Introduction
Hydrogen (H2) occurs safely in the air
with a concentration of <4.7%, and can be used as an inert gas
at body temperature. H2 selectively quenches detrimental
reactive oxygen species (ROS), and it has become a novel
anti-oxidant due to its anti-apoptotic, antioxidant,
anti-inflammatory and anti-allergy effects (1,2). ROS
increase cell migration and enhance tumor invasion and metastasis
(3). Antioxidants have been
demonstrated to effectively protect against cell damage, and
H2 effectively decreases radicals (·OH) and
peroxynitrite (ONOO−) in living cells without disrupting
the ROS that are involved in normal metabolic oxidation reduction
reactions in cell signaling. Therefore, H2 can be used
as an anti-inflammatory and anti-tumorigenic agent in clinical
practice. The present review focuses on the association between
H2 and ROS in inflammatory disease and cancer.
H2 usage method
Inhalation
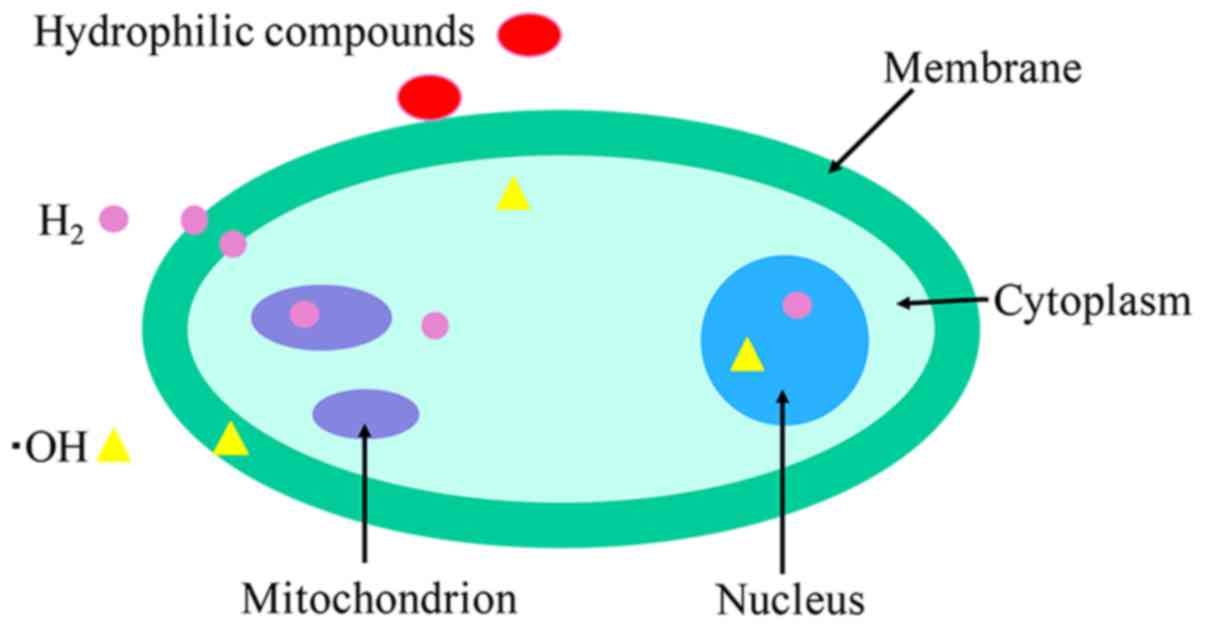
H2 has capability to penetrate
biomembranes and diffuse into the cytosol, mitochondria and nuclei
due to its distribution characteristics, including being able to
rapidly penetrate vessel walls and being able to dissolve in water
or saline (Fig. 1) (4,5). By
contrast, the majority of hydrophilic antioxidants cannot penetrate
biomembranes and remain on the surface. Inhalation of H2
or the administration of H2 water can increase the
concentration of H2 in arterial and venous blood
(6).
Oral administration
There are several methods to produce H2
water, including infusing H2 gas into water up to 0.8 mM
(1.6 ppm) under atmospheric pressure or dissolving electrolyzed
H2 into pure water to form H2 bubbled water.
H2 rapidly penetrates the glass and plastic walls of any
vessels, but has a half-time of 0–2 h and almost disappears after 8
h, so aluminum containers with no dead volume are usually used to
reserve H2 gas (7).
Intravenous drip
In contrast to H2 gas, H2
saturated in saline (HS) is easy to administer by dissolving
H2 in physiological saline for 6 h under 0.4 MPa
pressure to a supersaturated level (8). HS can be stored in an aluminum bag under
atmospheric pressure at 4°C, with a >0.6 mmol/l concentration of
H2 (8). HS can be infused
into the stomachs of rats for experimental and clinical treatments
(8,9).
