Introduction
Gastric cancer (GC) is the second most common cause
of cancer-associated mortality worldwide. There are ~100,0000 new
cases of GC annually, and >700,000 cases of GC-associated
mortality (1). It is reported that
~50% of patients with GC exhibit metastasis at the time of
diagnosis (2). Therefore, it is
important to identify novel early diagnostic markers and
therapeutic targets of tumor invasion and metastasis.
Long non-coding RNAs (lncRNAs) have been identified
as a class of non-protein-coding transcripts, which are 200
nucleotides in length. Previous studies have reported that the
abnormal expression of lncRNAs is significantly involved in cancer
development and progression (3,4). In GC,
the overexpression of lncRNA SPRY4-IT1 promotes tumor cell
proliferation and invasion by activating enhancer of zeste homolog
2 in hepatocellular carcinoma (HCC) (5). The decreased expression of lncRNA
LINC00261 predicts a poor prognosis in patients with GC and
suppresses tumor metastasis by affecting the epithelial-mesenchymal
transition (6). The overexpression of
metastasis-associated lung adenocarcinoma transcript 1 promotes
cell invasion and metastasis of GC by increasing the expression of
epidermal growth factor-like domain 7 (7). The increased expression of lncRNA
taurine upregulated 1 also predicts a poor prognosis of GC and
regulates cell proliferation by epigenetically silencing p57
(8).
Previous studies have shown that TP73-AS1 is
involved in tumor progression. For example, the silencing of lncRNA
TP73-AS1 inhibits cell proliferation and induces apoptosis in
esophageal squamous cell carcinoma (9). The TP73-AS1 interacts with microRNA
(miR)-142 to modulate brain glioma growth through the high mobility
group box 1 (HMGB1)/receptor for advanced glycation end products
(RAGE) pathway (10). lncRNA TP73-AS1
modulates HCC cell proliferation through miR-200a-dependent
HMGB1/RAGE regulation (11); however,
the expression and molecular mechanism underlying lncRNA TP73-AS1
in GC remains to be elucidated.
In the present study, it was shown that TP73-AS1 was
upregulated in GC tissues. The higher expression of TP73-AS1 was
associated with poor prognosis in patients with GC. The
downregulation of TP73-AS1 suppressed cell proliferation and
invasion. Additionally, the knockdown of TP73-AS1 suppressed the
WNT/β-catenin signaling pathway in GC. These results indicated that
TP73-AS1 may be a target for GC treatment.
Materials and methods
Patient tissue specimens
A total of 64 paired human GC tissue specimens and
corresponding adjacent normal tissues were obtained from patients
who underwent surgical resection between January 2010 and March
2013 in the Department of General Surgery of Shanghai Tongji
Hospital (Shanghai, China). All GC tissue specimens were
immediately snap-frozen in liquid nitrogen until total RNA
extraction. Informed consent was obtained from all patients and the
study was approved by the Medical Ethics Committee of Shanghai
Tongji Hospital.
Cell culture
The human GC, MGC-803, the BGC-823 cell lines, and a
normal gastric epithelium cell line (GES-1) were purchased from the
Shanghai Institute of Biochemistry and Cell Biology (Shanghai,
China). All cells were cultured in DMEM supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 5%
CO2 at 37°C.
Cell transfection
The small interfering RNA si-TP73-AS1-1 sense,
5′-GATCGCGTTCTGTGTGGAACTTACTGGATCAAGAGTCCAGTAAGTTCCACACAGAATTTTTTCCAAA-3′
and antisense,
5′-AGCTTTTGGAAAAAATTCTGTGTGGAACTTACTGGACTCTTGATCCAGTAAGTTCCACACAGAACGC-3′;
si-TP73-AS1-2 sense,
5′-GATCGCGTAGACAGAGGTCATCAGCCAGTCAAGAGCTGGCTGATGACCTCTGTCTATTTTTTCCAAA-3′
and antisense,
5′-AGCTTTTGGAAAAAATAGACAGAGGTCATCAGCCAGCTCTTGACTGGCTGATGACCTCTGTCTACGC-3′;
and si-negative control (NC) were synthesized and purchased from
GenePharma Co. Ltd. (Shanghai, China). Cell transfection was
performed using Lipofectamine™ 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The GC tissues and cells were used to extract total
RNA using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
The RNA was measured using the Nano Drop 2000 Spectrophotometer
(Nano Drop Technologies; Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA) and the ratio of absorbance was at 260 to 280
nm (260/280 ratio). The 1 µg RNA samples were transcribed to cDNA
using a First Strand cDNA Synthesis kit (Tiangen Biotech Co., Ltd.,
Beijing, China). The reverse transcription reaction condition was
as follows: 37°C for 15 min, 85°C for 5 sec and 4°C for 2 min. The
mRNA was detected using TaqMan MicroRNA PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China) on ABI 7500 fast system
(Applied Biosystems; Thermo Fisher Scientific, Inc., Carlsbad, CA,
USA). To quantify the mRNA expression, the SYBR Premix Ex Taq
(Takara Biotechnology Co., Ltd., Dalian, China) was used to
amplifying the mRNA expression. The qRT-PCR reaction conditions
were as follows: 95°C for 30 sec, followed by 40 cycles at 95°C for
5 sec and 60°C for 34 sec. GAPDH was used as the internal control.
