Introduction
Electrochemotherapy (ECT) is an emerging
skin-directed therapy with elevated cytotoxic activity and
antivascular effects (1). ECT was
first introduced in the late 1980s and has now evolved into a
clinically verified treatment approach for cutaneous and
subcutaneous tumors and has recently been extended to the treatment
of deep-seated tumors (1,2). ECT is the combination of electroporation
(EP), as a mean to facilitate transporting low-permeant or
nonpermeant anticancer drugs into tumor cells, and chemotherapy
(3). EP is based on the application
of intense and short electric pulses that make the cell membrane
permeable, allowing for the penetration of different substances
directly into the cytosol (4,5). This technique has also been used in
vitro to load dyes, DNA, RNA, ions and proteins into cells
(6,7).
Radiotracers, drugs and oligonucleotides have been loaded into
cells in vivo (8) and EP is
used as an efficient non-viral approach for gene therapy (9,10). The
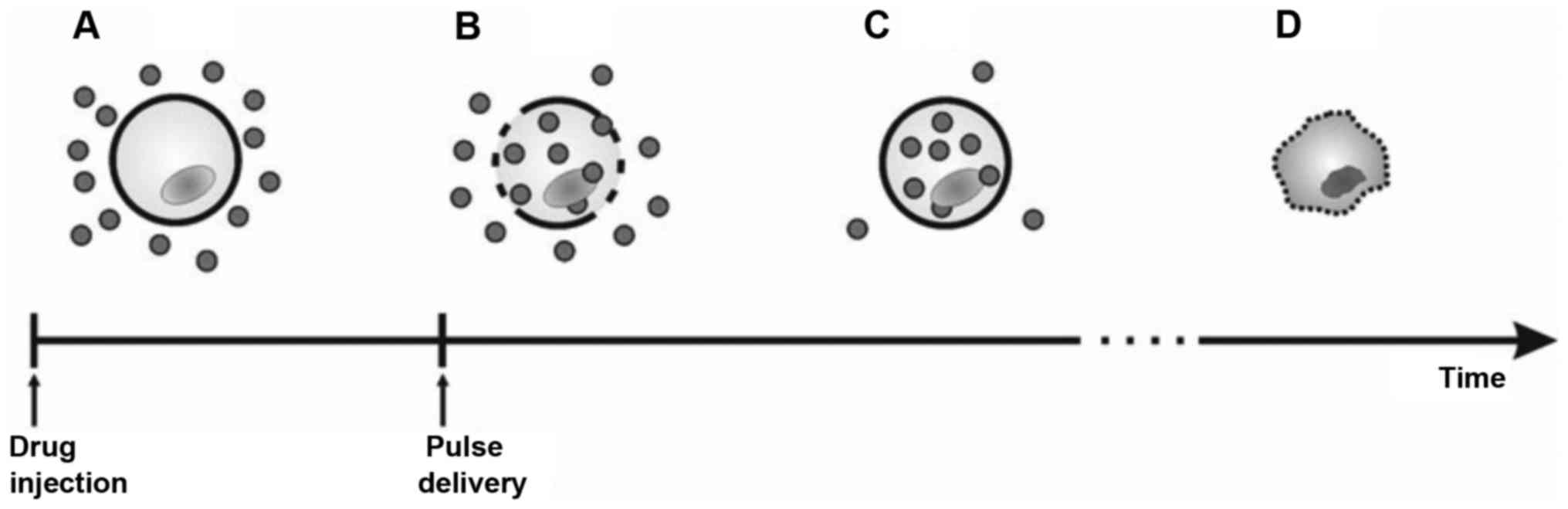
basic concept of ECT is shown in Fig.
1.
ECT is routinely used for cutaneous and subcutaneous
tumors, including skin metastases of head and neck neoplasms, basal
cell and Merkel cell carcinoma, Kaposi sarcoma, chronic lymphocytic
leukemia infiltration and melanoma metastases due to its safety,
effectiveness, organ sparing due to limited toxicity,
cost-effectiveness, low-risk repeatability, and ready integration
as neoadjuvant treatment (11).
This review paper aims to discuss advantages and
limitations of the applications of ECT in head and neck cancer,
with special attention to future clinical and research
perspectives.
Electroporation and antineoplastic agents
for head and neck cancer
EP and irreversible cell death are possible outcomes
of cells or tissues being exposed to direct currents (12). If a cell is exposed to an electric
field that is too large, the electroporated state is irreversible,
and cell death follows (13). The
application of electric pulses with higher amplitudes (14) or nanopulses (15) leads to the irreversible
electroporation of cells and, consequently, to cell death, and has
been suggested instead of ECT for tumor ablation; however, this
technology is still under development.
The description of the phenomena occurring at the
cell membrane level is still incomplete. One of the most accepted
theories posits the generation of hydrophilic pores that transport
molecules across cell membrane (12).
However, these pores have yet to be observed in the membranes of
cells submitted to effective electric pulses. Other theories
hypothesize that there are no pores, and water enters the membrane
through defects in its structure; the permeability coefficient of
hydrophilic molecules such as bleomycin or cisplatin is favored by
hydration of the membranes (16).
Through these permeation structures, diffusion enables cell entry
by non-permeant molecules (16,17).
Bleomycin is a medication commonly used for the
treatment of head and neck cancer. The study of Pron et al
(18) identified a bleomycin-binding
plasma membrane protein complex that entered the cell through
endocytotic vesicles; however, it is still unknown as to why only a
few molecules of bleomycin reach the DNA (19). The cytotoxicity of bleomycin can be
enhanced 300 to 700-fold by EP (20,21) and,
once in the cytosol, bleomycin has been reported to damage the DNA
of target cells (22,23).
Cisplatin, another common antineoplastic agent, has
a permeabilization coefficient through the plasma membrane by
passive diffusion of <50%, whereas the remainder is transported
by carrier molecules (24). EP
enables an increase of cisplatin cytotoxicity up to 80-fold by
increasing the flux and accumulation of the drug in cells (25). This increase in cisplatin uptake and
DNA adduct generation is significant; however, it is still lower
than bleomycin (26). Consequently,
bleomycin is the drug most commonly used in clinical trials
(27).
