Introduction
An estimated 1.8 million novel lung cancer cases
occurred in 2012, accounting for ~13% of total cancer diagnoses
worldwide (1). Sundar et al
(2) demonstrated that there are two
main types of lung cancer, small-cell lung cancer (SCLC) and
non-small-cell lung cancer (NSCLC), with >80% of lung cancer
cases being NSCLC in 2014. The combination of the traditional
methods of surgery, chemotherapy and radiotherapy with
immunotherapy is a novel modality for anti-cancer therapies to
reduce the mortality of patients with cancer. However, there are
still unacceptably high rates of relapse and mortality in patients
with early-stage, surgically-resectable lung cancer (3). Currently, chemotherapy is the standard
treatment for advanced stage and metastatic NSCLC (4). However, chemotherapy is associated with
a decline in sensitivity over time and often has a toxicity profile
that reduces the overall quality of life of the patients, without
significantly improving prognosis (5). Despite numerous advances in treatment
modalities, the treatment and mechanism for NSCLC progression
remains unclear.
Dendritic cells (DCs) are the most effective
antigen-presenting cells and have been applied in cellular
immunotherapy research worldwide (6).
Since the first DC vaccine for prostate cancer was approved by the
FDA, DC-based immunotherapy has become an increasingly promising
novel therapeutic option. Cytokine-induced killer cells (CIK) are
well known for their antitumor activity (7). In recent years, there has been an
upsurge of interest in unraveling the roles of combined DC-CIK
therapy on NSCLC, with numerous results indicating that DC/CIK
immunotherapy combined with other treatments has a good clinical
efficacy and prospects for the treatment of NSCLC (8–11).
However, the mechanism by which DC-CIK cells can specifically kill
NSCLC remains unclear. Therefore, the present study used DCs to
induce CIK cells specifically targeted to NSCLC.
The family of 14-3-3 proteins serve key roles in
integrating cellular survival signaling. 14-3-3ζ is a member of a
family highly conserved proteins that control key aspects of
cellular function, including proliferation, apoptosis, and cell
survival (12). Experimental and
clinical results from previous studies have suggested that 14-3-3
proteins represent an addiction for numerous cancers and
consequently are an attractive target for anti-cancer therapeutics
(13,14). The protein has been identified as a
putative oncoprotein in several cancers, including NSCLC, liver
cancer, head and neck squamous cell carcinoma, and is a potential
target for developing a prognostic biomarker and therapeutics that
may enhance the antitumor activity of cisplatin for the treatment
of NSCLC (15).
An increasing amount of evidence has indicated that
dysregulation of apoptosis contributes to the development of human
cancers (16). Bad, a proapoptotic
Bcl-2 family protein regulates the intrinsic apoptosis pathway, Bad
is also regulated by phosphorylation, which leads to its
sequestration by 14-3-3 scaffold proteins (17). Phosphorylated (p)-Bad dissociates from
Bcl-2 and is sequestered in the cytosol to promote cell survival
(18).
In the present study the antitumor effects of DC-CIK
cells in Lewis lung cancer (LLC) cell lines were evaluated and the
underlying mechanism of these effects was investigated. The DC-CIK
cells effects on cytotoxic potentiation and apoptosis were
investigated and the cytotoxic effects were evaluated using an MTT
assay and apoptosis morphology observation. Expression of 14-3-3ζ
and p-Bad were measured by western blot analysis.
Materials and methods
Culture of CIK
Peripheral blood mononuclear cells (PBMCs) were
collected from healthy blood donors (2 males, 2 females; median
age, 39 years; age range, 28–50 years) with no clinical symptoms of
any disease. A total of 10 ml of blood was collected from each
donor in evacuated tubes containing 0.1 mg/ml heparin. Mononuclear
cells (MNC) were isolated with lymphocyte separating medium (Wuhan
Huamei Biotech Co., Ltd., Wuhan, China) and washed by normal saline
(centrifugation, 1,341.48 × g for 15 min). Mononuclear cells were
cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented
with 10% fetal calf serum (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) and recombinant human (rh) interferon (IFN)-γ
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) 1,000 U/ml, CD3
monoclonal antibody (sc-20047; 1:4,000, final concentration, 50
ng/ml; Santa Cruz Biotechnology, Inc.) and 300 units/ml of
recombinant human interleukin-2 (rh IL-2) were added to CIK cells
and cultured at 37°C in 5% CO2 incubator.