Clinical application of H2-enriched
glucose-electrolyte solution can be used for acute cerebral
infarction and in patients treated with t-PA (9). The solution can be produced at 1.6 ppm
H2 concentrations using H2 adding equipment.
Administration of 500 ml intravenous H2-enriched fluid
over 30 min for >3 days could relieve the associated symptoms of
fever and pain in patients with acute erythematous skin diseases,
but does not change physiological parameters in the blood (10).
External use
H2 penetrates the skin easily and is
distributed throughout the whole body via the blood in 10 min, as
measured by H2 gas content in expired breath. Submersion
in a warm water bath with dissolved H2 is a method of
absorbing H2 into the body in daily life. Hydrogen-water
bathing therapy (hydrogen-water was provided by Shanghai Yiquan
Investment Limited Partnership Company, Shanghai, China) has a
significant and rapid improvement on disease severity and the
quality of life for patients with psoriasis and parapsoriasis en
plaques (11). Additionally,
H2-loaded eye drops can be made by dissolving
H2 in saline and can be directly dropped onto the ocular
surface (12,13).
ROS in inflammatory disease and cancer
Cancer is a multi-stage process defined by
initiation, promotion and progression (14–16), and
oxidative stress interacts with all three stages of this process.
ROS can increase tumor cell proliferation, survival and cellular
migration in animal models and humans by inducing cellular signal
transduction pathways (17,18).
What are ROS? ROS are formed as a result of
an imbalance between free radical and reactive metabolite
production, and can potentially exhibit a negative impact on the
organism (19). ROS are products of
oxygen-derived small molecules involved in normal cellular
metabolism, including oxygen radicals such as superoxide anion
(O2•−), hydroxyl (·OH), peroxyl
(RO2•), and alkoxyl (RO•), as well
as non-radicals, which can be converted to radicals or function as
oxidizing agents, including H2 peroxide
(H2O2), hypochlorous acid (HOCl), ozone
(O3) and singlet oxygen (1O2). ROS
promote DNA synthesis, cell proliferation, cell survival, cellular
migration and invasion, tumor metastasis and angiogenesis (20). Aerobic cells produce ROS, including
O2•−, H2O2 and ·OH, in
endogenous metabolic reactions (21).
Mitochondria are constantly exposed to high levels of ROS, which
cause mitochondrial DNA damage and increase O and ·OH levels in
cellular apoptosis (2).
Reactive nitrogen species (RNS) are formed from
nitrogen-containing oxidants such as nitric oxide (NO). The
mitochondrial respiratory chain can generate RNS under hypoxic
conditions, while RNS can further generate other reactive species
(22), and continuous cellular ROS
and RNS generation is now known to be a consequence of numerous
factors, including carcinogen exposure, inflammation and
mitochondrial respiration (23).
ROS initiate tumor progression
Tumor cells generate ROS more abundantly than normal
cells and cause elevated oxidative stress (24). Damage to DNA by ROS is involved in
chronic inflammatory diseases and in a wide variety of cancer
types, including bladder cancer (25), brain tumors (26), breast cancer (27), cervical cancer (28), gastric cancer (29), liver cancer (30), lung cancer (31), melanoma (32), multiple myeloma (33), leukemia (34), lymphoma (35), oral cancer (36), ovarian cancer (37), pancreatic cancer (38), prostate cancer (39) and sarcoma (40).
ROS can initiate tumorigenicity and subsequent tumor
progression by inducing DNA damage (41). Oxidative stress interacts with the
initiation, promotion and progression of cancer. During the
initiation stage, ROS introduce gene mutations and structural
alterations into the DNA and produce DNA damage. In the promotion
stage, ROS increase cell proliferation or decrease apoptosis of the
initiated cell population by causing abnormal gene expression,
blocking cell communication and modifying second-messenger systems.
Finally, oxidative stress may add DNA alterations to the initiated
cell population and promote cancer progression (42).
Impact of ROS on cancer by regulation
of gene expression
ROS serve vital roles in stimulating cell signaling
pathways in intra- and extracellular environmental conditions
(43), regulating gene mutations, and
balancing cell proliferation and apoptosis (3,44). Cancer
signaling starts from the hypoxic microenvironment of the autocrine
and paracrine elements, including vascular endothelial growth
factor, hepatocyte growth factor, hypoxia-inducible factor-1α
(HIF-1α), NO and H2O2, which generate a
positive feedback loop to hyper-activate the protein kinase B (Akt)
locus. Oxidative stress can activate several transcription factors,
including nuclear factor (NF)-κB, activator protein 1, p53, HIF-1α,
matrix metalloproteinases, peroxisome proliferator-activated
receptor-γ, β-catenin/Wnt and nuclear factor erythroid 2-related
factor 2 (Nrf2). These effector molecules are activated under
prolonged ROS-related chronic inflammation and alter the malignant
transformation and the expression of genes involved in immune,
inflammatory responses, carcinogenesis and metastasis.