Bleomycin was isolated from the culture broth of the
fungus Streptomyces verticillus collected from the soil in a
coalmine in Japan (28). It is a
family of at least 13 small water-soluble glycopeptide antibiotics,
and the antineoplastic action of bleomycin is employed in the
treatment of, among others, Hodgkin's disease, non-Hodgkin's
lymphoma and testicular cancer (29,30).
Bleomycin breaks single- and double-stranded DNA, creating DNA
fragmentation, chromosomal gaps and deletions; a previous study
demonstrated 300-fold increased toxicity from double-strand DNA
breaks compared with single-strand breaks (31).
In 1991, the Institut Gustave Roussy (Paris)
conducted the first clinical trial of ECT with bleomycin, in 8
patients with progressive or recurrent head and neck squamous cell
carcinoma (32,33). Subsequent studies have focused on
treating head and neck cancer, including squamous cell carcinoma,
adenoid cystic carcinoma and adenocarcinoma, basal cell carcinoma,
malignant melanoma and adenocarcinoma of the breast (4,5,26,4).
Electric field and electrodes
The pulses used in ECT are typically square wave
electric pulses of ~100 µs using a field strength of 1300 V/cm
(34,35). Pulses are grouped in runs of 4, 6, or
8. Small tumors may be treated with a single run, while larger
tumors require moving the electrodes step-by-step according to the
permeabilization coefficient for EP of the whole target area
(35).
There are two types of electrodes, plate electrodes
and needle electrodes (35).
Treatment of superficial lesions, such as those of the skin, are
typically performed with plate electrodes. The depth of penetration
of the electric field is small and is subject to the distance
between the electrodes; the greater the distance is, the deeper the
penetration of the electric field into the tissue (36). Needle electrodes can be positioned
either in two parallel rows or in a circular array (37). In contrast to plate electrodes, needle
electrodes must be inserted throughout the tumor tissue up to the
deep tumor border (38).
With either electrode type, the highest electric
field can be located around and between electrodes, which reduces
quickly once outside of the electrode array. Such arrays may be
circular or consist of two parallel rows. Thus, if the tumor is
larger than the distance between the electrodes, moving and placing
electrodes adjacently for each consecutive electric pulse
application can efficiently treat the entire tumor (37). Deep-seated head and neck tumors
typically require long single-needle electrodes; tumor placement
and regional anatomy render covering the entire neoplasm with
standard electrodes impractical (2).
Injection site, timing and drug dosage
The efficacy of ECT depends on guaranteeing drug
presence in the tumor at the time of application. Bleomycin can be
delivered intratumorally or systemically, in one clinical study, an
intra-arterial injection of bleomycin was performed (39). The comparatively limited increase in
efficacy of ECT with intravenous cisplatin (40), especially in head and neck metastases,
has consequently limited clinical interest. Therefore, cisplatin
has hence been confined to administration via the intratumoral
route.
For systemic delivery, pulses require administration
during the pharmacokinetic peak, which is between 8–28 min
following drug administration (39).
Intratumoral delivery must be performed between 1–10 min from drug
delivery for maximum treatment efficacy (41,42).
The intravenous injection of bleomycin requires a
dose of 15,000 IU/m2; the neoplasm volume determines
doses for intratumoral injection comprising of ~500
IU/cm3 bleomycin, and 1 mg/cm3 cisplatin
(43). Intratumoral administration
requires a smaller dose of bleomycin than that required for
intravenous administration and larger-volume tumors are generally
thought to be more readily treated by intravenous than intratumoral
administration; however, intratumoral administration may provide
more efficacious treatment for poorly-vascularized tumors (39–43).
Vascular lock
Blood flow changes can occur following the in
vivo delivery of electric pulses (44). In normal tissues, these effects appear
as a transient hypoperfusion. The vascular effect implies that at
the time of cell permeabilization, the drug is withheld within the
electroporated area by the ‘vascular lock’ (44–46).
Furthermore, the return to baseline blood flow levels in tumors may
take hours, a much longer time than for normal tissue in which it
may take minutes (44).
These short-term vascular effects caused by the
electric pulses are amplified in antitumor ECT. This is because the
tumor endothelial cells are also dividing cells that can be
destroyed by a combination of the drugs and the electric pulses
(46). The mid- and long-term
antivascular effects of ECT could therefore result from the killing
of tumor endothelial cells, which would prevent the rapid
reorganization of the tumor vasculature. Consequently, an almost
permanent extremely hypoxic environment is created following nodule
treatment by ECT (44–46), which may also contribute to the highly
efficient antitumor effects observed.
Immune response
In the early 1990s, shortly following the initial
preclinical trials on ECT, host immune response demonstrated an
involvement in post-electrochemotherapy cures (47). At that time, immunotherapy was based
on the administration of biological response modifiers such as
cytokines or lymphokines (47).
Several additional preclinical trials have
demonstrated that ECT combined with immunotherapy enhanced local
antitumor effects and achieved systemic effects in several
experimental models, including a metastasizing murine tumor model
(48), a non-metastasizing murine
tumor model (49) and a carcinoma
model transplanted into the liver of rabbits (50).
This combination therapy is an attractive
possibility that requires further experimental and clinical
development. Other immunostimulatory approaches can be considered;
for example, Heller et al (51) evaluated the transfer of the
interleukin (IL)-12 gene in combination with bleomycin delivery in
mice. Other combinations with chemokines have been already
explored, such as the association of ECT and Tumour Necrosis Factor
α administration (52).
Selectivity towards tumor cells, treatment
protocols and follow up
In the presently available literature, the majority
of tumors treated with ECT were primary tumors or metastases; the
majority are cutaneous nodules or subcutaneous tumors (26); however, treatments have also been
reported for recurrent head and neck cancer (53) and neck lymph node metastases (54). The tumor size in the examined
literature ranged from malignant melanomas of ~0.3 cm (55) to cutaneous nodules in head and neck
squamous cell carcinomas of up to 5 cm (32), in addition to breast metastases with
cutaneous infiltration measuring 8×20 cm (39).