Medium was changed every three days for half dose
and added with 300 U/ml rh IL-2 to maintain the cell concentration
at 1×106/ml. On day 14, CIK cells were harvested with
trypsin and washed twice with PBS. Ethical approval for the present
study was obtained from the Ethics and Welfare Committee of Jinzhou
Medical University (Jinzhou, China). Written informed consent was
obtained from all participants.
Culture of DC
PBMC were cultured in serum-free RPMI-1640 medium
(Hyclone; GE Healthcare Life Sciences) at 37°C in 5% CO2
for 2 h and washed by serum-free RPMI 1640 culture medium twice.
Adherent cells were suspended in RPMI 1640 culture medium which
contained rh Granulocyte-macrophage colony-stimulating factor
(GM-CSF) (1,000 U/ml), rhIL-4 (500 U/ml), rh tumor necrosis factor
(TNF)-α (200 U/ml) for 7 days.
DC co-cultured with CIK
Mature DCs were obtained from the aforementioned
culture of DC 7 days after GM-CSF TNF-α induction, then CIK and DCs
were co-cultured in RPMI-1640 at 37°C in 5% CO2 in a
proportion of 1:5. Cells were cultured together for 3 days.
Culture of LLC cells
LLC cells were obtained from Shanghai Institute of
Biochemistry and Cell Biology (Chinese Academy of Sciences,
Shanghai, China). Cells were cultured in RPMI-1640 medium
supplemented with 10% heat inactivated fetal bovine serum (Hyclone;
GE Healthcare Life Sciences) and antibiotic solution (penicillin
100 U/ml and streptomycin 100 µg/ml). (Sigma-Aldrich; Merck KGaA;
Darmstadt, Germany). Cells were cultured under standard conditions
in a 5% CO2 humidified incubator at 37°C. LLC cells
alone were cultured as control group.
Cell migration assay
The cells were divided into 4 groups: DC-CIK, CIK,
PBS and the control group (RPMI-1640 medium with 10% fetal calf
serum). RPMI-1640 medium with 10% fetal calf serum was added in the
upper transwell insert chamber with the control group. DC-CIK, CIK
cells or PBS were seeded at the concentration of 0.6×105
cells/well in the upper transwell insert chamber containing a
polycarbonate filter (6.5 mm diameter, 0.4 µm pores; Corning
Costar, Corning, NY, USA). LLC cells was added to the lower chamber
at 2.0×105 cells/500 µl/well in all groups, and the
plates were incubated for 7 days at 37°C in 5% CO2. Both
upper and lower chamber cells were cultured in RPMI-1640 medium
with 10% fetal calf serum. Images of the LLC cells in each group
were captured under an inverted microscope (Olympus Corporation,
Tokyo, Japan) to evaluate the number and morphology of cells for 20
min at room temperature at 3, 5 and 7 days (magnification, ×200).
LLC cells were used as target cells, and the CIK and DC-CIK cells
cultured for 7 days were used as effector cell mixed for the cell
viability assay and western blot analysis.
Cell viability assay
The cell viability of LLC cells was measured in the
7th day. LLC cells were obtained from the aforementioned cell
migration assay were diluted with RPMI-1640 medium containing 10%
fetal calf serum at 1×104 cells/ml. There were three
parallel wells for DC-CIK, CIK, PBS and the control group. The MTT
assay was employed to examine cell viability. Briefly, MTT was
added to the culture medium at a final concentration of 0.5 mg/ml
and cells were incubated at 37°C for 3 h, the culture medium
containing MTT was removed. Dimethyl sulfoxide (100 µl) was then
added into each well to dissolve the formed blue formazan.
Absorbance (A) was read at 490 nm on a microplate reader. Cell
viability was calculated as follows: Cell viability (%) = A
experiment group/A control group ×100%.
Western blot analysis
LLC cells were lysed with RIPA lysis buffer [50 mM
of tris-HCl (pH 7.5), 150 mM of NaCl, 1% NP-40, 1% sodium
deoxycholate, 0.1% SDS, 5 mM of EDTA, 25 mM of NaF, 2 mM Na3VO4,
and 1 mM of PMST] containing 1:100 diluted protease inhibitor
cocktail (cat. no. P8340; Sigma-Aldrich; Merck KGaA) on ice. The
protein concentrations were measured using a Bradford assay
subsequent to centrifugation at 13,000 × g for 15 min at 4°C. Equal
amounts of proteins were separated on a 10% gel by SDS-PAGE and
transferred to a polyvinylidene fluoride membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). For western blot analysis
of 14-3-3ζ, (ab87361; 1:1,000; Abcam, Cambridge, MA, USA), p-bad
(ab171725; 1:1,000; Abcam) and β-actin (A5441; 1:10,000;
Sigma-Aldrich; Merck KGaA), the primary antibodies used in the
experiment were probed and incubated overnight at 4°C, followed by
secondary antibody reactions with horseradish peroxidase-cinjugated
goat anti-mouse IgG (ab205719; 1:5,000; Abcam) for 1.5 h. The
detection was evaluated by the 3-bromo-4-chloro-5-indolyl phosphate
and nitro blue tetrazolium reaction (Ameresco, Inc., Framingham,
MA, USA).
