Introduction
Colon cancer is one of the primary malignant tumor
types in the digestive system, with the highest incidence rate in
developed countries, of which the total number of mortality
(650,000) ranked second in China in 2012 (1). With the improvement in living standards,
changes in diet, aging of the population and the census of colon
cancer, colon cancer has been identified to exhibit an increasing
trend in incidence in China in 2010, and is a serious threat to the
health of the population (1). The
survival and prognosis of patients with colon cancer depends on the
time at which the tumor is detected (2). However, for >57% patients, the cancer
has already metastasized upon diagnosis (1). In the last 20 years, a large number of
studies regarding colon cancer have demonstrated favorable progress
in the diagnosis and treatment, and the 5-year survival rate for
the patients with an early stage of colon cancer is ~90%; however,
the overall survival rate of the patients with advanced and
metastatic colon cancer has not been increased significantly, at
only 15% (1).
microRNAs (miRNAs) are a class of short (typically
between 17 and 25 nucleotides) non-coding single-stranded RNAs,
which are evolutionarily conservative (3). miRNAs serve an important regulatory
function in cell metabolism, proliferation, differentiation,
apoptosis and other biological processes involved in viral
infections, as well as the occurrence, diagnosis and treatment of
cardiovascular disease, nerve and muscle disorders and numerous
other aspects (4). miRNAs also serve
an important function in tumor biology, including tumor evolution,
invasion, metastasis and angiogenesis (5).
Studies on the function of miRNA in diagnosis of
cancer are based on the miRNA expression marks, i.e. miRNA
expression profile studies (6). miRNA
expression profiles consist of determining different miRNA
expression levels in multiple tumor samples (7). Previous studies have demonstrated that
miRNA expression profiles are able to identify the tumor type, the
staging and the other clinical characteristics, in addition to
distinguishing between tumor and normal tissues, by which a variety
of tumor and normal tissue samples may be analyzed systematically
(8,9).
The diagnostic accuracy rate of a tumor based on specific miRNA
expression profiles is ≤70% (8).
With an improved understanding of the cancer
pathogenesis at the molecular level, an increasing number of
tumor-associated signaling pathways have been identified, and the
phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt) signaling pathway is important
(10). Gene expression of members of
this pathway has been proved to be one of the most important
prognostic markers for lung, breast and kidney cancer (11). To the best of our knowledge, there
have been relatively few studies in this area regarding colon
cancer, therefore research on the PTEN/PI3K/Akt signaling pathway
is expected to lead to an improved understanding of the occurrence,
individualized treatment and prognosis of colon cancer (10).
It has been indicated in previous research that
glycogen synthase kinase 3β (GSK3β) is the major regulatory enzyme
for numerous intracellular signal transduction pathways, including
Wnt/β-catenin and nuclear factor-κB (12). It is able to participate in regulating
cell proliferation and apoptosis by affecting the downstream
nuclear transcription factor (13).
However, the effect of GSK3β and GSK3β inhibitor on the biological
characteristics of tumor cell remains controversial (13). GSK3β is the major regulatory enzyme of
numerous intracellular signal transduction pathways (14). Regulating GSK3β activity is able to
affect the growth and apoptosis of different tumor types, including
colon, lung and breast cancer (15);
however, experiments on the effect of regulating GSK3β activity on
tumor cell proliferation have led to contrasting results (12–15).
The Wnt/β-catenin signaling pathway is associated
with tumor development (15). In
breast, liver, stomach, thyroid, lung and prostate cancer, as well
as melanoma and other malignant tumor types, the abnormal
activation of the Wnt/β-catenin signaling pathway and the
downregulation of expression or the inactivation of the pathway
inhibitory proteins, such as Dickkopf Wnt signaling pathway
inhibitor 1 (DKKI) or Wnt inhibitory factor 1 (WIF), have been
determined (15,16). The abnormal activation of the pathway
is an early event in colorectal cancer, and also indicates its
importance in development. The effects of miRNA-29a on colon cancer
cell viability and the molecular mechanisms underlying the effects
were investigated.
Materials and methods
Ethics statement
Serum samples from 12 patients (mean age 63.5±5.5
years, age range 58–69 years old, all male) and 6 normal healthy
volunteers (mean age 60±7 years, age range 53–67 years old, all
male) were obtained from General Surgery at June 2016 to July 2016,
Beijing Chao-Yang Hospital, Capital Medical University (Beijing,
China) and were stored at −70°C. The present study was approved by
the Ethical Board of Beijing Chao-Yang Hospital, Capital Medical
University. Written informed consent was provided by all patients
and healthy volunteers for the use of their samples.
RNA isolation and quantification of
mRNA expression
Total RNA was isolated from serum samples and
HCT-116 (purchased from the Type Culture Collection of the Chinese
Academy of Sciences, Shanghai, China) using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA
(1 µg) was used for cDNA synthesis using a PrimeScript First Strand
cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan). The reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
performed using Power SYBR® Green PCR Master Mix
(Invitrogen; Thermo Fisher Scientific, Inc.) using an ABI7700
system. PCR amplification was performed at 95°C for 3 min prior to
40 cycles of 95°C for 30 sec, 58°C for 30 sec and 72°C for 60 sec,
followed by a final incubation at 72°C for 5 min. The primers for
miRNA-29a were: 5′-GAGGATCCCCTCAAGGATACCAAGGGATGAAT-3′ (forward)
and 5′-CTTCTAGAAGGAGTGTTTCTAGGTTCCGTCA-3′ (reverse). The primers
for U6 were: 5′-CTCGCTTCGGCAGCACATATAC-3′ (forward) and
5′-GGAACGCTTCACGAATTTGC-3′ (reverse). Relative miRNA-29a expression
was calculated using the 2−∆∆Cq method (17).
