Introduction
Distant metastasis represents the outcome with the
worst prognosis for various types of malignant tumors (1–5), but
little is known regarding the impact of interacting epithelial and
mesenchymal phenotypic cancer cells within its etiopathogenesis.
Oral squamous cell carcinoma, particularly when localized at the
tongue base, with >5 cm of tumor thickness and histologically
confirmed angiogenesis has been reported to exhibit regional and/or
distant metastasis (6) more
frequently than those located at other tongue sites.
Metastasis is defined as the breakaway of cancer
cells from a primary tumor site and their spread via blood or
lymphatic fluid to other parts or organs in the body (7). This process includes the following
steps: i) Penetration of cancer cells through the epithelial
basement membrane and invasion of surrounding tissues, ii)
intravasation of blood and/or lymphatic vessels, iii) spread
through circulation, and iv) extravasation and growth in distant
sites or organs (8).
Based on a hamster cheek pouch carcinoma (HCPC)
model, Tsuji et al (9)
proposed a ‘cooperation theory’, suggesting that cancer cells
undergo a phenotypic change to accomplish the various steps of
metastasis (9,10). It was proposed that only coaction of
epithelial and mesenchymal cells may lead to spontaneous
metastasis. Mesenchymal phenotypic cancer cells degrade the
extracellular matrix, thereby enabling cancer cell invasion,
intravasation and transport, epithelial phenotypic cancer cells
eventually reestablish colonies at distant sites. Although this
animal trial provided insight into certain cancer metastasis
phases, particularly emphasizing the different roles of two cancer
cell phenotypes, its predictive power is limited due to an inherent
5% genomic difference between rodent and human cells (11).
In order to investigate the interaction of human
mesenchymal and epithelial tongue cancer cell lines in cancer
metastasis, such phenotypic cancer cell lines stably labeled with
two different fluorescent proteins (12) were injected into 48 male athymic
Balb/c nude mice (13). In
vivo and ex vivo analyses were performed to investigate
whether or not lung metastasis occurred following subcutaneous
injections due to phenotypic interaction, and whether or not
metastasis after intravenous injection into the tail vein could be
observed under a fluorescence microscope and in histopathological
analyses.
Materials and methods
Animal care
A total of 48 male athymic Balb/c nude mice (4 weeks
old; 12–14 g; Charles River Lab, Wilmington, USA) were supplied by
the Laboratory Animal Unit (LAU) of The University of Hong Kong.
LAU also provided daily animal care with a standard rodent diet (a
complete life cycle diet; LabDiet, St. Louis, MO, USA) and
autoclaved water. The animals were housed in a 12 h light/dark
cycle at a temperature between 16 and 22°C in a 5% carbon dioxide,
80% oxygen, 15% nitrogen humidified atmosphere. All mice were
acclimatized to their new surroundings for two days prior to the
start of the experiment.
Animal experiment
Experimental metastasis (10) was induced by subcutaneous or
intravenous injection of fluorescence-labelled human tongue cancer
UM1 and UM2 cell lines and their mixture labelled with a green
fluorescent protein (GFP) and a red fluorescent protein (RFP),
respectively, to obtain spontaenous, direct metastasis. The cell
lines were cultured in a mixture of Dulbecco's modified Eagle's
medium and Ham's F-12 medium at a ratio of 1:1 with 10% fetal
bovine serum and 100 U/ml penicillin-streptomycin. The parental UM1
and UM2 cells were donated by Dr David Wong, School of Dentistry,
University of California (LA, USA). The manufacturing process and
the validity examination of the tumorigenicity of these two cell
lines in nude mice have already been published elsewhere (12,13). The
number of injected cancer cells and the volume of
phosphate-buffered saline (PBS) added were determined according to
the technique described by Nakayama et al (14). While the mice in groups A, B and C
underwent subcutaneous injection with 0.3 ml PBS (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) containing 1×107 cancer
cells (UM1-GFP cells in Group A, UM2-RFP cells in Group B, the 1:1
mixture in Group C), those in groups D, E and F received
intravenous injection with 0.2 ml PBS containing 5×105
cancer cells (UM1-GFP cells in Group D, UM2-RFP cells in Group E,
the 1:1 mixture in Group F) into the tail vein. Table I provides a survey of the experiment.
Injected dosages of cancer cells in each group correspond to those
used in previous experiments (14).
 | Table I.Experimental metastasis cancer cell
line injection scheme. |
Table I.
