Introduction
In the past 20 years, gastric cancer has remained
one of the most common types of malignant tumor and is a common
cause of cancer-associated mortality worldwide (1). Data from the National Central Cancer
Registry in 2013 indicated that the incidence of gastric cancer in
China was 0.042%, which was second only to lung cancer (2). For those patients suffering with
unresectable tumors or recurrent disease, it is generally
acknowledged that comprehensive treatment with systemic medication,
including chemotherapy, targeted drugs and immunotherapy, should be
adopted (3,4).
The overexpression rates of human epidermal growth
factor receptor-2 (HER2) in gastric cancer have been demonstrated
to range between 7.3 and 22.1% (5,6). A
retrospective study demonstrated that HER2 expression is associated
with aggressive biological behavior (7). HER2 expression is an indicative factor
of the efficacy of trastuzumab treatment. In the Trastuzumab for
Gastric Cancer (ToGA) study, targeting HER2 with trastuzumab in
first-line therapy combined with standard chemotherapy
significantly improved the overall survival of patients compared
with standard chemotherapy alone (16.0 vs. 11.8 months) (8). Results from certain phase II clinical
trials revealed longer overall survival and reduced side effects
resulting from trastuzumab in combination with other chemotherapy
regimens (9,10). However, the present literature is
incomplete and lacks data from randomized controlled clinical
trials. Additionally, there are no guidelines to aid physicians in
reaching a decision for patients who require third-line or further
chemotherapy. Only apatinib is approved for use as a third-line
treatment of refractory gastric cancer in China (11). Other studies on irinotecan or
docetaxel are limited by small sample sizes (12–14). Thus,
there is currently a lack of an international standard for patients
following second-line treatment.
The present study reports the case of a patient with
