Introduction
Prostate cancer (PCa) is the most common form of
cancer among males in developed countries, with an estimated
648,400 new cases and 136,500 mortalities in 2008 (1). Currently, androgen-deprivation therapy
is a key treatment for metastatic PCa. However, many patients
develop castration-resistant PCa, which is a major cause of male
mortality in developed countries (2).
Further research is urgently required to develop more effective
therapies for this disease.
MicroRNA (miRNA) is a class of non-coding
single-stranded RNA that regulates tumor-associated genes via
binding the 3′-UTR of target gene mRNA (3–5). Previous
research has reported that miRNAs (miRs) serve important roles in
human cancer biological processes, including initiation,
development, migration and metastasis (6–8). In terms
of the development of PCa, miR-34a inhibits prostate cancer stem
cell (CSC) features and metastasis by directly repressing CD44
(9). Upregulation of miR-132/212
expression inhibits TGF-β-mediated epithelial-mesenchymal
transition (EMT) of PCa cells by targeting SOX4 (10). Tumor-suppressive miR-29 inhibits
cancer cell migration and invasion via targeting LAMC1 in PCa
(11). Previous studies have also
reported that miR-218 serves a tumor-suppressive role in PCa.
However, the role of miR-218 in regulating PCa stemness and EMT
remains uncharacterized.
In the present study, the expression of miR-218 in
PCa cell lines was investigated, and the impact of miR-218 on tumor
migration, EMT and CSC properties in PCa was investigated in
vitro. The results demonstrate that miR-218 was downregulated
in PCa cell lines and was able to suppress PCa cell migration, EMT
and cancer stem cell properties. The expression of Gli1 was
inhibited by miR-218 overexpression, suggesting a role for this
protein in the mechanism of tumor-suppression of miR-218 in PCa.
Altogether, the present study indicates that miR-218 served a
critical role in inhibiting PCa development, providing new insights
into clarifying the potential mechanisms of PCa oncogenesis and
revealing that miR-218 may be a novel therapeutic target for
PCa.
Materials and methods
Cell lines and cell culture
PCa cell lines LNCaP and C4-2 were obtained from the
American Type Culture Collection (Manassas, VA, USA). BPH-1 cells
were provided by Dr Jer-Tsong Hsieh (University of Texas
Southwestern Medical Center, Dallas, TX, USA). These three cell
lines were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum
(FBS; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) in a
humidified chamber at 37°C in 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from harvested cells using
TRIzol (Thermo Fisher Scientific, Inc.) and reverse transcribed to
cDNA using the miScript II RT kit (Qiagen GmbH, Hilden, Germany),
according to the manufacturers' protocols. The CFX96 PCR system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) with SYBR-Green PCR
Master Mix (Takara Bio, Inc., Otsu, Japan) was used to detect the
transcriptional expression of miR-218. The thermocycling conditions
were as follows: 95°C for 30 sec, followed by 40 cycles at 95°C for
5 sec, and then 60°C for 30 sec. U6 was used as an internal
control, and relative gene expression was calculated using the
2−ΔΔCq method (12). The
primer sequences used were as follows: miR-218 forward,
5′-CGAGTGCATTTGTGCTTGATCTA-3′ and reverse,
5′-TAATGGTCGAACGCCTAACGTC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-TGGTGTCGTGGAGTCG-3′.
Lentivirus transfection
LNCaP and C4-2 cells were seeded and cultured for 24
h and to 40-50% confluence. The lentiviral vector 3 (LV3)-miR-218,
constructed by GenePharma Co., Ltd. (Shanghai, China), was used to
transfect cells in an overnight incubation. LV3 scrambled
lentiviral vector (LV3-NC; GenePharma Co., Ltd.) was used as a
negative control. At 48 h after infection, the stable clones were
maintained by puromycin (2-3 µg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany)-resistant culturing.
Transwell migration assay
Complete growth medium (RPMI-1640 with 10% FBS, 1
ml) was added to each lower chamber as a chemoattractant. Transwell
inserts with a pore diameter of 8-µm were used (Millipore; Merck
KGaA). Cells, suspended in serum-free medium at 5-8×104
cells/ml, were seeded into the upper chamber at 400 µl/well. After
an incubation of 20 h, the upper surface of the insert was wiped
and cells that had migrated to the lower surface were fixed using
4% paraformaldehyde for 30 min and stained with 0.1% crystal violet
for 20 min at room temperature. Cell number was counted in 6 random
fields per well (magnification, ×200).
