. 91:75–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Torosyan Y, Dobi A, Glasman M, Mezhevaya
K, Naga S, Huang W, Paweletz C, Leighton X, Pollard HB and
Srivastava M: Role of multi-hnRNP nuclear complex in regulation of
tumor suppressor ANXA7 in prostate cancer cells. Oncogene.
29:2457–2466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu PI, Huang MS, Chen HC, Hsu PN, Lai TC,
Wang JL, Lo GH, Lai KH, Tseng CJ and Hsiao M: The significance of
ANXA7 expression and its correlation with poor cellular
differentiation and enhanced metastatic potential of gastric
cancer. J Surg Oncol. 97:609–614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang M and Liang Q: Study the relationship
between the expression of Annexin A7 and CT of nasopharyngeal
carcinoma. J Chin Clin Med Imaging. 22:6–9. 2011.
|
|
23
|
Alfonso P, Canamero M, Fernández-Carbonié
F, Núñez A and Casal JI: Proteome analysis of membrane fractions in
colorectal carcinomas by using 2D-DIGE saturation labeling. J
Proteome Res. 7:4247–4255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Gao FL, Chang YZ and Li X:
Expression of Annexin A7 in human uterine cervical squamous
carcinomas and normal tissues. Acta Anatomica Sinica. 41:603–605.
2010.
|
|
25
|
Leighton X, Srikantan V, Pollard HB,
Sukumar S and Srivastava M: Significant allelic loss of ANX7region
(10q21) in hormone receptor negative breast carcinomas. Cancer
Lett. 210:239–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan HF, Li Y, Zhao Q, Fan LQ, Tan BB and
Ye WH: Expression of Annexin A7 and its clinical significance in
differentiation and metastasis of gastric carcinoma. Int J Clin Exp
Pathol. 7:6567–6574. 2014.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karube A, Shidara Y, Hayasaka K, Maki M
and Tanaka T: Suppression of calphobindin I (CPB I) production in
carcinoma of uterine cervix and endometrium. Gynecol Oncol.
58:295–300. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanat O, O'Neil B and Shahda S: Targeted
therapy for advanced gastric cancer: A review of current status and
future prospects. World J Gastrointest Oncol. 7:401–410. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xi JM and Zhao Q: Expression of Annexin A7
in gastric cancer tissues and their effects on the differentiation
and metastasis of gastric cancer. J Exp Clin Med. 9:726–727.
2010.
|
|
31
|
Gong X, Tang J and Geng X: Expression and
significance of Annexin 7 in gastric cancer and lymphatic
metastasis. Inter J Pathol Clin Med. 29:369–373. 2009.
|
|
32
|
Giansanti V, Torriglia A and Scovassi AI:
Conversation between apoptosis and autophagy: ‘Is it your turn or
mine?’. Apoptosis. 16:321–333. 2011. View Article : Google Scholar : PubMed/NCBI
|