gastric cancer who received multi-line chemotherapy, including
treatment with trastuzumab. The course of treatment is presented in
Fig. 1.
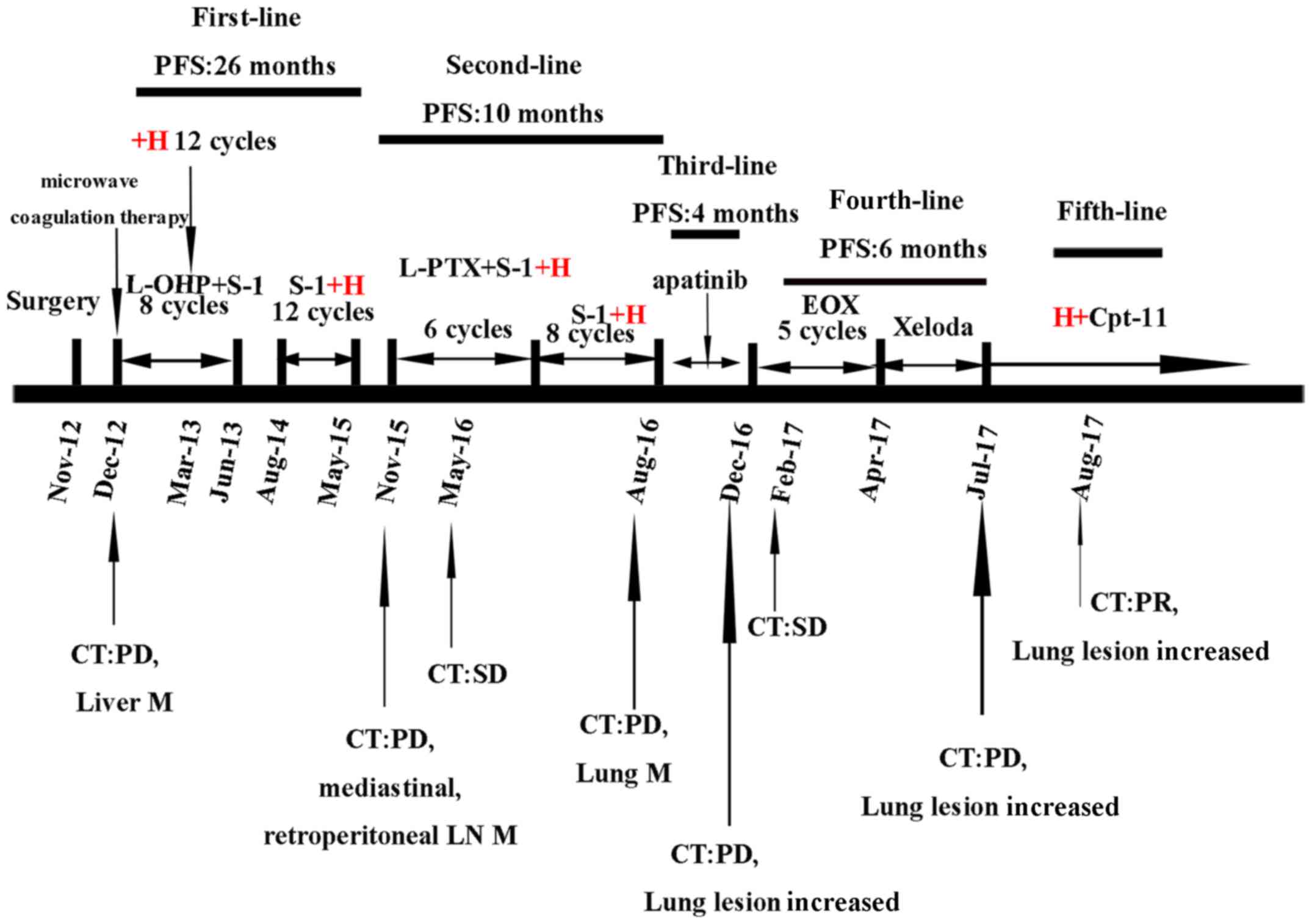 | Figure 1.Multi-line treatment schedule. The
patient received multi-line chemotherapy combined with trastuzumab
following disease progression. CT, computed tomography; PD,
progressive disease; SD, stable disease; PR, partial response; PFS,
progression-free survival; M, metastasis; LN, lymph node; H,
trastuzumab; L-OHP, oxaliplatin; S-1, tegafur gimeracil oteracil
potassium; L-PTX, paclitaxel liposome; Cpt-11, irinotecan; EOX,
epirubicin/oxaliplatin/xeloda; XP, xeloda/cisplatin; CEA,
carcino-embryonic antigen; AFP, α fetoprotein. |
Case report
A 55-year-old male patient visited the First
Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China) in
December 2012 with the diagnosis of gastric tumor from Xijing
Hospital, the Fourth Military Medical University. The patient
underwent radical gastrectomy in November 2011. Surgical specimens
were fixed with 4% neutral buffered formaldehyde for 8 h at room
temperature. The postoperative pathological results revealed
moderately and poorly differentiated adenocarcinoma. Metastatic
disease was observed in nodes at the lesser curvature (1/1) and
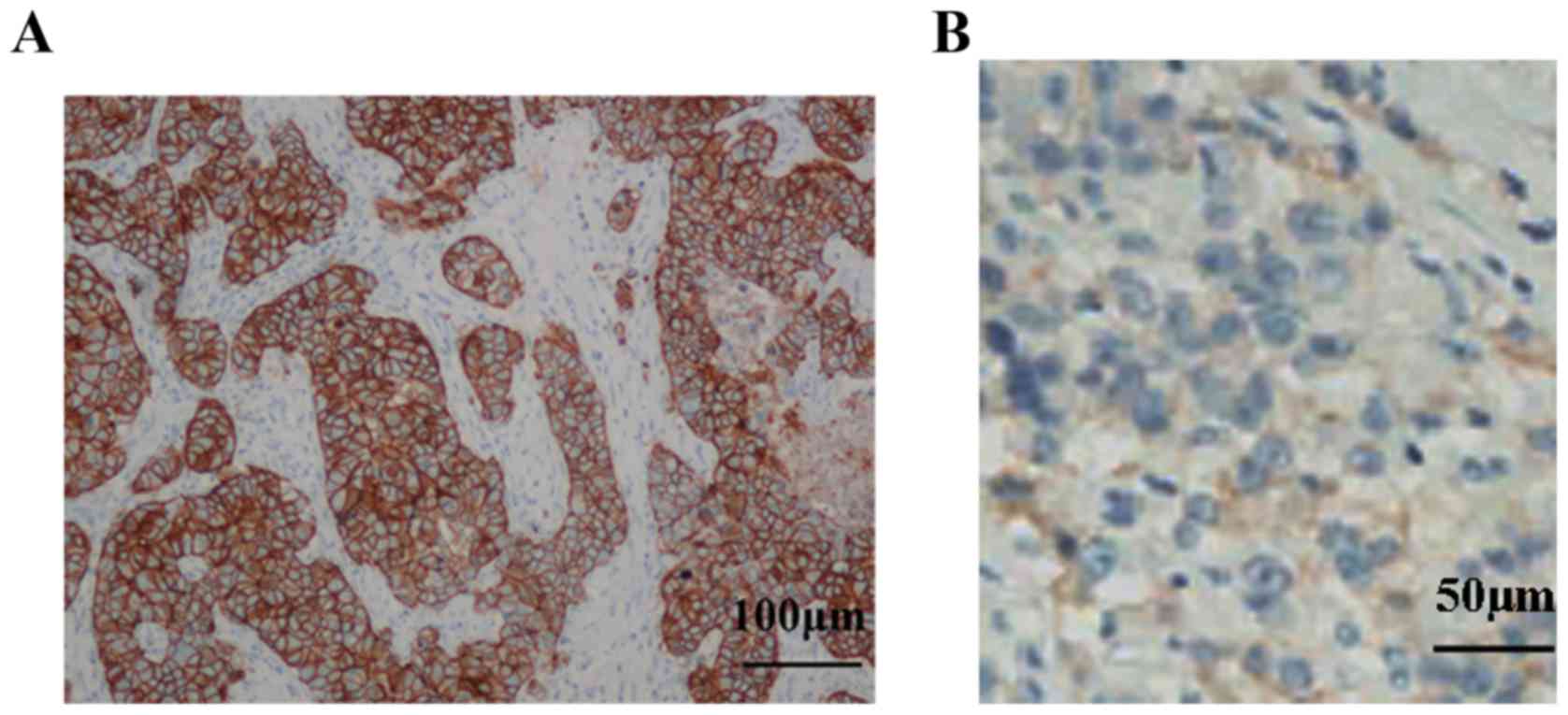
greater curvature (5/13) of the stomach. Immunohistochemistry
indicated that the samples were epidermal growth factor receptor
(EGFR)(+) and HER-2(3+), with Ki-67 (a cellular marker for
proliferation, 20%) (Fig. 2). The
immunohistochemical detection process was based on to the 2011
version of HER2 detection guidelines for gastric cancer (15,16).
Envision method was used for HER2 staining. Surgical specimens were
the selected samples for HER2 staining which were fixed with 4%
neutral buffered formaldehyde for 8 h at room temperature, then
embedded in paraffin wax. Slice thickness was between 3 and 5 µm.