Experimental metastasis cancer cell
line injection scheme.
|
| Animals | Age, weeks | Cells | Injection site | Sacrifice time |
|---|
| A | 8 | 4 | UM1-GFP | Dorsal site | Week 8 |
| B | 8 | 4 | UM2-RFP | Dorsal site | Week 8 |
| C | 8 | 4 | Mixture | Dorsal site | Week 8 |
| D | 8 | 4 | UM1-GFP | Tail vein | Week 8 |
| E | 8 | 4 | UM2-RFP | Tail vein | Week 8 |
| F | 8 | 4 | Mixture | Tail vein | Week 8 |
Assessment of experimental
metastasis
All animals were screened for fluorescent singals
one week after the injection and once every week thereafter until
their euthanasia in week 8 under anesthesia. The latter was
performed using ketamine (100 mg/kg; Alfasan Diergeneesmiddelen BV,
Woerden, Netherlands) and xylazine (10 mg/kg; Alfasan
Diergeneesmiddelen BV). All mice underwent in vivo imaging
in a supine position using the IVIS® Spectrum in
vivo imaging system (PerkinElmer Inc., Waltham, MA, USA). The
system captured fluorescent light in targeted organs using GFP and
DsRed filter sets with excitation wave lengths of 465 and 535 nm,
respectively, and emission wave length widths of 500–580 and
