Introduction
Carbon ion beam radiotherapy is regarded as a
cutting-edge technology for the treatment of a number of types of
human malignancy, including bone and soft-tissue sarcoma of the
head, neck and pelvis, and locally recurrent rectal and pancreatic
cancer, following surgery (1).
Whereas a systemic review indicated that the mechanism by which
ionized radiation therapy may induce apoptosis of cancer cells
involves redox regulation (2), one
study revealed that X-rays induced the production of reactive
oxygen species (ROS), and that cancer stem cells and other small
fractions of tumor tissues were able to survive following therapy
(3). By contrast, carbon ion beam
radiotherapy typically exerts antitumor effects directly on
cellular organelles, including the nucleus, in which the DNA may be
exposed to double-stranded breaks (4). As DNA double-stranded breaks are harmful
to cells, this presents a promising therapeutic mechanism via which
efficient eradication of tumors is expected (5).
Previous studies have indicated that cancer stem
cells serve a function in the therapeutic resistance to X-ray
radiation via redox regulation; for example, cancer stem cells
express cell surface markers, including cluster of differentiation
(CD)13 (6) and CD44 variants
(7), and survive by controlling
intracellular ROS metabolism following chemotherapy (6,7). CD13
functions as an aminopeptidase N in the antioxidant pathway,
whereas CD44 variants bound to the amino acid transporter xCT
subunit contribute to the maintenance of reduced conditions, which
enable cancer cell survival (7).
Novel therapeutic approaches targeting redox controls in cancer
stem cells have been developed, including the use of sulfasalazine,
an inhibitor of xCT-dependent cystine transport (8), and ubenimex, an inhibitor of CD13
(6). In particular, genetic ablation
of CD44 or treatment with sulfasalazine was demonstrated to
suppress the development of spasmolytic polypeptide-expressing
metaplasia and subsequent gastric tumor growth in mouse models of
gastric carcinogenesis (8).
Carbon ion beam radiotherapy resistance mechanisms
are not fully understood. Although the anticancer mechanism of
X-ray radiation (ROS production) facilitates the survival of cancer
stem cells, the unique mechanisms involved in the process of cancer
cell survival are not mutually exclusive (4). In the present study, an X-ray-resistant
cell line (×60) and a carbon ion beam-resistant cell line (C30)
were studied using mice. It was revealed that ×60 cells were
resistant to X-rays and carbon ion beams, whereas C30 cells were
not resistant to X-rays, suggesting that X-ray resistance and
carbon ion beam resistance exhibit distinct mechanisms. As carbon
ion beam radioresistant cells have not been extensively studied to
date, the association between carbon ion beam and chemotherapy
resistance remains unknown. Evaluation of optimal chemotherapy
regimens is important for improving treatment outcomes, as it
serves an important function in eliminating metastases and
microinvasions, which may cause tumor recurrence (4). For X-ray radioresistance, clinical
trials have examined a number of drugs and treatments in order to
determine the most effective combination and types; for example,
cisplatin (CDDP) and 5-fluorouracil (5-FU) are typically used in
combination with X-ray radiotherapy (2–4). A
previous study demonstrated that a combination of
trifluorothymidine (FTD) and the thymidine phosphorylase inhibitor,
tipiracil HCl (TAS-102), was effective against 5-FU-resistant
cancer (9). However, there is not
much evidence associated with carbon ion beam radiation, and
previous clinical studies have focused on an optimal treatment
dose, but not drug selection (1,10–16). In the present study, the effect of
FTD, a derivative of deoxythymidine, on carbon ion beam
radioresistant cells from rodents was studied. The results of the
present study revealed previously uncharacterized features of
carbon ion beam radioresistance, suggested the good efficacy of
FTD, and encouraged further study of the mechanism of cellular
resistance to charged particle therapy and the effects in
humans.
Materials and methods
Cell lines
The NR-S1 mouse squamous cell carcinoma cell line
(control) was provided by Dr Koichi Ando (Medicine and Biology
Division, Gunma University Heavy Ion Medical Center, Maebashi,
Japan). The ×60 (X-ray radioresistant) and C30 (carbon ion
radioresistant) cells were grown from NR-S1 as described (17) and maintained in Dulbecco's modified
Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) with 10% fetal bovine serum (FBS; HyClone; GE Healthcare,
Logan, UT, USA) and penicillin/streptomycin (Sigma-Aldrich; Merck
KGaA), and maintained at 37°C in an incubator containing 5%
CO2.
