Introduction
Choriocarcinoma is a highly malignant trophoblastic
pregnancy-associated tumor that often occurs with complete
hydatidiform mole (1,2). It grows rapidly and is able to
metastasize widely to other organs or tissues through the venous
and lymphatic system. Although the complete recovery rate has
improved owing to advances in chemotherapy, the toxicity and side
effects of the drugs used are poorly tolerated (3). Furthermore, ~7% of low-risk patients and
27% of high-risk patients may exhibit an incomplete response to
first-line, single-agent or multi-agent chemotherapy, and may
relapse from remission (4). Thus, it
remains necessary to develop novel, specific and low-toxicity drugs
for the treatment of choriocarcinoma.
Dihydromyricetin (DMY;
C15H12O8), a natural flavonoid, is
an active component of extracts from Ampelopsis
grossedentata (5). Numerous
pharmacological functions of DMY have been reported, including
antioxidant, antibacterial, anti-inflammatory, antihypertensive,
hepatoprotective and anticancer effects (6–10). The
potent in vitro antitumor activity of DMY has also been
revealed through the induction of apoptosis in various cell lines,
including HepG2 cells, head and neck squamous cell carcinoma, human
non-small cell lung cancer and gastric cancer cells (11–14). DMY
has been indicated to have antitumor effects in nude mice
inoculated with GLC-82 lung cancer cells, as well as nude mice
inoculated with Bel-7402 hepatocellular carcinoma cells (15,16).
Furthermore, DMY could suppress distant pulmonary metastasis of 4T1
mouse breast carcinoma (17). DMY has
been demonstrated to exert a strong antitumor effect with low
toxicity (18,19) with a maximum tolerated dose of 5.0
g/kg in Wistar mice (20). DMY has
been indicated to exhibit antitumor activity in vitro and
in vivo without evident toxicity. However, the effects of
DMY on human choriocarcinoma remain to be described. In the present
study it was revealed that DMY inhibited JAr cell viability in
vitro, which indicated that DMY may be a novel drug for the
treatment of choriocarcinoma. Subsequently, the antitumor activity
of DMY in human choriocarcinoma was determined.
Materials and methods
Reagents
DMY (>99% purity) was purchased from the Beijing
Hengyuan Qitian Research Institute of Chemical Technology (Beijing,
China) and dissolved in DMSO (<0.05%, v/v, without detectable
effects) for all study experiments.
Cell culture
Human fetally derived trophoblast choriocarcinoma
JAr cells were obtained from the State Key Laboratory of
Reproductive Biology, Institute of Zoology, Chinese Academy of
Sciences (Shanghai, China). JAr cells were cultured at 37°C in 5%
CO2 in DMEM medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.). The cells were then
passaged using 0.25% trypsin and 0.02% EDTA (Gibco; Thermo Fisher
Scientific, Inc.) when the confluency reached 70–80%.
MTT assay
JAr cells were seeded in 96-well plates and allowed
to adhere overnight. When the confluency reached 30–40%, the cells
were incubated for 24 or 48 h with 0, 20, 40, 80 or 100 mg/l of DMY
in 200 µl. Each treatment was performed in 6 wells. The cell
viability was determined using MTT reagent (Gibco; Thermo Fisher
Scientific, Inc.), according the manufacturer's protocol, and the
absorbance was determined at a wavelength of 492 nm by using a
Multiskan MK3 microplate reader (Thermo Fisher Scientific Inc.,
Waltham, MA, USA). The experiment was repeated three times.
Flow cytometry assay
JAr cells were incubated for 48 h with DMY at the
designated concentrations (0, 40, 60 and 100 mg/l) and then
processed with an AV-FITC kit (BioBox, Ba11100, Nanjing, China), in
accordance with the manufacturer's protocol. The samples were
analyzed by a FACSCalibur flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) at 488 nm, in order to quantify the apoptotic
rate.
TUNEL assay
Apoptosis of JAr cells was determined using a TUNEL
detection kit (Roche Applied Science, Penzberg, Germany) in
accordance with the manufacturer's protocol. The JAr cells were
incubated for 48 h with DMY at the designated concentrations (0,
40, 60 and 100 mg/l), fixed with 4% paraformaldehyde in PBS for 1 h
at room temperature and permeabilized with 0.1% Triton X-100 in
0.1% sodium citrate for 2 min on ice. According to the
manufacturer's protocol, a positive control was permeabilized with
DNase I recombinant for 10 min at 15–25°C to induce DNA strand
breaks. Then the cells were washed in PBS, incubated with the TUNEL
reaction mixture for 1 h at 37°C (a negative control incubated with
label solution instead of TUNEL reaction mixture), washed again
with PBS, and incubated with 0.1 µg/ml DAPI in PBS at 30°C for
15–30 min. The samples were analyzed, following a final wash with
PBS, under a fluorescence microscope (IX73; Olympus, Tokyo, Japan).