Western blot assay
Cells were washed 3 times in PBS before the protein
was extracted using radioimmunoprecipitation assay buffer [50 mM
Tris (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% NP-40 and 0.5% sodium
deoxycholate] with protease inhibitors. The concentration of
protein was detected by Bradford assay protein quantitation kit
(Abcam, Cambridge, UK). Proteins (30 µg) were separated by 12%
SDS-PAGE and transferred into nitrocellulose membranes. Following
blocking in 5% skim milk at room temperature for 1 h, the membranes
were incubated with primary antibodies at 4°C overnight. Primary
antibodies used were as follows: GAPDH (1:10,000; cat. no. KC-5G4;
Kangchen Bio-tech Co., Ltd., Shanghai, China); E-cadherin (1:1,000;
cat. no. sc-8426; Santa Cruz Biotechnology, Inc., Dallas, TX, USA);
Vimentin (1:200; cat. no. sc-6260; Santa Cruz Biotechnology, Inc.);
CD44 (1:800; cat. no. 3570; Cell Signaling Technology, Inc.,
Danvers, MA, USA); Oct4 (1:500; cat. no. ab18976; Abcam); Nanog
(1:200; cat. no. ab21624; Abcam); and Gli1 (1:1,000; cat. no. 2643;
Cell Signaling Technology, Inc.). The membranes were then washed in
Tris-buffered saline with 0.1% Tween and incubated with horseradish
peroxidase-conjugated secondary antibodies [goat anti-rabbit IgG
(1:2,000; cat. no. ZB-2301); and goat anti-mouse IgG (1:2,000; cat.
no. ZB-2305) (all from OriGene Technologies, Inc., Beijing, China)