X-ray radioresistant cells
X60 cells were established by irradiating NR-S1
cells with 10 Gy X-ray radiation once every 2 weeks. The cells were
irradiated with a total dose of 60 Gy and cultured in DMEM with 10%
FBS at 37°C to ~70% confluency for 4 weeks following the final
irradiation (17).
Carbon ion beam radioresistant cells
of rodents
C30 cells were established by irradiating NR-S1
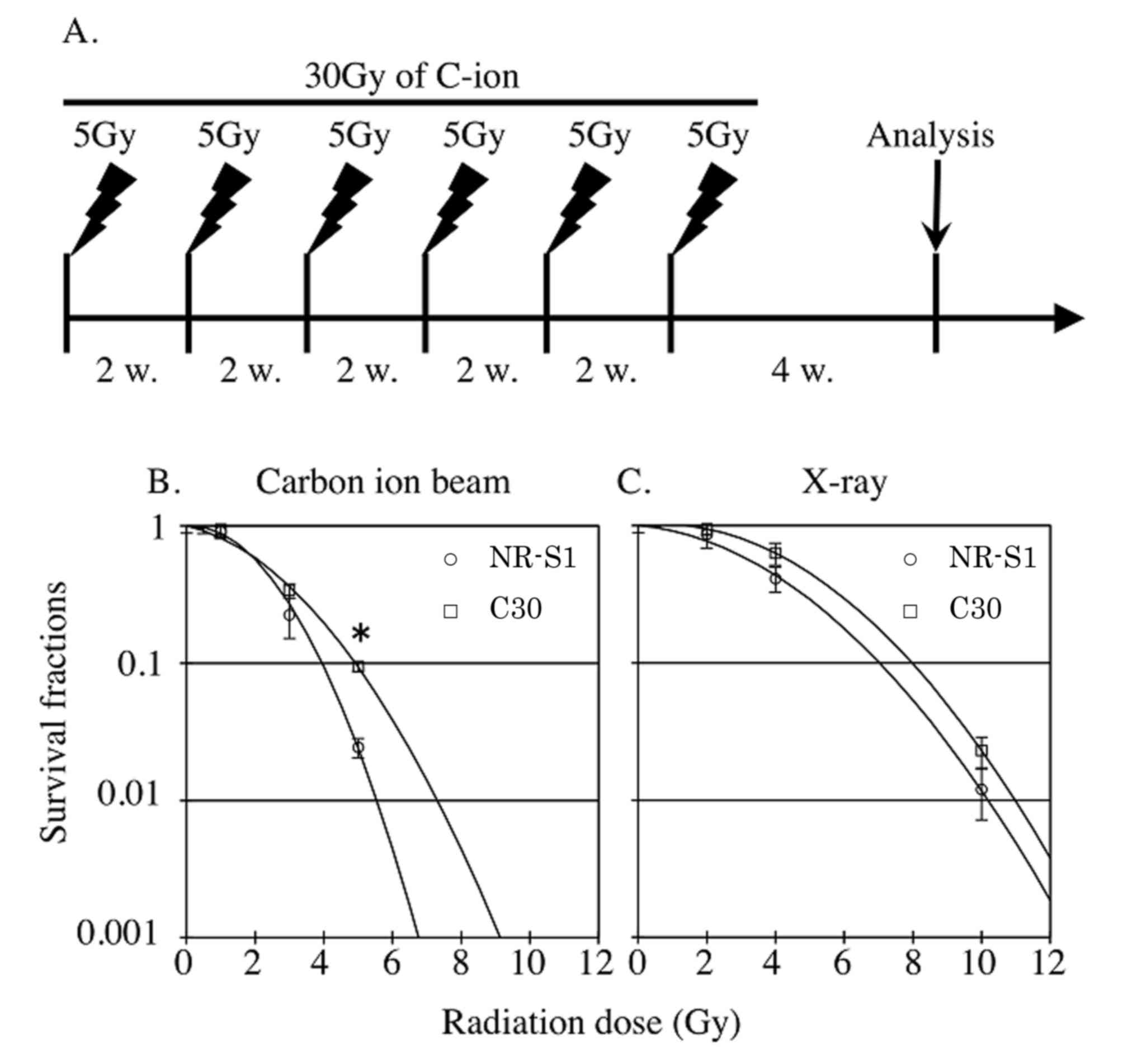
cells with 5 Gy carbon ion beam radiation once every 2 weeks
(Fig. 1). The cells were irradiated
with a total dose of 30 Gy and cultured in DMEM with 10% FBS at
37°C at ~70% confluency for 4 weeks following the final
irradiation.