Cooking boiling and heating methods were used for antigen retrieval
(dewaxed tissue section was placed into 0.01 mol/l citrate buffer
heated to 95°C for 15 min; then keep warm for 10 min; finally
naturally cooled to room temperature). Then sample were incubated
with 3% H2O2 for 10 min at room temperature
to block the interstitial source hyperparathyroid activity. Primary
antibody (Anti-ERBB2; cat. no. ab134182) was obtained from Abcam
(Cambridge, USA) and diluted into working fluids (1:500), which
bound to the extracellular domain of human ErbB2. A total of 50 µl
primary antibody working fluids were added for 30 min at room
temperature. DAB working fluid was added at room temperature for
5–30 min. Then the HER2 expression was observed under a bright
field of vision under a light microscope at a magnification ×400.
The HER2 scoring system was as follows: HER2 (−), no positive cells
or membrane-positive cells <10%; HER2 (1+), 10% of cells have
faint/inappreciable membrane staining or only partial cell membrane
staining; HER2 (2+), >10% of cells have weak, or
medium-intensity, complete U-type film or II-type coloring; HER2
(3+), >10% of cells have medium or strong intact U-type
membranes or II-type coloring.
From abdominal computed tomography (CT) and
contrast-enhanced ultrasound scanning following surgery, hepatic
metastasis was identified. The patient then received microwave
thermocoagulation therapy. Between December 2012 and June 2013, the
patient received chemotherapy with the SOX regimen for 8 cycles
[oxaliplatin (L-OHP), 130 mg/m2 on day 1, and
tegafur/gimeracil/oteracil (S-1) 50 mg twice daily on days 1–14,
every 3 weeks]. During this period, combined treatment with
trastuzumab was performed 12 times (8 mg/kg first dose and 6 mg/kg
followed on day 0, every 3 weeks) and the disease remained stable.
Trastuzumab combined with S-1 was used as the maintenance regimen
of the first-line therapy, which lasted 12 cycles.
In November 2015, the patient returned and was
reexamined. At this time, CT examination revealed mediastinal and
retro-peritoneal lymphadenectasis, which indicated disease
progression. Considering the benefit from the use of trastuzumab in
first-line chemotherapy (disease control was maintained for 26
months), a second-line regimen of 6 cycles of paclitaxel liposome
(L-PTX, 175 mg/m2 on day 1) and S-1 (50 mg twice daily
on days 1–14) together with trastuzumab (8 mg/kg first dose and 6
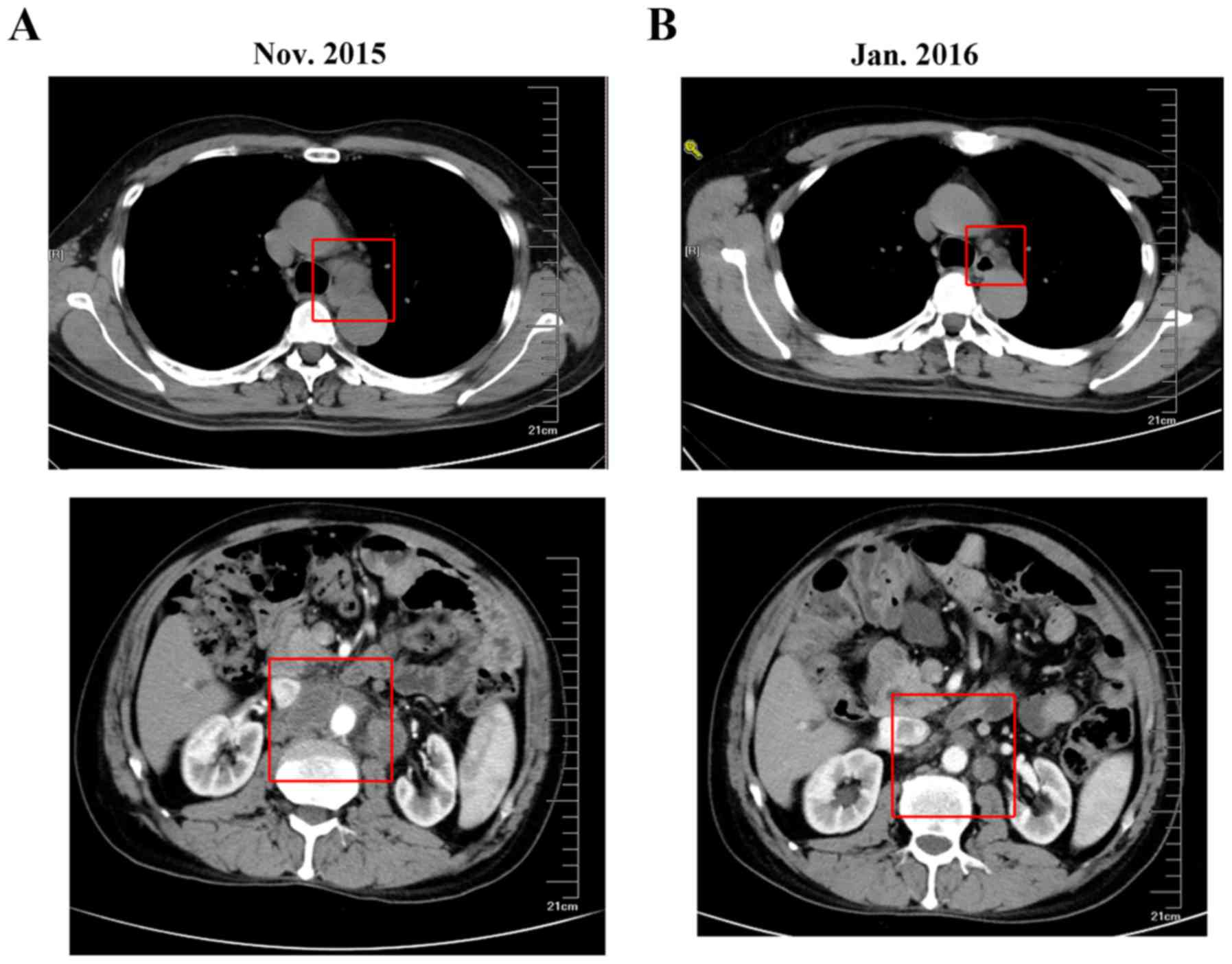
mg/kg followed on day 0) was selected. A CT scan in January 2016
revealed that the mediastinal and retro-peritoneal lymphadenectasis
had decreased in size (Fig. 3).