The RT-qPCR results were calculated using the 2−∆∆Cq
method (12). The primer sequences
were as follows: LnRNATP73-AS1 forward, 5′-CCGGTTTTCCAGTTCTTGCAC-3′
and reverse, 5′-GCCTCACAGGGAAACTTCATGC-3′; GAPDH forward,
5′-GGTGAAGGTCGGAGTCAACG-3′ and reverse,
5′-CAAAGTTGTCATGGATGHACC-3′.
Cell proliferation and cell colony
formation assay
Cell proliferation was evaluated with Cell Counting
kit-8 (CCK-8) solution (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) in accordance with the manufacturer's protocol.
Briefly, the MGC-803 and BGC-823 cells (2×103
cells/well) were seeded into 96-well plates in triplicate. The
proliferative activity of cells was detected at 0, 24, 48, and 72
h, and 10 µl of CCK-8 solution was added to each well and cultured
for 2 h at room temperature. Subsequently, cellular proliferation
was detected at the absorbance of the converted dye at 450 nm. For
the cell colony assay, the MGC-803 and BGC-823 cells (200
cells/well) were seeded into 6-well plates in triplicate. The cells
were cultured for 3 weeks at 37°C in 5% CO2. The cells
were then fixed with 100% methanol for 20 min at room temperature
and stained with 0.1% crystal violet for 10 min at room
temperature, and the numbers of cell colonies were calculated under
a light microscope (DP72; Olympus Corporation, Tokyo, Japan).
Cell invasion assay
The cell invasion assays were performed using
Transwell chambers with a pore size of 8-µm (Corning Incorporated,
Cambridge, MA, USA) and coated with Matrigel (BD Biosciences, San
Jose, CA, USA). A total of 5×104 transfected MGC-803 and
BGC-823 cells were resuspended in 300 µl of medium and seeded in
the upper chamber, with 500 µl of medium supplemented with 10% FBS
added to the lower chamber. Following culture of the cells for 24
h, the cells in the lower chamber were fixed with methanol for 10
min (Beyotime Institute of Biotechnology, Shanghai, China) and
stained with 0.1% crystal violet (Beyotime Institute of
Biotechnology) for 10 min. The cells were counted under an inverted
microscope in five randomly selected fields (Olympus Corporation,
Tokyo, Japan).
Western blot analysis
The cells were lysed with RIPA buffer (Beyotime
Institute of Biotechnology). Protein quantity was detected using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology),
according to the manufacture's protocol. The cell protein lysates
(40 µg) were separated via 10% SDS-polyacrylamide gel
electrophoresis and transferred onto PVDF membranes (EMD Millipore,
Billerica, MA, USA). The PVDF membranes were blocked with 5%
skimmed milk for 2 h at room temperature. The PVDF membranes were
then incubated with the following specific antibodies:
Transcription factor 4 (TCF4; cat. no. 2566; 1:1,000 dilution);
β-catenin (cat. no. 9562; 1:1,000 dilution); and GAPDH (cat. no.
5174; 1:3,000 dilution; all Cell Signaling Technology, Inc.,
Beverly, MA, USA) overnight at 4°C. Subsequently, the secondary
antibody (goat anti-rabbit IgG; cat. no. sc-2004; 1:1,000 dilution;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was added to
the PVDF membranes and for incubated for 1.5 h at room temperature.