The apoptotic rate was quantified by counting the apoptotic cells
in six random fields.
Western blot analysis
The JAr cells were incubated for 48 h with DMY at
the designated concentrations (0, 40, 60 and 100 mg/l), collected,
and lysed on ice with radioimmunoprecipitation assay buffer (Thermo
Fisher Scientific, Inc.). The cell lysates were centrifuged at
14,000 × g for 10 min at 4°C. The protein concentration was
quantified by the bicinchoninic acid assay (BCA; Pierce; Thermo
Fisher Scientific, Inc.). Equal amounts (30 µg) of protein were
separated by 12% SDS-PAGE and then transferred onto a
polyvinylidene difluoride (PVDF) membrane. The membranes were
blocked with 5% non-fat milk and incubated with the following
primary antibodies (diluted 1:1,000) overnight at 4°C: β-actin
(mouse anti-human monoclonal; cat. no. sc-130065; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), Bax (mouse anti-human
monoclonal; cat. no. ab77566; Abcam, Cambridge, UK), Bcl-2 (rabbit
anti-human polyclonal; cat. no. E1A6139; Enogene, Nanjing, China),
poly (ADP-ribose) polymerase (Parp), pro-caspase-3 and cleaved
caspase-3 (rabbit anti-human polyclonal; cat. nos. 9661s and
14220s, respectively; Cell Signaling Technology, Inc., Danvers, MA,
USA). The membranes were then washed in TBS-Tween (0.05%) buffer
and incubated with a secondary antibody (peroxidase-conjugated
Affinipure goat anti-mouse IgG, cat. no. ab6728;
peroxidase-conjugated Affinipure goat anti-rabbit IgG, cat. no.
ab6721; dilution 1:5,000; Abcam) for 1 h at room temperature.
Immunoreactive bands were detected by enhanced chemiluminescence
(Pierce; Thermo Fisher Scientific, Inc.) and imaged using the
Tanon-6100 Chemiluminescent Imaging system (Tanon Science and
Technology Co., Ltd., Shanghai, China). The band densities were
calculated with Quantity One software 4.6.2 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(IBM Corp., Armonk, NY, USA). All data are expressed as the mean ±
standard deviation. One-way analysis of variance was used to make
comparisons between groups. The pairwise comparison of means among
groups was performed using the Student-Newman-Keuls method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
DMY inhibits the viability of JAr
cells
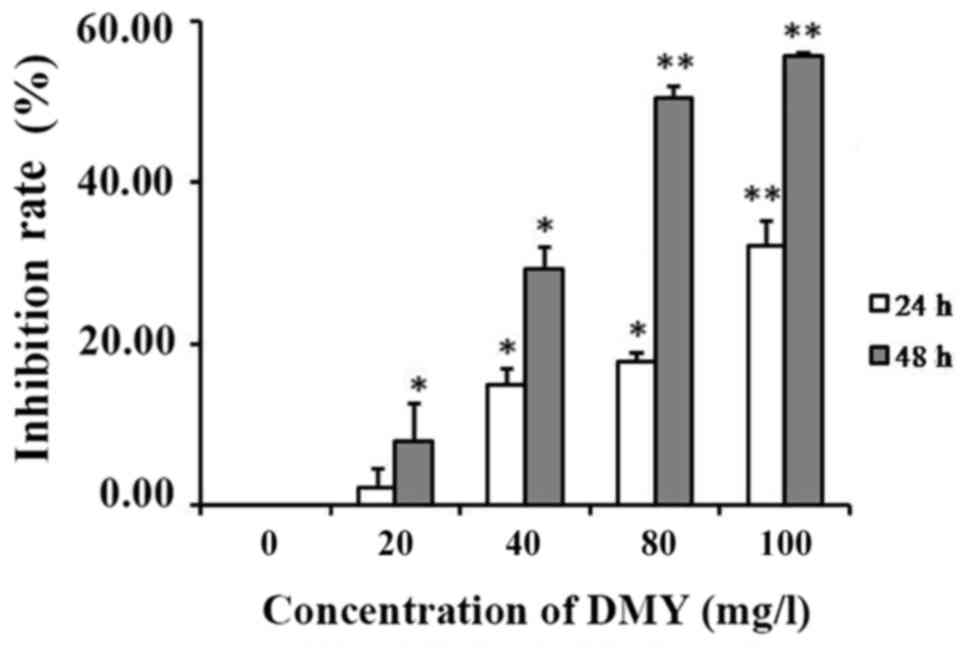
An MTT proliferation assay was performed to evaluate
the influence of 0, 20, 40, 80 and 100 mg/l DMY on the cellular
viability of JAr cells at 24 and 48 h (Fig. 1). As indicated, the viability of JAr
cells was time- and dose-dependently reduced following treatment
with DMY in comparison with the control cells (P<0.05; Fig. 1).