Cell viability assay with FTD, 5-FU
and CDDP
Cell viability assays were performed using Cell
Counting Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) according to the manufacturer's protocol. As presented in
Fig. 2, cells were plated into
96-well plates at 1,000 cells/well and cultured at 37°C overnight.
Subsequently, the cells were treated with various concentrations of
5-FU (10, 5, 2.5, 1.25, 6.25×10−1, 3.12×10−1,
1.56×10−1, 7.81×10−2, 3.9×10−2 and
1.95×10−2 µM), FTD (2×102, 40, 8, 1.6,
3.2×10−1, 6.4×10−2, 1.28×10−2,
2.56×10−3, 5.12×10−4 and 1.02×10−4
µM), and CDDP (2.5, 1.25, 6.25×10−1,
3.12×10−1, 1.56×10−1, 7.81×10−2,
3.9×10−2, 1.95×10−2, 9.76×10−3 and
4.88×10−3 mM) at 37°C for 72 h with the comparison of
parental NR-S1 control. Viable cells were counted using Cell
Counting Kit-8 after 2 h of incubation at 37°C.
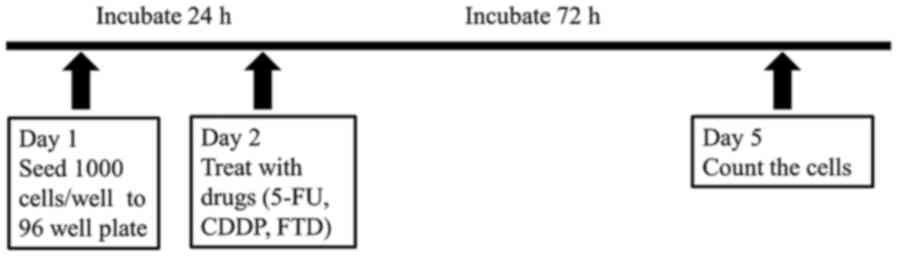 | Figure 2.Schema of the protocol. Viability
assays were performed with three replicates/assay. NR-S1, ×60 and
C30 cells were seeded at 1,000 cells/well. After 24 h of
incubation, the specified chemotherapeutic drugs were added in
various concentrations (10, 5, 2.5, 1.25, 6.25×10−1,
3.12×10−1, 1.56×10−1, 7.81×10−2,
3.9×10−2 and 1.95×10−2 µM of 5-FU;
2×102, 40, 8, 1.6, 3.2×10−1,
6.4×10−2, 1.28×10−2, 2.56×10−3,
5.12×10−4 and 1.02×10−4 µM of FTD; 2.5, 1.25,
6.25×10−1, 3.12×10−1, 1.56×10−1,
7.81×10−2, 3.9×10−2, 1.95×10−2,
9.76×10−3 and 4.88×10−3 mM of CDDP. Survival
was evaluated by counting the number of viable cells using Cell
Counting Kit-8 72 h after the addition of the drugs. 5-FU,
5-fluorouracil; CDDP, cisplatin; FTD, trifluorothymidine. |
Statistical analysis
Results are expressed as mean ± standard deviation
based on 3 independent experiments. The statistical significance of
the results was evaluated using a pairwise t-test with Bonferroni's
correction. Analysis was performed using R package software
(v3.4.3; date accessed, 30/11/2017; http://www.R-project.org/). P<0.05 was considered
to indicate a statistically significant difference.
Results
Radiation sensitivity of carbon ion
beam irradiated radiation-resistant cancer cells
Although it is known that repeated X-ray irradiation
results in cancer cells developing X-ray resistance, cancer cells
developing resistance following repeated C-ion irradiation has not
been demonstrated. In the present study, C-ion-induced
radioresistant cancer cells (C30) were established using repetition
of C-ion irradiation, and their X-ray and C-ion sensitivity was
then evaluated (Fig. 1B). The C-ion
resistance of the C30 cells was significantly increased
(P<0.01), compared with that of the parental NR-S1 cells. The
C30 cells were 3.9-fold resistant to C-ion at 5 Gy compared with
the NR-S1 cells. The D10 dose, the radiation dose required to
decrease the survival fraction by 0.1, of C-ion irradiation for the
C30 and NR-S1 cells was 4.9 and 3.9 Gy, respectively. Furthermore,
the X-ray sensitivity of the C30 cells was determined (Fig. 1C). Notably, the C30 cells did not
acquire significant X-ray resistance compared with the NR-S1 cells.