In the head and neck, a single ECT treatment may be
sufficient for single or multiple tumor nodules, including head and
neck metastases (56). This treatment
may completely eradicate tumor nodules. Less dramatically effective
treatment can be repeated as often as monthly. Larger tumors >3
cm in size can be successfully treated by repetitive application of
electric pulses to the tumor until the whole tumor area is covered
(56).
The size or the volume of the tumor is necessary to
evaluate response and to calculate the amount of bleomycin to
inject when using intratumoral injection. Allegretti et al
(53) measured tumor volumes with the
formula for an ellipse V=abc π/6, which some authors have reported
to be the most accurate formula for estimating tumor volume
(57,58).
Different methods are used to evaluate the tumor
response following ECT. A clinical evaluation of tumor size or
tumor volume is often used (59,60), while
many authors supplement this with biopsy (61) and, in some cases, Computed Tomography,
Positron Emission Tomography, or Magnetic Resonance Imaging
(55–64).
Some authors have treated patients under general
anesthesia or neuroleptanalgesia due to tumor localization, size or
number of tumors. Head and neck tumors often require general
anesthesia when localization is in a sensitive area or open surgery
is indicated (56–58). When smaller tumors, such as skin
metastases, are treated with ECT, local anesthesia may be
sufficient; epinephrine may be administered with local anesthesia
to add a beneficial vasoconstriction that impedes washout of any
drug injected prior to EP (59).
Side effects
A common side effect during the ECT procedure is an
involuntary muscle contraction when the electric pulse is applied,
especially when the lesion is in the neck area. The contractions
cease at the end of the pulse; it is generally painless, but
patients may feel some discomfort (11). Occasionally, mild burning of the skin
has been observed in patients treated with plate electrodes;
however, this type of burning has not been observed with needle
electrodes (59). Crusting over the
treated area is part of the natural healing process.
When lesions are located in crucial functional head
and neck areas, extensive tumor necrosis can be responsible for
functional deficits such as dysphagia, fistula formation and loss
of oral competence (65).
Furthermore, repeated treatment of the scalp has been reported to
be associated with poor spontaneous healing rates (65,66).
Minor side effects of ECT include skin ulceration
and hyperpigmentation, maculopapular rash, skin suppuration and
odor, and headaches (66).
Treatment outcomes
The response of individual tumors was classified by
Mali et al (62) as complete
response (CR), partial response (PR), no change (NC) or progressive
disease (PD), according to the data reported in a systematic review
and meta-analysis performed by the authors on 44 studies involving
1,894 tumors (62). In addition, the
concept of objective response (OR, including CR and PR) and no
response were introduced. However, the studies were performed with
variable treatment protocols, different electrodes and electric
pulse generators. Therefore, it was recommended to perform a
prospective non-randomized multi-institutional study.
In 2006, the results of a consortium of four cancer
centers gathered together in a European Standard Operating
Procedures of Electrochemotherapy (ESOPE) project were published
(63). The response to treatment
following ECT was tested according to tumor type, drug used, route
of administration and type of electrode. The study (63) provided the following results: i) A
response rate of 85% was achieved for ECT-treated tumor nodules,
irrespective of tumor histology, drug or route of administration
used. The carcinomas treated most frequently were cutaneous and
subcutaneous melanoma nodules, followed by breast cancer, colon
cancer, squamous cell carcinoma of the skin, squamous cell
carcinoma of the cervix, Kaposi and cutaneous leiomyosarcoma and
subcutaneous tumor nodules. ii) The local tumor control rate for
ECT 150 days after treatment was 88% with bleomycin administered
intravenously, 73% with bleomycin administered intratumorally and
75% with cisplatin administered intratumorally. This demonstrated
that the three approaches were equally effective in local tumor
control (63).
The European Research on Electrochemotherapy in Head
and Neck Cancer (EURECA) group has achieved promising results with
ECT in skin and mucosal tumors of the head and neck. In fact, after
one year of follow-up, a global Disease-Free Survival (DFS) of 89%
was detected with different percentages depending on the
histological type of the tumor. The DFS for malignant melanoma was
100%, the DFS for basal cell carcinoma was 89% and the DFS for
squamous cell carcinoma was 87% (64,65).
Another clinical study of EURECA group on recurrent
mucosal head and neck tumors reported positive results with an
objective response to ECT of 56% (CR, 19%; PR, 37%) (66). Table I
presents the principal findings of ECT treatment in head and neck
cancer (2,27,32,2).
 | Table I.Electrochemotherapy outcomes
review. |
Table I.
Electrochemotherapy outcomes
review.