Cell culture and transfection
The HCT-116 cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
at 37°C in a humidified atmosphere containing 5% CO2. A
total of 100 ng Negative control (5′-CCCCCCCCCC-3′) and 100 ng
miRNA-29a mimic (5′-ATGACTGATTTCTTTTGGTG-3′) were transfected into
cells using Lipofectamine® RNAiMax reagent (Invitrogen;
Thermo Fisher Scientific, Inc.).
Cell viability assay and lactate
dehydrogenase (LDH) activity
Cells were plated at [(1–2)x103 cells/well] in 96-well
plates, incubated overnight and transfected with negative control
and miRNA-29a mimic at 37°C. After 24, 48 and 72 h, cell viability
were determined using an MTT assay (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C for 4 h. Dimethylsulfoxide was added to
dissolve the resultant formazan crystals. Absorbance at 492 nm was
determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc., Pittsburg, PA,
USA).
Cells were plated at [(1–20×103
cells/well] in 96-well plates, incubated overnight at 37°C and
transfected with negative control and miRNA-29a mimic. After 48 h,
the LDH activity of cells was determined using LDH activity kits
(C0016; Beyotime Institute of Biotechnology, Haimen, China).
Absorbance at 450 nm was determined using a NanoDrop ND-1000
spectrophotometer.
Apoptosis assay and caspase 3/9
activity assay
Cells were plated at [(1–2)x106 cells/well] in 6-well
plates, incubated overnight and transfected with negative control
and miRNA-29a mimic at 37°C. After 48 h, cells were stained with 5
µl annexin V-FITC and 10 µl propidium iodide (BD Biosciences,
Franklin Lakes, NJ, USA) for 30 min at 37°C. Apoptotic cells were
measured using a Flow Cytometer (c6; BD Biosciences, Franklin
Lakes, NJ, USA) and analyzed using Flowjo 7.6.1 software (FlowJo
LLC, Ashland, OR, USA). Cells were plated at [(1–2)x106 cells/well] in 6-well
plates, incubated overnight at 37°C and transfected with negative
control and miRNA-29a mimic. After 48 h, total protein extracts
were prepared by lysing cells in Cell Lysis buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA). Protein concentrations in the
lysates were determined using a bicinchoninic acid (BCA) protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). Protein
extracts (50 µg) were incubated with caspase-3 (C1116) and
caspase-9 activity kits (C1158; Beyotime Institute of
Biotechnology) for 1 h at 37°C. Absorbance at 405 nm was determined
using a NanoDrop ND-1000 spectrophotometer.
Western blot analysis
Total protein extracts were prepared by lysing cells
in Cell Lysis buffer. Protein concentrations in the lysates were
determined using a BCA protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein extracts (50 µg) were separated by
SDS-PAGE (8–12% gel) and transferred onto nitrocellulose membranes.
The membranes were incubated with primary antibodies against B-cell
lymphoma 2-associated X protein (Bax; 1:500; cat. no. sc-6236),
PTEN (1:500; cat. no. sc-6817-R), phosphorylated (p)-Akt (1:300;
cat. no. sc-7985-R), p-GSK3β (1:300; cat. no. sc-81496), Wnt
(1:500; cat. no. sc-13962), β-catenin (1:500; cat. no. sc-515105)
and GAPDH (1:500; cat. no. sc-25778) (all Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at 4°C overnight. Following washing with
Tris-buffered saline containing Tween-20, membranes were probed
with goat anti-rabbit or anti-mouse immunoglobulin G horseradish
peroxidase-conjugated secondary antibodies (cat. nos. 7074 and
7076; 1:5,000; Cell Signaling Technology, Inc.) at 37°C for 1 h.
The proteins designated were visualized using an enhanced
chemiluminescence detection kit (GE Healthcare Life Sciences,
Little Chalfont, UK) and quantified using Image_Lab_3.0 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Results are expressed as the mean ± standard
deviation using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Comparison
among groups was performed by one-way analysis of variance followed
by Dunnett's t-test. P<0.01 was considered to indicate a
statistically significant difference.
Results
Expression of miRNA-29a in colon
cancer serum samples
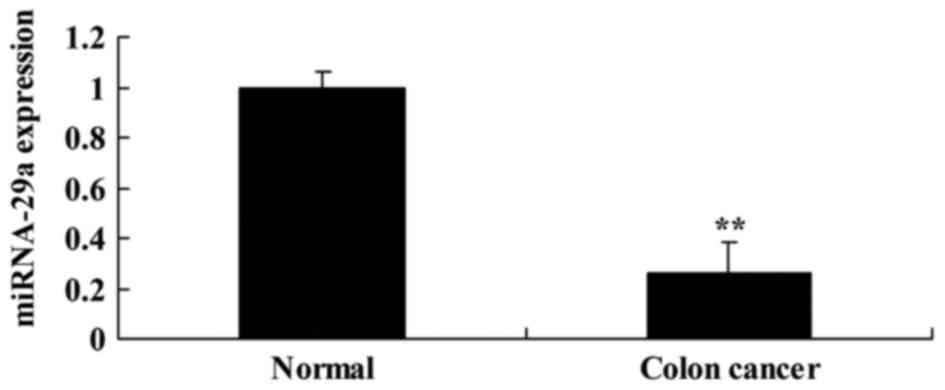
First, miRNA-29a expression was analyzed in serum
samples from patients with colon cancer. As presented in Fig. 1, miRNA-29a in colon cancer serum
samples was significantly downregulated, compared with the normal
group (P<0.01).
