Introduction
Low-grade myofibroblastic sarcoma (LGMS) is a rare
sarcoma tumor. Atypical myofibroblasts have been shown to be
involved in LGMS, such as in the femur (1), vulva (2)
and phalanx (3). In the head and neck
region, LGMS frequently occurs in the tongue (4). Myofibroblasts, which show
characteristics of both fibroblasts and smooth muscle cells, were
firstly identified in granulation tissue in 1971 (5). Fibroblasts differentiate into
myofibroblasts during wound healing and tissue repair (6). According to the 2013 World Health
Organization (WHO) classification (7), LGMS is categorized as a
fibroblastic/myofibroblastic tumor; intermediate tumors (rarely
metastasizing). Clinically, LGMS has a propensity for local
recurrence and is associated with a low risk of metastatic spread.
Immunohistochemically, LGMS tumor cells are positive for at least
one myogenic marker, such as alpha-smooth muscle actin (α-SMA),
desmin or muscle actin (HHF-35). As no definitive markers of LGMS
have been found to date, it is difficult to make a diagnosis based
on the pathological findings alone. In addition, whether or not the
genetic mutations of cancer-related genes shown in sarcomas are
involved in LGMS is unclear.
In the present study, we carried out
immunohistochemical analyses in LGMS arising in the tip of the
tongue in a sporadic case, examined genomic rearrangements, such as
SS18-SSXs (SS18-SSX1, SS18-SSX2, SS18-SSX4, SS18-SSX4V,
SS18p-SSXp or SS18-SSXp) and MYH9-USP6s
(MYH9 exon 1 and USP6 exon 1 or MYH9 exon 1