Anti-oxidative characteristic of
H2
It has been demonstrated that a number of factors,
including intense exercise, cardiac infarction (45), cessation of blood flow, organ
transplantation and inflammation (46), can cause acute oxidative stress.
H2 is able to reduce the risk of life style-related
diseases and cancer (7,47–49), and
thus can be used to treat various diseases using its characteristic
of protecting nuclear DNA and mitochondria.
H2 reduces oxidants in
ROS
H2 dissolved in culture medium
selectively reduces the strongest oxidants, such as OH and
ONOO−, in cell signaling, but does not disturb the
cellular levels of ·O2, NO· or
H2O2, as well as ROS involved in metabolic
oxidation-reduction reactions in cell-free systems (Fig. 2). As ·OH is strong enough to react
with H2, it can be a marker of the oxidative strength of
ROS. It was previously reported that H2 treatment
significantly reduced ·OH produced by radiolysis or photolysis of
water and decreased the levels of ·OH in cultured cells, thus
protecting the mitochondria from OH (1). Since H2 penetrates
biomembranes and diffuses into organelles, it can decrease cellular
levels of ATP synthesized in the mitochondria and nucleus (1). Ren et al (50) demonstrated that treatment with 5%
H2-rich water led to a significant decrease in the level
of ROS, maintained the biomass and polar growth morphology of the
mycelium, and decreased the secondary metabolism under acetic
acid-induced oxidative stress (50).
H2 also decreased the levels of ROS and promoted the
chronic ultraviolet exposure-induced expression of phosphoinositide
3-kinase, Akt and Nrf2 in HaCaT cells (51). Since H2 treatment exhibited
anti-oxidant and anti-inflammatory neuroprotective effects, it
essentially decreased cyclooxygenase-2 (oxidative stress markers)
in immune-positive neurons (52).
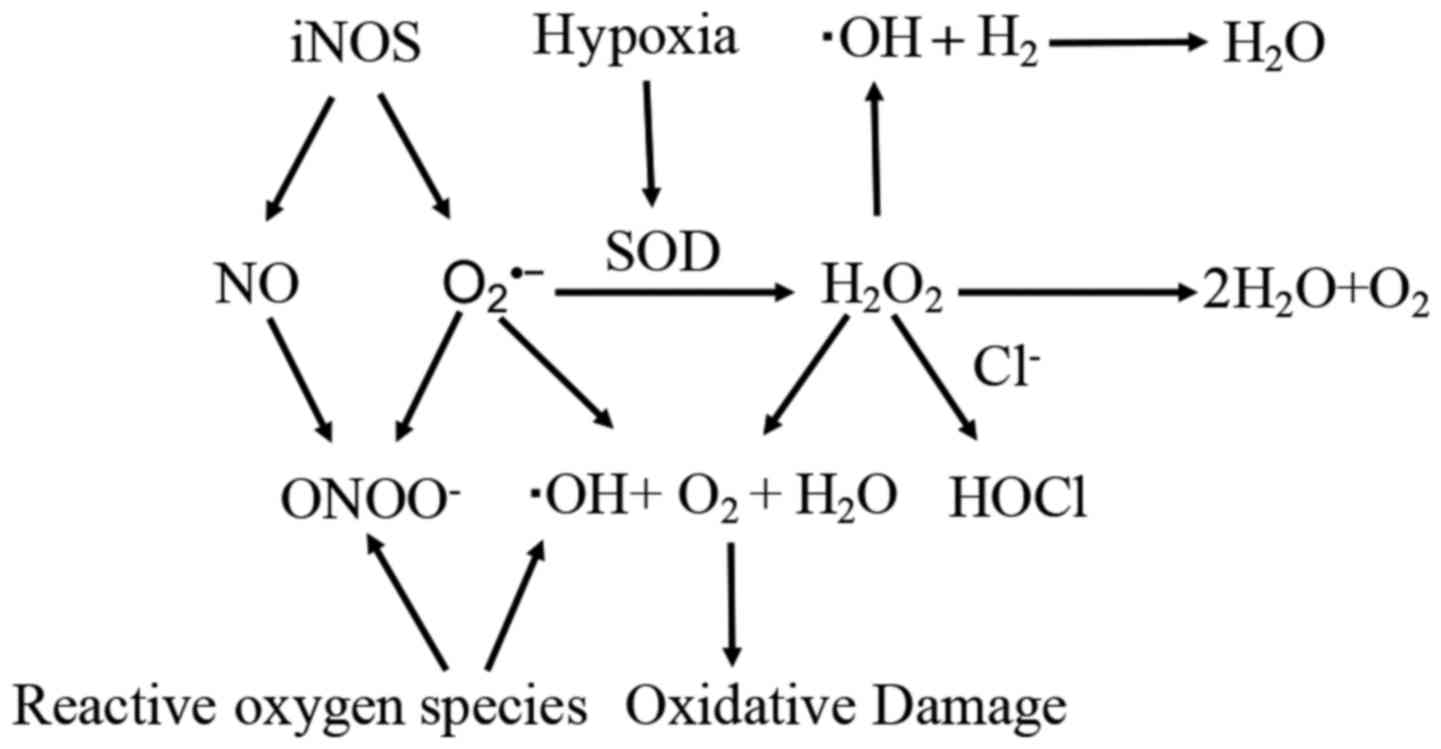 | Figure 2.Impact of key oxidants of
H2 in cancer: ·OH and ONOO− are highly
reactive to damaged cells, while ·O2, NO· and
H2O2 have physiological roles as signaling
molecules. H2, hydrogen; OH, hydroxyl radicals;
ONOO−, peroxynitrite; ·O2, superoxide anion;
NO, nitric oxide; H2O2, H2
peroxide; Cl−, chloride; H2O, water; iNOS,
inducible nitric oxide synthase; SOD, superoxide dismutase. |
Anti-inflammatory and antitumor
activity of H2
H2 anti-inflammatory and anti-allergic
features that function via the induction of inflammatory cytokines
and the inhibition of cell signal factors. H2 has been
shown to decrease the expression of a number of pro-inflammatory
factors, including tumor necrosis factor-α (TNF-α), interleukin
(IL)-6, IL-1β, IL-10, IL-12, chemokine ligand 2 (CCL2),
intercellular adhesion molecule 1, NF-κB, high mobility group box 1
protein and prostaglandin E2. Furthermore, H2-rich
saline reduced serum diamine oxidase, TNF-α, IL-1β, IL-6, tissue
malondialdehyde, protein carbonyl and myeloperoxidase activity, and
also inhibited pro-apoptotic players, including JNK and caspase-3
(53,54).
In a previous study, H2 gas inhalation
significantly reduced the number of total cells, eosinophils and
lymphocytes in the bronchial alveolar lavage fluid, and increased
the level of IL-4, IL-13, TNF-α and chemokine (C-X-C motif) ligand
15. The IL-4 serum level was significantly decreased following
inhalation. H2 gas inhalation markedly upregulated the
activity of superoxide dismutase and significantly attenuated the
increased level of malondialdehyde and myeloperoxidase in allergic
asthmatic mice (55).