|
|
|
|
|
| Outcome (%) |
|
|---|
| Author, year | Patients | Histology | Anesthesia | Drug | CR | PR | OR | NR | (Refs.) |
|---|
| Belehradek et
al (1993) | 8 | SCC | S: 8 | Bleomycin IV 10–15
mg/m2 | 57 | 17 | 72 | 28 | (32) |
|
|
|
| GA: 2 | Bleomycin IV: 3, IV
+IA:1 |
|
|
|
|
|
| Domenge et
al (1996) | 4 | SCC | S: 2 | 10–15
mg/m2 | 0 | 0 | 0 | 100 | (39) |
|
|
| BCC: 10 |
|
| 75 | 25 | 100 | 0 |
|
|
|
| SCC: 17 |
|
| 42.8 | 19.5 | 62.3 | 37.7 |
|
| Mir et al
(1998) | 17 | MM: 20 | – | Bleomycin IV 10–15
mg/m2 | 52.8 | 39.4 | 92.2 | 7.8 | (36) |
| Panje et al
(1998) | 8 | SCC | – | Bleomycin IT 1
UI/cm3 of 4 IU/ml solution | 50 | 25 | 75 | 25 | (67) |
| Allegretti et
al (2001) | 4 | SCC | GA: 4 | Bleomycin IT 4
UI/ml solution | 50 | 50 | 100 | 0 | (53) |
| Rabussay et
al (2002) |
|
|
|
|
|
|
|
| (68) |
| North
Am I | 17 |
|
|
| 30 | 25 | 55 | 45 |
|
| North
Am II | 25 |
|
|
| 19 | 39 | 58 | 42 |
|
| Eu | 12 | SCC | – | Bleomycin IT 1
IU/cm3 of 4 IU/ml solution | 28 | 28 | 56 | 44 |
|
| Burian et al
(2003) | 12 | SCC | GA: 12 | Bleomycin IT 1
UI/cm3 of 4 IU/ml solution | 83.3 | 16.7 | 100 | 0 | (27) |
| Bloom and Goldfarb
(2005) | 54 | SCC | – | Bleomycin IT 1
UI/cm3 of 4 IU/ml solution | 24.6 | 31.9 | 56.5 | 43.5 | (69) |
| Larkin et al
(2007) | 3 | SCC | – | Bleomycin | 33.3 | 0 | 33.3 | 66.6 | (70) |
|
|
| SCC: 13 |
|
|
|
|
|
|
|
|
|
| BCC: 9 | GA: 23 |
|
|
|
|
|
|
| Gargiulo et
al (2012) | 24 |
ADENOCARCINOMA:2 | S: 1 | Bleomycin IV 15,000
IU/m2 | 72 | 28 | 100 | 0 | (71) |
|
|
| SCC: 13 |
|
|
|
|
|
|
|
| Mevio et al
(2012) | 14 | BCC:1 | GA: 14 | Bleomycin IV 15,000
IU/m2 | 61.5 | 32.5 | 94 | 6 | (72) |
| Seccia et al
(2014) | 8 | SCC | GA: 8 | Bleomycin IV 15,000
IU/m2 | 37.5 | 50 | 87.5 | 12.5 | (73) |
| Campagna et
al (2014) | 2 | SCC | GA: 28 | Bleomycin IV 15,000
IU/m2 | 0 | 100 | 100 | 0 | (74) |
|
|
|
|
| Bleomycin IV 15,000
IU/m2: 7 |
|
|
|
|
|
|
|
| SCC: 24 |
| Bleomycin IT 1,000
UI/cm3: 7 |
|
|
|
|
|
|
|
| BCC:9 | S: 29 | Bleomycin IT 1,000
UI/cm3 + |
|
|
|
|
|
| Campagna et
al (2014) | 39 |
ADENOCARCINOMA:6 | GA: 19 | Bleomycin IV 15,000
IU/m2: 25 | 38 | 21 | 59 | 41 | (75) |
| Macri et al
(2014) | 1 | SCC | S: 1 | Bleomycin IV 15,000
IU/m2 | 100 | 0 | 100 | 0 | (76) |
| Domanico et
al (2015) | 4 | SCC | GA: 4 | Bleomycin IV 15,000
IU/m2 | 0 | 75 | 75 | 0 | (77) |
| Landstrom et
al (2015) | 4 | SCC | GA: 4 | Bleomycin IT 1,000
UI/cm3 | 100 | 0 | 100 | 0 | (78) |
| Landstrom et
al (2015) | 19 | SCC | GA: 19 | Bleomycin IT 1,000
UI/cm3 | 100 | 0 | 100 | 0 | (79) |
| Groselj et
al (2015) | 1 | SCC | GA: 1 | Bleomycin IV 15,000
IU/m2 | 100 | 0 | 100 | 0 | (2) |
| Rotunno et
al (2016) | 55 |
|
|
| 60 | 31 | 91 | 9 | (80) |
|
|
| BCC: 34 |
|
|
|
|
|
|
|
|
|
| SSC: 50 |
| Bleomycin IV 15,000
IU/m2: 97 |
|
|
|
|
|
|
|
| MM: 10 | S: 46 | Bleomycin IT 1
UI/cm3 of 4 |
|
|
|
|
|
| Bertino et
al (2016) | 105 | OTHERS: 11 | GA: 59 | IU/ml solution:
8 | 50.25 | 24 | 74.25 | 25.75 | (65) |
|
|
|
|
| Bleomycin IV 15,000
IU/m2: 41 |
|
|
|
|
|
| Plaschke et
al (2017) | 43 | SCC | S: 1 | Bleomycin IT 1
UI/cm3 of 4 | 19 | 37 | 56 | 44 | (66) |
| Montuori et
al (2018) | 15 | BCC | GA: 42 | IU/ml solution:
2 | 100 | 0 | 100 | 0 | (81) |
|
|
|
|
| Bleomycin IV 15,000
IU/m2: 16 | 96 | 4 | 100 | 0 |
|
| Groselj et
al (2018) | 28 | SCC | GA: 28 | Bleomycin IV 15,000
IU/m2: 12 | 100 | 0 | 100 | 0 | (82) |
Discussion
Based on the presently available evidence, ECT may
be considered for treating a large range of tumors, including skin
metastases of the head and neck, treatment restrictions of primary
cutaneous and subcutaneous tissue, as in melanoma and squamous cell
carcinoma, means a viable therapeutic alternative is being
overlooked (1–4). Examples for application include the oral
and nasal cavity and pharyngeal-laryngeal lumen, and long
single-needle electrodes allow for ECT of deep-seated tumors of the
head and neck (2,13–15).
Electric pulses can permeabilize any living cell, and bleomycin and
cisplatin may therefore be applied to DNA molecules, irrespective
of the onco- or antioncogenes expressed by tumor cells (5,6).
Recently, a multicenter retrospective analysis
reviewed the cases of 19 patients who underwent ECT from July 2007
to May 2014 for superficial advanced angiosarcomas. The authors
reported that after 2 months, an objective response was observed in
63% patients, with 6-month disease stabilization in 47% of
patients. Treatment was generally well tolerated; local symptom
improvement included palliation of bleeding (26%) and pain relief
(32%). Based on the results obtained in these patients, the authors
concluded that ECT may represent a promising treatment for
providing local tumor control and symptom palliation in patients
with superficial angiosarcomas (83).