and USP6 exon 2) for differential diagnostic supports and
investigated whether or not these LGMS tumor cells harbored point
mutations in the APC, CTNNB1, EGFR, KRAS, PIK3CA and
p53 genes for detection of the malignant tumor behavior.
Case report
A 38-year-old woman visited at the Department of
Oral and Maxillofacial Surgery at Kyushu University Hospital
(Fukuoka, Japan) with a 2-month history of a painless mass showing
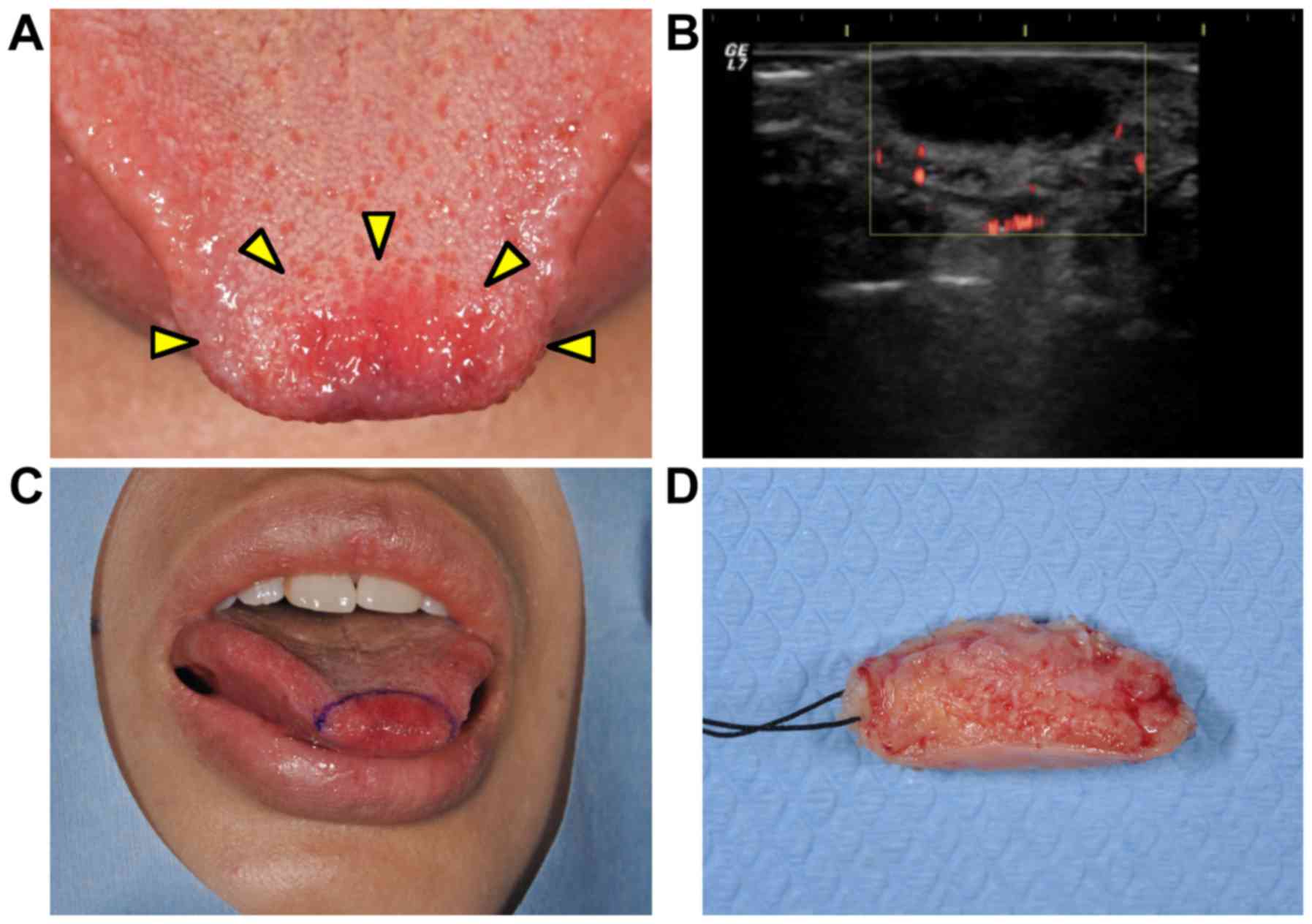
gradual growth in the tip of the tongue (Fig. 1A). An oral examination revealed that
the tumorous mass was soft-elastic and well-circumscribed, and the
overlying mucosal surface showed redness and a normal texture.
There was no clinical evidence of cervical lymphadenopathy. She had
a medical history of idiopathic thrombocytopenic purpura (cured)
and fibroma of her right breast (under observation). An
ultrasonogram examination revealed that the mass had a hypoechoic
area with undetectable vascularization (Fig. 1B). Based on these findings, the
tumorous mass was clinically diagnosed as not a malignant tumor. An
excisional biopsy was performed (Fig. 1C
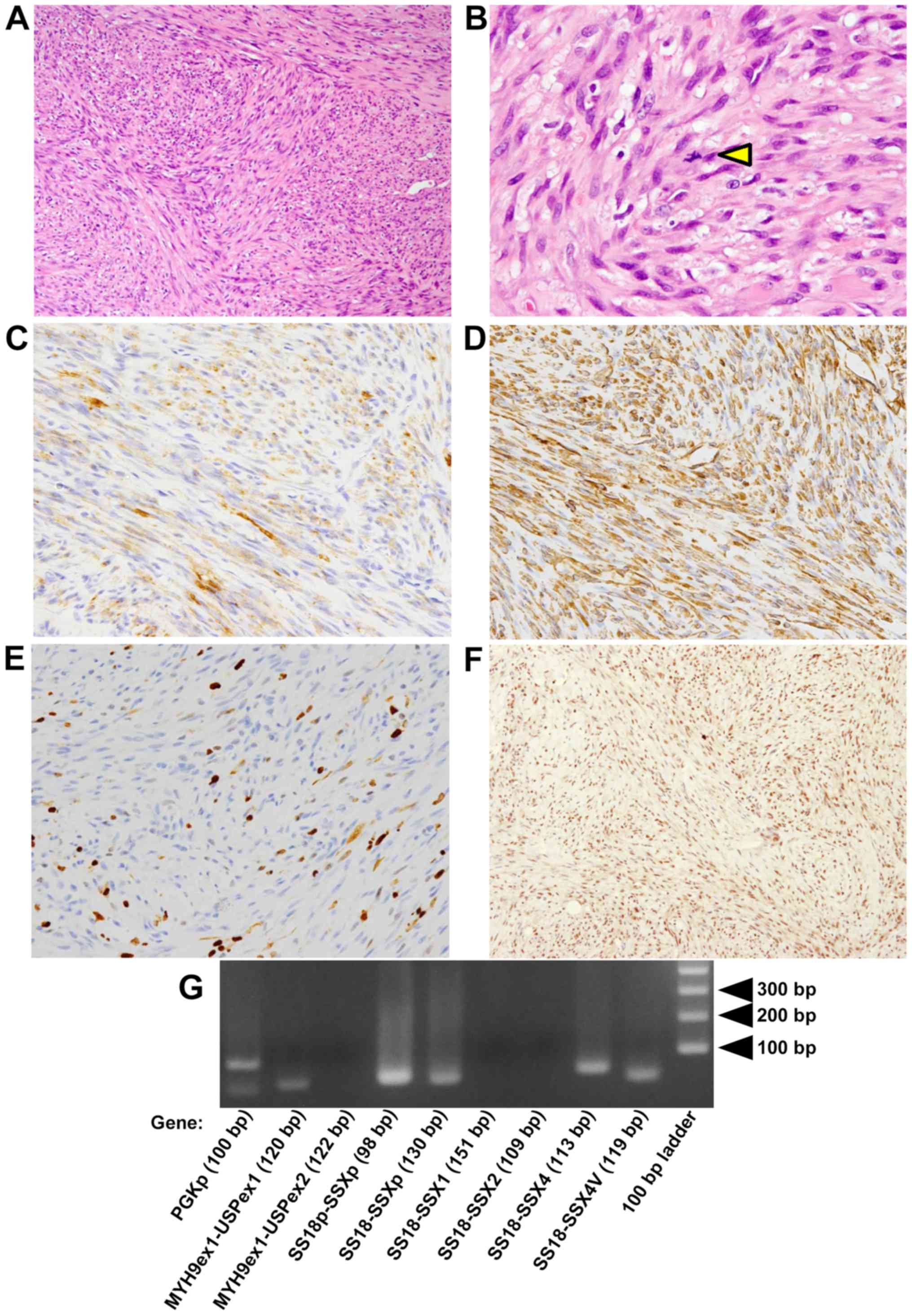
and D), and the mass showed proliferation of spindle-shaped
cells with fascicular and/or storiform patterns in the
subepithelial nodular tumor lesion (Fig.
2A). Nuclear pleomorphism was shown in the tumor cells. The
number of mitotic figures was <1 per 10 high-power fields
(Fig. 2B).
Immunohistochemical examination was performed as