H2 can function as an anti-tumorigenic
agent due to its preventive effect against tumor progression and
invasion. Accordingly, neutral pH H2-enriched
electrolyzed (NHE) water as an anti-oxidant was previously shown to
counteract ROS, inhibiting tumor cell proliferation and invasion
together with scavenging of intracellular oxidants. NHE water
preferentially inhibited clonal growth of human tongue carcinoma
cells, inhibited tumor invasion of human fibrosarcoma cells
concurrently with intracellular oxidant repression, and scavenged
intracellular oxidant H2 peroxides (48). Additionally, nano-bubble H2
water with platinum colloid is more attractive as a novel antitumor
regiment, as it reduces the side effects in normal tissues; it was
reported that decreased cell numbers, cell shrinkage, cell
apoptosis, cell deformation and microvilli on the membrane surface
were observed in Ehrlich ascites tumors, as H2 water
erased the ROS that were indispensable for cell growth. These
antitumor effects were promoted by combination with hyperthermia at
42°C (49) (Table I).
 | Table I.Summary of various preventive and
therapeutic effects of hydrogen by clinical examinations or by
animal experiments. |
Table I.
Summary of various preventive and
therapeutic effects of hydrogen by clinical examinations or by
animal experiments.
| Category |
Disease/condition | Preventive
treatment | (Refs.) |
|---|
| Metabolic
syndrome | Diabetes | Improve the
impaired sugar tolerance abilities of obese insulin-resistant type
2 diabetic mice. | (47) |
|
| Hypertension and
dyslipidemia | Increase the level
of antioxidant enzyme SOD and decrease total
cholesterol/HDL-cholesterol level. | (7) |
|
Ischemia-reperfusion injuries | Cerebral
infarction | Protect brain
ischemia and reperfusion injuries against inflammation and
oxidative stress. | (1) |
|
| Liver
cirrhosis | Prevent ROS-induced
cell death and inflammation in the liver. | (53) |
|
| Myocardial
infarction | Reduce infarct size
in the rat model of myocardial ischemia-reperfusion injury. | (45) |
|
| Organ
transplantation | Reduce
ischemia-reperfusion injury in the intestinal graft injury. | (46) |
|
Neuroprotection | Parkinson's
disease | Reduce dopaminergic
neuronal loss in 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine in
Parkinson's disease. | (60) |
|
| Cognitive
impairment | Ameliorate
cognitive impairment in senescence-accelerated mice. | (59) |
| Inflammation | Septic
appendicitis | Reduce early and
late pro-inflammatory cytokine levels in the serum and tissues of
appendicitis rats. | (57) |
|
| Intestine
disease | Reduce
ischemia-reperfusion injury in the intestinal graft injury. | (61) |
| Cancer therapy | Fibrosarcoma | Inhibit tumor
invasion of human fibrosarcoma cells. | (48) |
|
| Tongue
carcinoma | Inhibit clonal
growth of human tongue carcinoma cells. | (48) |
|
| Ehrlich ascites
tumor | Erase the ROS in
Ehrlich ascites tumor types. | (49) |
H2 treats disease via an
antioxidant effect
In previous studies, the beneficial effects of
treatment with H2 on organ damage were associated with
decreased oxidative product levels, increased antioxidant enzyme
activities, and reduced early and late pro-inflammatory cytokine
levels in the serum and tissue. Brain damage followed by cerebral
ischemia/reperfusion (I/R) injuries generated ROS, while the
antioxidant effect of H2 gas inhalation was able to
reduce brain, liver and heart ischemia-reperfusion injury, and
intestinal graft injury (1,45,46,56).