Statistical analysis
All statistical analyses were performed using SPSS
Statistics 17.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed
as the mean ± standard deviation. One-way analysis of variance was
used followed by Fisher's Least Significant Difference test for
homogeneous data and followed by Dunnett's T3 for the
heteroscedastic data. P<0.05 was considered to indicate a
statistically significant difference.
Results
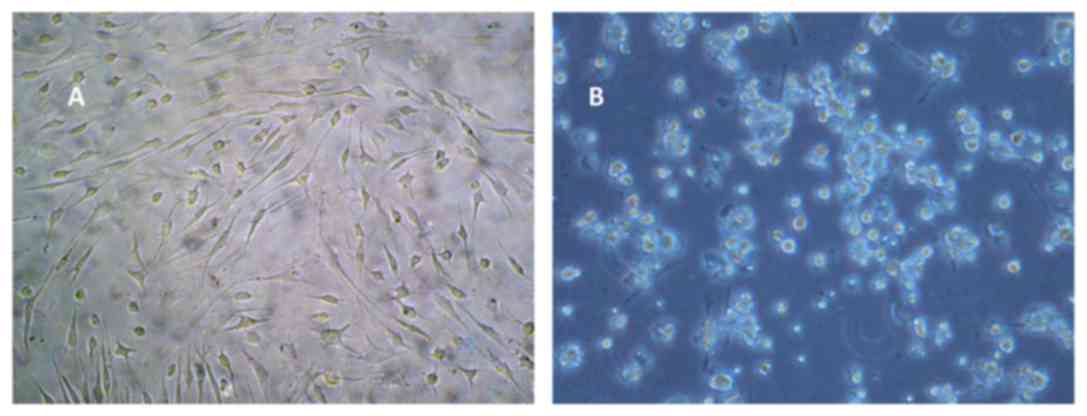
LLC proliferation
Adherent LLC cells that exhibited a round or
semi-shuttle shape growth were examined under an inverted
microscope (magnification, ×200) at 24–48 h after they were
passaged (Fig. 1A). Proliferation
began at the first three days following culturing. The cells of
passaged 2 exhibited a shuttle or fibroblast shape, with the cells
being fully extended (magnification, ×400; Fig. 1B).
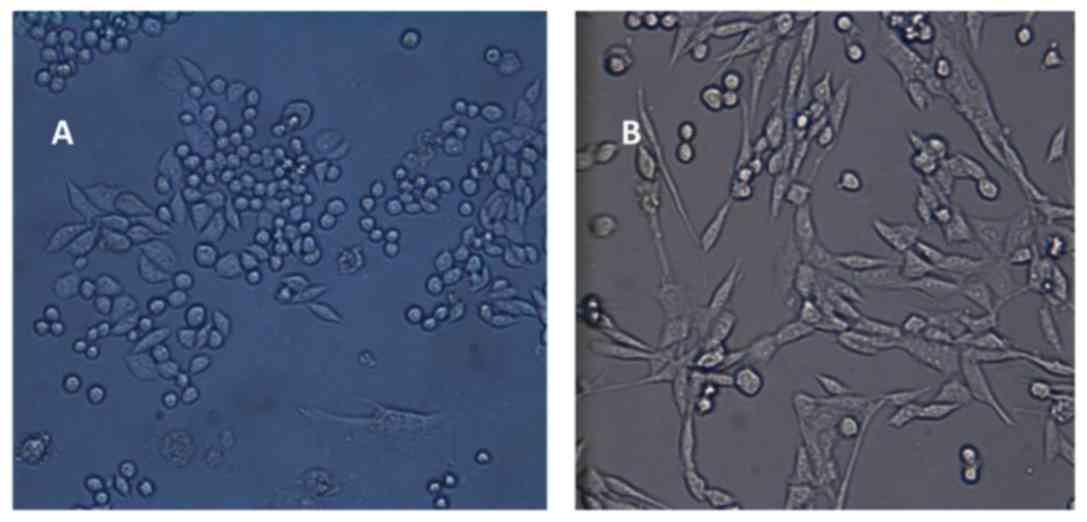
Morphology of DCs and CIK cells
Microscopic observation revealed that adherent DCs
were visible at the bottom of the plate. On day 7, the cells
exhibited an irregular, fusiform or stellate shape that is
characteristic of mature DCs. The DCs were thriving, with evident
thick and long dendritic protrusions (Fig. 2A). CIK cells were spherical, uniform
and transparent, cells were increased in size and small colonies
had formed. On day 12, the number of colonies had markedly
increased, exhibiting cellular proliferation (Fig. 2B).