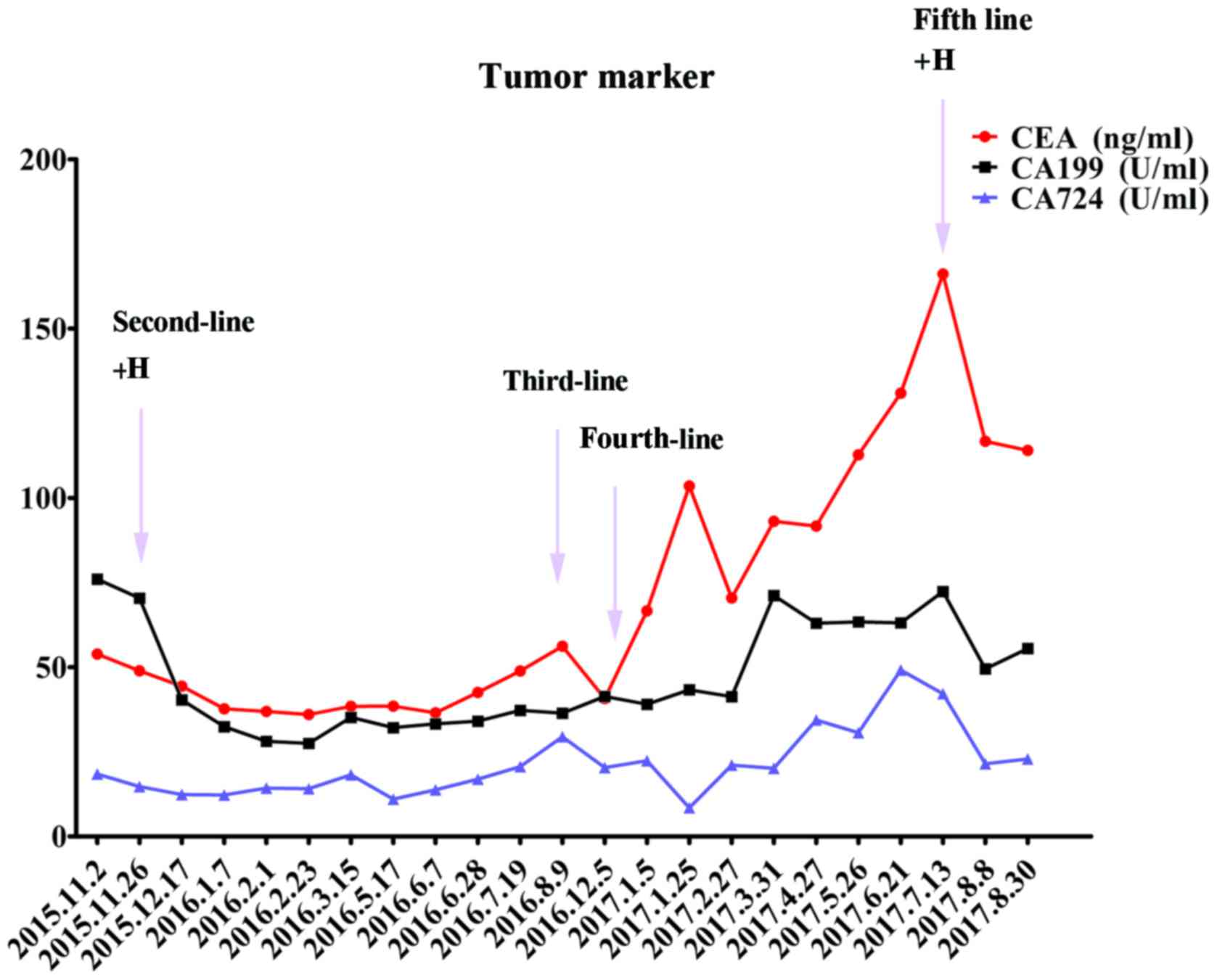
Furthermore, the tumor marker levels from the serum sample
(carcinoembryonic antigen and cancer antigen) were markedly
decreased (Fig. 4). In March 2016,
10-MV radiotherapy (tumor absorption dose 50 Gy/25 fraction)
targeting abdominal lymph node lesions was performed. Between March
2016 and August 2016, maintenance treatment of trastuzumab combined
with S-1 was administered. The disease was kept stable and the
tumor markers exhibited little fluctuation until a new pulmonary
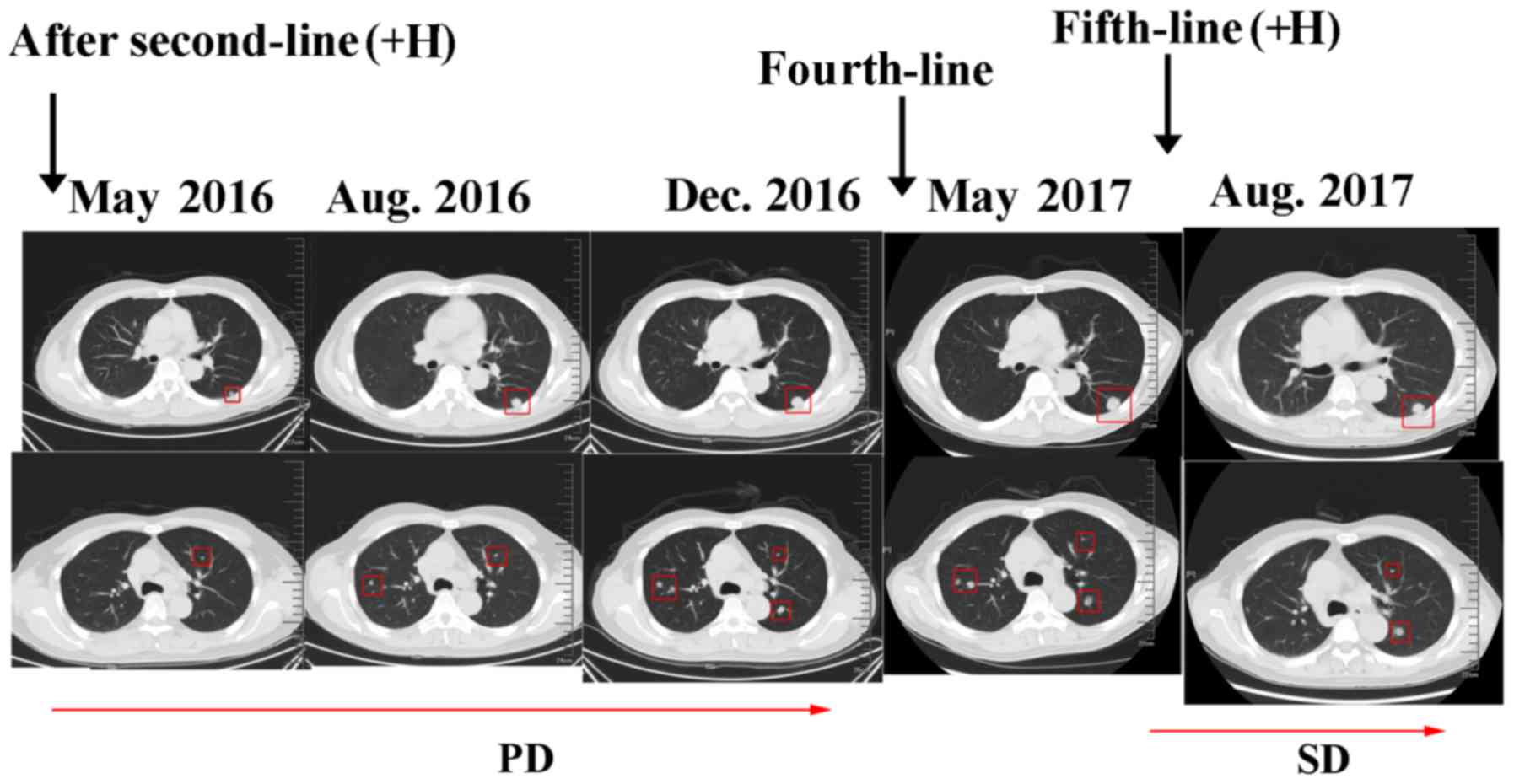
nodule was observed during a CT scan in August 2016. In the
subsequent 5 months, the targeted drug was changed to apatinib (250
mg twice daily, from August 2016 to December 2016) due to newly
observed pulmonary lesions. In December 2016, a further CT scan
revealed disease progression resulting from the pulmonary lesions
(Fig. 5).
There is no widely-accepted agreement on the
fourth-line therapy that should be used to treat advanced gastric
tumors. In the present study, the EOX chemotherapy regimen was
utilized for the patient (epirubicin, 50 mg/m2 on day 1;
L-OHP, 130 mg/m2 on day 1; xeloda 1,000 mg/m2
on days 1–14, every 3 weeks) for 5 cycles. The tumor marker levels
declined after 2 cycles and the disease was controlled stably for 6
months. However, a CT scan in May 2017 revealed multiple new
lesions in the lung (Fig. 5).
Up to this point, the patient had been treated with