The D10 dose of X-ray for C30 and NR-S1 cells was 7.9 and 7.0 Gy,
respectively. The C30 cells were 1.9-fold resistant to X-ray at 10
Gy compared with the NR-S1 cells.
Sensitivity of carbon ion beam
radioresistant cells
The present study focused on the difference in
radioresistance between ×60 and C30 cells, and hypothesized that
C30 cells exhibited different radioresistances themselves to that
of ×60. If ×60 and C30 cells exhibited distinct characteristics,
the chemosensitivity of the two types of cell may additionally be
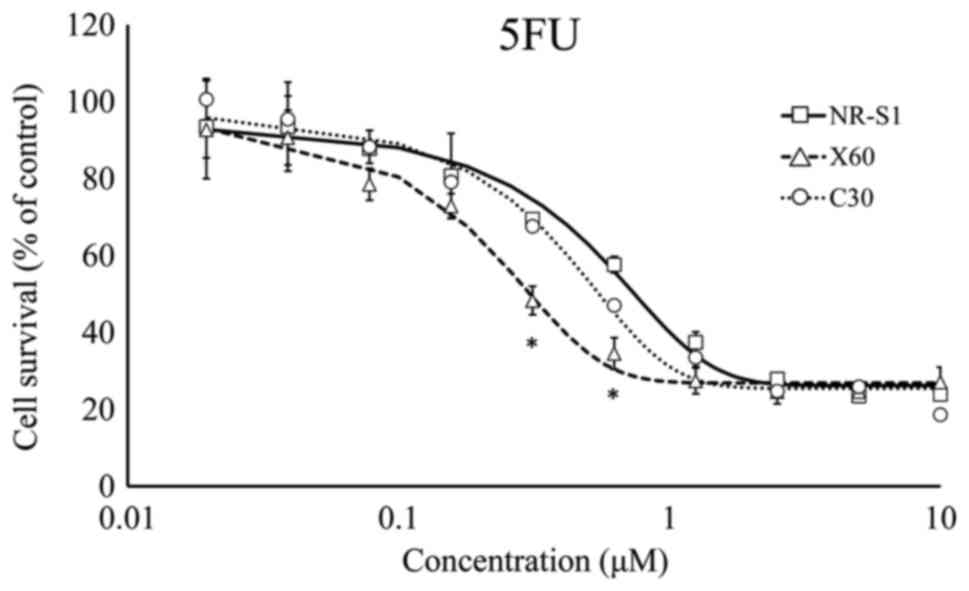
different. To evaluate the chemosensitivity of ×60 and C30 cells,
viability assays using CDDP, 5-FU and FTD were performed. Each
group was compared with the control NR-S1 group by pairwise t-test
with Bonferroni's correction. The analysis revealed that ×60 cells
were more sensitive to 5-FU compared with the C30 and NR-S1 cells
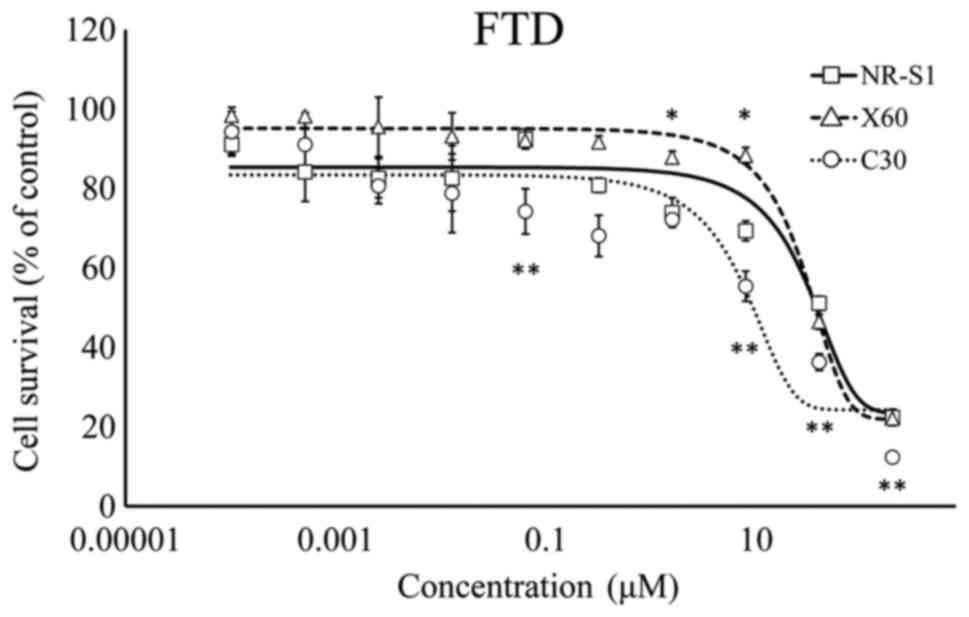
at two points (Fig. 3). C30 cells
were significantly more sensitive to FTD compared with the NR-S1
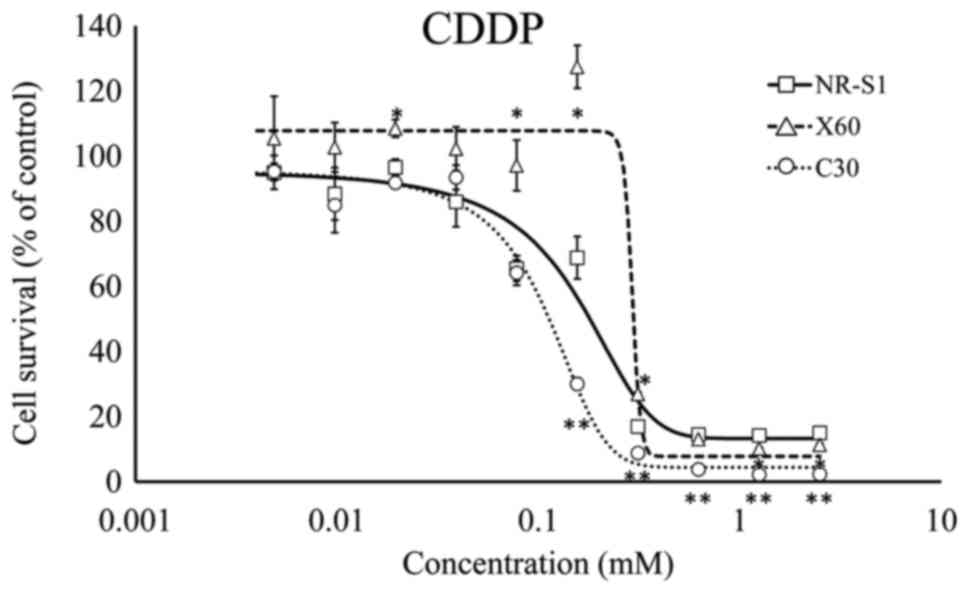
cells (Fig. 4). The cell survival was
increased in ×60 cells in the concentration of 0.1 mM of CDDP
compared with the NR-S1 cells (Fig.
5).
Sensitivity of X-ray radioresistant
cells
The chemosensitivity of X-ray radioresistant (×60)
cells was evaluated. ×60 cells were more sensitive to 5-FU,
compared with NR-S1 and C30 cells (Fig.
3). By contrast, ×60 cells were less sensitive to CDDP and FTD
compared with NR-S1 and C30 cells (Figs.
4 and 5). Therefore, ×60 and C30