The blots were determined using a chemiluminescence detection kit
(GE Healthcare Life Sciences, Piscataway, NJ, USA). The gray bands
were analyzed using Image J software (version 1.46; National
Institutes of Health, Bethesda, MD, USA). The GAPDH protein
expression was used as the internal control.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). All data are
shown as the mean ± standard deviation. Comparison of means between
different samples was analyzed using Student's t-test and one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of TP73-AS1 is upregulated
in GC tissues
The present study first detected the expression of
TP73-AS1 in 64 paired human GC tissue specimens and corresponding
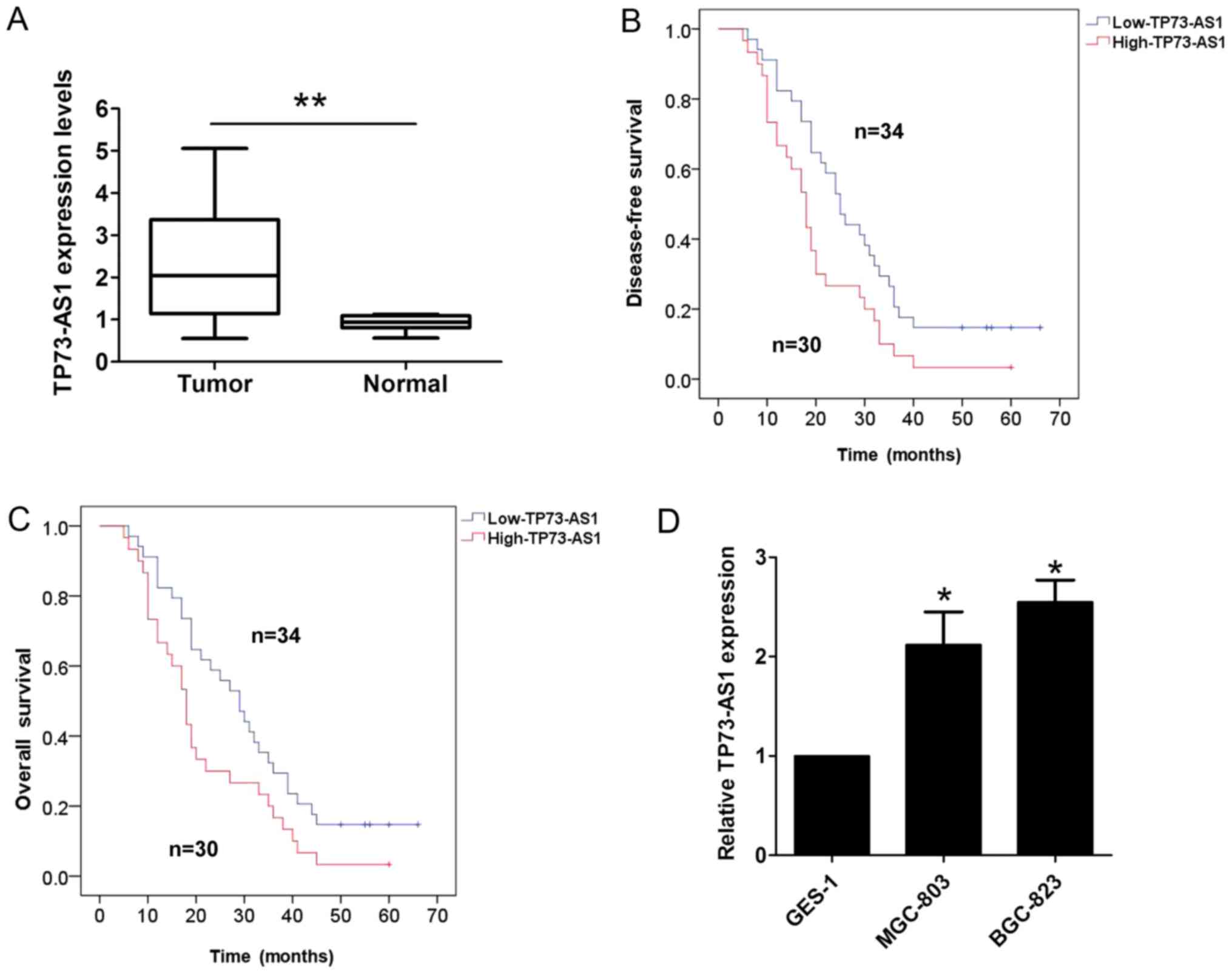
adjacent normal tissues using RT-qPCR analysis. As shown in
Fig. 1A, the expression of TP73-AS1
was higher in the GC tissues than the corresponding adjacent normal
tissues. Furthermore, the association between the expression of
TP73-AS1 and clinicopathologic factors in patients with GC was
evaluated. The results of the statistical analysis showed that a
higher expression of TP73-AS1 was correlated with lymph node
metastasis (P=0.001) and tumor-node-metastasis (TNM) stage
(P=0.003; Table I); however, no
association existed with other clinicopathologic features,
including sex, age and tumor size (P>0.05; Table I). The results of the Kaplan-Meier
analysis indicated that a higher expression of TP73-AS1 in patients
with GC predicted a shorter disease-free survival (DFS; log
rank=5.412, P<0.05; Fig. 1B) and
overall survival (OS; log rank=4.506, P<0.05; Fig. 1C). In addition, it was demonstrated
that the expression of TP73-AS1 was significantly higher in two GC
cell lines (MGC-803 and BGC-823), compared with that in the normal
gastric epithelium cell line (GES-1; Fig.