620–680 nm, respectively, once a week following cell injection.
Following euthanisation at week 8, the lungs were harvested and
physically examined for metastatic tumors. Thereafter, the lungs
were immerged in PBS, followed by ex vivo imaging using the
IVIS® Spectrum in vivo imaging system
(PerkinElmer Inc.). Imaging data were recorded and analyzed using
the Living Image 4.4 software package (PerkinElmer Inc.).
Histology
Subsequently, the lung tissue underwent cryosection
and paraffin embedding for histological examination. The specimens
were inserted in a 2:1 mixture of 20% sucrose (Electron Microscopy
Sciences; EMS, Hatfield, PA, USA) and Tissue-Tek® O.C.T.
(EMS) for 30 min. Next, the specimens were submersed in a 1:1
mixture of 20% sucrose (EMS) and Tissue-Tek® O.C.T (EMS)
and mixed for another 30 min. Finally, all specimens were embedded
in Tissue-Tek® O.C.T. (EMS), laid in a tissue mold (EMS)
and cut into slices of 6 µm thickness which were examined under a
green or/and red fluorescent light detecting microscope (Nikon
Corporation, Tokyo, Japan) at a magnification of ×20.
The remaining lung tissue was washed three times for
5 min each time with 1X PBS and fixed in 4% paraformaldehyde
overnight at room temperature (Sigma-Aldrich; Merck KGaA).
Following abundant rinsing under tap water, the fixed tissue was
embedded in paraffin, using a Shandon Excelsior ES®
Operator (Thermo Fisher Scientific Inc., Waltham, MA, USA) that
automatically performed the paraffin infiltration process. Tissue
slices of 6 µm thickness were cut with a microtome (Leica RM2155;
Leica Microsystems GmbH, Wetzlar, Germany) and stained with
hematoxylin for 3 min and eosin for 1 min (H&E) at room
temperature prior to being examined under a light microscope (Leica
Microsystems GmbH) at a magnification of ×200.
Results
In vivo physical examination of
spontaneous metastasis groups
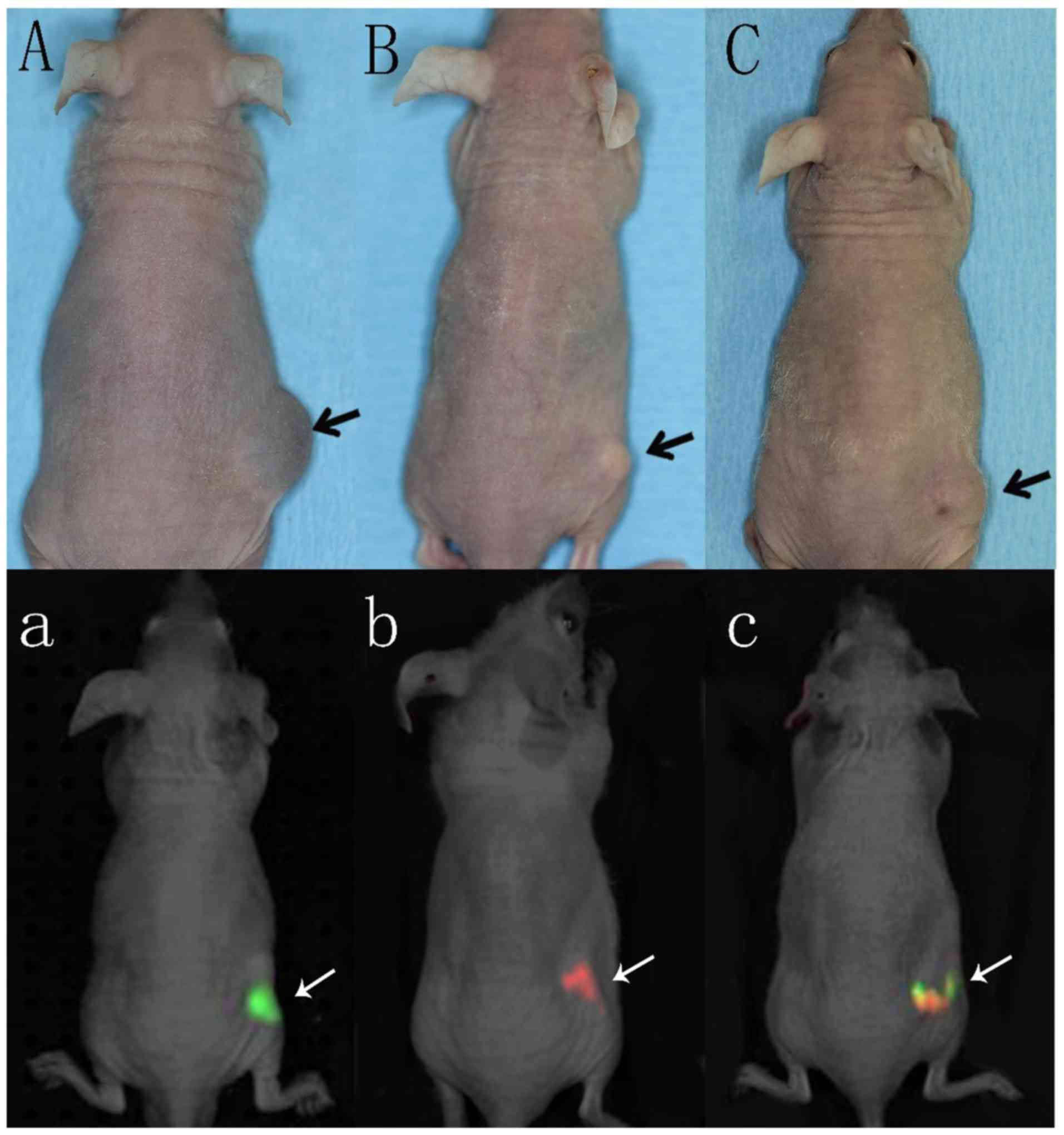
Mice in groups A, B and C that underwent
subcutaneous injection with cancer cells at the dorsal site
developed progressive tumor growth at the location of injection.
Regular physical examination of the mice revealed palpable tumors
at the end of the first week after injection (Fig. 1A-C).
Ex vivo lung examination in all
groups