exhibited different chemosensitivity and radiosensitivity
characteristics. As previously described (9), 5-FU resistance was not associated with
FTD resistance and FTD effectively sensitized C30 cells.
Discussion
Previous studies have demonstrated that X-ray
radiation results in replication stress and arrest, stalling of
replication forks and single-strand DNA breaks occur when cell
cycle checkpoints are activated (4,5). These
biological effects are associated with the production of ROS from
cellular organelles such as mitochondria (18). Charged particle therapy, including
carbon ion beam radiation, may induce double-strand DNA breaks, and
at an increased frequency compared with X-ray radiation, although
additive anti-cancer effects are typically observed (4). Carbon ion beam radiotherapy is a useful
treatment option for X-ray-resistant cancer (4). For retractable cancer, carbon ion beam
radiotherapy does not replace conventional X-ray radiotherapy, as
it exhibits an increased risk of damage to normal tissues and has a
higher cost (4). However, the
molecular mechanisms involved in this type of therapeutic
resistance remain unknown. The current working model of therapeutic
resistance [i.e. the mechanism of charged particle therapy-induced
double-strand DNA breaks (4)] and its
associated genomic effects should be considered, as charged
particles exert biological effects on DNA. This difference in
carbon ion beam mechanism may affect the cellular characteristics
of carbon ion-resistant cells, which as a result may have different
chemosensitivity.