described below. Paraformaldehyde-fixed paraffin-embedded (PFPE)
tissues were cut at 4-µm thickness. Immunohisto-chemical staining
was performed using BondIII (Leica Microsystems GmbH, Wetzlar,
Germany) according to the manufacturer's instructions in the
Division of Diagnostic Pathology in Kyushu University Hospital. The
following monoclonal antibodies were used: Mouse anti-AE1/AE3
(1:100; cat. no. M 3515; Agilent Technologies, Inc., Santa Clara,
CA, USA), mouse anti-EMA (1:100; cat. no. M 0613; Agilent
Technologies, Inc.), mouse anti-CAM5.2 (1:100; cat. no. 349205; BD
Biosciences, Franklin Lakes, NJ, USA), mouse anti-α-SMA (1:500;
cat. no. M 0851; Agilent Technologies, Inc.), mouse anti-CD34
(1:1,000; cat. no. PA0212; Leica Biosystems, Wetzlar, Germany),
mouse anti-MIB-1 (1:100; cat. no. M 7240; Agilent Technologies,
Inc.), and mouse anti-vimentin (1:5; cat. no. 760-2512; Ventana
Medical Systems, Inc., Tucson, AZ, USA). Immunohistochemical
staining was also performed with the universal immunoperoxidase
polymer method (Envision-kit; Agilent Technologies, Inc.) using
monoclonal mouse anti-BAF47 (INI1 gene product) (1:250; cat. no.
612110; BD Biosciences) antibody. Immunohistochemical analyses
showed that the spindle-shaped cells were positive for α-SMA
(Fig. 2C) and vimentin (Fig. 2D) and negative for epithelial markers;
AE1/AE3, EMA and CAM5.2 (data not shown). The MIB-l (monoclonal
antibody against Ki-67; cell proliferation marker) labeling index
was 10% in the hot spot (Fig. 2E).