An effective ECT requires that electrodes are
applied to ensure a complete cover of the entire neoplasm; this may
result in the serial administration of pulses for complete
coverage. Similarly, drug concentration and delivery must be
calibrated for maximum clinical efficacy, including the effects of
the electric pulses. ECT effectiveness therefore requires
administration protocols that are systematically planned and
scrupulously executed and accompanied by well-designed checklists
and follow-up to ensure and measure effectiveness. These principles
are irrespective of tumor or electrode type, or whether drugs are
delivered systemically or locally (44–46).
ECT treatment is intrinsically local and effective
treatment for the local control of tumor growth and is important in
the treatment of cancer. Surgery and/or radio-chemotherapy remain
the primary treatments for head and neck cancer; however, as
previously demonstrated at the clinical stage, adjuvant ECT
provides effective cytoreduction, allowing for subsequent surgeries
to potentially become less invasive and traumatic (39). Other therapeutic combinations, such as
radiotherapy, are also possible. In cases of local recurrences
where no further curative treatment options are available, ECT may
serve a central therapeutic role (54–56).
Recently, our group reported an interesting case of squamous cell
carcinoma of the head and neck with extensive skin metastases that
was successfully treated with ECT, demonstrating that ECT may be an
effective therapy for metastases or local squamous cell carcinoma
recurrence (54). ECT can typically
be applied in the outpatient setting with a favorable cost-benefit
ratio as bleomycin and cisplatin are relatively low-cost, and ECT
equipment is less expensive than ionizing radiation devices
(1–4,19,21).
Treatment of internal tumors using endoluminal
electrodes is currently being explored (84). This technological development has the
potential for treating head and neck tumors located in the parotid,
submandibular and thyroid glands or in the latero-cervical space
(84,85).
Challenges in applying ECT to deep-seated tumors are
represented by tissue conductivity, these include vasculature,
necrosis, micro-heterogeneities, to the effects of EP on
conductivity, determining the electrophoretic threshold (1). All such considerations dictate precise
treatment planning and design, down to electrode placement, drug
delivery, dosage and timing, to ensure the accuracy and robustness
of a treatment plan (1).
Current evidence provides a basis for combining ECT
with immunotherapy (49–52,86).
Immunomodulatory agents or the electro-transfer of genes coded for
immunoregulatory proteins suggest the potential of a safer systemic
cancer treatment, which is free from the adverse effects of current
therapeutic modalities. ECT provides its own therapeutic and
enhanced delivery benefits to this discussion, starting with
current protocols and adapting them as the future demands. A
broadening and extending of the range of indications for ECT can be
anticipated for primary curative approaches (87).
Conclusions
ECT has demonstrated its safety and efficacy in skin
metastases of head and neck tumors and, with some limitations, in
primary and relapsing neoplasms of this region. ECT can be repeated
as needed and does not interfere with or preclude subsequent
therapy with primary treatment modes. Although at present, ECT is a
palliative treatment, the high success rates of ECT and good level
of tolerability, considered against the scarcity of alternative
treatments in advanced stage cancers, make it worth consideration
among treatment options in selected patients.
Acknowledgements
No applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ADV and GA were responsible for the conception and
design of the present study. MR undertook drafting of the
manuscript, and LL and PM performed the analysis and interpretation
of literature and data. FA performed manuscript revisions and MDV
and AG were responsible for study design, critical revision of the
manuscript, provided final approval.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miklavcic D and Davalos RV:
Electrochemotherapy (ECT) and irreversible electroporation (IRE)
-advanced techniques for treating deep-seated tumors based on
electroporation. Biomed Eng Online. 14 Suppl 3:I12015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Groselj A, Kos B, Cemazar M, Urbancic J,
Kragelj G, Bosnjak M, Veberic B, Strojan P, Miklavcic D and Sersa
G: Coupling treatment planning with navigation system: A new
technological approach in treatment of head and neck tumors by
electrochemotherapy. Biomed Eng Online. 14 Suppl 3:S22015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mir LM, Orlowski S, Belehradek J Jr and
Paoletti C: Electrochemotherapy potentiation of antitumour effect
of bleomycin by local electric pulses. Eur J Cancer. 27:68–72.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heller R, Gilbert R and Jaroszeski MJ:
Clinical applications of electrochemotherapy. Adv Drug Deliv Rev.
35:119–129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heller R, Gilbert R and Jaroszeski MJ:
Electrochemotherapy: An emerging drug delivery method for the
treatment of cancer. Adv Drug Deliv Rev. 26:185–197. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mir LM, Banoun H and Paoletti C:
Introduction of definite amounts of nonpermeant molecules into
living cells after electropermeabilization: direct access to the
cytosol. Exp Cell Res. 175:15–25. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jaroszeski MJ, Dang V, Pottinger C, Hickey
J, Gilbert R and Heller R: Toxicity of anticancer agents mediated
by electroporation in vitro. Anticancer Drugs. 11:201–208. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rols MP, Delteil C, Golzio M, Dumond P,
Cros S and Teissie J: In vivo electrically mediated protein and
gene transfer in murine melanoma. Nat Biotechnol. 16:168–171. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mir LM, Bureau MF, Gehl J, Rangara R, Rouy
D, Caillaud JM, Delaere P, Branellec D, Schwartz B and Scherman D:
High-efficiency gene transfer into skeletal muscle mediated by
electric pulses. Proc Natl Acad Sci USA. 96:4262–4267. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heller R, Jaroszeski M, Atkin A, Moradpour
D, Gilbert R, Wands J and Nicolau C: In vivo gene electroinjection
and expression in rat liver. FEBS Lett. 389:225–228. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heller R, Jaroszeski MJ, Reintgen DS,
Puleo CA, DeConti RC, Gilbert RA and Glass LF: Treatment of
cutaneous and subcutaneous tumors with electrochemotherapy using
intralesional bleomycin. Cancer. 83:148–157. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orlowski S and Mir LM: Cell
electropermeabilization: A new tool for biochemical and
pharmacological studies. Biochim Biophys Acta. 1154:51–63. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weaver JC: Electroporation: A general
phenomenon for manipulating cells and tissues. J Cell Biochem.
51:426–435. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edd JF, Horowitz L, Davalos RV, Mir LM and
Rubinsky B: In vivo results of a new focal tissue ablation
technique: irreversible electroporation. IEEE Trans Biomed Eng.