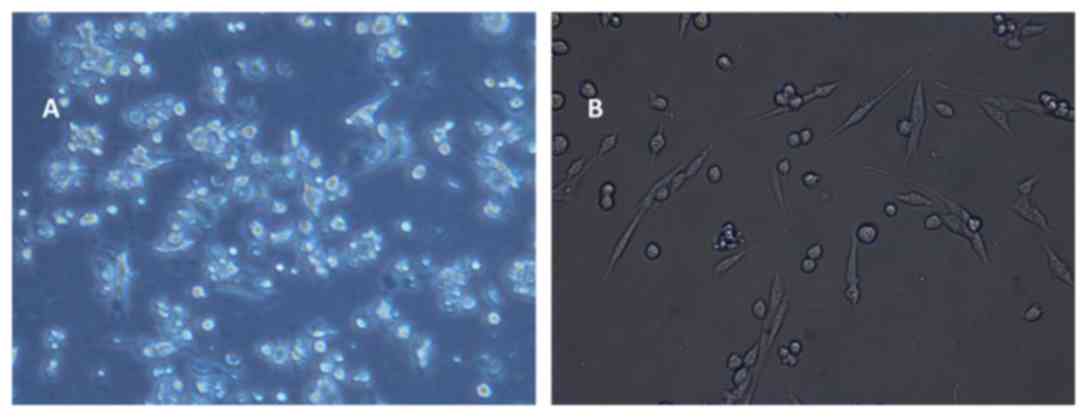
Morphology of LLC cells following
co-culture with DC-CIK
LLC cellular morphology did not change significantly
in all groups at 3 day of co-culture. Transparent LLC cells with a
uniform size in the CIK group were superior to the DC-CIK group.
LLC cells exhibited inflation, cytoplasmic contraction and an
increase in intercellular space in the DC-CIK group at 5 day of
cell co-culture (Fig. 3A). LLC cells
shrank in size and the intercellular space continued to increase in
the CIK cells group at 7 day (data not shown). A proportion of LLC
cells exhibited cell death in the DC-CIK group. Cell morphology
change was evident, and cell size became smaller and further away
from the surrounding cells. Connections between cells also
disappeared (Fig. 3B). These images
demonstrate that DC-CIKs induced morphological changes in LLC
cells.
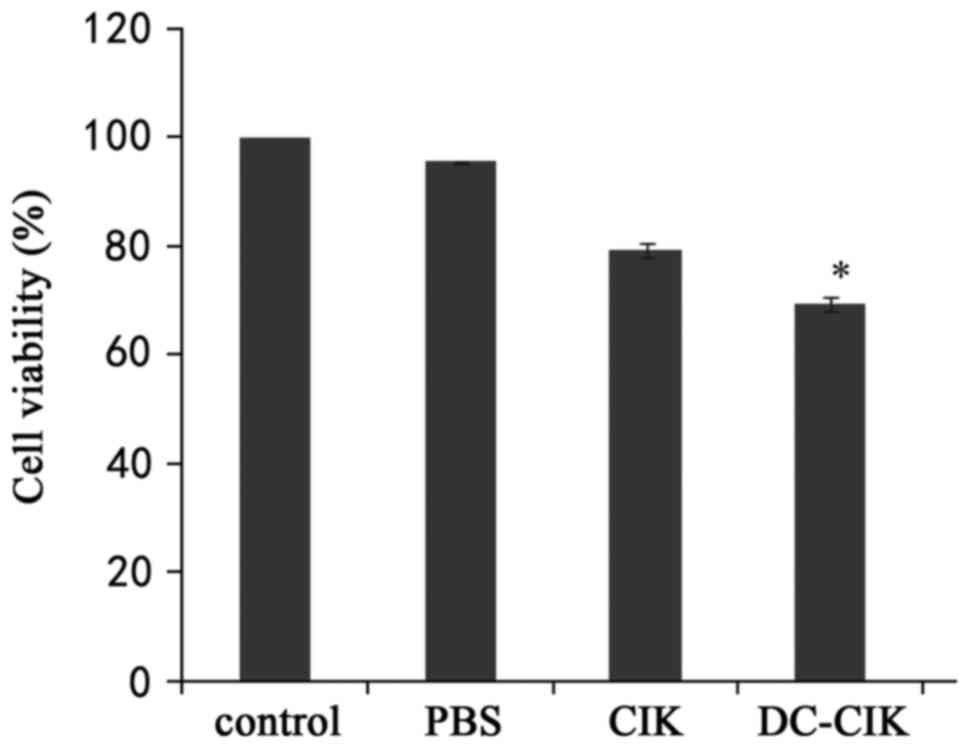
DC-CIK inhibited LLC cell
viability
In order to examine the cytotoxic effects of DC-CIK
on LLC cells, cell viability was examined by an MTT assay after LLC
cells were incubated with PBS, CIK or DC-CIK for 7 days. The DC-CIK
group was statistically different from the other groups. The
results demonstrated that DC-CIK inhibited the proliferation of LLC
cells (P<0.01; Fig. 4).