Almost all spindle-shaped cells were positive for BAF47 similar to
the cells in non-tumor tissues, such as inflammatory cells and
endothelial cells (Fig. 2F).
In addition, genomic rearrangements, such as
SS18-SSXs (SS18-SSX1, SS18-SSX2, SS18-SSX4, SS18-SSX4V,
SS18p-SSXp or SS18-SSXp) and MYH9-USP6s
(MYH9 exon 1 and USP6 exon 1 or MYH9 exon 1
and USP6 exon 2), for differential diagnostic supports, were
not detected (Fig. 2G). Total RNA was
extracted from PFPE sections with the RNeasy FFPE kit (Qiagen GmbH,
Hilden, Germany) according to the manufacturer's instructions. Five
micrograms of RNA was reverse-transcribed using ReverTra Ace
transcriptase (Toyobo Life Science, Osaka, Japan) in order to
prepare the first-strand cDNA. Each polymerase chain reaction (PCR)
product (10 µl) was directly loaded onto 2% agarose gel, stained
with ethidium bromide, and directly visualized under UV
illumination. Amplification of 100 base-pair (bp) products of PGKp
was positive control of this PCR. Forward and reverse primers and
annealing temperatures were summarized in Table I.
 | Table I.Forward and reverse primers for
genomic rearrangement analysis. |
Table I.
Forward and reverse primers for
genomic rearrangement analysis.
| Gene | Primer sets | Product size
(bp) |
|---|
| SS18-SSX1 | F:
5′-AGACCAACACAGCCTGGACCAC-3′ | 151 |
|
| R:
5′-GGTGCAGTTGTTTCCCATCG-3′ |
|
| SS18-SSX2 | F:
5′-AGACCAACACAGCCTGGACCAC-3′ | 109 |
|
| R:
5′-GCACTTCCTCCGAATCATTTC-3′ |
|
| SS18-SSX4 | F:
5′-AGACCAACACAGCCTGGACCAC-3′ | 113 |
|
| R:
5′-TCTGGCACTTCCTTCAAACC-3′ |
|
| SS18-SSX4V | F:
5′-AGACCAACACAGCCTGGACCAC-3′ | 119 |
|
| R:
5′-CCAGCTGCTTTCTCTTACGC-3′ |
|
| SS18p-SSXp | F:
5′-CCAGCAGAGGCCTTATGGATA-3′ | 98 |
|
| R:
5′-TTTGTGGGCCAGATGCTTC-3′ |
|
| SS18-SSXp | F:
5′-AGACCAACACAGCCTGGACCAC-3′ | 130 |
|
| R:
5′-TTTGTGGGCCAGATGCTTC-3′ |
|
| MYH9 (exon 1)-USP6
(exon 1) | F:
5′-GGGGCAGATCCAGGTTCAG-3′ | 120 |
|
| R:
5′-GAAACTGGGCATCTCTGTGGC-3′ |
|
| MYH9 (exon 1)-USP6
(exon 2) | F:
5′-GGGGCAGATCCAGGTTCAG-3′ | 122 |
|
| R:
5′-GATGGACATGGTAGAGAATGC-3′ |
|
Furthermore, PCR amplification and sequencing
analysis were carried out for assessing mutations. We extracted
genomic DNAs from macrodissected PFPE sections using the WaxFree™
Paraffin Sample DNA Extraction kit (Trimgen, Sparks Glencoe, MD,
USA) according to the manufacturer's instructions (8). The mutational status of APC,
CTNNB1 (9), EGFR (10), KRAS, PIK3CA (11) and p53 (12) genes was investigated using a PCR and
direct sequencing, and no genetic mutations of these cancer-related
genes were detectable. In Table II,
forward and reverse primers for indicated exons in this sequencing
analysis and annealing temperatures were shown. Copy number
variations (CNVs) of the chromosomal intervals and/or candidate
genes were not examined in the present study.
 | Table II.Forward and reverse primers for
genetic mutation analysis of cancer-related genes. |
Table II.