for 1 h at room temperature. Protein bands were visualized using a
Molecular Imager ChemiDoc XRS System (Bio-Rad Laboratories).
Colony-forming and tumor sphere
formation assays
For the colony-forming assay, 2×103 cells
were seeded into each well of a 6-well plate and incubated for
10-14 days. Following 3 washes in PBS, cells were fixed using 4%
paraformaldehyde for 30 min at room temperature and stained with
0.1% crystal violet for 20 min at room temperature. The tumor
sphere formation assay was performed by seeding 1×104
cells to each well of low-adhesion 6-well plate in serum-free
Dulbecco's modified Eagle/F12 medium supplemented with 20 ng/ml
epidermal growth factor, 10 ng/ml basic fibroblast growth factor
and 2% B27 (all Invitrogen; Thermo Fisher Scientific, Inc.). After
2 weeks, plates were analyzed for tumor sphere formation using an
inverted microscope (magnification, ×200).
Statistical analysis
All statistical analysis was performed using
GraphPad Prism version 6.0 (GraphPad Software, Inc., La Jolla, CA,
USA). Differences between 2 groups were compared using the
Student's t-test. For comparisons of ≥3 groups, one-way analysis of
variance followed by Tukey's post hoc test was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-218 expression is downregulated in
PCa cells
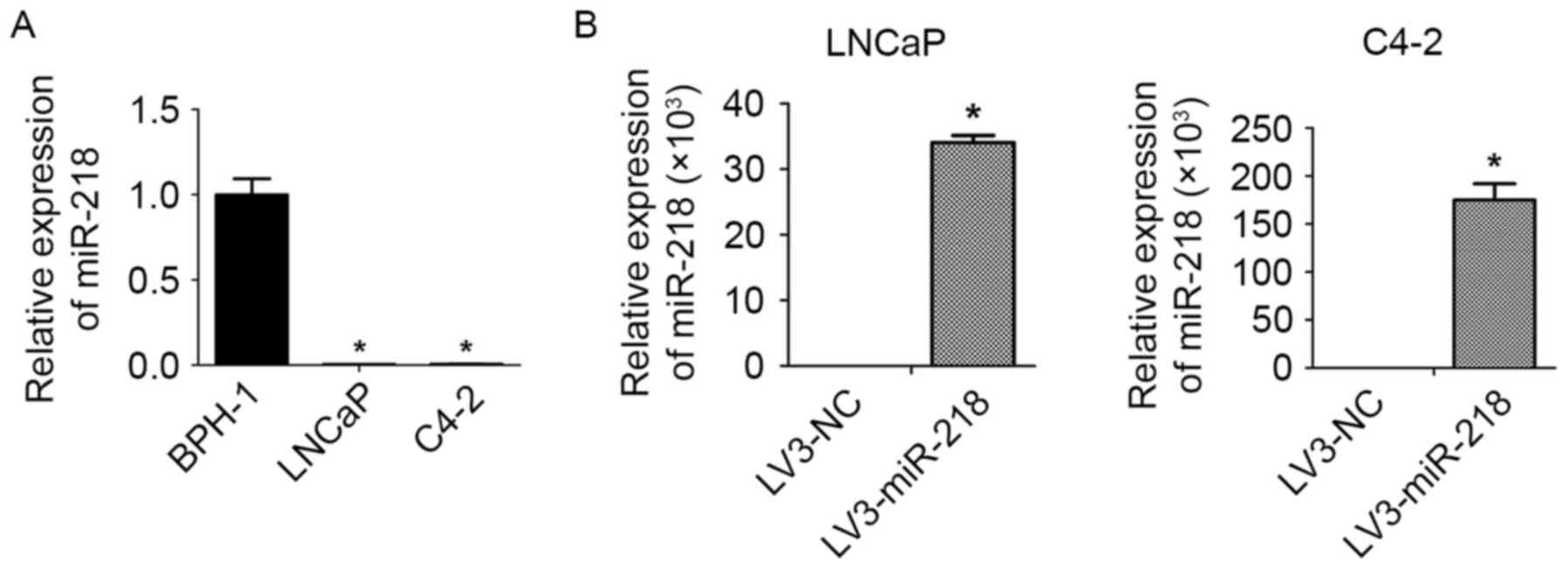
RT-qPCR was performed to compare the expression of