H2 protected neurons from ischemia and reperfusion, and
was efficacious for cerebral infarction. Furthermore, H2
gas suppressed the progression of hepatic ischemia and reperfusion
injury (1). Inhalation of
H2 gas significantly lessened the damage to the organs
of septic mice with moderate or severe appendicitis by reducing
early and late pro-inflammatory cytokine levels in the serum and
tissues, thus increasing the survival rate (57).
Ingestion of H2 water can eliminate ROS
and confer antitumor activity (48);
it represents a novel method of H2 administration and
has greater advantages over other forms of antioxidant therapy.
Consumption of H2-enriched water has beneficial effects
in clinical practice, including the treatment of atherosclerosis,
metabolic syndrome, type 2 diabetes, and cognitive impairment
during aging and Parkinson's disease (7,47,58–60).
It was previously reported that HS protected brain
ischemia and reperfusion injuries against inflammation and
oxidative stress, as well as improving function in a neonatal
hypoxia-ischemia rat model (59). HS
prevented early pathological changes in acute hepatic injury and
was able to prevent ROS-induced cell death and inflammation in the
liver by inhibiting the processes of liver cirrhosis and hepatocyte
compensatory proliferation (53).
Additionally, HS has protective effects on small intestine
ischemia/reperfusion injuries (8).
These advantages of HS elucidate the clinical potential for
preventive and therapeutic anti-oxidative applications (Table I).
Therapeutic and protective function of
H2 in chemotherapy and radiotherapy
Radiotherapy and chemotherapy are major treatment
types for cancer. H2 diffuses rapidly to reduce
cytotoxic radicals and inflammation in tissues. H2 gas
or H2 water has been shown to improve the quality of
life (QOL) of patients during chemotherapy via its antioxidant
properties. Inhalation of 1% H2 gas or drinking
H2 water alleviated the nephrotoxicity, mortality and
body-weight loss caused by cisplatin. Drinking H2 water
also decreased the level of apoptosis in the kidney. Despite
possessing protective effects against cisplatin-induced toxicity,
H2 did not compromise the antitumor effects of cisplatin
against cancer cell lines in vitro and in tumor-bearing mice
in vivo (4,61).
It was hypothesized that the majority of
radiation-induced symptoms associated with increased ROS and
inflammation during radiotherapy would significantly affect the
patient's QOL (62). The biological
reaction to radiation-induced oxidative stress is reduced by the
consumption of H2-rich water, without antitumor
activities being impaired. In one study, consumption of
H2-rich water for 6 weeks during radiotherapy
significantly improved the QOL scores of patients with malignant
liver tumors, and the levels of reactive oxygen metabolites in the
blood were reduced (63).
Overall, H2 reduces the risk of life
style-related oxidative stress by reacting with strong reactive
oxygen/nitrogen species in cell-free reactions. It is easily to
apply H2 in cases of oxidative stress, inflammation and
tumors. Due to the lack of adverse effects and the high efficacy
for the majority of pathogenic statuses involved, H2
gas, H2 water and HS are increasingly being accepted as
promising candidates for therapeutic approaches. We hypothesize
that H2 gas inhalation and oral administration of
H2 water could protect against inflammation in oxidative
stress-related cancer, and thus improve the antitumor effect in the
clinical management of cancer.
References
|
1
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sato Y, Kajiyama S, Amano A, Kondo Y,
Sasaki T, Handa S, Takahashi R, Fukui M, Hasegawa G, Nakamura N, et
al: Hydrogen-rich pure water prevents superoxide formation in brain
slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem
Biophys Res Commun. 375:346–350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rojas V, Hirshfield KM, Ganesan S and
Rodriguez-Rodriguez L: Molecular characterization of epithelial
ovarian cancer: Implications for diagnosis and treatment. Int J Mol
Sci. 17:pii: E2113. 2016. View Article : Google Scholar
|
|
4
|
Nakashima-Kamimura N, Mori T, Ohsawa I,
Asoh S and Ohta S: Molecular hydrogen alleviates nephrotoxicity
induced by an anti-cancer drug cisplatin without compromising
anti-tumor activity in mice. Cancer Chemother Pharmacol.