In the present study, rodent cell lines that were
resistant to carbon ion beam radiation were examined. The results
indicated that carbon ion beam radioresistant cells were more
sensitive to FTD exposure compared with X-ray radioresistant cells.
FTD, a derivative of deoxythymidine, is an antitumor and antiviral
agent. As antitumor agents, FTD combined with tipiracil
hydrochloride (1:0.5 ratio; TAS-102) are available for use with
5-FU refractory unresectable colorectal cancer (19). Tipiracil may inhibit thymidine
phosphorylase in the liver and intestine, resulting in slow FTD
degradation and augmentation of FTD bioavailability (19). A previous randomized trial has
demonstrated the efficacy of TAS-102 for refractory metastatic
colorectal cancer (20). In the
present study, FTD was used in an in vitro assay; however,
as there are differences between rodents and humans, clinical
trials are required to validate the in vitro results.
Furthermore, administration of FTD may effectively sensitize cells
to carbon ion beam irradiation, although clinical studies in humans
are required.
FTD may be converted to FTD monophosphate via
thymidine kinase 1. The FTD monophosphate may be phosphorylated to
FTD triphosphate, which has been suggested to integrate into DNA
(19). These biological effects have
been demonstrated to lead to apoptosis and the inhibition of viral
replication (19). We hypothesize
that carbon ion beam radiation induces random double-stranded DNA
breaks in multiple genomic regions; in response, the remaining
unaffected coding and non-coding regions, including microRNA, are
upregulated to compensate for the damage caused, resulting in
resistance to charged particle radiation. However, the potentially
active genomic region may incorporate FTD triphosphate, resulting
in the induction of apoptosis.
FTD is hypothesized to be effective against
5-FU-resistant cancer (9), and the
present study revealed that FTD was effective against carbon ion
beam-resistant cancer cells. Treatment with 5-FU was more effective
in X-ray-resistant cancer cells than control cells, and 5-FU is
typically used with X-ray radiotherapy. FTD may become a good
option to use in combination with carbon ion beam radiotherapy
instead of 5-FU. Additional in vivo and clinical studies are
required to validate these results.
The results of the present study demonstrated that
carbon ion beam radioresistant rodent cells are sensitized to FTD
exposure. In future, the following studies are required: i)
Clinical trials to evaluate charged particle therapy combined with
FTD treatment in humans; ii) biomarker identification for the
prediction of carbon ion beam radioresistance, including microRNAs
in liquid biopsy; and iii) a mechanistic study of carbon ion beam
radioresistance. A similar clinical setting may be useful to
investigate advanced gastrointestinal cancer following carbon ion
beam radiation.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a
Grant-in-Aid for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology (Japan),
P-DIRECT (to HI), a Grant-in-Aid from the Ministry of Health, Labor
and Welfare (MM), a grant from the National Institute of Biomedical
Innovation (MM), and a grant from the Osaka University Drug
Discovery Funds (HI). Partial support was received from Taiho
Pharmaceutical Co., Ltd. (MM and HI), Evidence Based Medical
Research Center through institutional endowments (MM and HI). Part
of the study was performed as a research project with heavy ions at
NIRS-HIMAC (grant no. 15J183) and supported by the Grants-in-Aid
for Scientific Research in Japan Society for the Promotion of
Science (grant no. 15K15467).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The experiments were performed by SJB, KS, JK, KH,
KK and MK. The analysis of data was performed by SJB, NN, JK and
MK. The manuscript was written by SJB, KS, JK, KH, KK, MK. YD, MM,
KO and HI. The study was designed by SJB, YD, MM, KO and HI.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Taiho Pharmaceutical Co., Ltd. and Evidence Based
Medical Research Center funding had no role in the study design,
data collection or data analysis. However, the company performed a
pre-submission review of the manuscript.