1D).
 | Table I.Association between the expression of
TP73-AS1 and the clinicopathological factors of 64 patients with
gastric cancer. |
Table I.
Association between the expression of
TP73-AS1 and the clinicopathological factors of 64 patients with
gastric cancer.
|
| Expression of
TP73-AS1 |
|---|
|
|
|
|---|
| Clinicopathologic
factor | Patients (n) | Lower (n=34) | Higher (n=30) | P-value |
|---|
| Sex |
|
|
| 0.223 |
| Male | 42 | 20 | 22 |
|
|
Female | 22 | 14 | 8 |
|
| Age (years) |
|
|
| 0.934 |
| ≤55 | 43 | 23 | 20 |
|
|
>55 | 21 | 11 | 10 |
|
| Tumor size |
|
|
| 0.924 |
| <3
cm | 38 | 20 | 18 |
|
| >3
cm | 26 | 14 | 12 |
|
| Histological
differentiation |
|
|
| 0.375 |
| High,
middle | 39 | 23 | 16 |
|
| Low | 25 | 11 | 14 |
|
| Lymph node
metastasis |
|
|
|
|
| No | 36 | 26 | 10 | 0.001a |
| Yes | 28 | 8 | 20 |
|
| Local invasion |
|
|
| 0.090 |
|
T1-T2 | 37 | 23 | 14 |
|
|
T3-T4 | 27 | 11 | 16 |
|
| TNM stage |
|
|
| 0.003a |
| I/II | 38 | 26 | 12 |
|
|
III/IV | 26 | 8 | 18 |
|
Downregulation of the expression of
TP73-AS1 decreases cell proliferation in GC
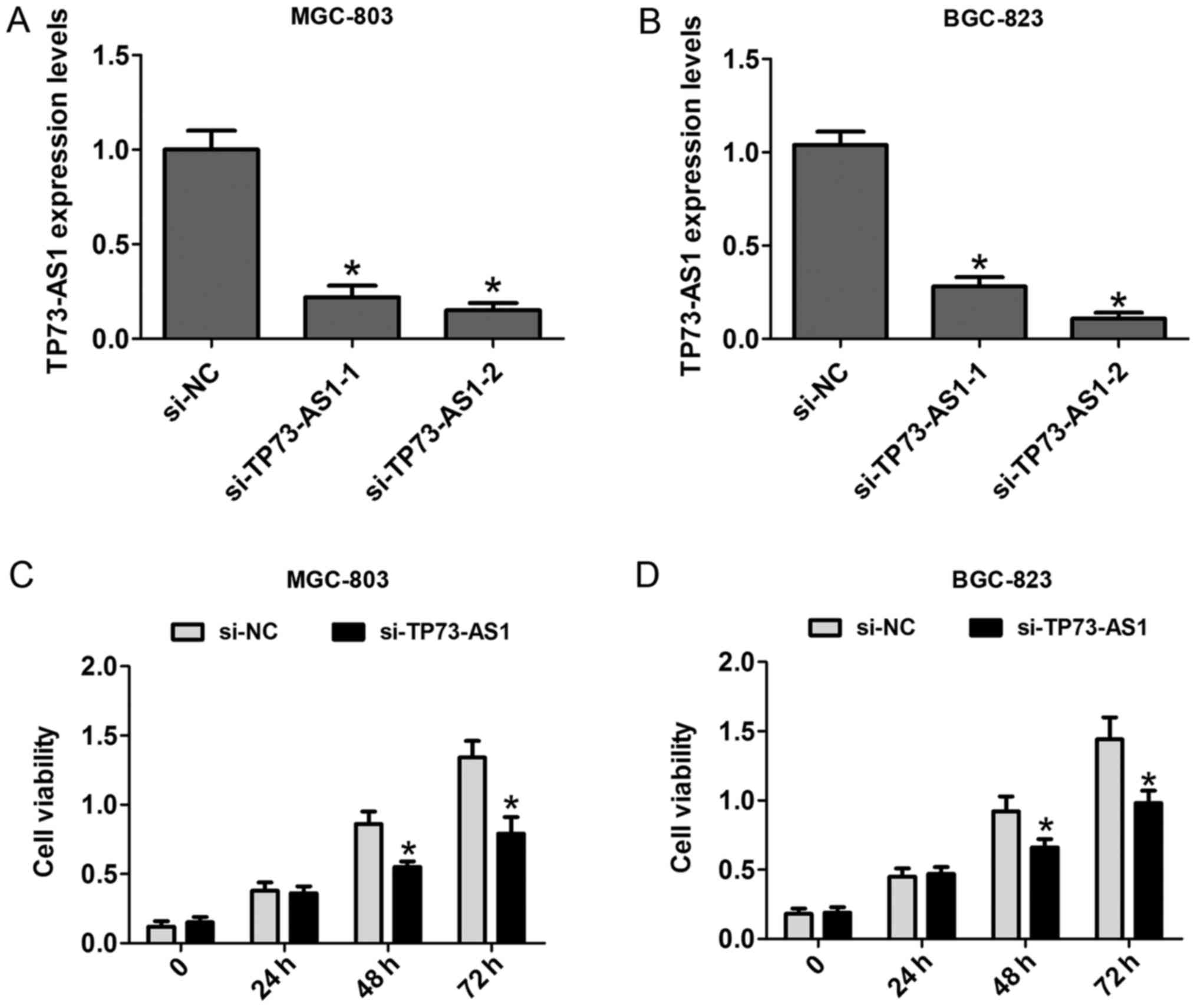
To clarify the functional effects of the expression
of TP73-AS1 on cell proliferation, CCK-8 and cell colony formation
assays were performed. First, two siRNAs against TP73-AS1 were
applied to knock down TP73-AS1 in MGC-803 and BGC-823 cells. The
results showed that si-TP73-AS1-2 had a higher silencing efficiency