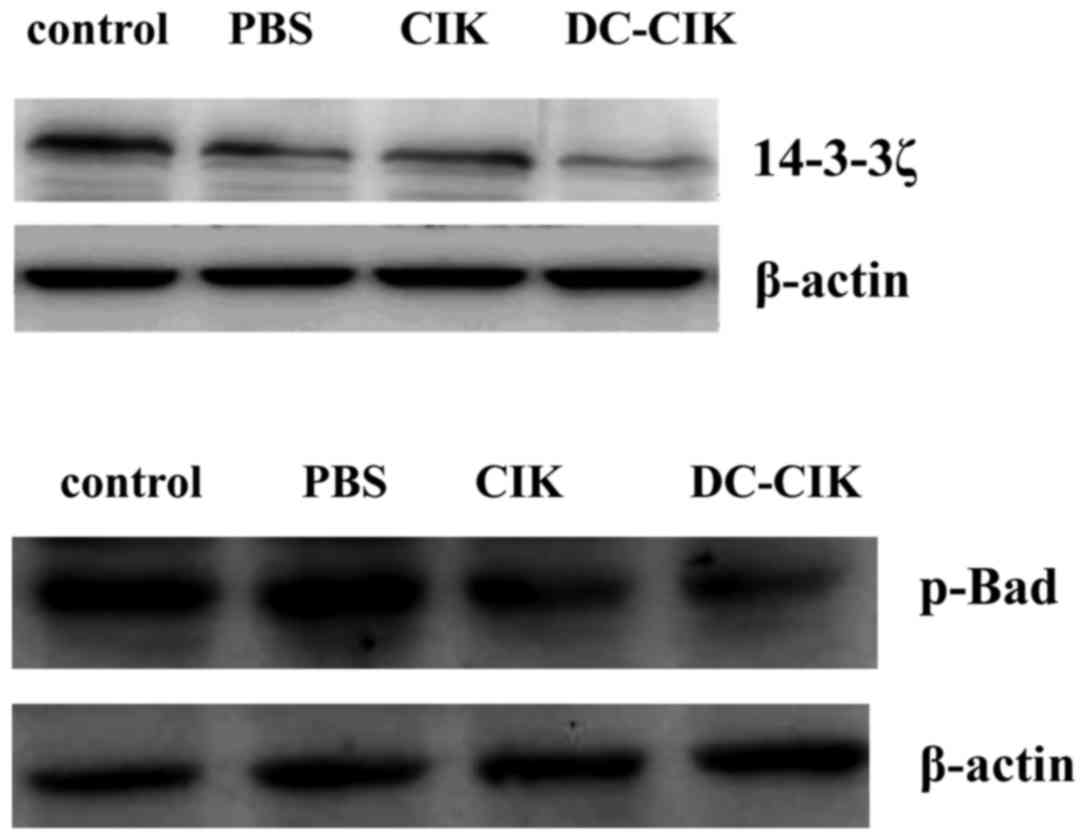
Effect of DC-CIK on expression levels
of 14-3-3ζ and p-Bad
To illustrate the mechanism of DC-CIK induced
apoptosis, the involvement of 14-3-3ζ and p-Bad were investigated
by western blot analysis. As depicted in Fig. 5, compared with the control group, the
protein level of 14-3-3ζ and p-Bad was slightly decreased in the
DC-CIK group. The results demonstrate that DC-CIK reduced the
expression of 14-3-3ζ and p-Bad protein in LLC cells.