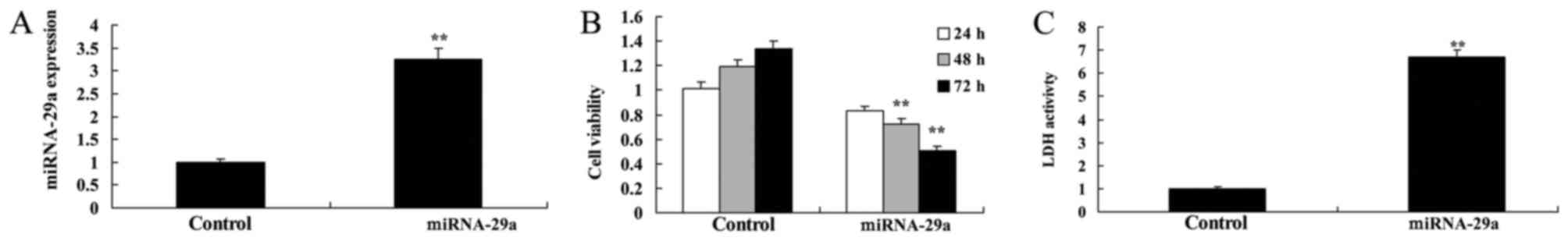
Upregulation of miRNA-29a suppresses
viability of HCT-116 cells
To further assess the effects of transfecting
miRNA-29a into HCT-116 cells, cell viability and LDH activity were
determined. miRNA-29a mimic led to increase of miRNA-29a
expression, suppression of cell viability and an increase in LDH
activity in HCT-116 cells, compared with the control group
(Fig. 2).
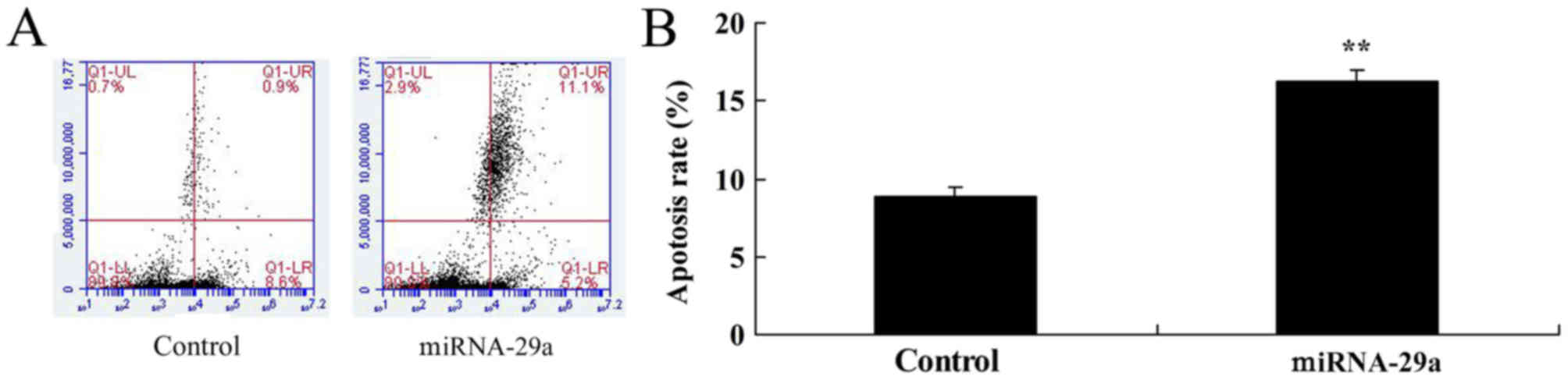
Upregulation of miRNA-29a increases
apoptosis of HCT-116 cells
Furthermore, flow cytometry demonstrated that
upregulation of miRNA-29a significantly increased apoptosis of
HCT-116 cells, compared with the control group (P<0.01; Fig. 3).
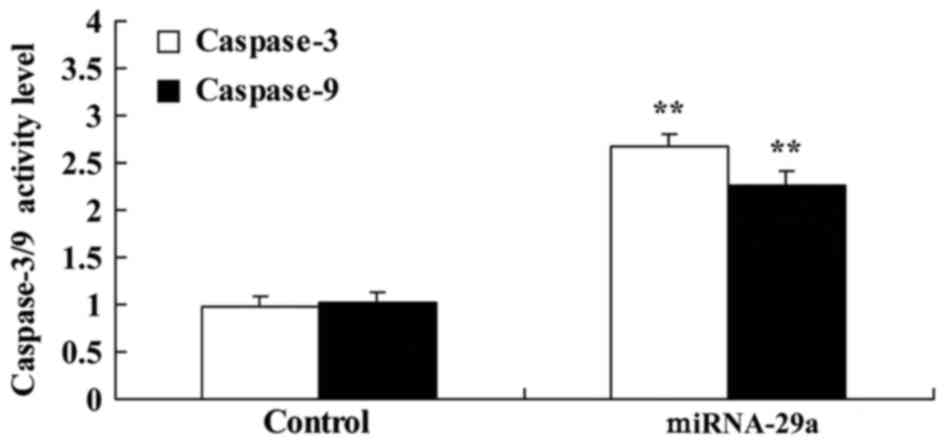
Upregulation of miRNA-29a promotes
caspase-3/9 activities of HCT-116 cells
Caspase-3/9 activities in HCT-116 cells by miRNA-29a
were examined. As presented in Fig.
4, upregulation of miRNA-29a significantly promoted the
caspase-3/9 activities of HCT-116 cells, compared with the control
group (P<0.01).