than TP73-AS1-2, and was selected for use in the following
experiments (Fig. 2A and B). The
results of the CCK-8 assays showed that the cell proliferation rate
was significantly reduced at 48 and 72 h in the MGC-803 and BGC-823
cells following knockdown (Fig. 2C and
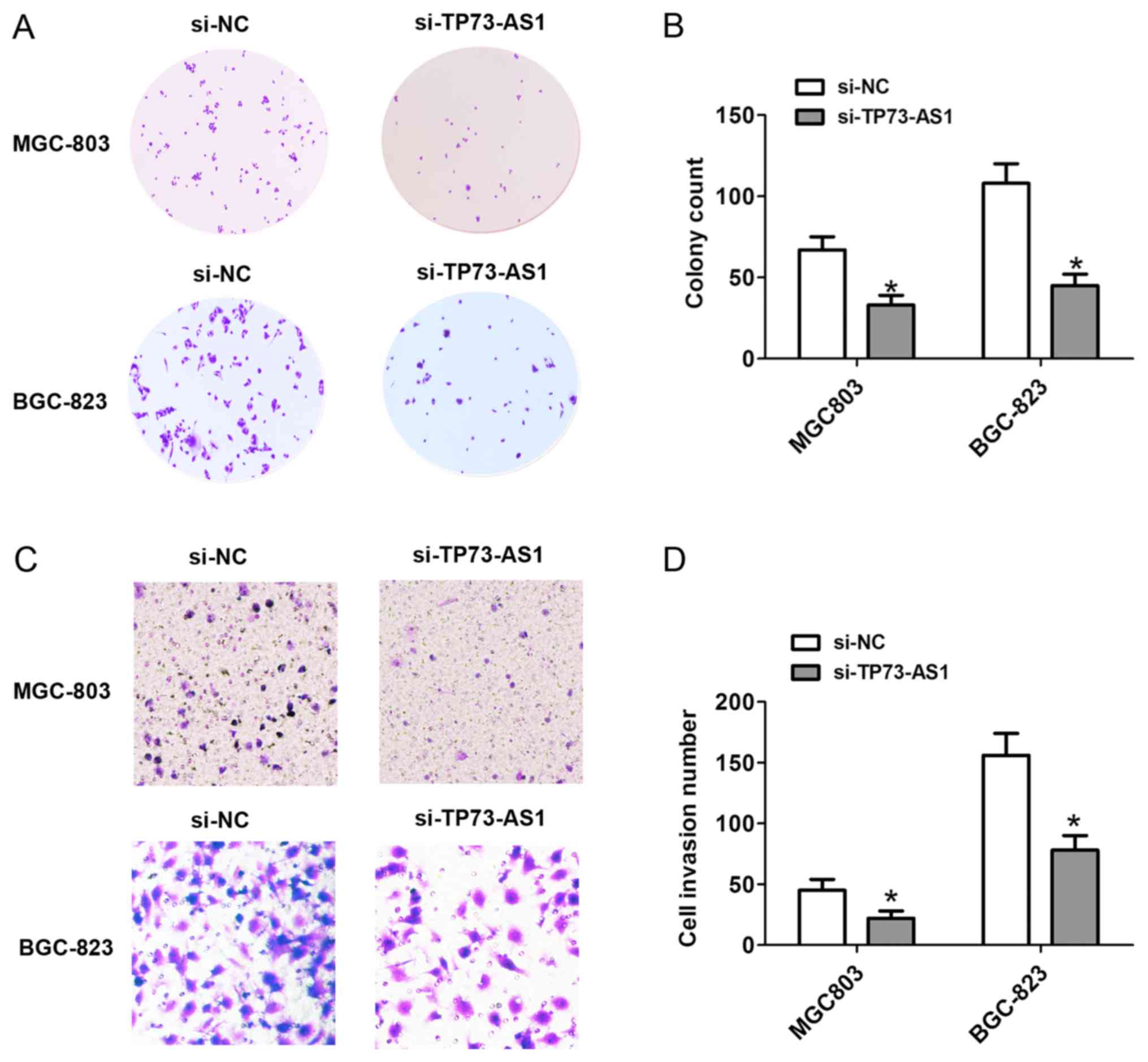
D). Additionally, the number of cell colonies following cell
transfection with si-TP73-AS1 on day 14 was markedly reduced in the
MGC-803 and BGC-823 cells (Fig. 3A and
B). These results showed that the downregulation of TP73-AS1
decreased the proliferation of GC cells.
Downregulation of the expression of
TP73-AS1 decreases the invasion of GC cells
To detect the effects of the expression of TP73-AS1
on cell invasion, Transwell invasion assays were performed.
Following cell transfection with si-NC or si-TP73-AS1 for 48 h, the
cell invasion was significantly decreased, compared with that in
the si-NC group in the MGC-803 and BGC-823 cells (Fig. 3C and D). Therefore, downregulation of
the expression of TP73-AS1 decreased cell invasion in GC.
Downregulation of the expression of
TP73-AS1 inhibits the WNT/β-catenin signaling pathway in GC
cells
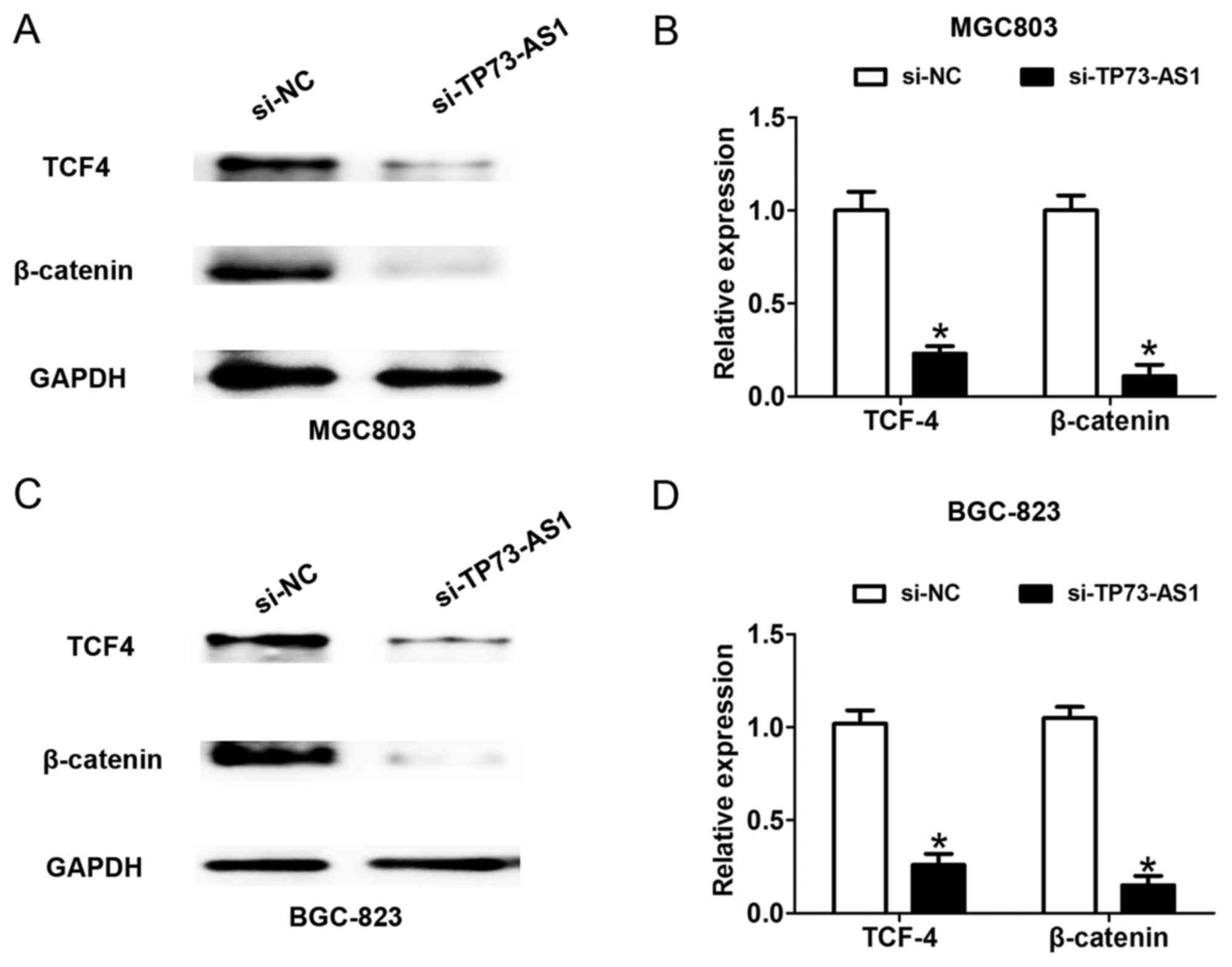
According to the above findings, a higher level of
TP73-AS1 contributed to the progression of GC. The present study
examined the potential mechanism by which TP73-AS1 regulates GC
cell proliferation and invasion. The WNT/β-catenin signaling