Macroscopical lung metastasis was only detected in
the mice in groups E (5/8; 63%) and F (6/8; 75%), but not in any of
the other groups (Fig. 2).
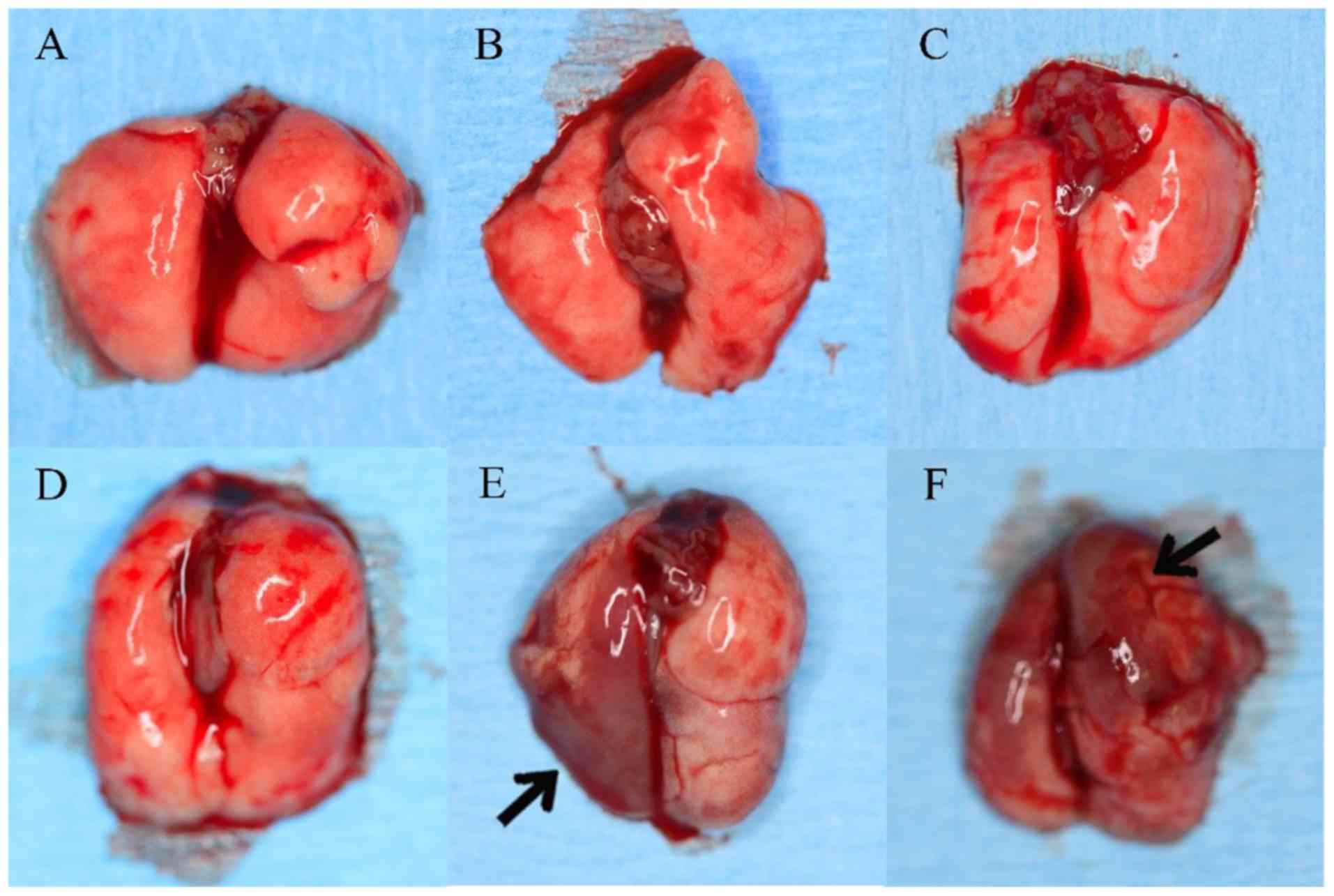 | Figure 2.Ex vivo examination of the
lungs. Group A, UM1-GFP s.c.; Group B, UM2-RFP s.c.; Group C, 1:1
mixture s.c.; Group D, UM1-GFP t.v.; Group E, UM2-RFP t.v.; and
Group F, 1:1 mixture t.v.; GFP, green fluorescent protein; RFP, red
fluorescent protein; s.c., subcutaneous; t.v., tail vein. |
In vivo and ex vivo fluorescent
imaging
While in vivo imaging did not detect any
fluorescent signals except in those animals with the primary dorsal
tumors (Fig. 1A-C), ex vivo
imaging examination revealed red fluorescent signals in lungs of
the mice in groups E and F (Fig. 3).
Any harvested lung tissue, however, revealed green fluorescence
signals.
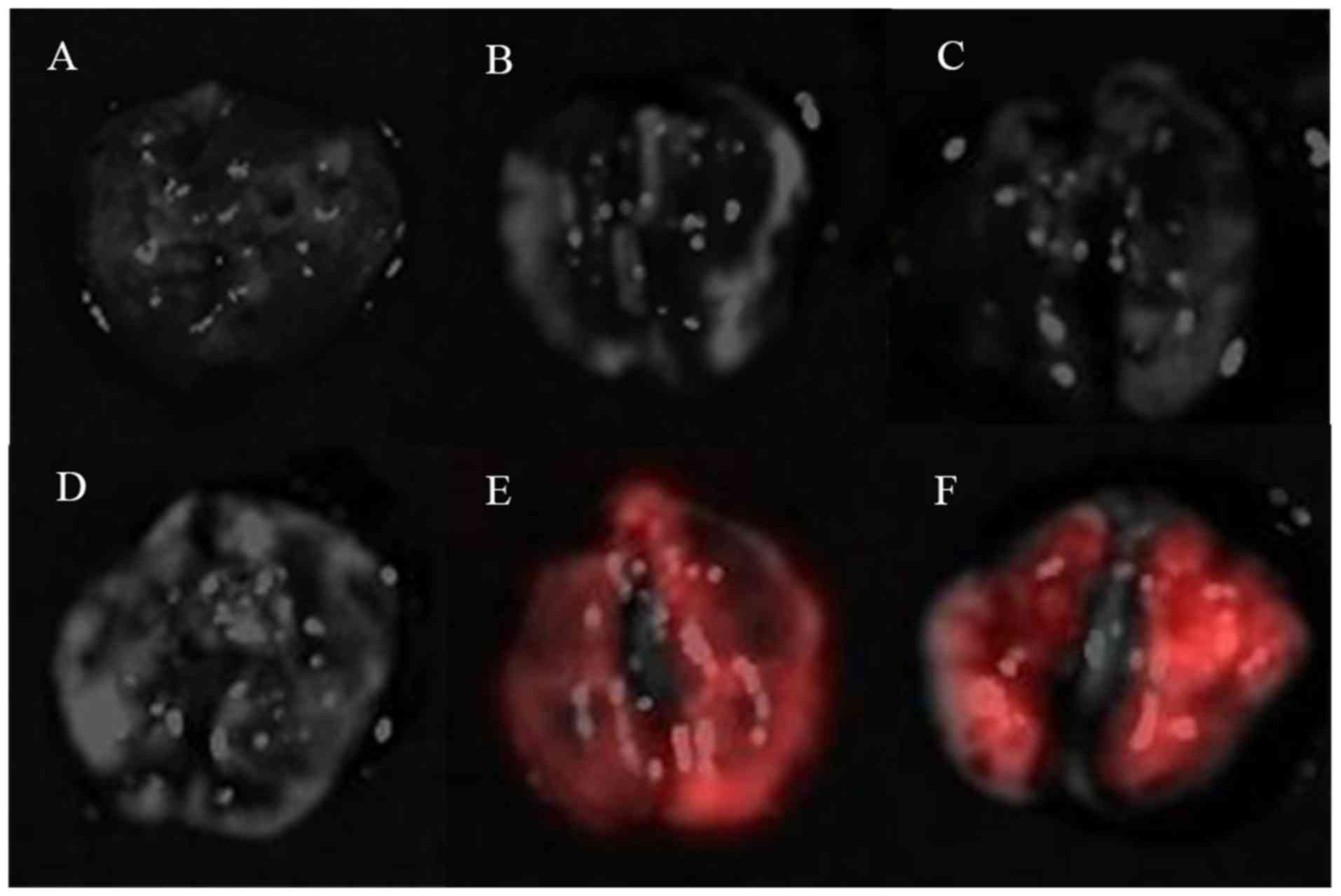 | Figure 3.Ex vivo fluorescent imaging of
the lungs. Group A, UM1-GFP s.c.; Group B, UM2-RFP s.c.; Group C,
1:1 mixture s.c.; Group D, UM1-GFP t.v.; Group E, UM2-RFP t.v.; and
Group F, 1:1 mixture t.v.; GFP, green fluorescent protein; RFP, red
fluorescent protein; s.c., subcutaneous; t.v., tail vein. |