a combined chemotherapy regimen with a HER2-targeting strategy
using multiple lines of therapy. When faced with the choice of the
fifth-line therapy, trastuzumab together with irinotecan (120
mg/m2, days 1 and 8) was selected. The lung lesion was
reduced in size and the tumor marker level was markedly decreased
(Fig. 5). The left ventricular
ejection fraction (LVEF), the most considerable toxic reaction of
trastuzumab, was kept stable during the therapy period (range,
62–74%) and the maximum decrease in LVEF was <16%. The
hematological toxicity was primarily leucopenia. Currently, the
patient remains under treatment in a stable condition.
Discussion
The present study reports the case of a patient with
HER2-overexpressing metastatic gastric cancer experiencing survival
benefits from multi-line chemotherapy combined with trastuzumab. At
the time of writing, fifth-line chemotherapy has been administered
to the patient and the treatment with trastuzumab has been utilized
three times.
Previously, a subgroup analysis of ToGA demonstrated
that patients with immunohistochemistry staining scores of 3+ or 2+
together with positive fluorescence in situ hybridization
results could experience survival benefits from trastuzumab
combined with chemotherapy (17). The
comparison of the median overall survival (mOS) was more
predominant (16.0 vs. 11.8 months) than that in the total cohort
(13.8 vs. 11.1 months) (8).