pathway is significantly associated with GC cell proliferation and
invasion (13). Following cell
transfection with si-NC or si-TP73-AS1 for 48 h, the downregulated
expression of TP73-AS1 suppressed the protein expression of TCF4
and β-catenin in the MGC-803 and BGC-823 cells (Fig. 4A-D), which suggested that the
knockdown of TP73-AS1 suppressed the WNT/β-catenin signaling
pathway in GC.
Discussion
Deregulation of the expression of lncRNA in various
types of cancer is essential for tumorigenesis and development.
Previously, studies have demonstrated that lncRNAs may be applied
to serve as prognostic markers and therapeutic targets in tumors.
In GC, the lncRNA, highly upregulated in liver cancer, acts as a
novel serum biomarker for the diagnosis and prognostic prediction
of GC (14). The overexpression of
lncRNA homeobox A transcript at the distal tip enhances tumor
invasion and predicts poor prognosis in GC (15). The plasma level of H19 is also
significantly higher in patients with GC and serves as a potential
biomarker for diagnosis (16). The
decreased expression of the lncRNA LINC00261 indicates poor
prognosis in GC and suppresses GC metastasis by affecting the
epithelial-mesenchymal transition (6). In the present study, it was shown that
TP73-AS1 was upregulated in GC tissues compared with adjacent
normal tissues. The higher expression if TP73-AS1 was associated
with lymph node metastasis, TNM stage, and poor DFS and OS rates in