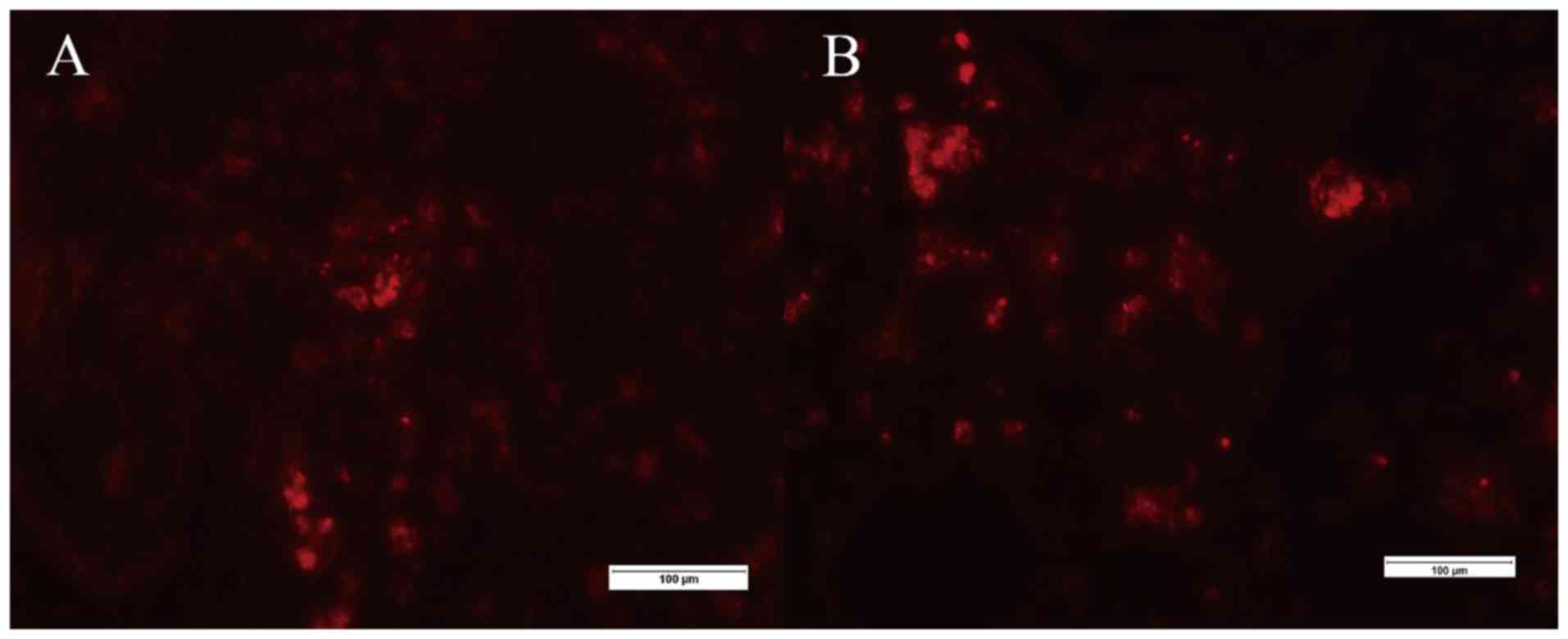
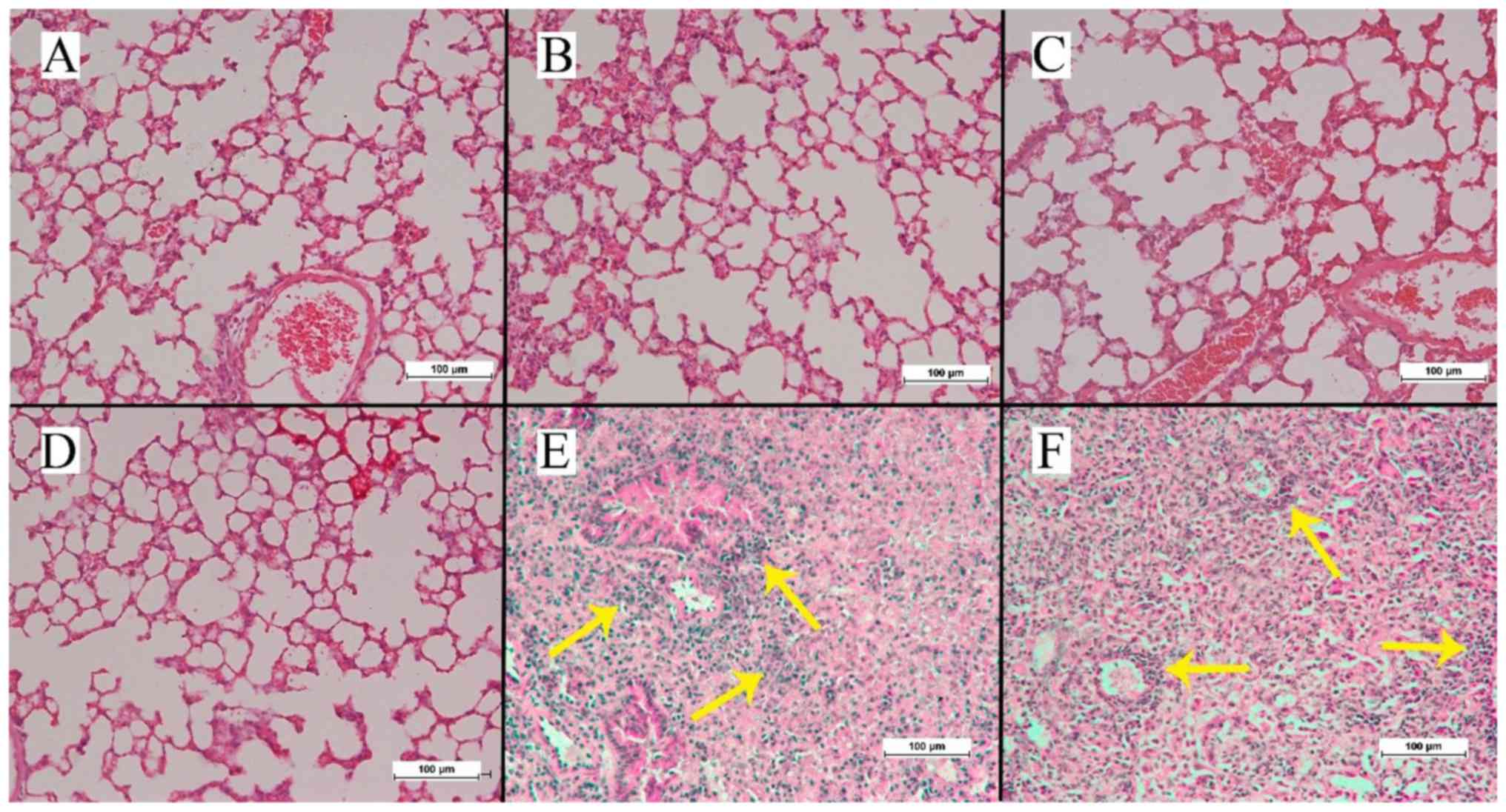
Histological analysis
While no cryosection specimen harvested from the
animals of the spontaneous metastasis groups A, B and C revealed
any fluorescent signals other than the primary tumor, red
fluorescent signals were observed in the harvested specimens of
animals from the direct metastasis groups E and F (Fig. 4). By contrast, no specimen from any
animal in the direct metastasis groups displayed green fluorescent
signals. The H&E stained lung tissue slices (Fig. 5) were inconspicuous, except for in the
animals in groups E and F where metastatic lung foci could be
observed.
Discussion
While the epithelial phenotypic human tongue cancer
UM2-RFP cell line is able to release direct metastasis in lungs
when injected into the tail vein of athymic Balb/c nude mice, the
mesenchymal phenotypic UM1-GFP cell line did not lead to metastasis
following intravenous injection. Epithelial and mesenchymal
phenotypic cancer cell lines were unable to establish distant
metastasis once injected via the subcutaneous pathway.