53:1409–1415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nuccitelli R, Pliquett U, Chen X, Ford W,
Swanson James R, Beebe SJ, Kolb JF and Schoenbach KH: Nanosecond
pulsed electric fields cause melanomas to self-destruct. Biochem
Biophys Res Commun. 343:351–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weaver JC: Electroporation theory.
Concepts and mechanisms. Methods Mol Biol. 55:3–28. 1995.PubMed/NCBI
|
|
17
|
Neumann E, Schaefer-Ridder M, Wang Y and
Hofschneider PH: Gene transfer into mouse lyoma cells by
electroporation in high electric fields. EMBO J. 1:841–845.
1982.PubMed/NCBI
|
|
18
|
Pron G, Belehradek J Jr and Mir LM:
Identification of a plasma membrane protein that specifically binds
bleomycin. Biochem Biophys Res Commun. 194:333–337. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mir LM, Tounekti O and Orlowski S:
Bleomycin: Revival of an old drug. Gen Pharmacol. 27:745–748. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gehl J, Skovsgaard T and Mir LM:
Enhancement of cytotoxicity by electropermeabilization: An improved
method for screening drugs. Anticancer Drugs. 9:319–325. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orlowski S, Belehradek J Jr, Paoletti C
and Mir LM: Transient electropermeabilization of cells in culture.
Increase of the cytotoxicity of anticancer drugs. Biochem
Pharmacol. 37:4727–4733. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poddevin B, Orlowski S, Belehradek J Jr
and Mir LM: Very high cytotoxicity of bleomycin introduced into the
cytosol of cells in culture. Biochem Pharmacol. 42 Suppl:S67–S75.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hall SW, Strong JE, Broughton A, Frazier
ML and Benjamin RS: Bleomycin clinical pharmacology by
radioimmunoassay. Cancer Chemother Pharmacol. 9:22–25. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eljack ND, Ma HY, Drucker J, Shen C,
Hambley TW, New EJ, Friedrich T and Clarke RJ: Mechanisms of cell
uptake and toxicity of the anticancer drug cisplatin. Metallomics.
6:2126–2133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sersa G, Cemazar M and Miklavcic D:
Antitumor effectiveness of electrochemotherapy with
cis-diamminedichloroplatinum(II) in mice. Cancer Res. 55:3450–3455.
1995.PubMed/NCBI
|
|
26
|
Rodriguez-Cuevas S, Barroso-Bravo S,
Almanza-Estrada J, Cristobal-Martinez L and Gonzalez-Rodriguez E:
Electrochemotherapy in primary and metastatic skin tumors: Phase II
trial using intralesional bleomycin. Arch Med Res. 32:273–276.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burian M, Formanek M and Regele H:
Electroporation therapy in head and neck cancer. Acta Otolaryngol.
123:264–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Umezawa H, Maeda K, Takeuchi T and Okami
Y: New antibiotics, bleomycin A and B. J Antibiot (Tokyo).
19:200–209. 1966.PubMed/NCBI
|
|
29
|
Dillman RO: Rationales for combining
chemotherapy and biotherapy in the treatment of cancer. Mol
Biother. 2:201–207. 1990.PubMed/NCBI
|
|
30
|
Tounekti O, Pron G, Belehradek J Jr and
Mir LM: Bleomycin, an apoptosis-mimetic drug that induces two types
of cell death depending on the number of molecules internalized.
Cancer Res. 53:5462–5469. 1993.PubMed/NCBI
|
|
31
|
Tounekti O, Kenani A, Foray N, Orlowski S
and Mir LM: The ratio of single- to double-strand DNA breaks and
their absolute values determine cell death pathway. Br J Cancer.
84:1272–1279. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Belehradek M, Domenge C, Luboinski B,
Orlowski S, Belehradek J Jr and Mir LM: Electrochemotherapy, a new
antitumor treatment. First clinical phase I–II trial. Cancer.
72:3694–3700. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mir LM, Belehradek M, Domenge C, Orlowski
S, Poddevin B, Belehradek J Jr, Schwaab G, Luboinski B and Paoletti
C: Electrochemotherapy, a new antitumor treatment: first clinical
trial. C R Acad Sci III. 313:613–618. 1991.(In French). PubMed/NCBI
|
|
34
|
Sersa G, Cufer T, Cemazar M, Rebersek M
and Zvonimir R: Electrochemotherapy with bleomycin in the treatment
of hypernephroma metastasis: Case report and literature review.
Tumori. 86:163–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Puc M, Corovic S, Flisar K, Petkovsek M,
Nastran J and Miklavcic D: Techniques of signal generation required
for electropermeabilization. Survey of electropermeabilization
devices. Bioelectrochemistry. 64:113–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mir LM, Glass LF, Sersa G, Teissié J,
Domenge C, Miklavcic D, Jaroszeski MJ, Orlowski S, Reintgen DS,
Rudolf Z, et al: Effective treatment of cutaneous and subcutaneous
malignant tumours by electrochemotherapy. Br J Cancer.
77:2336–2342. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mir LM, Gehld J, Sersae G, et al: Standard
operating procedures of the electrochemotherapy: Instructions for
the use of bleomycin or cisplatin administered either systemically
or locally and electric pulses delivered by the Cliniporator by
means of invasive or non-invasive electrodes. EJC Supplements.
4:14–25. 2006. View Article : Google Scholar
|
|
38
|
Miklavcic D, Corovic S, Pucihar G and
Pavselj N: Importance of tumour coverage by sufficiently high local
electric field for effective electrochemotherapy. Eur J Cancer
Suppl. 4:45–51. 2006. View Article : Google Scholar
|
|
39
|
Domenge C, Orlowski S, Luboinski B, De
Baere T, Schwaab G, Belehradek J Jr and Mir LM: Antitumor
electrochemotherapy: New advances in the clinical protocol. Cancer.