patients with GC. Therefore, TP73-AS1 functions as a biomarker for
predicting the prognosis of patients with GC.
Previous studies have indicated that decreased
TP73-AS1 inhibits cell proliferation and induces apoptosis in
esophageal squamous cell carcinoma (9). The knockdown of TP73-AS1, an lncRNA of
one triplet, not only reduced the expression of regulatory factor
×1, an mRNA of this triplet, but also induced apoptosis in U87
cells (17). In the present study, it
was demonstrated that the knockdown of TP73-AS1 suppressed cell
proliferation, cell colony formation and cell invasion, which
suggested that TP73-AS1 exerts a tumor-promoting function in GC
cells. Studies have found that the Wnt/β-catenin signaling pathway
is involved in oncogenesis, including the initiation and
progression of GC (18).
Wnt/β-catenin signaling enhances the transcriptional activity of
cyclooxygenase-2 in GC cells (19),
and aquaporin 3 promotes the stem-like properties of GC cells via
the Wnt/glycogen synthase kinase-3β/β-catenin pathway (20). In the present study, downregulation of
the expression of TP73-AS1 suppressed the expression of TCF4 and
β-catenin in GC cells, which suggested that the reduction in
TP73-AS1 suppressed the WNT/β-catenin signaling pathway in GC.
These results indicated that TP73-AS1 promotes cell proliferation
and invasion via regulation of the WNT/β-catenin signaling pathway
in GC.