64:753–761. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
James AM, Cochemé HM and Murphy MP:
Mitochondria-targeted redox probes as tools in the study of
oxidative damage and ageing. Mech Ageing Dev. 126:982–986. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cardinal JS, Zhan J, Wang Y, Sugimoto R,
Tsung A, McCurry KR, Billiar TR and Nakao A: Oral hydrogen water
prevents chronic allograft nephropathy in rats. Kidney Int.
77:101–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakao A, Toyoda Y, Sharma P, Evans M and
Guthrie N: Effectiveness of hydrogen rich water on antioxidant
status of subjects with potential metabolic syndrome-an open label
pilot study. J Clin Biochem Nutr. 46:140–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng X, Mao Y, Cai J, Li Y, Liu W, Sun P,
Zhang JH, Sun X and Yuan H: Hydrogen-rich saline protects against
intestinal ischemia/reperfusion injury in rats. Free Radic Res.
43:478–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagatani K, Nawashiro H, Takeuchi S,
Tomura S, Otani N, Osada H, Wada K, Katoh H, Tsuzuki N and Mori K:
Safety of intravenous administration of hydrogen-enriched fluid in
patients with acute cerebral ischemia: Initial clinical studies.
Med Gas Res. 3:132013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ono H, Nishijima Y, Adachi N, Sakamoto M,
Kudo Y, Nakazawa J, Kaneko K and Nakao A: Hydrogen(H2) treatment
for acute erythymatous skin diseases. A report of 4 patients with
safety data and a non-controlled feasibility study with H2
concentration measurement on two volunteers. Med Gas Res. 2:142012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu Q, Wu Y, Li Y, Chen Z, Wang L, Xiong
H, Dai E, Wu J, Fan B, Ping L and Luo X: Positive effects of
hydrogen-water bathing in patients of psoriasis and parapsoriasis
en plaques. Sci Rep. 8:80512018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kubota M, Shimmura S, Kubota S, Miyashita
H, Kato N, Noda K, Ozawa Y, Usui T, Ishida S, Umezawa K, et al:
Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced
angiogenesis in a mouse corneal alkali-burn model. Invest
Ophthalmol Vis Sci. 52:427–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oharazawa H, Igarashi T, Yokota T, Fujii
H, Suzuki H, Machide M, Takahashi H, Ohta S and Ohsawa I:
Protection of the retina by rapid diffusion of hydrogen:
Administration of hydrogen-loaded eye drops in retinal
ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 51:487–492.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ames BN and Gold LS: Animal cancer tests
and cancer prevention. J Natl Cancer Inst Monogr. 125–132.
1992.PubMed/NCBI
|
|
15
|
Guyton KZ and Kensler TW: Oxidative
mechanisms in carcinogenesis. Br Med Bull. 49:523–544. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schulte-Hermann R, Timmermann-Trosiener I,
Barthel G and Bursch W: DNA synthesis, apoptosis, and phenotypic
expression as determinants of growth of altered foci in rat liver
during phenobarbital promotion. Cancer Res. 50:5127–5135.
1990.PubMed/NCBI
|
|
17
|
Trush MA and Kensler TW: An overview of
the relationship between oxidative stress and chemical
carcinogenesis. Free Radic Biol Med. 10:201–209. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cerutti PA: Prooxidant states and tumor
promotion. Science. 227:375–381. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duracková Z: Some current insights into
oxidative stress. Physiol Res. 59:459–469. 2010.PubMed/NCBI
|
|
20
|
Zhang G, Miura Y and Yagasaki K:
Suppression of adhesion and invasion of hepatoma cells in culture
by tea compounds through antioxidative activity. Cancer Lett.
159:169–173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fridovich I: The biology of oxygen
radicals. Science. 201:875–880. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hussain SP, Hofseth LJ and Harris CC:
Radical causes of cancer. Nat Rev Cancer. 3:276–285. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lei XG, Zhu JH, Cheng WH, Bao Y, Ho YS,
Reddi AR, Holmgren A and Arnér ES: Paradoxical roles of antioxidant
enzymes: Basic mechanisms and health implications. Physiol Rev.