miR-218 in PCa cells with that in healthy prostate epithelial
cells. This revealed that the expression of miR-218 was notably
downregulated in PCa cell lines, LNCaP and C4-2, compared with the
normal prostate epithelial cells (BPH-1; Fig. 1A).
Construction of miR-218-overexpressing
PCa cells
In order to reveal the effects of miR-218 on PCa
migration, EMT and CSC properties, miR-218-overexpressing
LNCaP/C4-2 cells were constructed by transfecting the cells with
LV3-miR-218 lentiviral vectors. Subsequently, cells were analyzed
by RT-qPCR to confirm miR-218-overexpression (Fig. 1B).
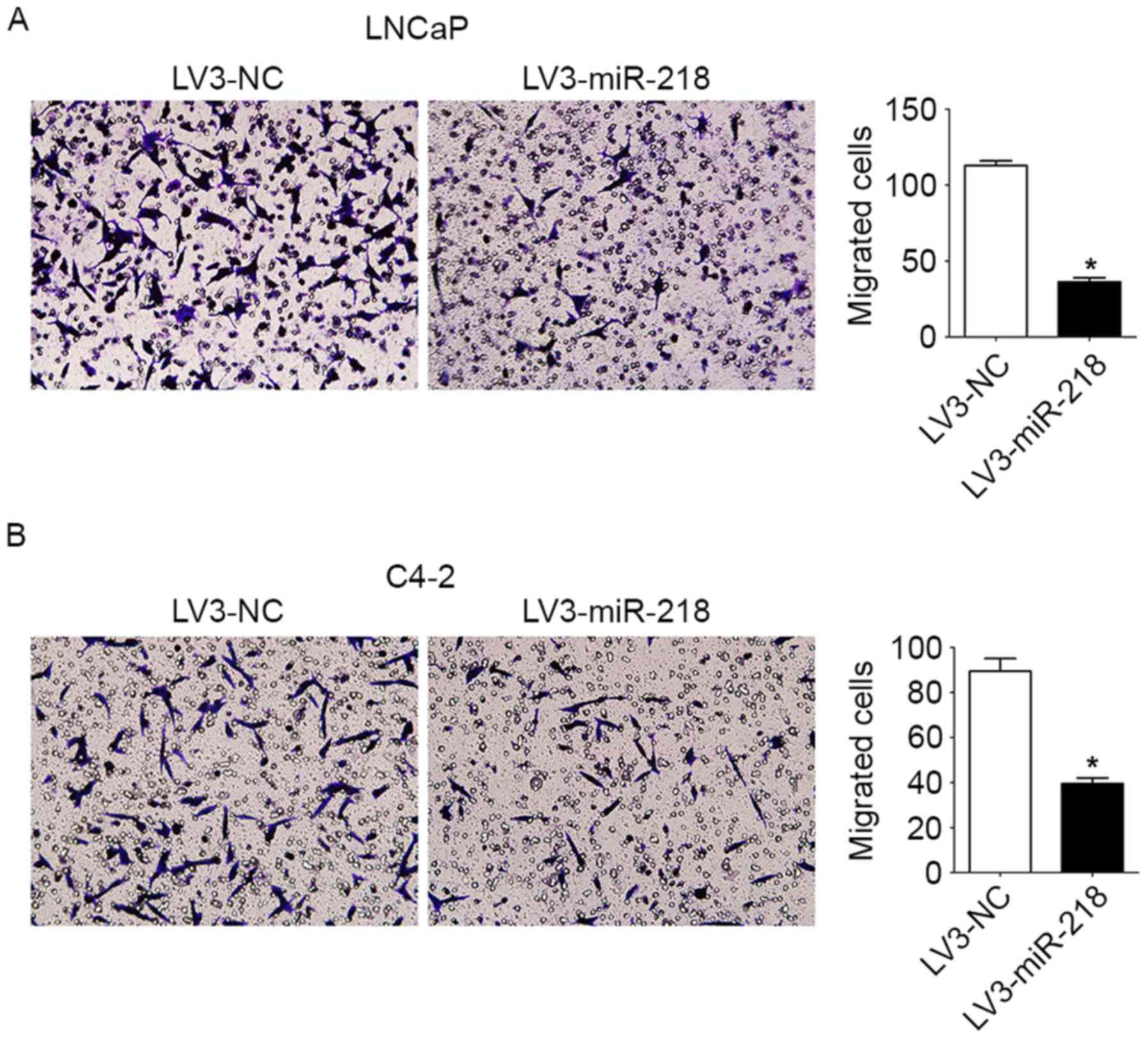
Overexpression of miR-218 inhibits PCa
cell migration and EMT
The Transwell migration assays demonstrated that the
migration of LNCaP/C4-2 cells was suppressed by miR-218
overexpression (Fig. 2). Western
blotting of EMT markers demonstrated that miR-218 overexpression
caused a slight increase in the expression of E-cadherin and a
significant decrease in the expression of vimentin (Fig. 3A). These results suggest that
overexpression of miR-218 inhibits PCa cell migration and EMT.
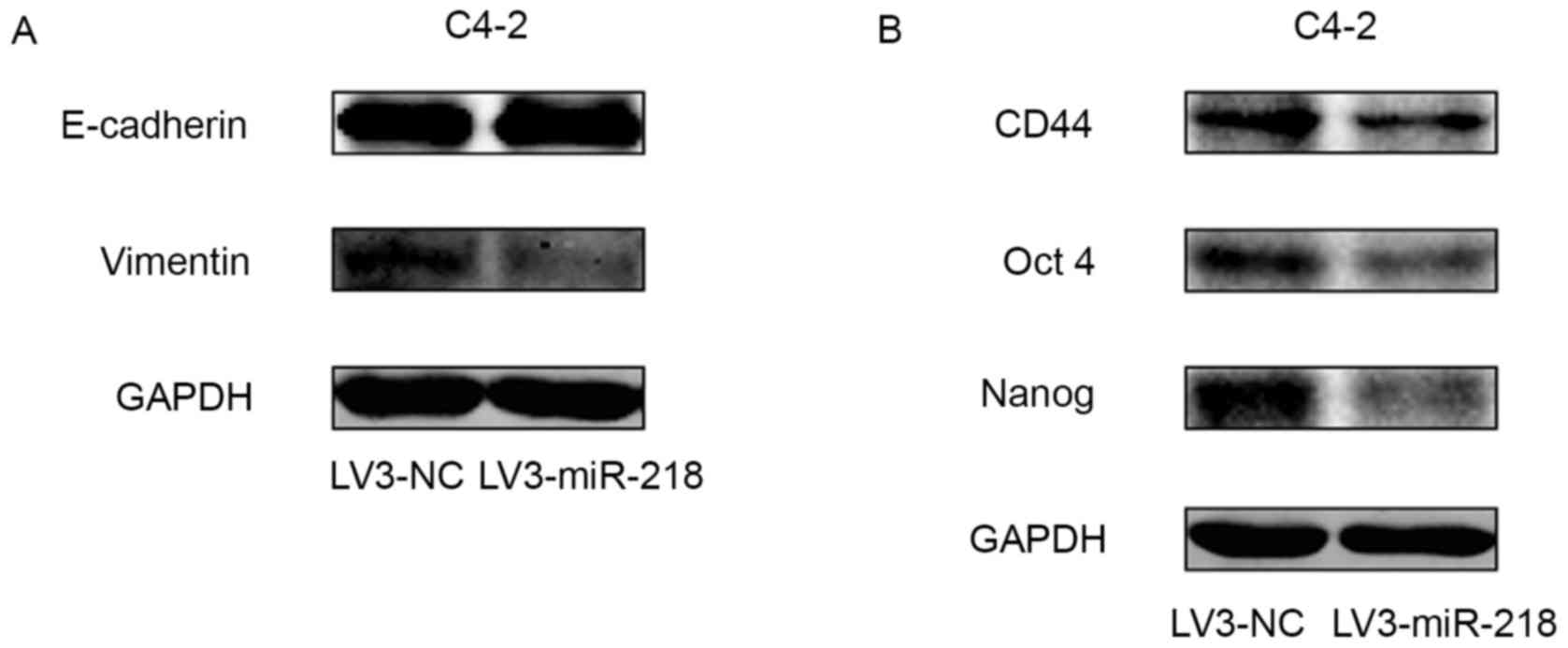
Overexpression of miR-218 diminishes
PCa cell stemness properties
Protein expression profiling of cancer stemness
markers was performed by western blotting. The results indicate
that overexpression of miR-218 downregulated the expression of
CD44, Oct4 and Nanog (Fig. 3B).