Forward and reverse primers for
genetic mutation analysis of cancer-related genes.
| Gene | Primers | Product size
(bp) |
|---|
| APC-1-Exon 16 | F:
5′-TAGGATGTAATCAGACGACAC-3′ | 152 |
|
| R:
5′-CAGTCTGCTGGATTTGGTTC-3′ |
|
| APC-2-Exon 16 | F:
5′-GAAGTTCCAGCAGTGTCACA-3′ | 162 |
|
| R:
5′-GTGTTCAGGTGGACTTTTGG-3′ |
|
| APC-3-Exon 16 | F:
5′-CAAAAGTGGTGCTCAGACAC-3′ | 164 |
|
| R:
5′-TGCCACTTACCATTCCACTG-3′ |
|
| APC-4-Exon 16 | F:
5′-TCCGTTCAGAGTGAACCATG-3′ | 149 |
|
| R:
5′-GGTACTTCTCGCTTGGTTTG-3′ |
|
| APC-5-Exon 16 | F:
5′-GTAAAACACCTCCACCACCT-3′ | 151 |
|
| R:
5′-TCAGCATCTGGAAGAACCTG-3′ |
|
| APC-6-Exon 16 | F:
5′-AATGCTGCAGTTCAGAGGGT-3′ | 172 |
|
| R:
5′-CCTGAACTGGAGGCATTATTC-3′ |
|
| APC-7-Exon 16 | F:
5′-CTCGATGAGCCATTTATACAG-3′ | 158 |
|
| R
5′-AGGTCCTTTTCAGAATCAATAG-3′ |
|
| CTNNB1-Exon 3 | F:
5′-GAAAAGCGGCTGTTAGTCAC-3′ | 133 |
|
| R:
5′-GAGAAAATCCCTGTTCCCAC-3′ |
|
| EGFR-Exon 18 | F:
5′-TGTCTCTGTGTTCTTGTCCC-3′ | 162 |
|
| R:
5′-CCAGGGACCTTACCTTATAC-3′ |
|
| EGFR-Exon 19 | F:
5′-GCCAGTTAACGTCTTCCTTC-3′ | 157 |
|
| R:
5′-CCACACAGCAAAGCAGAAAC-3′ |
|
| KRAS-Exon 1 | F:
5′-GGTACTGGTGGAGTATTTGA-3′ | 158 |
|
| R:
5′-CAAGATTTACCTCTATTGTTGG-3′ |
|
| PIK3CA-Exon 20 | F:
5′-AACTGAGCAAGAGGCTTTGG-3′ | 122 |
|
| R:
5′-CTTTTCAGTTCAATGCATGCTG-3′ |
|
| p53-Exon 5 | F:
5′-CTCTTCCTGCAGTACTCCCCTGC-3′ | 211 |
|