Discussion
NSCLC is the leading cause of cancer-associated
mortality in the USA (15). To date,
surgery, radiotherapy and chemotherapy are still the principal
therapeutic regimens; however, radiotherapy and chemotherapy
exhibit heavy toxic side effects, which are detrimental to the
survival rate of patients (19). The
study of Zhou et al (20)
demonstrated that a DC vaccine combined with CIK cells induced a
T-cell-mediated immune response that included activating native T
cells, which serve a critical role in the innate and adaptive
immune responses. In 2005, Lee et al (21) confirmed the curative effect of a DC
vaccine pulsed with autologous tumor lysate in patients with
hepatocellular carcinoma. These findings demonstrate that DC/CIK
cells can be effective against a variety of cancers through
immunotherapy. A previous study was conducted with the goal of
improving the efficacy of DC-based immunotherapy (7). The combined DC-CIK therapy, with
synchronous radiotherapy and chemotherapy to treat stage IIIB
non-small cell lung cancer was superior to single synchronous
radiotherapy and chemotherapy (22).
DC-CIK combined with concurrent radiochemotherapy was demonstrated
to improve the life quality and prolong the survival time of
patients (22). In the present study,
DC-CIK treatment clearly reduced cell viability on LLC cells by a
cell viability assay-this was consistent with the study of Cui
et al (23). The present study
identified that co-cultured DCs and CIK cells inhibited the
proliferation LLC cells by downregulating 14-3-3ζ and p-Bad. DC/CIK
cell therapy may be an effective treatment strategy. Similar
results have been published by a previous study regarding liver
cancer cells (24).
14-3-3 proteins bind to a number of functionally
diverse signaling proteins including protein kinases and protein
phosphatases, and are involved in important cellular processes such
as signal transduction, cell cycle control, and apoptosis (15,25).
Ectopic expression of 14-3-3 has been discovered in various
malignancies, including lung cancer, liver cancer and head and neck
squamous cell carcinoma. Enhanced expression of 14-3-3 proteins
have been detected in human cancers including lung cancer (26), which correlates with more aggressive
tumors and a poor prognosis (17).
Downregulation of 14-3-3ζ in head and neck cancer cells and lung
cancer cells renders cells more sensitive to chemotherapy (27,28). The
over-expression 14-3-3ζ in NSCLC tissues is associated with the
severity of disease and similarly targeted knock-down of 14-3-3ζ
using RNAi in A549 cells, and also increased the sensitivity of
cells to cisplatin (29). These
findings suggest that 14-3-3ζ serves an important role in promoting
tumor aggressiveness. As 14-3-3ζ expression is closely associated
with NSCLC disease, the present study examined DC-CIK for
anti-cancer effects on LLC cells. It was determined that DC-CIK
reduced the expression of 14-3-3ζ protein. It is supported by the
study of Lin et al (30).
The mitochondria-dependent (type II) apoptotic
pathway begins with the apoptosis-regulating protein Bcl-2 family,
including the anti-apoptotic proteins Bcl-2 and Bcl-xL in addition
to the pro-apoptotic proteins Bad and Bax (31). Previous studies have demonstrated that
the effect of apoptosis and repressed cell viability may be due to
the decreased levels of p-Bad (29,32).
Previous report indicated that the expression of p-Bad was
increased in colorectal cancer cells, and suggested that the
increased expression of the protein in malignant colorectal
epithelial cells compared with the normal mucosal epithelial cells
may possibly alter the regulation of cell death during colorectal
tumorigenesis (33). However the
association between p-Bad and cancers remains controversial. A
previous study reported this beauvericin-induced apoptosis in human
NSCLC A549 cells was also accompanied by the upregulation of p-Bad
(30). P-Bad expression was detected
well in normal gastric mucosal epithelial cells, whereas it was
detected in only 51% (31 of the 60) of the cancers. The decreased
expression of p-Bad in malignant gastric epithelial cells compared
with normal mucosal epithelial cells suggested that loss of p-Bad
expression may serve a role in gastric tumorigenesis (34). In the present study, the results
demonstrated that LLC cells treated with DC-CIK resulted in
decreased cell adherence and abnormal morphological changes which
are characteristics of apoptosis and reduced cell proliferation. In
addition, DC-CIK treatment resulted in decreased expression of
p-Bad protein in LLC cells. Depending on the nature of its target
proteins, 14-3-3 binding impacts multiple signaling pathways that
determine cell fate and organ development. For example, 14-3-3
associations control Raf signaling fidelity and neutralizes
Bad-mediated apoptosis (35).