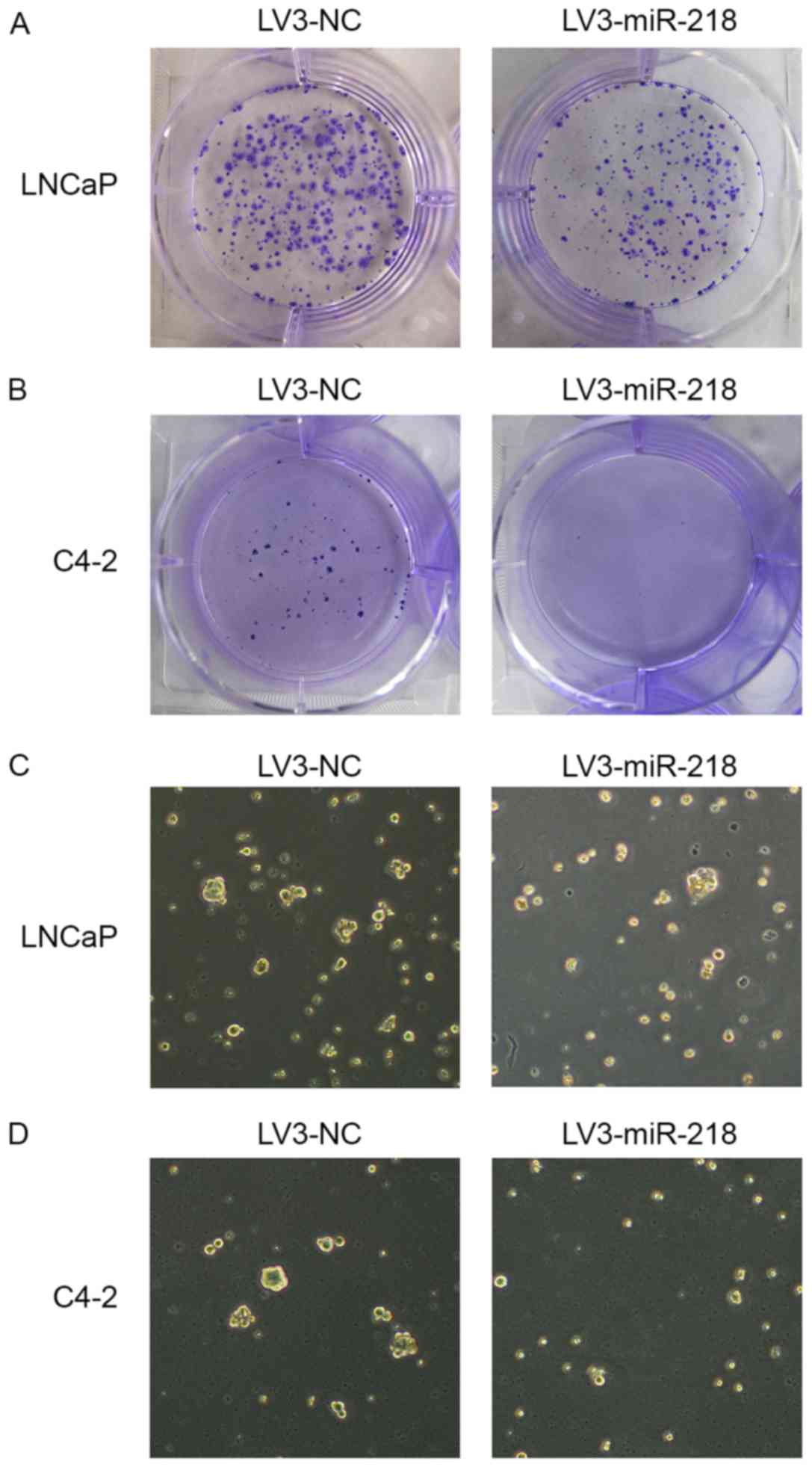
Colony forming assays were performed to assess the self-renewal
capacity of PCa cells. It was demonstrated that the
miR-218-overexpressing LNCaP cells formed fewer and smaller
colonies than the control cells (Fig.