| R:
5′-GCCCCAGCTGCTCACCATCGCTA-3′ |
|
| p53-Exon 6 | F:
5′-GTGCAGCTGTGGGTTGATT-3′ | 182 |
|
| R:
5′-GGCCACTGACAACCACCCTTAACC-3′ |
|
| p53-Exon 7 | F:
5′-GCTTGCCACAGGTCTCCCCAAG-3′ | 192 |
|
| R:
5′-AGGCTGGCAAGTGGCTCCTGAC-3′ |
|
| p53-Exon 8 | F:
5′-TGGTAATCTACTGGGACGGA-3′ | 134 |
|
| R:
5′-GCTTAGTGCTCCCTGGGGGC-3′ |
|
| p53-Exon 9 | F:
5′-GCCTCTTTCCTAGCACTGCCCAAC-3′ | 102 |
|
| R:
5′-CCCAAGACTTAGTACCTGAAGGGTG-3′ |
|
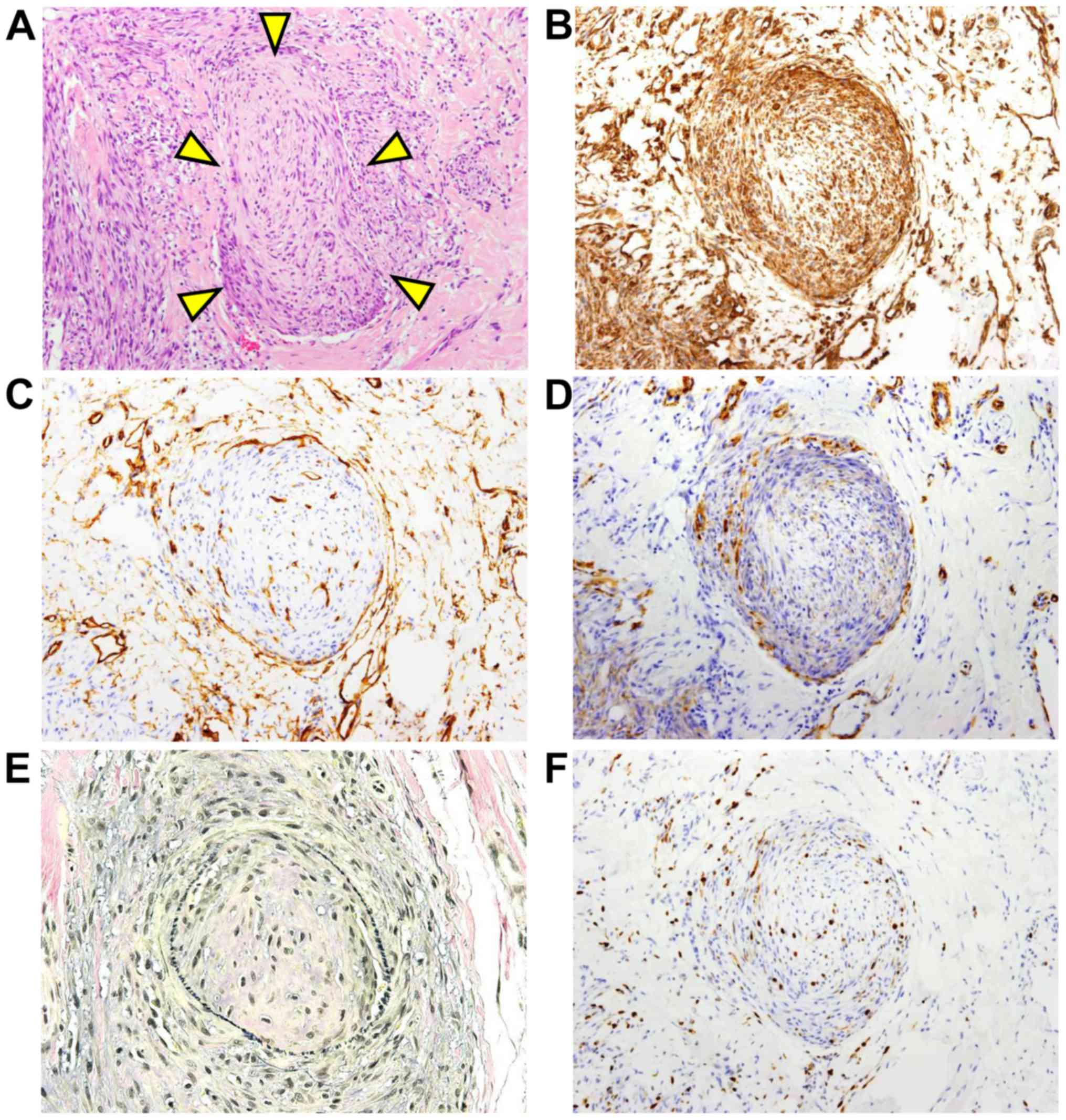
Several intravascular invasions of the tumor cells
were found (Fig. 3A). Intravascular
invading tumor cells were positive for vimentin (Fig. 3B). The vasculature was lined by
CD34-positive vascular endothelial cells (Fig. 3C) and supported by α-SMA-positive
(Fig. 3D) muscular tissue.
Furthermore, elastic fibers were positive for Elastica van Gieson
(EVG) staining (Fig. 3E). EVG
staining was performed according to the standard protocol. The
MIB-l labeling index of the tumor cells noted in the vascular
tissue was 2–10% (Fig. 3F). Since the
histological findings showed a tumor-free region with 2–3 mm normal
tissue in the extirpated sample, re-resection of the tongue was
performed to obtain a tumor-free region with 10 mm normal tissue.
The patient had no evidence of local recurrence or metastasis in
the two and a half years since the first operation.