Therefore, further study is required to examine the effects of
p-Bad and the association between 14-3-3ζ and p-Bad in LLC
cells.
Taken together, the results of the present study
indicate that DC-CIK induced cell apoptosis in LLC cells, which was
associated with the downregulation of 14-3-3ζ and p-Bad. Whether
the effects were directly associated with the modulation of
autophagy, requires further study. The data presented provides
evidence that DC-CIK may have the potential to serve as a promising
adjuvant in the combination therapy for the treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Student's
Platform for Innovation and Entrepreneurship Training Program
funded by Liaoning (grant no. 201610160044).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ and XL conducted the conception and design of the
present study. YH and FL performed the experiments.
Ethics approval and consent to
participate
Ethical approval for the present study was obtained
from the Ethics and Welfare Committee of Jinzhou Medical University
(Jinzhou, China). Written informed consent was obtained from
participants.
Consent for publication
Written informed consent was obtained from
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sundar R, Soong R, Cho BC, Brahmer JR and
Soo RA: Immunotherapy in the treatment of non-small cell lung
cancer. Lung Cancer. 85:101–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choudhury A, Palma M and Mellstedt H: The
future of cancer vaccines for non-small-cell lung cancer: Ongoing
trials. Clin Lung Cancer. 9(Suppl 1): S37–S44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma A, Moore WH, Lanuti M and Shepard
JA: How I do it: Radiofrequency ablation and cryoablation of lung
tumors. J Thorac Imaging. 26:162–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koski GK, Cohen PA, Roses RE, Xu S and
Czerniecki BJ: Reengineering dendritic cell-based anti-cancer
vaccines. Immunol Rev. 222:256–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie S, Wu X, Zhang G, Xu K, Bian X, Zhang
S and Ye Y: Remarkable regression of a lung recurrence from an
undifferentiated embryonal sarcoma of the liver treated with a DC
vaccine combined with immune cells: A case report. Cell Immunol.
290:185–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao P, Bu X, Wei X, Sun W, Xie X, Li C,
Guo Q, Zhu D, Wei X and Gao D: Dendritic cell immunotherapy
combined with cytokine-induced killer cells promotes skewing toward
Th2 cytokine profile in patients with metastatic non-small cell
lung cancer. Int Immunopharmacol. 25:450–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shan CC, Shi LR, Ding MQ, Zhu YB, Li XD,
Xu B, Jiang JT and Wu CP: Cytokine-induced killer cells co-cultured
with dendritic cells loaded with the protein lysate produced by
radiofrequency ablation induce a specific antitumor response. Oncol
Lett. 9:1549–1556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao M, Li H, Li L and Zhang Y: Effects of
a gemcitabine plus platinum regimen combined with a dendritic
cell-cytokine induced killer immunotherapy on recurrence and
survival rate of non-small cell lung cancer patients. Exp Ther Med.
7:1403–1407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Ren B, Li H, Yu J, Cao S, Hao X
and Ren X: Enhanced antitumor effects of DC-activated CIKs to
chemotherapy treatment in a single cohort of advanced
non-small-cell lung cancer patients. Cancer Immunol Immunother.
62:65–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tzivion G, Gupta VS, Kaplun L and Balan V:
14-3-3 proteins as potential oncogenes. Semin Cancer Biol.
16:203–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neal CL and Yu D: 14-3-3ζ as a prognostic
marker and therapeutic target for cancer. Expert Opin Ther Targets.
14:1343–1354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao J, Meyerkord CL, Du Y, Khuri FR and
Fu H: 14-3-3 proteins as potential therapeutic targets. Semin Cell
Dev Biol. 22:705–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan T, Li R, Todd NW, Qiu Q, Fang HB, Wang
H, Shen J, Zhao RY, Caraway NP, Katz RL, et al: Up-regulation of
14-3-3zeta in lung cancer and its implication as prognostic and
therapeutic target. Cancer Res. 67:7901–7906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lowe SW, Cepero E and Evan G: Intrinsic
tumour suppression. Nature. 432:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zha J, Harada H, Yang E, Jockel J and
Korsmeyer SJ: Serine phosphorylation of death agonist BAD in
response to survival factor results in binding to 14-3-3 not
BCL-XL. Cell. 87:619–628. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
del Peso L, González-García M, Page C,
Herrera R and Nuñez G: Interleukin-3-induced phosphorylation of BAD
through the protein kinase Akt. Science. 278:687–689. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manegold C, Vansteenkiste J, Cardenal F,
Schuette W, Woll PJ, Ulsperger E, Kerber A, Eckmayr J and von Pawel
J: Randomized phase II study of three doses of the integrin
inhibitor cilengitide versus docetaxel as second-line treatment for
patients with advanced non-small-cell lung cancer. Invest New
Drugs. 31:175–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou P, Liang P, Dong B, Yu X, Han Z and
Xu Y: Phase I clinical study of combination therapy with microwave
ablation and cellular immunotherapy in hepatocellular carcinoma.