4A). Similar results were obtained from the clonogenic assay
performed in C4-2 cells (Fig. 4B),
indicating that miR-218 may serve a critical role in tumor growth
inhibition. The tumor sphere formation assay is well established
for measuring the self-renewing capability of stem cells. In these
experiments, the control cells generated more tumor-spheres than
the miR-218-overexpressing cells in both cell lines (Fig. 4C and D). Therefore, these results
suggest that overexpression of miR-218 diminished PCa cell stemness
properties.
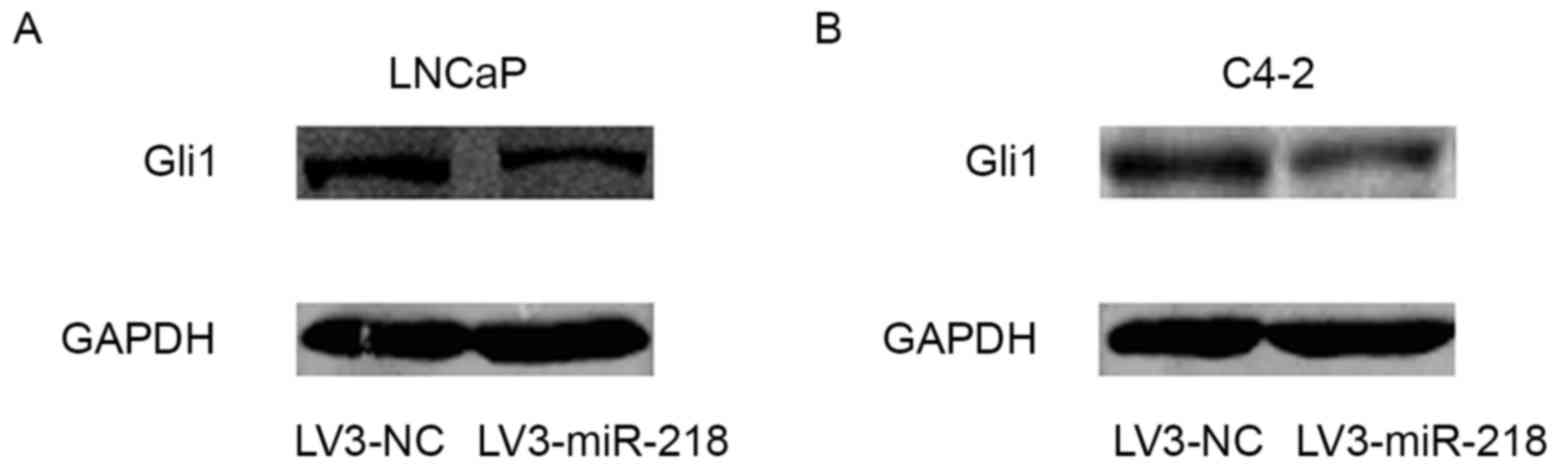
Glil expression is downregulated by
miR-218 overexpression
Numerous studies have indicated that the Hedehog-Gli
signaling pathway serves a critical role in cancer cell EMT
occurrence and CSC generation (13–15). In
the preset study, western blot analysis indicated that the
expression of Gli1 was inhibited by miR-218 overexpression
(Fig. 5), indicating that miR-218
suppression of Gli1 may be a mechanism for the anticancer effect of
miR-218 in PCa.
Discussion
Advances have been made in PCa diagnosis and
treatment in recent years (16).
However, management of PCa remains a challenge. It has been widely
reported that EMT and CSCs are critical for cancer initiation and
development (17–21). However, the molecular mechanisms by
which EMT and CSCs execute their effects require further
investigation.
Previous studies have demonstrated that miRNAs serve
important roles in human cancer biological processes, including
initiation, development, migration and metastasis (22–26).
miR-218 can act as a tumor suppressor and is downregulated in
various types of human cancer (27–31).
miR-218 can inhibit cancer cell proliferation, invasion, migration,
EMT, lymph node metastasis and self-renewal in glioma, cervical
cancer, gastric cancer and bladder cancer, among others (32–36). One
study indicated that miR-218 expression is decreased in PCa, and
impedes IL-6-induced PCa cell proliferation and invasion via
suppression of LGR4 expression (37).
miR-218 has also been demonstrated to inhibit PCa cell growth and
promote apoptosis by repressing TPD52 expression (38), as well as inhibit PCa cell migration
and invasion via targeting LASP1 (39). We hypothesize that miR-218 may also
inhibit PCa cell migration, EMT and CSC properties. However, the
underlying role of miR-218 in regulating PCa stemness maintenance
and EMT is poorly characterized at present.