Discussion
LGMS is known to be a rare sarcoma tumor (1–3). In the
head and neck region, LGMS frequently occurs in the tongue,
especially in the lateral edge of the tongue (13) compared with that in the skin and
gastrointestinal tract. To our knowledge, this is the first report
of LGMS arising in the tip of the tongue. In the 2013 WHO
classification, LGMS is an intermediate-rarely metastasizing tumor
mainly composed of atypical myofibroblasts. Myofibroblasts share
immunohistochemical and ultrastructural features of both
fibroblasts and smooth muscle cells and play a role in wound and
tissue repair (14). However, the
function of myofibroblasts in LGMS has been unclear. LGMS tends to
be found in local recurrence rather than metastasis (15). The current case showed a low MIB-l
labeling index in the tumor cells and several intravascular
invasions of the tumor cells. The MIB-l labeling index of the
intravascular tumor cells was 2–10%, which was almost the same as
that in the original tumor (Fig. 3E).
There has been no evidence of local recurrence or metastasis in the
two and a half years since the first operation. Therefore, these
findings were consistent with the description by the WHO that LGMS
has a malignant potential and is an intermediate-rarely
metastasizing tumor.
The differential diagnosis of LGMS should include
other spindle cell tumors, such as benign tumors; inflammatory
myofibroblastic tumors and nodular fasciitis; such as intermediate
tumor; desmoplastic fibroma or malignant tumors, such as synovial
sarcoma (12), leiomyosarcoma and
osteosarcoma (1). Inflammatory
myofibroblastic tumor shows infiltration of inflammatory cells. As
the genomic rearrangement of MYH9-USP6s (MYH9 exon 1
and USP6 exon 1 or MYH9 exon 1 and USP6 exon
2), which is frequently observed in nodular fasciitis (16), was not shown in the current case (data
not shown), nodular fasciitis would be excluded. Synovial sarcoma
would be ruled out, because reduced expression of BAF47 (INI1 gene
product) protein (17), which is
shown in synovial sarcoma, was not observed. Neither rhabdoid
cells, which are characterized by the existence of a large
eosinophilic inclusion within the cytoplasm, eccentric nuclei and
prominent nucleoli, nor the genomic rearrangement of
SS18-SSXs (SS18-SSX1, SS18-SSX2, SS18-SSX4, SS18-SSX4V,
SS18p-SSXp or SS18-SSXp) were detected in the case
(Fig. 2G). In general, malignant
tumor cells frequently show increased number of mitotic figures and
point mutations of cancer-related genes. The tumor cells involved
in the current LGMS showed no point mutations in the APC,
CTNNBI, EGFR, KRAS, PIK3CA or p53 gene (data not shown).
Many cases of leiomyosarcoma (18)
and osteosarcoma (19) have been
reported to have a gene mutation in p53, and desmoplastic
fibroma has a point mutation in CTNNB1. Therefore, point
mutations of the investigated genes might not be involved in
intravascular invasion of myofibroblasts in LGMS. Because there are
no established treatments for LGMS at present, the mechanism
underlying the invasion of atypical myofibroblasts into vascular
tissue should be clarified in the future.
Acknowledgments
Not applicable.
Funding
The present study was supported by JSPS KAKENHI
(2016–2018; grant no. JP16K11501) and (2017–2018; grant no.
17H06947).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Author contributions
YM performed the immunohistochemial experiments. YM
and SF designed and wrote the manuscript. YM, MM, SK, and SN
performed the clinical diagnosis, treatment (including resection of
the tongue) and follow-up of the patient. KK, YY and YO performed
the mutations and genomic rearrangements analysis. YM, SF, KK, YY,
YO and KT made the histopathological diagnosis. KT interpreted
results and co-wrote the manuscript.
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee of Kyushu University (approval nos. 25-111, 26–257, 27–77
and 29–625). Written informed consent was obtained from the
patient.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saito T, Mitomi H, Kurisaki A, Torigoe T,
Takagi T, Suehara Y, Okubo T, Kaneko K and Yao T: Low-grade
myofibroblastic sarcoma of the distal femur. Int J Surg Case Rep.