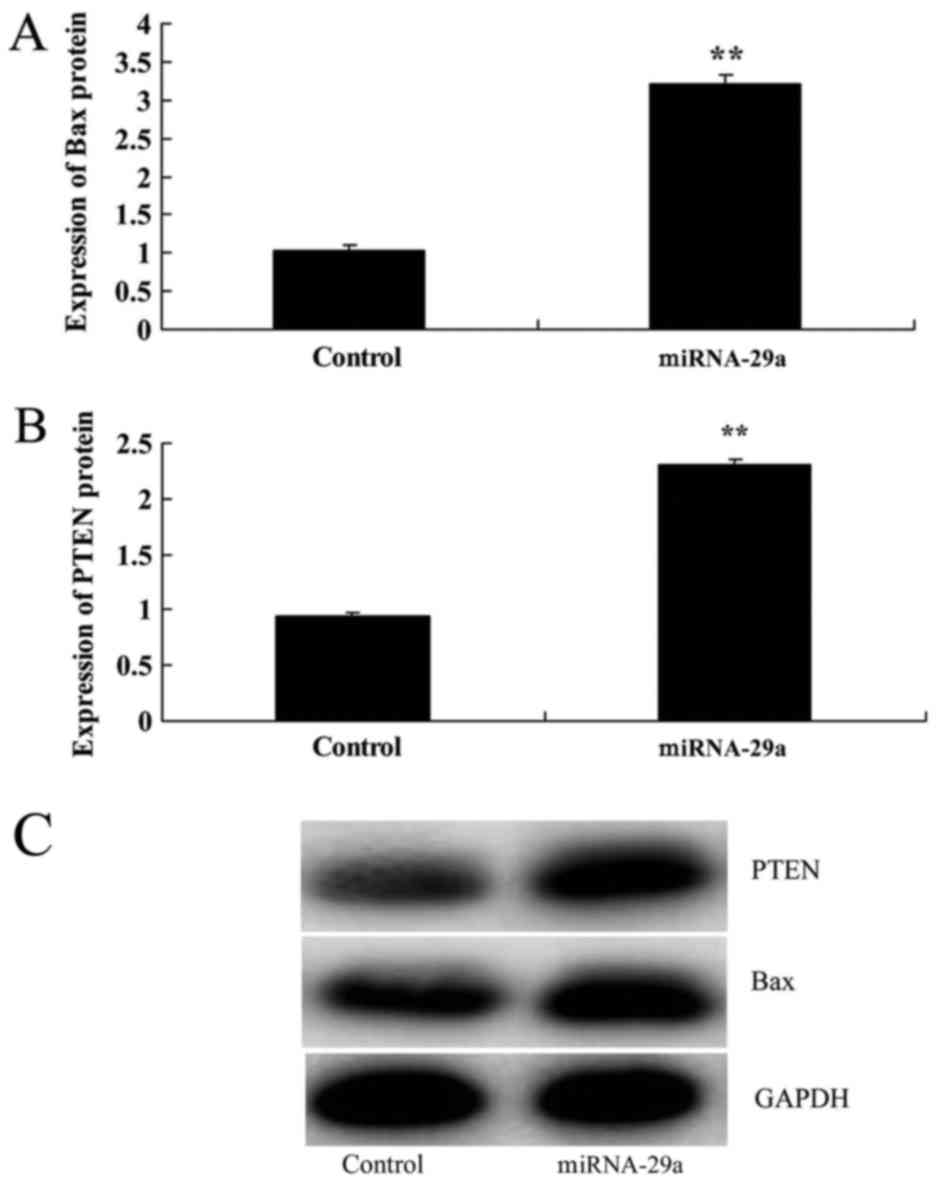
Upregulation of miRNA-29a promotes Bax
and PTEN protein expression in HCT-116 cells
Next, Bax and PTEN protein expression in HCT-116
cells were determined following miRNA-29a transfection for 48 h.
Bax and PTEN protein expression following miRNA-29a upregulation
were significantly increased, compared with the control group
(P<0.01; Fig. 5).
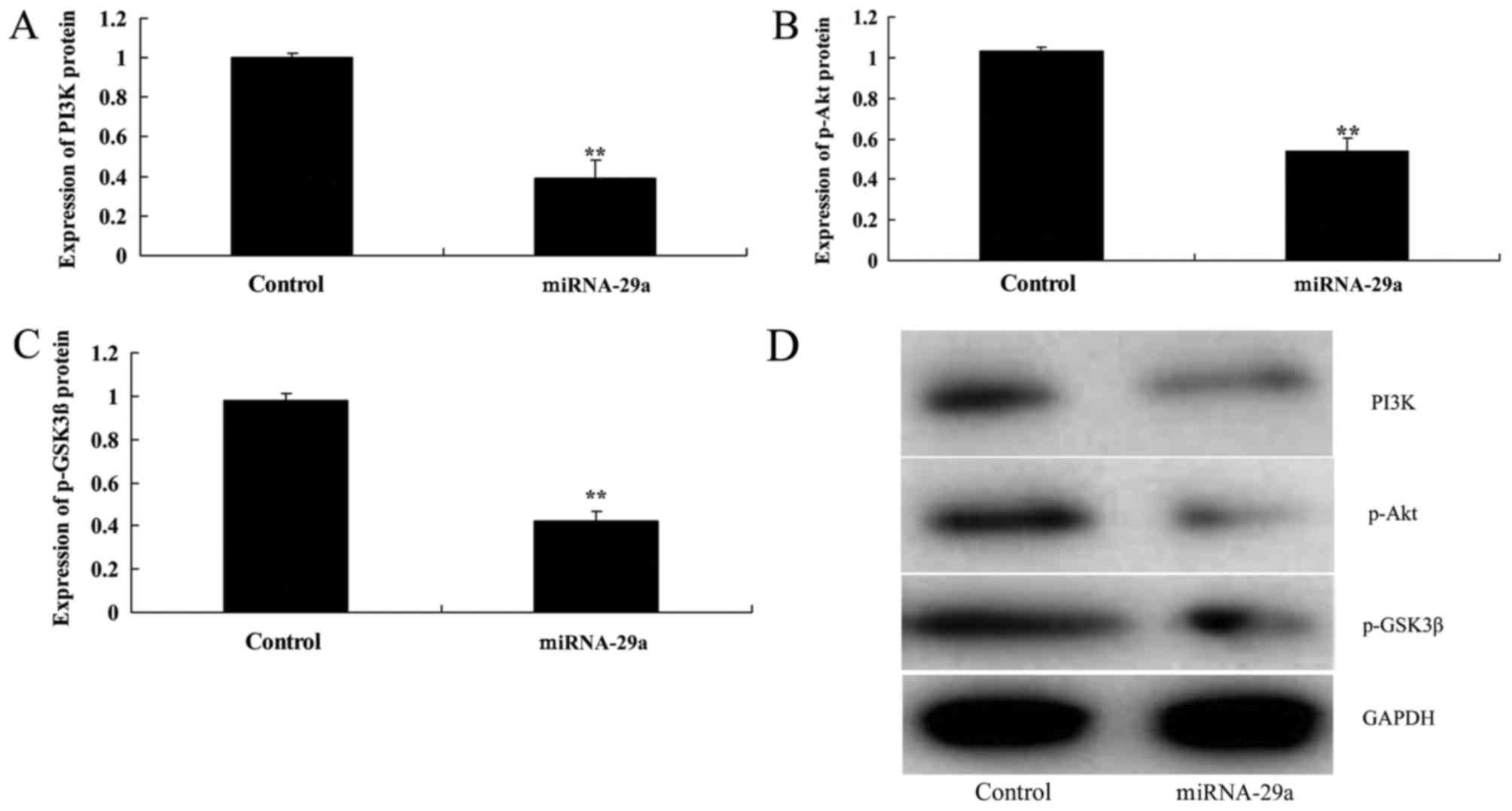
Upregulation of miRNA-29a decreases
PI3K, p-Akt and p-GSK3β expression in HCT-116 cells
Western blot analysis indicated that upregulation of
miRNA-29a significantly suppressed PI3K, p-Akt and p-GSK3β
expression levels in HCT-116 cells, compared with the control group
(P<0.01; Fig. 6).
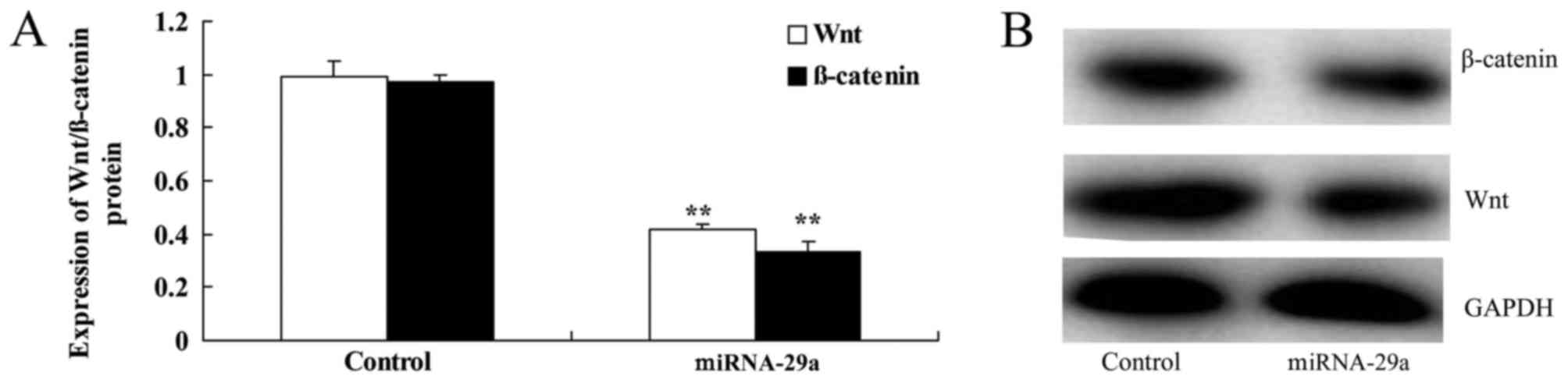
Upregulation of miRNA-29a suppresses
the Wnt/β-catenin signaling pathway of HCT-116 cells
As presented in Fig.
7, upregulation of miRNA-29a significantly suppressed Wnt and
β-catenin protein expression in HCT-116 cells, compared with the
control group (P<0.01).
Discussion
As a common malignant, colon cancer is a common
malignancy, which ranks third in total morbidity and mortalities
(2814,000) in the USA in 2015 (18).
In China, colon cancer is the third most common cancer and ranks
fifth for its mortality rate (19).
The average age for a patient with colon cancer is ~40 years, with
being between 50 and 60 years, 10 years less compared with the
average age of Western countries (19). It was demonstrated that miRNA-29a in
colon cancer serum samples was significantly downregulated,
compared with the normal group. These results revealed that
miRNA-29a may be an important element in colon cancer. The effect
of miRNAs on cancer makes them an important target for therapeutic
intervention. Gene therapy is able to prevent colon cancer cell
growth by modulating the expression of tumor suppressor miRNA or
miRNA promoters (20). This
adjustment is able to control the tumor growth rate, which has the
potential for the treatment of early- and late-stage cancer. This
indicates that a number of the factors may reverse miRNA
expression, and it may be possible to transform cancerous tissue
into normal tissue (21). miRNA may
serve a function in cancer chemoprevention, which may have the
ability to decrease tumor size and metastasis, to lead to the
discovery of novel therapeutic drugs (21). Although the experimental efficacy of
miRNA appears to be promising, it must also be validated with
different patients in future clinical practice (22). These results indicated that
upregulation of miRNA-29a suppressed cell viability, increased
apoptosis, and promoted caspase-3/9 activities and Bax protein
expression of colon cancer cells. Therefore, miRNA-29a may have
induced colon cancer cell death through Bax/caspase-3/9.