Cancer Biol Ther. 11:450–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee WC, Wang HC, Hung CF, Huang PF, Lia CR
and Chen MF: Vaccination of advanced hepatocellular carcinoma
patients with tumor lysate-pulsed dendritic cells: A clinical
trial. J Immunother. 28:496–504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu XP, Xu YH, Zhou J and Pan XF: A
clinical study evaluating dendritic and cytokine-induced killer
cells combined with concurrent radiochemotherapy for stage IIIB
non-small cell lung cancer. Genet Mol Res. 14:10228–10235. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui Y, Yang X, Zhu W, Li J, Wu X and Pang
Y: Immune response, clinical outcome and safety of dendritic cell
vaccine in combination with cytokine-induced killer cell therapy in
cancer patients. Oncol Lett. 6:537–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li QY, Shi Y, Huang DH, Yang T, Wang JH,
Yan GH, Wang HY, Tang XJ, Xiao CY, Zhang WJ, et al:
Cytokine-induced killer cells combined with dendritic cells
inhibited liver cancer cells. Int J Clin Exp Med. 8:5601–5610.
2015.PubMed/NCBI
|
|
25
|
Obsil T and Obsilova V: Structural basis
of 14-3-3 protein functions. Semin Cell Dev Biol. 22:663–672. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gardino AK and Yaffe MB: 14-3-3 proteins
as signaling integration points for cell cycle control and
apoptosis. Semin Cell Dev Biol. 22:688–695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matta A, DeSouza LV, Ralhan R and Siu KW:
Small interfering RNA targeting 14-3-3ζ increases efficacy of
chemotherapeutic agents in head and neck cancer cells. Mol Cancer
Ther. 9:2676–2688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Zhao J, Du Y, Park HR, Sun SY,
Bernal-Mizrachi L, Aitken A, Khuri FR and Fu H: Down-regulation of
14-3-3zeta suppresses anchorage-independent growth of lung cancer
cells through anoikis activation. Proc Natl Acad Sci USA.
105:162–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rong F, Li W, Chen K, Li DM, Duan WM, Feng
YZ, Li F, Zhou XW, Fan SJ, Liu Y, et al: Knockdown of RhoGDIα
induces apoptosis and increases lung cancer cell chemosensitivity
to paclitaxel. Neoplasma. 59:541–550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin HI, Lee YJ, Chen BF, Tsai MC, Lu JL,
Chou CJ and Jow GM: Involvement of Bcl-2 family, cytochrome c and
caspase 3 in induction of apoptosis by beauvericin in human
non-small cell lung cancer cells. Cancer Lett. 230:248–259. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bishopric NH, Andreka P, Slepak T and
Webster KA: Molecular mechanisms of apoptosis in the cardiac
myocyte. Curr Opin Pharmacol. 1:141–150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kavitha K, Kowshik J, Kishore TK, Baba AB
and Nagini S: Astaxanthin inhibits NF-κB and Wnt/β-catenin
signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to
induce intrinsic apoptosis in a hamster model of oral cancer.
Biochim Biophys Acta. 1830:4433–4444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim MR, Jeong EG, Chae B, Lee JW, Soung
YH, Nam SW, Lee JY, Yoo NJ and Lee SH: Pro-apoptotic PUMA and
anti-apoptotic phospho-BAD are highly expressed in colorectal
carcinomas. Dig Dis Sci. 52:2751–2756. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jeong EG and Lee SH, Kim SS, Ahn CH, Yoo
NJ and Lee SH: Immunohistochemical analysis of phospho-BAD protein
and mutational analysis of BAD gene in gastric carcinomas. APMIS.
115:976–981. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zippo A, Serafini R, Rocchigiani M,
Pennacchini S, Krepelova A and Oliviero S: Histone crosstalk
between H3S10ph and H4K16ac generates a histone code that mediates
transcription elongation. Cell. 138:1122–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|