In the present study, the expression of miR-218 was
notably downregulated in PCa cells and the LNCaP/C4-2 cell
constructs overexpressing miR-218 indicated that miR-218 inhibited
PCa cell migration. Results of western blotting revealed that the
overexpression of miR-218 downregulated the expression of vimentin,
CD44, Oct4 and Nanog. In colony-forming assays,
miR-218-overexpressing cells formed fewer and smaller colonies than
the control cells. Consistently, the control cells generated more
tumor-spheres than miR-218-overexpressing cells, suggesting that
overexpression of miR-218 inhibited PCa stemness properties.
Copious evidence has indicated that the Hedgehog-Gli signaling
pathway serves a critical role in cancer cell EMT and CSC
generation (13–15,40). In
the present study, the expression of Gli1 was inhibited by miR-218
overexpression. This indicates that miR-218 suppression of Gli1 may
serve in the mechanism by which miR-218 inhibits EMT and stemness
maintenance in PCa.
The regulatory mechanisms of human CSC maintenance
and EMT are very complex. If miR-218 targets Gli1 by binding its
3′-UTR, or other molecular mediators between miR-218 and Gli1, the
mechanism requires further investigation. In conclusion, the
present study indicates that miR-218 served a critical role in
inhibiting the migration, EMT and CSC properties of PCa cells. This
provides a new insight for clarifying the potential mechanisms of
PCa oncogenesis, and indicates that miR-218 may be a potential
therapeutic target for PCa.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China, (grant nos., 81372736 and
81272811) awarded to YD and LZ, respectively.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YD and DH designed and supervised all experiments
and contributed to the manuscript preparation. BG performed the
experiments, analyzed the data and contributed to the manuscript
preparation. LM and JT contributed to the cell culture and
lentivirus transfection. LZ and KW contributed to the data
analysis. SX and XW contributed to western blot assay and
manuscript preparation.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Egan A, Dong Y, Zhang H, Qi Y, Balk SP and
Sartor O: Castration-resistant prostate cancer: Adaptive responses
in the androgen axis. Cancer Treat Rev. 40:426–433. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Tang J, Zhi X, Xie K, Wang W, Li
Z, Zhu Y, Yang L, Xu H and Xu Z: miR-874 functions as a tumor
suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway
in gastric cancer. Oncotarget. 6:1605–1617. 2015.PubMed/NCBI
|
|
9
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu W, Tao T, Qi M, Wang L, Hu J, Li X,
Xing N, Du R and Han B: MicroRNA-132/212 upregulation inhibits
TGF-β-mediated epithelial-mesenchymal transition of prostate cancer
cells by targeting SOX4. Prostate. 76:1560–1570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishikawa R, Goto Y, Kojima S, Enokida H,
Chiyomaru T, Kinoshita T, Sakamoto S, Fuse M, Nakagawa M, Naya Y,
et al: Tumor-suppressive microRNA-29s inhibit cancer cell migration
and invasion via targeting LAMC1 in prostate cancer. Int J Oncol.
45:401–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu X, Su B, Xie C, Wei S, Zhou Y, Liu H,
Dai W, Cheng P, Wang F, Xu X and Guo C: Sonic hedgehog-Gli1
signaling pathway regulates the epithelial mesenchymal transition
(EMT) by mediating a new target gene, S100A4, in pancreatic cancer
cells. PLoS One. 9:e964412014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clement V, Sanchez P, de Tribolet N,
Radovanovic I and Ruiz i Altaba A: HEDGEHOG-GLI1 signaling
regulates human glioma growth, cancer stem cell self-renewal, and
tumorigenicity. Curr Biol. 17:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahmed A, Ali S and Sarkar FH: Advances in
androgen receptor targeted therapy for prostate cancer. J Cell
Physiol. 229:271–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: Migrating cancer stem cells-an integrated
concept of malignant tumour progression. Nat Rev Cancer. 5:744–749.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deshmukh A, Deshpande K, Arfuso F,
Newsholme P and Dharmarajan A: Cancer stem cell metabolism: A
potential target for cancer therapy. Mol Cancer. 15:692016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colak S and Medema JP: Cancer stem
cells-important players in tumor therapy resistance. FEBS J.