The PTEN/PI3K/Akt signaling pathway is composed of
PTEN, PI3K, Akt and its downstream effector molecules (11). PTEN is another important tumor
suppressor gene following p53, which has a phosphatase activity,
and serves an important role in regulating the cell cycle and
inducing the apoptosis of tumor cells (11). PI3K is an important intracellular
kinase, and excessive activation of which serves an important
function in the activation of tumor occurrence (11). Akt is markedly homologous with the
viral oncogene v-Akt, which induces leukemia in mice, and the major
effector molecules of PI3K, and overactivation may inhibit or
activate the downstream target proteins, leading to the infinite
proliferation of cells though numerous mechanisms (20). PI3K is activated by a variety of
mechanisms, resulting in the production of important molecules,
including phosphatidylinositiol 3,4,5-trisphosphate
(PIP3); binding Akt; phosphorylating Akt, and changing
the conformation as an intracellular second messenger, which
furthermore leads to the translocation of Akt from the cytoplasm to
the plasma membrane, thereby activating the downstream target
proteins and mediating growth factors, such as insulin, promoting
cell survival (20). Suppressor gene
PTEN generates phosphatidylinositol 4,5-bisphosphate through the
dephosphorylation by the catalysis of PIP3, to inhibit
the Akt translocation and conformational change so as to decrease
the activity of Akt, thereby antagonizing the signaling pathway to
serve a tumor-suppressing function (20). When PTEN is mutated or inactivated,
the suppressive effect on PIP3 ceases, leading to
intracellular accumulation of a large amount of PIP3,
Akt overactivation, cell immortalization (23,24). In
this report, upregulation of miRNA-29a decreased PTEN, PI3K, p-Akt
and p-GSK3β protein expression in colon cancer cells. Li et
al (25) indicated that miRNA-29a
induced apoptosis of papillary thyroid carcinoma cells through Akt
expression.
GSK3β is a multifunctional serine/threonine kinase.
It is involved in numerous important physiological processes,
including intracellular glycometabolism, cell proliferation,
differentiation and apoptosis (26).
Abnormal GSK3β expression and dysfunction may induce a series of
insuperable diseases, including cancer, diabetes and Alzheimer's
disease (27). Therefore, GSK3β has
become a focus of research. The present study determined that
upregulation of miRNA-29a suppressed p-GSK3β protein expression in
colon cancer cells. Shen et al (28) demonstrated that miRNA-29a contributes
to drug resistance of breast cancer cells to Adriamycin via the
GSK3β signaling pathway. There results indicated that miRNA-29a
regulates the PTEN/PI3K/Akt/GSK3β signaling pathway to induce the
apoptosis of colon cancer cells.
The Wnt/β-catenin signaling pathway is markedly
conserved during evolution, serving an important function during
embryonic development from fruitflies to humans (26). The Wnt/β-catenin pathway has multiple
sites of action, which is subject to the regulation of multiple
signaling pathways (27). The change
in any component in this pathway may cause an abnormality in the
signal transduction, resulting in body dysplasia or neoplasia
(27). In a variety of tumor types,
including breast cancer, prostate cancer, melanoma, colorectal
cancer and lung cancer, abnormalities have been determined in this
pathway (29). The downregulation in
expression of signaling pathway antagonist proteins, such as DKKI
or WIF, may be determined in a variety of human tumor types,
indirectly causing abnormal regulation of Wnt/β-catenin, which
serves an important function in the occurrence and the development
of colorectal cancer (30). The
abnormal regulation of Wnt/β-catenin and its upstream signals
result in intracellular accumulation of β-catenin and
translocation, and, following translocation into the nucleus, it
activates T-cell factor/lymphoid enhancer factor transcriptional
activity, causing the abnormal expression of downstream genes
(31). For >90% patients with
colorectal cancer, the activation of the Wnt/β-catenin signaling
pathway can be determined, ~80% exhibited increased expression of
β-catenin and abnormal expression in the cytoplasm and nucleus, and
the key protein β-catenin that inhibits the Wnt/β-catenin pathway
in colorectal cells is able to inhibit tumor growth and progression
(32). The results of the present
study also indicated that upregulation of miRNA-29a suppressed the
Wnt/β-catenin signaling pathway in colon cancer cells. Nagano et
al (33) indicated that miRNA-29a
induces resistance to gemcitabine of pancreatic cancer cells
through the Wnt/β-catenin signaling pathway, therefore it was
considered that miRNA-29a induced apoptosis of colon cancer through
the suppression of the Wnt/β-catenin signaling pathway.