Therefore, the combination of trastuzumab with cisplatin and
fluorouracil (FU) or capecitabine (CAP) is considered the standard
first-line chemotherapeutic regimen of advanced HER2-positive
gastric cancer. Certain other phase II clinical trials have
evaluated the safety and efficiency of trastuzumab combined with
other chemotherapy regimens, including CAP plus L-OHP (9), S-1 plus cisplatin (10,18) and
CAP plus L-OHP (19). Although
evidence suggests that a platinum-based drug combination is more
likely to improve the survival of gastric cancer patients, the SOX
plus trastuzumab regimen was selected in favor of 5FU or
capecitabin plus CDDP as the first-line therapy due to the age and
physical condition of the patient described in the present study.
Compared with cisplatin and carboplatin, L-OHP has a reduced toxic
effect. In clinical trials, the L-OHP contained regimens, including
EOF, EOX and SOX, which demonstrated good efficiency and safety
(20,21). In the present case, first-line use of
trastuzumab with the SOX chemotherapy regimen achieved successful
stabilization of the disease for >2 years.
For those patients who experience progression
following first-line treatments, large randomized clinical trials
are lacking, and no consistent treatment recommendation is
available. Based on the results of the COUGAR-02 (22) and WJOG 4007 (23) studies, second-line chemotherapeutic
drugs for advanced gastric cancer patients are predominantly
taxanes or irinotecan. In the National Comprehensive Cancer Network
Clinical Practice Guidelines for Gastric Cancer, it is recommended
to use trastuzumab for posterior line therapy in patients with
HER2-positive advanced gastric cancer who do not use trastuzumab in
first-line therapy (24). The
JFMC45-1102 phase II clinical trial demonstrated the satisfactory
survival benefit of a weekly dose of paclitaxel combined with
trastuzumab, including progression-free survival (PFS) of 5.1
months) and mOS of 16.8 months, in patients with HER2-positive
cancer who did not receive trastuzumab treatment previously
(25). These data all support the use
of trastuzumab in second-line therapy. However, the prospective
data about cross-line application of trastuzumab treatment is
lacking. Whether trastuzumab may be used effectively alongside
another chemotherapy regimen following disease progression is
unknown. Based on the good reactivity of the present patient to
trastuzumab treatment in first-line therapy, trastuzumab plus
Tegafur Gimeracil Oteracil Potassium (S-1) and paclitaxel liposome
was attempted in second-line therapy (26). The disease remained stable for 10
months. Table summarizes the regimens and survival duration of
patients in clinical trials with second-line treatment reported in
the literature (12,22,23,27–29).
The PFS ranged from 2.2 to 3.6 months and the OS ranged from 4.0 to
9.5 months (Table I). The survival
time of the present patient exceeded the median survival time
presented in those studies that contained only chemotherapy as the
second-line therapy. Thus, we hypothesize that the targeted agent
may bring benefits to patients with advanced disease even
subsequent to first-line therapy. Other drugs targeting HER2,
including lapatinib, trastuzumab emtansine (T-DM1) and
anti-angiogenic drugs, such as ramucirumab and bevacizumab, are
also alternative treatments in the case of first-line treatment
failure (30,31). The REGARD (32) and RAINBOW (33) trials analyzed the efficiency of
ramucirumab in the second-line treatment of HER2-amplified advanced
gastric cancer. No matter the single-agent use or combination with
paclitaxel, ramucirumab significantly improved the mOS for nearly 2
months. Therefore, ramucirumab in combination with taxane drugs is
approved by the Food and Drug Administration for the second-line
treatment of advanced gastric cancer. This provides novel options
for physicians to treat cancer, which will be of benefit to
patients.
 | Table I.Summary of clinical trials containing
second-line chemotherapy. |
Table I.
Summary of clinical trials containing
second-line chemotherapy.
|
|
|
| Survival time,
months |
|
|---|
|
|
|
|
|
|
|---|
| First author | Year | Regimen | PFS | OS | (Refs.) |
|---|
| Thuss-Patience et
al | 2011 | Irinotecan | / | 4.0 | (27) |
| Kang et
al | 2012 | Irinotecan, | / | 6.5 | (12) |
|
|
| Docetaxel |
| 5.2 |
|
| Meads et
al | 2014 | Docetaxel | / | 5.2 | (23) |
| Hironaka et
al | 2013 | Paclitaxel | 3.6 | 9.5 | (22) |
|
|
| Irinotecan | 2.3 | 8.4 |
|
| Sym et
al | 2013 | Irinotecan | 2.2 | 5.8 | (28) |
|
|
| Irinotecan plus
5-FU/leucovorin | 3.0 | 6.7 |
|
| Maruta et
al | 2007 | Docetaxel | / | 4.0 | (29) |
|
|
| Docetaxel plus
5-FU |
| 7.6 |
|
To date, there has been a lack of randomized
controlled clinical trials to support third-line treatment, but
certain retrospective studies have identified that patients
receiving multi-line treatment have experienced improved survival
times (34,35). A phase III trial verified that
apatinib treatment significantly improved OS (6.5 vs. 4.7 months)
and PFS (2.6 vs. 1.8 months) times following two or more prior
lines of failed chemotherapy (36). A
number of associated studies regarding immune checkpoint
inhibitors, including pembrolizumab and nivolumab, have been
performed (37,38). In the present study, when taking the
previous treatment and the condition of the patient into account,
it was decided that continuing therapy was possible and apatinib
was selected as the third-line therapy, which lasted for 4 months
and was then changed to the EOX regimen. The fourth-line therapy
controlled the disease for 6 months. The disease then progressed
further and the regimen was changed to trastuzumab plus irinotecan.