96:307–364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
25
|
Miyajima A, Nakashima J, Yoshioka K,
Tachibana M, Tazaki H and Murai M: Role of reactive oxygen species
in cis-dichlorodiammineplatinum-induced cytotoxicity on bladder
cancer cells. Br J Cancer. 76:206–210. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salganik RI, Albright CD, Rodgers J, Kim
J, Zeisel SH, Sivashinskiy MS and Van Dyke TA: Dietary antioxidant
depletion: Enhancement of tumor apoptosis and inhibition of brain
tumor growth in transgenic mice. Carcinogenesis. 21:909–914. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brown NS and Bicknell R: Hypoxia and
oxidative stress in breast cancer. Oxidative stress: Its effects on
the growth, metastatic potential and response to therapy of breast
cancer. Breast Cancer Res. 3:323–327. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sharma A, Rajappa M, Satyam A and Sharma
M: Oxidant/anti-oxidant dynamics in patients with advanced cervical
cancer: Correlation with treatment response. Mol Cell Biochem.
341:65–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oliveira CP, Kassab P, Lopasso FP, Souza
HP, Janiszewski M, Laurindo FR, Iriya K and Laudanna AA: Protective
effect of ascorbic acid in experimental gastric cancer: Reduction
of oxidative stress. World J Gastroenterol. 9:446–448. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Calvisi DF, Ladu S, Hironaka K, Factor VM
and Thorgeirsson SS: Vitamin E down-modulates iNOS and NADPH
oxidase in c-Myc/TGF-alpha transgenic mouse model of liver cancer.
J Hepatol. 41:815–822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azad N, Rojanasakul Y and Vallyathan V:
Inflammation and lung cancer: Roles of reactive oxygen/nitrogen
species. J Toxicol Environ Health B Crit Rev. 11:1–15. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fruehauf JP and Trapp V: Reactive oxygen
species: An Achilles' heel of melanoma? Expert Rev Anticancer Ther.
8:1751–1757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuku I, Aydogdu I, Bayraktar N, Kaya E,
Akyol O and Erkurt MA: Oxidant/antioxidant parameters and their
relationship with medical treatment in multiple myeloma. Cell
Biochem Funct. 23:47–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sumi D, Shinkai Y and Kumagai Y: Signal
transduction pathways and transcription factors triggered by
arsenic trioxide in leukemia cells. Toxicol Appl Pharmacol.
244:385–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van de Wetering CI, Coleman MC, Spitz DR,
Smith BJ and Knudson CM: Manganese superoxide dismutase gene dosage
affects chromosomal instability and tumor onset in a mouse model of
T cell lymphoma. Free Radic Biol Med. 44:1677–1686. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bahar G, Feinmesser R, Shpitzer T,
Popovtzer A and Nagler RM: Salivary analysis in oral cancer
patients: DNA and protein oxidation, reactive nitrogen species, and
antioxidant profile. Cancer. 109:54–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chan DW, Liu VW, Tsao GS, Yao KM, Furukawa
T, Chan KK and Ngan HY: Loss of MKP3 mediated by oxidative stress
enhances tumorigenicity and chemoresistance of ovarian cancer
cells. Carcinogenesis. 29:1742–1750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang M, Topalovski M, Toombs JE, Wright
CM, Moore ZR, Boothman DA, Yanagisawa H, Wang H, Witkiewicz A,
Castrillon DH and Brekken RA: Fibulin-5 blocks microenvironmental
ROS in pancreatic cancer. Cancer Res. 75:5058–5069. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shan W, Zhong W, Swanlund J and Oberley
TD: Oxidative stress in prostate cancer. Oxidative Stress Cancer
Biol Ther. 301–331. 2011.
|
|
40
|
Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM,
Wang H, Hale LP, Dong C, Cesarman E, Mesri EA and
Goldschmidt-Clermont PJ: Antitumorigenesis of antioxidants in a
transgenic Rac1 model of Kaposi's sarcoma. Proc Natl Acad Sci USA.