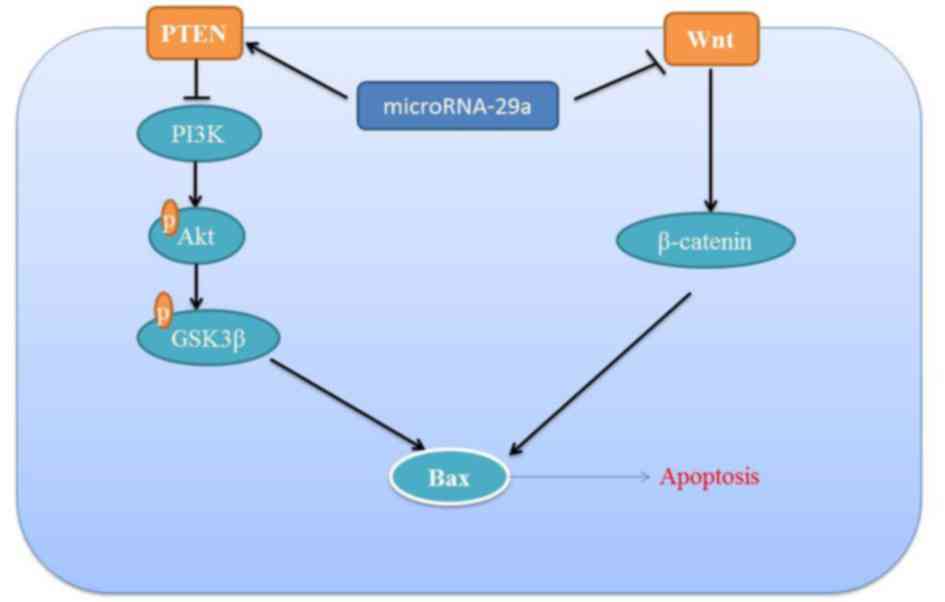
In conclusion, the results of the present study
demonstrated a significant association between miRNA-29a expression
and the response to apoptosis in colon cancer cell. The results
demonstrated that the miRNA-29a-induced apoptosis of colon cancer
is mediated by activation of the PTEN/PI3K/Akt/GSK3β signaling
pathway and suppression of the Wnt/β-catenin signaling pathway
(Fig. 8).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZG designed the experiment. XH, JZ and YW performed
the experiment. ZG and XH analyzed the data. ZG wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical Board
of Beijing Chao-Yang Hospital. Written informed consent was
provided by all patients and healthy volunteers for the use of
their samples.
Consent for publication
Consent was received from the patients for
publication of this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang HX, Wang ZT, Lu XX, Wang YG, Zhong J
and Liu J: NLRP3 gene is associated with ulcerative colitis (UC),
but not Crohn's disease (CD), in Chinese Han population. Inflamm
Res. 63:979–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaki MH, Lamkanfi M and Kanneganti TD: The
Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends
Immunol. 32:171–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bank S, Andersen PS, Burisch J, Pedersen
N, Roug S, Galsgaard J, Turino SY, Brodersen JB, Rashid S,
Rasmussen BK, et al: Genetically determined high activity of IL-12
and IL-18 in ulcerative colitis and TLR5 in Crohns disease were
associated with non-response to anti-TNF therapy. Pharmacogenomics
J. 18:87–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bauer C, Duewell P, Mayer C, Lehr HA,
Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E and Schnurr M:
Colitis induced in mice with dextran sulfate sodium (DSS) is
mediated by the NLRP3 inflammasome. Gut. 59:1192–1199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Engler DB, Leonardi I, Hartung ML, Kyburz
A, Spath S, Becher B, Rogler G and Müller A: Helicobacter
pylori-specific protection against inflammatory bowel disease
requires the NLRP3 inflammasome and IL-18. Inflamm Bowel Dis.
21:854–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu
C, Chen Y, Cai W and Wu J: Chenodeoxycholic acid activates NLRP3
inflammasome and contributes to cholestatic liver fibrosis.
Oncotarget. 7:83951–83963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ten Hove T, Corbaz A, Amitai H, Aloni S,
Belzer I, Graber P, Drillenburg P, van Deventer SJ, Chvatchko Y and
Te Velde AA: Blockade of endogenous IL-18 ameliorates TNBS-induced
colitis by decreasing local TNF-alpha production in mice.
Gastroenterology. 121:1372–1379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inoue A, Mizushima T, Wu X, Okuzaki D,
Kambara N, Ishikawa S, Wang J, Qian Y, Hirose H, Yokoyama Y, et al:
A miR-29b byproduct sequence exhibits potent tumor-suppressive
activities via inhibition of NF-kappaB signaling in KRAS-mutant
colon cancer cells. Mol Cancer Ther. 17:977–987. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang N, Zhang Y and Liang H: microRNA-598
inhibits cell proliferation and invasion of glioblastoma by
directly targeting metastasis associated in colon cancer-1. Oncol
Res. 2018, Feb 14. Doi: 10.3727/096504018X15185735627746.
View Article : Google Scholar
|
|
10
|
Bruusgaard A and Andersen RB:
Chenodeoxycholic-acid treatments of rheumatoid arthritis. Lancet.