The use of trastuzumab combined with different chemotherapy
regimens in cross-line treatment has contributed to the survival
benefit of this patient and demonstrated the efficiency of
trastuzumab with good safety.
The present study also has a number of limitations.
Firstly, although the expression of HER2 was determined using
immunohistochemistry, the absence of corroborating western blotting
data and the lack of immunohistochemistry on non-cancerous tissue
samples are limitations of this study. Secondly, the effect of this
cross-line application of trastuzumab should be evaluated in
numerous situations in the future, as opposed to in a single
patient. Although the disease was controlled well, cardiac and
hematological toxicities were also observed in this case.
Therefore, the long-term safety and effectiveness of this therapy
require evaluation in subsequent follow-ups.
In conclusion, the application of trastuzumab in
multi-line treatment for a patient with HER2-positive advanced
gastric cancer appears to have benefited the survival of the
present patient, and may be of benefit to others in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the SEERdatabase (National Cancer
Institute) repository, http://seer.cancer.gov/seerstat.
Authors' contributions
LZ and LW gave contributions to conception and
design. LZ, PL, XZ and LW reviewed the literature and designed the
article structure. JinY, AZ and MZ contributed to the acquisition
and analysis of data. JY gave interpretation of data. ZC was a
major contributor in writing the manuscript. XZ, JY and JinY
revised and edited the manuscript critically for important
intellectual content. ZC, LW, JY and JinY gave final approval of
the version to be published.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
First Affiliated Hospital of Xi'an Jiaotong University Ethics
Committee.
Patient consent for publication
The patient provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng R, Zeng H, Zhang S and Chen W:
Estimates of cancer incidence and mortality in China, 2013. Chin J
Cancer. 36:662017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukushima R: Recent progress in the
treatment of advanced gastric cancer. Nihon Geka Gakkai Zasshi.
113:32012.(In Japanese). PubMed/NCBI
|
|
4
|
Xu JM: Comments on the seven clinical
questions & answers in Japanese gastric treatment guidelines of
the 4th edition. Zhonghua Zhong Liu Za Zhi (Chinese J Oncol).
39:236–238. 2017.
|
|
5
|
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM,
Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, et
al: HER2 screening data from ToGA: Targeting HER2 in gastric and
gastroesophageal junction cancer. Gastric Cancer. 18:476–484. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho J, Jeong J, Sung J, Sung CO, Kim KM,
Park CK, Choi MG, Sohn TS, Bae JM and Kim S: A large cohort of
consecutive patients confirmed frequent HER2 positivity in gastric
carcinomas with advanced stages. Ann Surg Oncol. 20 Suppl
3:S477–S484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim KC, Koh YW, Chang HM, Kim TH, Yook JH,
Kim BS, Jang SJ and Park YS: Evaluation of HER2 protein expression
in gastric carcinomas: Comparative analysis of 1,414 cases of
whole-tissue sections and 595 cases of tissue microarrays. Ann Surg
Oncol. 18:2833–2840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S,
Wang J, Xu N, Cheng Y, Bai Y, et al: Optimal regimen of trastuzumab
in combination with oxaliplatin/capecitabine in first-line
treatment of HER2-positive advanced gastric cancer (CGOG1001): A
multicenter, phase II trial. BMC Cancer. 16:682016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chua C, Tan IB, Yamada Y, Rha SY, Yong WP,
Ong WS, Tham CK, Ng M, Tai DW, Iwasa S, et al: Phase II study of
trastuzumab in combination with S-1 and cisplatin in the first-line
treatment of human epidermal growth factor receptor HER2-positive
advanced gastric cancer. Cancer Chemother Pharmacol. 76:397–408.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brower V: Apatinib in treatment of
refractory gastric cancer. Lancet Oncol. 17:e1372016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang JH, Lee SI, Lim DH, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang EJ, Im SA, Oh DY, Han SW, Kim JS,
Choi IS, Kim JW, Kim YJ, Kim JH, Kim TY, et al: Irinotecan combined
with 5-fluorouracil and leucovorin third-line chemotherapy after
failure of fluoropyrimidine, platinum, and taxane in gastric
cancer: Treatment outcomes and a prognostic model to predict
survival. Gastric Cancer. 16:581–589. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tarazona N, Smyth EC, Peckit C, Chau I,
Watkins D, Rao S, Starling N and Cunningham D: Efficacy and
toxicity of salvage weekly paclitaxel chemotherapy in non-Asian
patients with advanced oesophagogastric adenocarcinoma. Ther Adv
Med Oncol. 8:104–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guideline Recommendations for HER2
Detection in Gastric Cancer Group: Guidelines for HER2 detection in
gastric cancer (2016). Zhonghua Bing Li Xue Za Zhi. 45:528–532.
2016.PubMed/NCBI
|
|
16
|
Guideline Recommendations for HER2
Detection in Gastric Cancer Group: Guidelines for HER2 detection in
gastric cancer. Zhonghua Bing Li Xue Za Zhi. 40:553–557.