77:956–963. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sersa G, Stabuc B, Cemazar M, Miklavcic D
and Rudolf Z: Electrochemotherapy with cisplatin: The systemic
antitumour effectiveness of cisplatin can be potentiated locally by
the application of electric pulses in the treatment of malignant
melanoma skin metastases. Melanoma Res. 10:381–385. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cemazar M, Milacic R, Miklavcic D, Dolzan
V and Sersa G: Intratumoral cisplatin administration in
electrochemotherapy: Antitumor effectiveness, sequence dependence
and platinum content. Anticancer Drugs. 9:525–530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heller R, Jaroszeski M, Perrott R, Messina
J and Gilbert R: Effective treatment of B16 melanoma by direct
delivery of bleomycin using electrochemotherapy. Melanoma Res.
7:10–18. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mir LM: Electroporation-based gene
therapy: Recent evolution in the mechanism description and
technology developments. Methods Mol Biol. 1121:3–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sersa G, Cemazar M, Parkins CS and Chaplin
DJ: Tumour blood flow changes induced by application of electric
pulses. Eur J Cancer. 35:672–677. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gehl J and Geertsen PF: Efficient
palliation of haemorrhaging malignant melanoma skin metastases by
electrochemotherapy. Melanoma Res. 10:585–589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cemazar M, Parkins CS, Holder AL, Chaplin
DJ, Tozer GM and Sersa G: Electroporation of human microvascular
endothelial cells: Evidence for an anti-vascular mechanism of
electrochemotherapy. Br J Cancer. 84:565–570. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mir LM, Orlowski S, Poddevin B and
Belehradek J Jr: Electrochemotherapy tumor treatment is improved by
interleukin-2 stimulation of the host's defenses. Eur Cytokine
Netw. 3:331–334. 1992.PubMed/NCBI
|
|
48
|
Orlowski S, An D, Belehradek J Jr and Mir
LM: Antimetastatic effects of electrochemotherapy and of
histoincompatible interleukin-2-secreting cells in the murine Lewis
lung tumor. Anticancer Drugs. 9:551–556. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mir LM, Roth C, Orlowski S,
Quintin-Colonna F, Fradelizi D, Belehradek J Jr and Kourilsky P:
Systemic antitumor effects of electrochemotherapy combined with
histoincompatible cells secreting interleukin-2. J Immunother
Emphasis Tumor Immunol. 17:30–38. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ramirez LH, Orlowski S, An D, Bindoula G,
Dzodic R, Ardouin P, Bognel C, Belehradek J Jr, Munck JN and Mir
LM: Electrochemotherapy on liver tumours in rabbits. Br J Cancer.
77:2104–2111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Heller L, Pottinger C, Jaroszeski MJ,
Gilbert R and Heller R: In vivo electroporation of plasmids
encoding GM-CSF or interleukin-2 into existing B16 melanomas
combined with electrochemotherapy induces long-term antitumour
immunity. Melanoma Res. 10:577–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sersa G, Cemazar M, Menart V,
Gaberc-Porekar V and Miklavcic D: Anti-tumor effectiveness of
electrochemotherapy with bleomycin is increased by TNF-alpha on
SA-1 tumors in mice. Cancer Lett. 116:85–92. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Allegretti JP and Panje WR:
Electroporation therapy for head and neck cancer including carotid
artery involvement. Laryngoscope. 111:52–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
De Virgilio A, Fusconi M, Greco A and de
Vincentiis M: The role of electrochemotherapy in the treatment of
metastatic head and neck cancer. Tumori. 99:6342013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rols MP, Bachaud JM, Giraud P, Chevreau C,
Roche H and Teissie J: Electrochemotherapy of cutaneous metastases
in malignant melanoma. Melanoma Res. 10:468–474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Snoj M, Cemazar M, Kolar Slekovec B and
Sersa G: Effective treatment of multiple unresectable skin melanoma
metastases by electrochemotherapy. Croat Med J. 48:391–395.
2007.PubMed/NCBI
|
|
57
|
Wapnir IL, Wartenberg DE and Greco RS:
Three dimensional staging of breast cancer. Breast Cancer Res
Treat. 41:15–19. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tomayko MM and Reynolds CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Heller R, Jaroszeski MJ, Glass LF, Messina
JL, Rapaport DP, DeConti RC, Fenske NA, Gilbert RA, Mir LM and
Reintgen DS: Phase I/II trial for the treatment of cutaneous and
subcutaneous tumors using electrochemotherapy. Cancer. 77:964–971.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kubota Y, Mir LM, Nakada T, Sasagawa I,
Suzuki H and Aoyama N: Successful treatment of metastatic skin
lesions with electrochemotherapy. J Urol. 160:14261998. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Glass LF, Pepine ML, Fenske NA, Jaroszeski
M, Reintgen DS and Heller R: Bleomycin-mediated electrochemotherapy
of metastatic melanoma. Arch Dermatol. 132:1353–1357. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mali B, Jarm T, Snoj M, Sersa G and
Miklavcic D: Antitumor effectiveness of electrochemotherapy: A
systematic review and meta-analysis. Eur J Surg Oncol. 39:4–16.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Marty M, Sersa G and Garbay JR:
Electrochemotherapy-An easy, highly effective and safe treatment of
cutaneous and subcutaneous metastases: Results of ESOPE (European
Standard Operating Procedures of Electrochemotherapy) study. Eur J
Cancer Suppl. 4:3–13. 2006. View Article : Google Scholar
|
|
64
|
Sersa G: The state-of-the-art of
electrochemotherapy before the ESOPE study: Advantages and clinical
uses. Eur J Cancer Suppl. 4:52–59. 2006. View Article : Google Scholar
|
|
65
|
Bertino G, Sersa G, De Terlizzi F, Occhini
A, Plaschke CC, Groselj A, Langdon C, Grau JJ, McCaul JA, Heuveling
D, et al: European research on electrochemotherapy in head and neck
Cancer (EURECA) project: Results of the treatment of skin cancer.