281:4779–4791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dave B, Mittal V, Tan NM and Chang JC:
Epithelial-mesenchymal transition, cancer stem cells and treatment
resistance. Breast Cancer Res. 14:2022012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miska EA: How microRNAs control cell
division, differentiation and death. Curr Opin Genet Dev.
15:563–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Yu Z, Li Y, Liu S, Gao C, Hou X, Yao
R and Cui L: The tumor suppressor miR-124 inhibits cell
proliferation by targeting STAT3 and functions as a prognostic
marker for postoperative NSCLC patients. Int J Oncol. 46:798–808.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng Z, Ding M, Ni J, Song D, Huang J and
Wang J: MiR-142 acts as a tumor suppressor in osteosarcoma cell
lines by targeting Rac1. Oncol Rep. 33:1291–1299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou N, Fei D, Zong S, Zhang M and Yue Y:
MicroRNA-138 inhibits proliferation, migration and invasion through
targeting hTERT in cervical cancer. Oncol Lett. 12:3633–3639. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davidson MR, Larsen JE, Yang IA, Hayward
NK, Clarke BE, Duhig EE, Passmore LH, Bowman RV and Fong KM:
MicroRNA-218 is deleted and downregulated in lung squamous cell
carcinoma. PLoS One. 5:e125602010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu H, Gao G, Jiang L, Guo L, Lin M, Jiao
X, Jia W and Huang J: Decreased expression of miR-218 is associated
with poor prognosis in patients with colorectal cancer. Int J Clin
Exp Pathol. 6:2904–2911. 2013.PubMed/NCBI
|
|
29
|
Guan H, Wei G, Wu J, Fang D, Liao Z, Xiao
H, Li M and Li Y: Down-regulation of miR-218-2 and its host gene
SLIT3 cooperate to promote invasion and progression of thyroid
cancer. J Clin Endocrinol Metab. 98:E1334–E1344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang XX, Ge SJ, Wang XL, Jiang LX, Sheng
MF and Ma JJ: miR-218 tissue expression level is associated with
aggressive progression of gastric cancer. Genet Mol Res.
15:2016.
|
|
31
|
Wang HT, Liu AG, Luo DS, Zhou ZN, Lin HG,
Chen RZ, He JS and Chen K: miR-218 expression in osteosarcoma
tissues and its effect on cell growth in osteosarcoma cells. Asian
Pac J Trop Med. 7:1000–1004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang G, Fu Y, Liu G, Ye Y and Zhang X:
miR-218 inhibits proliferation, migration, and EMT of gastric
cancer cells by targeting WASF3. Oncol Res. 25:355–364. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang Z, Song Q, Zeng R, Li J, Li J, Lin
X, Chen X, Zhang J and Zheng Y: MicroRNA-218 inhibits EMT,
migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical
cancer. Oncotarget. 7:45622–45636. 2016.PubMed/NCBI
|
|
34
|
Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin
W and Zhang Y: MicroRNA-218 inhibits glioma invasion, migration,
proliferation, and cancer stem-like cell self-renewal by targeting
the polycomb group gene Bmi1. Cancer Res. 73:6046–6055. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kogo R, How C, Chaudary N, Bruce J, Shi W,
Hill RP, Zahedi P, Yip KW and Liu FF: The microRNA-218~Survivin
axis regulates migration, invasion, and lymph node metastasis in
cervical cancer. Oncotarget. 6:1090–1100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng Y, Yang X, Deng X, Zhang X, Li P,
Tao J and Lu Q: MicroRNA-218 inhibits bladder cancer cell
proliferation, migration, and invasion by targeting BMI-1. Tumour
Biol. 36:8015–8023. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li F, Gu C, Tian F, Jia Z, Meng Z, Ding Y
and Yang J: MiR-218 impedes IL-6-induced prostate cancer cell
proliferation and invasion via suppression of LGR4 expression.
Oncol Rep. 35:2859–2865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han G, Fan M and Zhang X: MicroRNA-218
inhibits prostate cancer cell growth and promotes apoptosis by
repressing TPD52 expression. Biochem Biophys Res Commun.
456:804–809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nishikawa R, Goto Y, Sakamoto S, Chiyomaru
T, Enokida H, Kojima S, Kinoshita T, Yamamoto N, Nakagawa M, Naya
Y, et al: Tumor-suppressive microRNA-218 inhibits cancer cell
migration and invasion via targeting of LASP1 in prostate cancer.
Cancer Sci. 105:802–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Inaguma S, Kasai K, Hashimoto M and Ikeda
H: GLI1 modulates EMT in pancreatic cancer-letter. Cancer Res.
72:3702–3703. 3704–3705. 2012. View Article : Google Scholar : PubMed/NCBI
|