Previous studies in nude mice made use of rodent
cancer cell lines to investigate various roles of cell phenotypes
in metastasis (9,10). The present study used human tongue
cancer mesenchymal (UM1) and epithelial (UM2) cell lines that
originated from a Japanese male patient who suffered from a
histopathologically confirmed tongue squamous cell cancer (14). The labelling method and the phenotypic
stability of the labelled cells in vitro and in vivo
were reported previously (12,13).
Xenograft models making use of human derived cancer cell lines are
considered more suitable for translational research, due to the
human nature of the injected cells and the fact that the majority
of human cancer cell lines form subcutaneous nodules that may serve
to measure cancer progression or regression (15).
It was unexpected to discover that the two
subcutaneously injected cancer cell lines did not lead to distant
metastasis. The following two explanations from previously
published literature may explain this: i) Compared with other types
of human solid cancer, including lung cancer, oral squamous cell
cancer is not notorious for distant metastases (16), and ii) innate and humeral adaptive
immunity may prevent local oral squamous cell cancer invasion and
metastasis (17). The fluorescent
proteins GFP and RFP may be integrated into the genetic information
of the human tongue cancer cell lines and became hereditary, as
previously described (12). Eventual
assumptions that these fluorescent proteins within the human cancer
cell line genomes may be responsible for generating an immune
response inhibiting secondary cancer growth in distant organs
(18), warrants further attention in
future research. Furthermore, this animal model may be useful in
future cancer cell studies investigating the involvement of
phenotypes in metastasis (9), as both
fluorescence-labelling and cell line phenotypes maintained stable
in vitro and in vivo following several cell cycles
(13).
At present, anticancer drugs are in clinical use
with the aim of promoting epithelial phenotypes of cancer cells. In
this animal trial, lung metastasis was only detected following
intravenous injection with the epithelial RFP-labelled phenotypic
cancer cell line into the tail vein, irrespective of whether
combined or not with mesenchymal GPF-labelled phenotypic cancer
cell lines. The intravenous inoculation of cancer cells is no
longer accepted as the ideal approach to evaluate metastasis
(19). However, the cancer cell
survival within the circulation and at secondary sites, including
the pulmonary niches, represent crucial steps for cancer metastasis
(20). It may be hypothesized that
epithelial phenotypic cancer cells in the circulation, as observed
in the present study, contribute to experimental metastasis. This
finding may be of importance in designing future anticancer drugs
(21). While this finding is opposite
to others (14), who detected
experimental metastasis into the lung of animals following
intravenous injection of mesenchymal phenotype cancer cell lines,
it is in line with several other animal experiments (9,10,22). A conclusion that mesenchymal cells do
not contribute to cancer metastasis requires to be confirmation in
future experiments. In future research, the lineage tracing method
(23) may be useful in determining
whether the transduction of fluorescent proteins influences the
disparity in these observations. Furthermore, a potential
cooperation of fluorescent epithelial and mesenchymal phenotypic
cancer cells in cancer metastasis (9)
may be investigated in this animal model using intravital
video-microscopic methods.
One shortcoming of the present study may lie in the
subcutaneous application site of the human tongue cancer cell
lines, as the microenvironments of the latter location and the
tongue tissue are heterogenous (15).
The fact that it was not possibile to monitor the metastatic
process in vivo represents a second shortcoming. Other than
in the spontaneous subcutaneous metastasis groups, in vivo
imaging of fluorescent signals was impossible, likely due to the
interference of the chest wall with the signal reception. An
eventual combination of similar animal models with intravital video
microscopy technique (24) may
provide a more promising method for investigating the dynamics of
metastatic diseases and angiogenic processes. A third shortcoming
is the fact that the liver, which is the first pass organ for the
blood flow of the inferior caval vein, was not investigated. Future
studies of tail vein injection may study the liver.
The results of the present study contributed
evidence to the role of epithelial phenotypic cancer cells in the
release of experimental metastasis following tail vein injection in
male athymic Balb/c nude mice, as well as proving that fluorescent
human tongue cancer cells may be reliably detected under the
fluorescence microscope for as long as 8 weeks after the two types
of injection.
Acknowledgements
The authors would like to thank Mrs. Hoi Yee Tong
and Mr. Yip Chui for their technical assistance in cell culture and
specimen preparation. They would also like to thank Dr W.K. Tse,
Ms. P.Y. Yuen, Mr. K.S. Cheung and Mr. C.F. Chan for the animal
care. Imaging data were acquired using equipment maintained by the
University of Hong Kong Li Ka Shing Faculty of Medicine, Faculty
Core Facility.
Funding
The present study was supported by the Seed Funding
Programme for Basic Research of the University Research Committee
of The University of Hong Kong (grant no.
104002330.054968.08002.301.01).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WXC, LWZ and RZ contributed to the study design.
WXC, RQY and LM performed the animal experiments and examined the
specimens. WXC, HZH and LWZ analyzed the data. WXC, LWZ and RZ were
major contributors in writing and revising the manuscript. All
authors read and approved the final manuscript.