References
|
1
|
Kamada T, Tsujii H, Blakely EA, Debus J,
De Neve W, Durante M, Jäkel O, Mayer R, Orecchia R, Pötter R, et
al: Carbon ion radiotherapy in Japan: An assessment of 20 years of
clinical experience. Lancet Oncol. 16:e93–e100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yasueda A, Urushima H and Ito T: Efficacy
and interaction of antioxidant supplements as adjuvant therapy in
cancer treatment: A systematic review. ntegr. Integr Cancer Ther.
15:17–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Skvortsova I, Debbage P, Kumar V and
Skvortsov S: Radiation resistance: Cancer stem cells (CSCs) and
their enigmatic pro-survival signaling. Semin Cancer Biol.
35:39–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baek SJ, Ishii H, Tamari K, Hayashi K,
Nishida N, Konno M, Kawamoto K, Koseki J, Fukusumi T, Hasegawa S,
et al: Cancer stem cells: The potential of carbon ion beam
radiation and new radiosensitizers (Review). Oncol Rep.
34:2233–2237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kunkel TA: DNA replication fidelity. J
Biol Chem. 279:16895–16898. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haraguchi N, Ishii H, Mimori K, Tanaka F,
Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, et al:
CD13 is a therapeutic target in human liver cancer stem cells. J
Clin Invest. 120:3326–3339. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishimoto T, Nagano O, Yae T, Tamada M,
Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al:
CD44 variant regulates redox status in cancer cells by stabilizing
the xCT subunit of system xc(−) and thereby promotes Tumor Growth.
Cancer Cell. 19:387–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wada T, Ishimoto T, Seishima R,
Tsuchihashi K, Yoshikawa M, Oshima H, Oshima M, Masuko T, Wright
NA, Furuhashi S, et al: Functional role of CD44v-xCT system in the
development of spasmolytic polypeptide-expressing metaplasia.
Cancer Sci. 104:1323–1329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peters CJ: Therapeutic potential of
TAS-102 in the treatment of gastrointestinal malignancies. Ther Adv
Med Oncol. 7:340–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uhl M, Herfarth K and Debus J: Comparing
the use of protons and carbon ions for treatment. Cancer J.
20:433–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui X, Oonishi K, Tsujii H, Yasuda T,
Matsumoto Y, Furusawa Y, Akashi M, Kamada T and Okayasu R: Effects
of carbon ion beam on putative colon cancer stem cells and its
comparison with X-rays. Cancer Res. 71:3676–3687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishikawa H, Tsuji H, Kamada T, Akakura K,
Suzuki H, Shimazaki J and Tsujii H: Working Group for Genitourinary
Tumors: Carbon-ion radiation therapy for prostate cancer. Int J
Urol. 19:296–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsujii H and Kamada T: A review of update
clinical results of carbon ion radiotherapy. Jpn J Clin Oncol.
42:670–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakano T, Suzuki Y, Ohno T, Kato S, Suzuki
M, Morita S, Sato S, Oka K and Tsujii H: Carbon beam therapy
overcomes the radiation resistance of uterine cervical cancer
originating from hypoxia. Clin Cancer Res. 12:2185–2190. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizoe JE, Hasegawa A, Jingu K, Takagi R,
Bessyo H, Morikawa T, Tonoki M, Tsuji H, Kamada T, Tsujii H, et al:
Results of carbon ion radiotherapy for head and neck cancer.
Radiother Oncol. 103:32–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jingu K, Tsujii H, Mizoe JE, Hasegawa A,
Bessho H, Takagi R, Morikawa T, Tonogi M, Tsuji H, Kamada T and
Yamada S: Organizing Committee for the Working Group for
Head-and-Neck Cancer: Carbon ion radiation therapy improves the
prognosis of unresectable adult bone and soft-tissue sarcoma of the
head and neck. Int J Radiat Oncol Biol Phys. 82:2125–2131. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sato K, Imai T, Okayasu R and Shimokawa T:
Heterochromatin domain number correlates with x-ray and carbon-ion
radiation resistance in cancer cells. Radiat Res. 182:408–419.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chandel NS: Evolution of Mitochondria as
Signaling Organelles. Cell Metab. 22:204–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Temmink OH, Emura T, de Bruin M, Fukushima
M and Peters GJ: Therapeutic potential of the dual-targeted TAS-102
formulation in the treatment of gastrointestinal malignancies.
Cancer Sci. 98:779–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919. 2015.
View Article : Google Scholar : PubMed/NCBI
|