106:8683–8688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J and Yi J: Cancer cell killing via
ROS: To increase or decrease, that is the question. Cancer Biol
Ther. 7:1875–1884. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Klaunig JE, Xu Y, Isenberg JS, Bachowski
S, Kolaja KL, Jiang J, Stevenson DE and Walborg EF Jr: The role of
oxidative stress in chemical carcinogenesis. Environ Health
Perspect. 106 Suppl 1:S289–S295. 1998. View Article : Google Scholar
|
|
43
|
Jabs T: Reactive oxygen intermediates as
mediators of programmed cell death in plants and animals. Biochem
Pharmacol. 57:231–245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roos WP, Thomas AD and Kaina B: DNA damage
and the balance between survival and death in cancer biology. Nat
Rev Cancer. 16:20–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hayashida K, Sano M, Ohsawa I, Shinmura K,
Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, et
al: Inhalation of hydrogen gas reduces infarct size in the rat
model of myocardial ischemia-reperfusion injury. Biochem Biophys
Res Commun. 373:30–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Buchholz BM, Kaczorowski DJ, Sugimoto R,
Yang R, Wang Y, Billiar TR, McCurry KR, Bauer AJ and Nakao A:
Hydrogen inhalation ameliorates oxidative stress in transplantation
induced intestinal graft injury. Am J Transplant. 8:2015–2024.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shirahata S, Hamasaki T, Haramaki K,
Nakamura T, Abe M, Yan H, Kinjo T, Nakamichi N, Kabayama S and
Teruya K: Anti-diabetes effect of water containing hydrogen
molecule and Pt nanoparticles. BMC Proc. 5 Suppl 8:P182011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saitoh Y, Okayasu H, Xiao L, Harata Y and
Miwa N: Neutral pH hydrogen-enriched electrolyzed water achieves
tumor-preferential clonal growth inhibition over normal cells and
tumor invasion inhibition concurrently with intracellular oxidant
repression. Oncol Res. 17:247–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Asada R, Kageyama K, Tanaka H, Matsui H,
Kimura M, Saitoh Y and Miwa N: Antitumor effects of nano-bubble
hydrogen-dissolved water are enhanced by coexistent platinum
colloid and the combined hyperthermia with apoptosis-like cell
death. Oncol Rep. 24:1463–1470. 2010.PubMed/NCBI
|
|
50
|
Ren A, Liu R, Miao ZG, Zhang X, Cao PF,
Chen TX, Li CY, Shi L, Jiang AL and Zhao MW: Hydrogen-rich water
regulates effects of ROS balance on morphology, growth and
secondary metabolism via glutathione peroxidase in Ganoderma
lucidum. Environ Microbiol. 19:566–583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang B, Zhao Z, Meng X, Chen H, Fu G and
Xie K: Hydrogen ameliorates oxidative stress via PI3K-Akt signaling
pathway in UVB-induced HaCaT cells. Int J Mol Med. 41:3653–3661.
2018.PubMed/NCBI
|
|
52
|
Varga V, Németh J, Oláh O, Tóth-Szűki V,
Kovács V, Remzső G and Domoki F: Molecular hydrogen alleviates
asphyxia-induced neuronal cyclooxygenase-2 expression in newborn
pigs. Acta Pharmacol Sin. 22–Mar;2018.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun H, Chen L, Zhou W, Hu L, Li L, Tu Q,
Chang Y, Liu Q, Sun X, Wu M and Wang H: The protective role of
hydrogen-rich saline in experimental liver injury in mice. J
Hepatol. 54:471–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hattori Y, Kotani T, Tsuda H, Mano Y, Tu
L, Li H, Hirako S, Ushida T, Imai K, Nakano T, et al: Maternal
molecular hydrogen treatment attenuates lipopolysaccharide-induced
rat fetal lung injury. Free Radic Res. 49:1026–1037. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang N, Deng C, Zhang X, Zhang J and Bai
C: Inhalation of hydrogen gas attenuates airway inflammation and
oxidative stress in allergic asthmatic mice. Asthma Res Pract.
4:32018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fukuda K, Asoh S, Ishikawa M, Yamamoto Y,
Ohsawa I and Ohta S: Inhalation of hydrogen gas suppresses hepatic
injury caused by ischemia/reperfusion through reducing oxidative
stress. Biochem Biophys Res Commun. 361:670–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L
and Wang G: Protective effects of hydrogen gas on murine
polymicrobial sepsis via reducing oxidative stress and HMGB1
release. Shock. 34:90–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ohsawa I, Nishimaki K, Yamagata K,
Ishikawa M and Ohta S: Consumption of hydrogen water prevents
atherosclerosis in apolipoprotein E knockout mice. Biochem Biophys
Res Commun. 377:1195–1198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gu Y, Huang CS, Inoue T, Yamashita T,
Ishida T, Kang KM and Nakao A: Drinking hydrogen water ameliorated
cognitive impairment in senescence-accelerated mice. J Clin Biochem
Nutr. 46:269–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fujita K, Seike T, Yutsudo N, Ohno M,
Yamada H, Yamaguchi H, Sakumi K, Yamakawa Y, Kido MA, Takaki A, et
al: Hydrogen in drinking water reduces dopaminergic neuronal loss
in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of
Parkinson's disease. PLoS One. 4:e72472009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kitamura A, Kobayashi S, Matsushita T,
Fujinawa H and Murase K: Experimental verification of protective
effect of hydrogen-rich water against cisplatin-induced
nephrotoxicity in rats using dynamic contrast-enhanced CT. Br J
Radiol. 83:509–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhao W and Robbins ME: Inflammation and
chronic oxidative stress in radiation-induced late normal tissue
injury: Therapeutic implications. Curr Med Chem. 16:130–143. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kang KM, Kang YN, Choi IB, Gu Y, Kawamura
T, Toyoda Y and Nakao A: Effects of drinking hydrogen-rich water on
the quality of life of patients treated with radiotherapy for liver
tumors. Med Gas Res. 1:112011. View Article : Google Scholar : PubMed/NCBI
|