Ethics statement and consent to
participate
The present study protocol was approved by the
Committee on the Use of Live Animals in Teaching and Research
(CULATR 3088-13), Li Ka Shing Faculty of Medicine, The University
of Hong Kong (Hong Kong, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kadakia S, Chan D, Mourad M and Ducic Y:
The prognostic value of age, sex, and subsite in cutaneous head and
neck melanoma: A clinical review of recent literature. Iran J
Cancer Prev. 9:e50792016.PubMed/NCBI
|
|
2
|
Cheng J and Yan S: Prognostic variables in
high-risk cutaneous squamous cell carcinoma: A review. J Cutan
Pathol. 43:994–1004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polireddy K and Chen Q: Cancer of the
pancreas: Molecular pathways and current advancement in treatment.
J Cancer. 7:1497–1514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Irani S: Distant metastasis from oral
cancer: A review and molecular biologic aspects. J Int Soc Prev
Community Dent. 6:265–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang B, Zhang S, Yue K and Wang XD: The
recurrence and survival of oral squamous cell carcinoma: A report
of 275 cases. Chin J Cancer. 32:614–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Massano J, Regateiro FS, Januario G and
Ferreira A: Oral squamous cell carcinoma: Review of prognostic and
predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 102:67–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mareel M and Leroy A: Clinical, cellular,
and molecular aspects of cancer invasion. Physiol Rev. 83:337–376.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuji T, Ibaragi S and Hu GF:
Epithelial-mesenchymal transition and cell cooperativity in
metastasis. Cancer Res. 69:7135–7139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuji T, Ibaragi S, Shima K, Hu MG,
Katsurano M, Sasaki A and Hu GF: Epithelial-mesenchymal transition
induced by growth suppressor p12CDK2-AP1 promotes tumor cell local
invasion but suppresses distant colony growth. Cancer Res.
68:10377–10386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Jong M and Maina T: Of mice and humans:
Are they the same?-Implications in cancer translational research. J
Nucl Med. 51:501–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai WX, Zheng LW, Huang HZ and Zwahlen RA:
Evidence of phenotypic stability after transduction of fluorescent
proteins in two human tongue cancer cell lines. Arch Oral Biol.
79:48–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai WX, Zheng LW, Ma L, Huang HZ, Yu RQ
and Zwahlen RA: Tumorigenicity and validity of fluorescence
labelled mesenchymal and epithelial human oral cancer cell lines in
nude mice. Biomed Res Int. 2016:48979862016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakayama S, Sasaki A, Mese H, Alcalde RE
and Matsumura T: Establishment of high and low metastasis cell
lines derived from a human tongue squamous cell carcinoma. Invasion
Metastasis. 18:219–228. 1999. View Article : Google Scholar
|
|
15
|
Teicher BA: Human tumor xenografts and
mouse models of human tumors: Re-discovering the models. Expert
Opin Drug Discov. 4:1295–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takes RP, Rinaldo A, Silver CE, Haigentz M
Jr, Woolgar JA, Triantafyllou A, Mondin V, Paccagnella D, de Bree
R, Shaha AR, et al: Distant metastases from head and neck squamous
cell carcinoma. Part I. Basic aspects. Oral Oncol. 48:775–779.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shultz LD, Goodwin N, Ishikawa F, Hosur V,
Lyons BL and Greiner DL: Human cancer growth and therapy in
immunodeficient mouse models. Cold Spring Harb Protoc.
2014:694–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steinbauer M, Guba M, Cernaianu G, Köhl G,
Cetto M, Kunz-Schughart LA, Geissler EK, Falk W and Jauch KW:
GFP-transfected tumor cells are useful in examining early
metastasis in vivo, but immune reaction precludes long-term tumor
development studies in immunocompetent mice. Clin Exp Metastasis.
20:135–141. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gomez-Cuadrado L, Tracey N, Ma R, Qian B
and Brunton VG: Mouse models of metastasis: Progress and prospects.
Dis Model Mech. 10:1061–1074. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonnomet A, Brysse A, Tachsidis A, Waltham
M, Thompson EW, Polette M and Gilles C: Epithelial-to-mesenchymal
transitions and circulating tumor cells. J Mammary Gland Biol
Neoplasia. 15:261–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu CY, Tsai YP, Wu MZ, Teng SC and Wu KJ:
Epigenetic reprogramming and post-transcriptional regulation during
the epithelial-mesenchymal transition. Trends Genet. 28:454–463.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lyons JG, Lobo E, Martorana AM and
Myerscough MR: Clonal diversity in carcinomas: Its implications for
tumour progression and the contribution made to it by
epithelial-mesenchymal transitions. Clin Exp Metastasis.
25:665–677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoffman RM: Green fluorescent protein
imaging of tumour growth, metastasis, and angiogenesis in mouse
models. Lancet Oncol. 3:546–556. 2002. View Article : Google Scholar : PubMed/NCBI
|