2011.PubMed/NCBI
|
|
17
|
Lei YY, Huang JY, Zhao QR, Jiang N, Xu HM,
Wang ZN, Li HQ, Zhang SB and Sun Z: The clinicopathological
parameters and prognostic significance of HER2 expression in
gastric cancer patients: A meta-analysis of literature. World J
Surg Oncol. 15:682017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kurokawa Y, Sugimoto N, Miwa H, Tsuda M,
Nishina S, Okuda H, Imamura H, Gamoh M, Sakai D, Shimokawa T, et
al: Phase II study of trastuzumab in combination with S-1 plus
cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer.
110:1163–1168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ryu MH, Yoo C, Kim JG, Ryoo BY, Park YS,
Park SR, Han HS, Chung IJ, Song EK, Lee KH, et al: Multicenter
phase II study of trastuzumab in combination with capecitabine and
oxaliplatin for advanced gastric cancer. Eur J Cancer. 51:482–488.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang FQ: A clinical study of DOF
regimenvsSOX regimen as first-line treatment in patients with
advanced gastric carcinoma. World Chin J Digestol. 22:48302014.
View Article : Google Scholar
|
|
21
|
Cunningham D, Okines AF and Ashley S:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 362:858–859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hironaka S, Ueda S, Yasui H, Nishina T,
Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki
T, et al: Randomized, open-label, phase III study comparing
irinotecan with paclitaxel in patients with advanced gastric cancer
without severe peritoneal metastasis after failure of prior
combination chemotherapy using fluoropyrimidine plus platinum: WJOG
4007 trial. J Clin Oncol. 31:4438–4444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meads DM, Marshall A, Hulme CT, Dunn JA
and Ford HE: The cost effectiveness of docetaxel and active symptom
control versus active symptom control alone for refractory
oesophagogastric adenocarcinoma: Economic analysis of the COUGAR-02
trial. PharmacoEconomics. 34:33–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ajani JA, D'Amico TA, Almhanna K, Bentrem
DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, et
al: Gastric cancer, version 3.2016, NCCN clinical practice
guidelines in oncology. J Natl Compr Cancer Netw. 14:1286–1312.
2016. View Article : Google Scholar
|
|
25
|
Nishikawa K, Takahashi T, Takaishi H, Miki
A, Noshiro H, Yoshikawa T, Nishida Y, Iwasa S, Miwa H, Masuishi T,
et al: Phase II study of the effectiveness and safety of
trastuzumab and paclitaxel for taxane- and trastuzumab-naive
patients with HER2-positive, previously treated, advanced, or
recurrent gastric cancer (JFMC45-1102). Int J Cancer. 140:188–196.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ford HE, Marshall A, Bridgewater JA,
Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S,
Middleton GW, et al: Docetaxel versus active symptom control for
refractory oesophagogastric adenocarcinoma (COUGAR-02): An
open-label, phase 3 randomised controlled trial. Lancet Oncol.
15:78–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thuss-Patience PC, Kretzschmar A, Bichev
D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G
and Reichardt P: Survival advantage for irinotecan versus best
supportive care as second-line chemotherapy in gastric cancer-a
randomised phase III study of the Arbeitsgemeinschaft
Internistische Onkologie (AIO). Eur J Cancer. 47:2306–2314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sym SJ, Hong J, Park J, Cho EK, Lee JH,
Park YH, Lee WK, Chung M, Kim HS, Park SH and Shin DB: A randomized
phase II study of biweekly irinotecan monotherapy or a combination
of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients
with metastatic gastric adenocarcinoma refractory to or progressive
after first-line chemotherapy. Cancer Chemother Pharmacol.
71:481–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maruta F, Ishizone S, Hiraguri M, Fujimori
Y, Shimizu F, Kumeda S and Miyagawa S: A clinical study of
docetaxel with or without 5′DFUR as a second-line chemotherapy for
advanced gastric cancer. Med Oncol. 24:71–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Satoh T, Xu RH, Chung HC, Sun GP, Doi T,
Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, et al: Lapatinib plus
paclitaxel versus paclitaxel alone in the second-line treatment of
HER2-amplified advanced gastric cancer in Asian populations:
TyTAN-a randomized, phase III study. J Clin Oncol. 32:2039–2049.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen SC, Kagedal M, Gao Y, Wang B,
Harle-Yge ML, Girish S, Jin J and Li C: Population pharmacokinetics
of trastuzumab emtansine in previously treated patients with
HER2-positive advanced gastric cancer (AGC). Cancer Chemother
Pharmacol. 80:1147–1159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan M, Shi Z, Wang Z, Lv W, Yang Y, Lu F,
Zhao Y and Zhong H: The significance of multi-line chemotherapy for
advanced gastric cancer: A retrospective analysis. Mol Clin Oncol.
6:606–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang W, Yu Y, Fang Y, Wang Y, Cui Y, Shen
K and Liu T: Systemic chemotherapy as a main strategy for liver
metastases from gastric cancer. Clin Transl Oncol. 17:888–894.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase iii trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moore KN, Martin LP, O'Malley DM,
Matulonis UA, Konner JA, Perez RP, Bauer TM, Ruiz-Soto R and Birrer
MJ: Safety and activity of mirvetuximab soravtansine (IMGN853), a
folate receptor alpha-targeting antibody-drug conjugate, in
platinum-resistant ovarian, fallopian tube, or primary peritoneal
cancer: A phase I expansion study. J Clin Oncol. 35:1112–1118.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamasaki M, Saito N, Hada Y, Miyamoto S,
Okanobu H, Ikeda N, Daido W, Ishiyama S, Deguchi N, Taniwaki M and
Ohashi N: Nivolumab therapy for synchronous ALK-positive lung
cancer and gastric cancer. Case Rep Oncol. 10:361–367. 2017.
View Article : Google Scholar : PubMed/NCBI
|