In conclusion, the present study showed that the
expression of TP73-AS1 was higher in GC, and that the knockdown of
TP73-AS1 suppressed cell proliferation and invasion. Furthermore,
the knockdown of TP73-AS1 suppressed the WNT/β-catenin signaling
pathway in GC. These results indicated that TP73-AS1 may be a
therapeutic target for GC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, BW and SX analyzed and interpreted the patient
data. QC and YL conceived and designed the study. YW, BW and SX
performed the experiments. QC rafted the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Shanghai Tongji Hospital and informed consent was
obtained from all participants.
Patient consent for publication
Informed consent was obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohtsu A: Chemotherapy for metastatic
gastric cancer: Past, present, and future. J Gastroenterol.
43:256–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y and Wang X: Role of long noncoding
RNAs in malignant disease (Review). Mol Med Rep. 13:1463–1469.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou M, Zhang XY and Yu X: Overexpression
of the long non-coding RNA SPRY4-IT1 promotes tumor cell
proliferation and invasion by activating EZH2 in hepatocellular
carcinoma. Biomed Pharmacother. 85:348–354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan Y, Wang YF, Su HF, Fang N, Zou C, Li
WF and Fei ZH: Decreased expression of the long noncoding RNA
LINC00261 indicate poor prognosis in gastric cancer and suppress
gastric cancer metastasis by affecting the epithelial-mesenchymal
transition. J Hematol Oncol. 9:572016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deng QJ, Xie LQ and Li H: Overexpressed
MALAT1 promotes invasion and metastasis of gastric cancer cells via
increasing EGFL7 expression. Life Sci. 157:38–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang E, He X, Yin D, Han L, Qiu M, Xu T,
Xia R, Xu L, Yin R and De W: Increased expression of long noncoding
RNA TUG1 predicts a poor prognosis of gastric cancer and regulates
cell proliferation by epigenetically silencing of p57. Cell Death
Dis. 7:e21092016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zang W, Wang T, Wang Y, Chen X, Du Y, Sun
Q, Li M, Dong Z and Zhao G: Knockdown of long non-coding RNA
TP73-AS1 inhibits cell proliferation and induces apoptosis in
esophageal squamous cell carcinoma. Oncotarget. 7:19960–19974.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang R, Jin H and Lou F: The long
non-coding RNA TP73-AS1 interacted with miR-142 to modulate brain
glioma growth through HMGB1/RAGE pathway. J Cell Biochem.
119:3007–3016. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li S, Huang Y, Huang Y, Fu Y, Tang D, Kang
R, Zhou R and Fan XG: The long non-coding RNA TP73-AS1 modulates
HCC cell proliferation through miR-200a-dependent HMGB1/RAGE
regulation. J Exp Clin Cancer Res. 36:512017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu F, Li J, Guo N, Wang XH and Liao YQ:
MiRNA-27a promotes the proliferation and invasion of human gastric
cancer MGC803 cells by targeting SFRP1 via Wnt/β-catenin signaling
pathway. Am J Cancer Res. 7:405–416. 2017.PubMed/NCBI
|
|
14
|
Jin C, Shi W, Wang F, Shen X, Qi J, Cong
H, Yuan J, Shi L, Zhu B, Luo X, et al: Long non-coding RNA HULC as
a novel serum biomarker for diagnosis and prognosis prediction of
gastric cancer. Oncotarget. 7:51763–51772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye H, Liu K and Qian K: Overexpression of
long noncoding RNA HOTTIP promotes tumor invasion and predicts poor
prognosis in gastric cancer. Onco Targets Ther. 9:2081–2088.
2016.PubMed/NCBI
|
|
16
|
Zhou X, Yin C, Dang Y, Ye F and Zhang G:
Identification of the long non-coding RNA H19 in plasma as a novel
biomarker for diagnosis of gastric cancer. Sci Rep. 5:115162015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang JB, Liu FH, Chen JH, Ge HT, Mu LY,
Bao HB and Lin ZG: Identifying survival-associated modules from the
dysregulated triplet network in glioblastoma multiforme. J Cancer
Res Clin Oncol. 143:661–671. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nuñez F, Bravo S, Cruzat F, Montecino M
and De Ferrari GV: Wnt/β-catenin signaling enhances
cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer
cells. PLoS One. 6:e185622011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Wang Y, Wen J, Zhao H, Dong X,
Zhang Z, Wang S and Shen L: Aquaporin 3 promotes the stem-like
properties of gastric cancer cells via Wnt/GSK-3β/β-catenin
pathway. Oncotarget. 7:16529–16541. 2016.PubMed/NCBI
|