1:7001976. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Q, Hu X, Xuan Y, Ying J, Fei Y, Rong J,
Zhang Y, Zhang J, Liu C and Liu Z: Kaempferol protects
ethanol-induced gastric ulcers in mice via pro-inflammatory
cytokines and NO. Acta Biochim Biophys Sin (Shanghai). 50:246–253.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park GB, Chung YH, Gong JH, Jin DH and Kim
D: GSK-3β-mediated fatty acid synthesis enhances epithelial to
mesenchymal transition of TLR4-activated colorectal cancer cells
through regulation of TAp63. Int J Oncol. 49:2163–2172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martínez-Martínez E, Martín-Ruiz A, Martín
P, Calvo V, Provencio M and García JM: CB2 cannabinoid receptor
activation promotes colon cancer progression via AKT/GSK3beta
signaling pathway. Oncotarget. 7:68781–68791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jendželovský R, Koval J, Mikeš J, Papčová
Z, Plšíková J and Fedoročko P: Inhibition of GSK-3beta reverses the
pro-apoptotic effect of proadifen (SKF-525A) in HT-29 colon
adenocarcinoma cells. Toxicol In Vitro. 26:775–782. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jamwal G, Singh G, Dar MS, Singh P, Bano
N, Syed SH, Sandhu P, Akhter Y, Monga SP and Dar MJ: Identification
of a unique loss-of-function mutation in IGF1R and a crosstalk
between IGF1R and Wnt/β-catenin signaling pathways. Biochim Biophys
Acta. 1865:920–931. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang W, Li Y, Chen Y, Chen H, Zhu P, Xu M,
Wang H, Wu M, Yang Z, Hoffman RM and Gu Y: Ethanolic extract of
traditional chinese medicine (TCM) gamboge inhibits colon cancer
via the Wnt/beta-catenin signaling pathway in an orthotopic mouse
model. Anticancer Res. 38:1917–1925. 2018.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lazaridis LD, Pistiki A,
Giamarellos-Bourboulis EJ, Georgitsi M, Damoraki G, Polymeros D,
Dimitriadis GD and Triantafyllou K: Activation of NLRP3
inflammasome in inflammatory bowel disease: Differences between
crohn's disease and ulcerative colitis. Dig Dis Sci. 62:2348–2356.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Itani S, Watanabe T, Nadatani Y, Sugimura
N, Shimada S, Takeda S, Otani K, Hosomi S, Nagami Y, Tanaka F, et
al: NLRP3 inflammasome has a protective effect against
oxazolone-induced colitis: A possible role in ulcerative colitis.
Sci Rep. 6:390752016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi EM: Kaempferol protects MC3T3-E1
cells through antioxidant effect and regulation of mitochondrial
function. Food Chem Toxicol. 49:1800–1805. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Yan L, Guo Z, Chen Y, Li M, Huang
C, Chen Z and Meng X: Chenodeoxycholic acid attenuates high-fat
diet-induced obesity and hyperglycemia via the G protein-coupled
bile acid receptor 1 and proliferator-activated receptor γ pathway.
Exp Ther Med. 14:5305–5312. 2017.PubMed/NCBI
|
|
22
|
Hirano Y, Hirano F, Fujii H and Makino I:
Fibrates suppress chenodeoxycholic acid-induced RANTES expression
through inhibition of NF-kappaB activation. Eur J Pharmacol.
448:19–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Qian J, Wang L, Li J, Zhao Y, Han
J, Khan Z, Chen X, Wang J and Liang G: Kaempferol attenuates
hyperglycemia-induced cardiac injuries by inhibiting inflammatory
responses and oxidative stress. Endocrine. 60:83–94. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SH, Park JG, Lee J, Yang WS, Park GW,
Kim HG, Yi YS, Baek KS, Sung NY, Hossen MJ, et al: The dietary
flavonoid Kaempferol mediates anti-inflammatory responses via the
Src, Syk, IRAK1, and IRAK4 molecular targets. Mediators Inflamm.
2015:9041422015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li R, Liu J, Li Q, Chen G and Yu X:
miR-29a suppresses growth and metastasis in papillary thyroid
carcinoma by targeting AKT3. Tumour Biol. 37:3987–3996. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhuang Z, Ye G and Huang B: Kaempferol
Alleviates the Interleukin-1β-induced inflammation in rat
osteoarthritis chondrocytes via suppression of NF-kappaB. Med Sci
Monit. 23:3925–3931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng X, Yang YL, Yang H, Wang YH and Du
GH: Kaempferol alleviates LPS-induced neuroinflammation and BBB
dysfunction in mice via inhibiting HMGB1 release and
down-regulating TLR4/MyD88 pathway. Int Immunopharmacol. 56:29–35.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen H, Li L, Yang S, Wang D, Zhong S,
Zhao J and Tang J: MicroRNA-29a contributes to drug-resistance of
breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling
pathway. Gene. 593:84–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Basu A, Das AS, Sharma M, Pathak MP,
Chattopadhyay P, Biswas K and Mukhopadhyay R: STAT3 and NF-kappaB
are common targets for kaempferol-mediated attenuation of COX-2
expression in IL-6-induced macrophages and carrageenan-induced
mouse paw edema. Biochem Biophys Rep. 12:54–61. 2017.PubMed/NCBI
|
|
30
|
Merhi A, de Mees C, Abdo R, Victoria
Alberola J and Marini AM: Wnt/β-catenin signaling regulates the
expression of the ammonium permease gene RHBG in human cancer
cells. PLoS One. 10:e01286832015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mervai Z, Sólyomváry A, Tóth G, Noszál B,
Molnár-Perl I, Baghy K, Kovalszky I and Boldizsár I: Endogenous
enzyme-hydrolyzed fruit of Cirsium brachycephalum: Optimal source
of the antiproliferative lignan trachelogenin regulating the
Wnt/beta-catenin signaling pathway in the SW480 colon
adenocarcinoma cell line. Fitoterapia. 100:19–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Voloshanenko O, Erdmann G, Dubash TD,
Augustin I, Metzig M, Moffa G, Hundsrucker C, Kerr G, Sandmann T,
Anchang B, et al: Wnt secretion is required to maintain high levels
of Wnt activity in colon cancer cells. Nat Commun. 4:26102013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagano H, Tomimaru Y, Eguchi H, Hama N,
Wada H, Kawamoto K, Kobayashi S, Mori M and Doki Y: MicroRNA-29a
induces resistance to gemcitabine through the Wnt/β-catenin
signaling pathway in pancreatic cancer cells. Int J Oncol.
43:1066–1072. 2013. View Article : Google Scholar : PubMed/NCBI
|