Eur J Cancer. 63:41–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Plaschke CC, Bertino G, McCaul JA, Grau
JJ, de Bree R, Sersa G, Occhini A, Groselj A, Langdon C, Heuveling
DA, et al: European Research on Electrochemotherapy in Head and
Neck Cancer (EURECA) project: Results from the treatment of mucosal
cancers. Eur J Cancer. 87:172–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Panje WR, Hier MP, Garman GR, Harrell E,
Goldman A and Bloch I: Electroporation therapy of head and neck
cancer. Ann Otol Rhinol Laryngol. 107:779–785. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rabussay DP, Nanda GS and Goldfarb PM:
Enhancing the effectiveness of drug-based cancer therapy by
electroporation (electropermeabilization). Technol Cancer Res
Treat. 1:71–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bloom DC and Goldfarb PM: The role of
intratumour therapy with electroporation and bleomycin in the
management of advanced squamous cell carcinoma of the head and
neck. Eur J Surg Oncol. 31:1029–1035. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Larkin JO, Collins CG, Aarons S, Tangney
M, Whelan M, O'Reily S, Breathnach O, Soden DM and O'Sullivan GC:
Electrochemotherapy: Aspects of preclinical development and early
clinical experience. Ann Surg. 245:460–479. 2007. View Article : Google Scholar
|
|
71
|
Gargiulo M, Papa A, Capasso P, Moio M,
Cubicciotti E and Parascandolo S: Electrochemotherapy for
non-melanoma head and neck cancers: Clinical outcomes in 25
patients. Ann Surg. 255:1158–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mevio N, Bertino G, Occhini A, Scelsi D,
Tagliabue M, Mura F and Benazzo M: Electrochemotherapy for the
treatment of recurrent head and neck cancers: Preliminary results.
Tumori. 98:308–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Seccia V, Muscatello L, Dallan I,
Bajraktari A, Briganti T, Ursino S, Galli L, Falcone A and
Sellari-Franceschini S: Electrochemotherapy and its controversial
results in patients with head and neck cancer. Anticancer Res.
34:967–972. 2014.PubMed/NCBI
|
|
74
|
Campana LG, Bertino G, Rossi CR, Occhini
A, Rossi M, Valpione S and Benazzo M: The value of
electrochemotherapy in the treatment of peristomal tumors. Eur J
Surg Oncol. 40:260–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Campana LG, Mali B, Sersa G, Valpione S,
Giorgi CA, Strojan P, Miklavcic D and Rossi CR: Electrochemotherapy
in non-melanoma head and neck cancers: A retrospective analysis of
the treated cases. Br J Oral Maxillofac Surg. 52:957–964. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Macri GF, Greco A, Gallo A, Fusconi M,
Marinelli C and de Vincentiis M: Use of electrochemotherapy in a
case of neck skin metastasis of oral squamous cell carcinoma: Case
report and considerations. Head Neck. 36:E86–E90. 2014.PubMed/NCBI
|
|
77
|
Domanico R, Trapasso S, Santoro M,
Pingitore D and Allegra E: Electrochemotherapy in combination with
chemoradiotherapy in the treatment of oral carcinomas in advanced
stages of disease: efficacy, safety, and clinical outcomes in a
small number of selected cases. Drug Des Devel Ther. 9:1185–1191.
2015.PubMed/NCBI
|
|
78
|
Landstrom FJ, Reizenstein JA, Nilsson CO,
Beckerath MV, Löfgren AL, Adamsson GB and Möller C:
Electrochemotherapy-possible benefits and limitations to its use in
the head and neck region. Acta Otolaryngol. 135:90–95. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Landstrom FJ, Reizenstein J, Adamsson GB,
Beckerath Mv and Möller C: Long-term follow-up in patients treated
with curative electrochemotherapy for cancer in the oral cavity and
oropharynx. Acta Otolaryngol. 135:1070–1078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Rotunno R, Marenco F, Ribero S, Calvieri
S, Amerio P, Curatolo P and Quaglino P: Electrochemotherapy in
non-melanoma head and neck skin cancers: a three-center experience
and review of the literature. G Ital Dermatol Venereol.
151:610–618. 2016.PubMed/NCBI
|
|
81
|
Montuori M, Santurro L, Feliziani A, DE
Sanctis F, Ricciardi E, Gaudio D, Campione E, Bianchi L, Silvi MB
and Rossi P: Electrochemotherapy for basocellular and
squamocellular head and neck cancer: preliminary experience in Day
Surgery Unit. G Ital Dermatol Venereol. 153:19–25. 2018.PubMed/NCBI
|
|
82
|
Groselj A, Bosnjak M, Strojan P, Krzan M,
Cemazar M and Sersa G: Efficiency of electrochemotherapy with
reduced bleomycin dose in the treatment of nonmelanoma head and
neck skin cancer: Preliminary results. Head Neck. 40:120–25. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Guida M, Campana LG, Curatolo P, Strippoli
S, Bonadies A, Grilz G, Cabula C, Rotunno R, Bucher S, Solari N, et
al: Local treatment with electrochemotherapy of superficial
angiosarcomas: Efficacy and safety results from a
multi-institutional retrospective study. J Surg Oncol. 114:246–253.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jahangeer S, Forde P, Soden D and Hinchion
J: Review of current thermal ablation treatment for lung cancer and
the potential of electrochemotherapy as a means for treatment of
lung tumours. Cancer Treat Rev. 39:862–871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sersa G, Cufer T, Paulin SM, Cemazar M and
Snoj M: Electrochemotherapy of chest wall breast cancer recurrence.
Cancer Treat Rev. 38:379–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Calvet CY and Mir LM: The promising
alliance of anti-cancer electrochemotherapy with immunotherapy.
Cancer Metastasis Rev. 35:165–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lenzi R, Muscatello L, Saibene AM,
Felisati G and Pipolo C: The controversial role of
electrochemotherapy in head and neck cancer: A systematic review of
the literature. Eur Arch Otorhinolaryngol. 274:2389–2394. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Miklavčič D, Serša G, Brecelj E, Gehl J,
Soden D, Bianchi G, Ruggieri P, Rossi CR, Campana LG and Jarm T:
Electrochemotherapy: technological advancements for efficient
electroporation-based treatment of internal tumors. Med Biol Eng
Comput. 50:1213–1225. 2012. View Article : Google Scholar : PubMed/NCBI
|