4:195–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roth TM, Fratkin J, Woodring TC and
McGehee RP: Low-grade myofibroblastic sarcoma of the vulva. Gynecol
Oncol. 92:361–364. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miguel San P, Fernández G, Ortiz-Rey JA
and Larrauri P: Low-grade myofibroblastic sarcoma of the distal
phalanx. J Hand Surg Am. 29:1160–1163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamada T, Yoshimura T, Kitamura N, Sasabe
E, Ohno S and Yamamoto T: Low-grade myofibroblastic sarcoma of the
palate. Int J Oral Sci. 4:170–173. 2013. View Article : Google Scholar
|
|
5
|
Gabbiani G, Ryan GB and Majne G: Presence
of modified fibroblasts in granulation tissue and their possible
role in wound contraction. Experientia. 27:549–550. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Follonier L, Schaub S, Meister JJ and Hinz
B: Myofibroblast communication is controlled by intercellular
mechanical coupling. J Cell Sci. 121:3305–3316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mentzel T: Low-grade myofibroblastic
sarcomaFletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: World
Health Organization Classification of Tumours: Pathology and
genetics of tumours of soft tissue and bone. IARC Press; Lyon,
France: pp. 85–86. 2013
|
|
8
|
Fujii S, Shinjo K, Matsumoto S, Harada T,
Nojima S, Sato S, Usami Y, Toyosawa S, Morii E, Kondo Y and Kikuchi
A: Epigenetic upregulation of ARL4C, due to DNA hypomethylation in
the 3′-untranslated region, promotes tumorigenesis of lung squamous
cell carcinoma. Oncotarget. 7:81571–81587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurihara S, Oda Y, Ohishi Y, Kaneki E,
Kobayashi H, Wake N and Tsuneyoshi M: Coincident expression of
beta-catenin and cyclin D1 in endometrial stromal tumors and
related high-grade sarcomas. Mod Pathol. 23:225–234. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishijima T, Yamamoto H, Nakano T,
Nakashima T, Taguchi K, Masuda M, Motoshita J, Komune S and Oda Y:
Dual gain of HER2 and EGFR gene copy numbers impacts the prognosis
of carcinoma ex pleomorphic adenoma. Hurn Pathol. 46:1730–1443.
2015.
|
|
11
|
Fujita K, Yamamoto H, Matsumoto T,
Hirahashi M, Gushima M, Kishimoto J, Nishiyama K, Taguchi I, Yao T
and Oda Y: Sessile serrated adenoma with early neoplastic
progression: A clinicopathologic and molecular study. Am J Surg
Pathol. 35:295–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oda Y, Sakamoto A, Saito T, Kawauchi S,
Iwamoto Y and Tstuneyoshi M: Molecular abnormalities of p53, MDM2,
and H-ras in synovial sarcoma. Mod Pathol. 13:994–1004. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Demarosi F, Bay A, Moneghini L and
Carrassi A: Low-grade myofibroblastic sarcoma of the oral cavity.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 108:248–254.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomasek JJ, Gabbianr G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niedzielska I, Janic T and Mrowiec B:
Low-grade myofibroblastic sarcoma of the mandible: A case report. J
Med Case Rep. 3:84582009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Erickson-Johnson MR, Chou MM, Evers BR,
Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X and Oliveira AM:
Nodular fasciitis: A novel model of transient neoplasia induced by
MYH9-USP6 gene fusion. Lab Invest. 91:1427–1433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kohashi K, Oda Y, Yamamoto H, Tamiya S,
Matono H, Iwamoto Y, Taguchi T and Tsuneyoshi M: Reduced expression
of SMARCB1/INI1 protein in synovial sarcoma. Mod Pathol.
23:981–990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee PJ, Yoo NS, Hagemann IS, Pfeifer JD,
Cottrell CE, Abel HJ and Duncavage EJ: Spectrum of mutations in
leiomyosarcomas identified by clinical targeted next-generation
sequencing. Erp Mol Pathol. 102:156–161. 2017. View Article : Google Scholar
|
|
19
|
Overholtzer M, Rao PH, Favis R, Lu XY,
Elowitz MB, Barany F, Ladanyi M, Gorlick R and Levine AJ: The
presence of p53 mutations in human osteosarcomas correlates with
high levels of genomic instability. Proc Natl Acad Sci USA.
100:11547–11552. 2003. View Article : Google Scholar : PubMed/NCBI
|