DMY induced JAr cell apoptosis
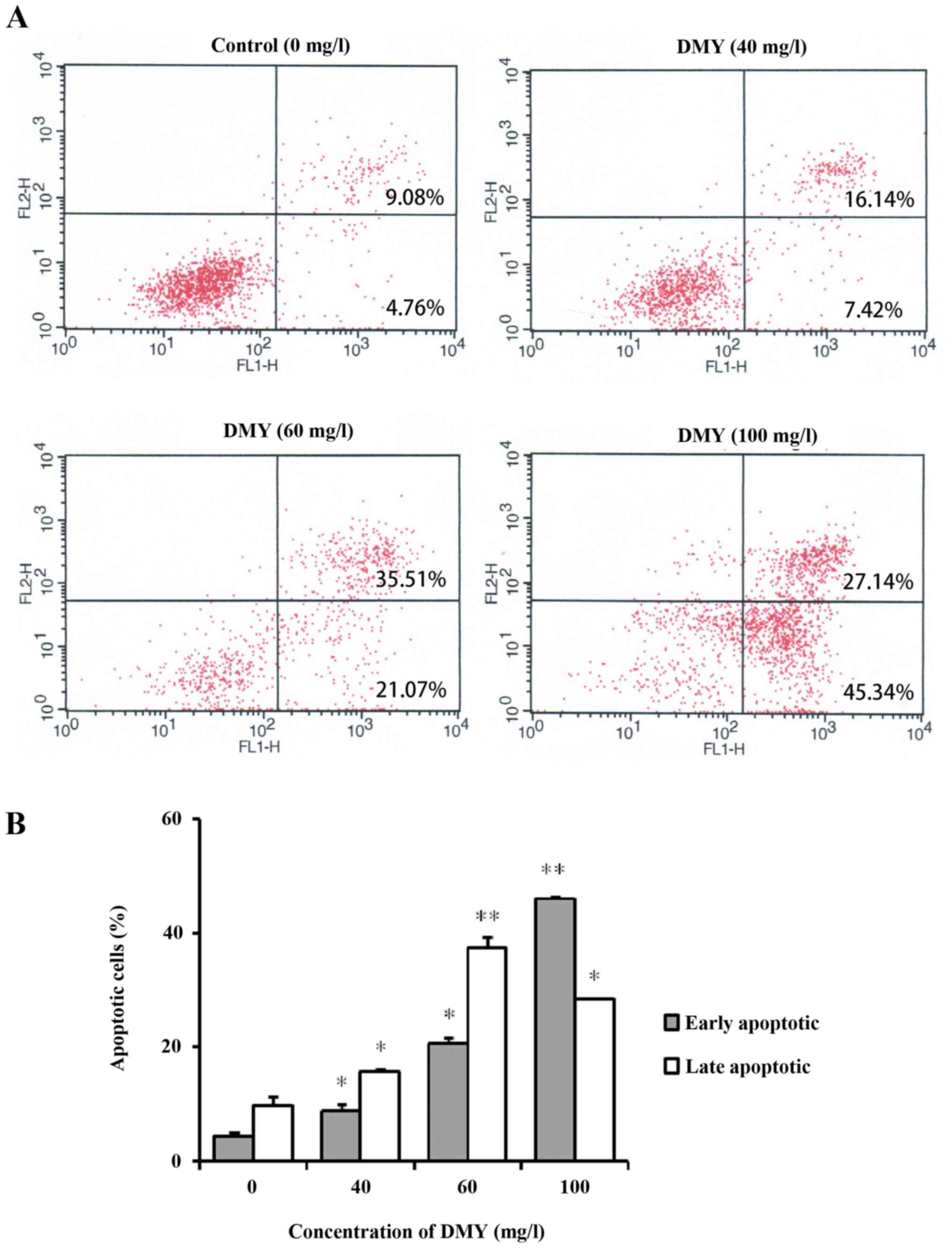
The present study investigated apoptosis following
incubation with different concentrations of DMY for 48 h. The
quantitative analysis of apoptosis by flow cytometry using Annexin
V/PI dual staining revealed that the apoptotic rate increased in a
dose-dependent manner (Fig. 2A). At
0, 40, 60 and 100 mg/l of DMY the proportion of apoptotic cells was
14.2±1.69, 24.43±1.72, 58±2.08 and 74.42±0.41%, respectively
(P<0.05; Fig. 2B).
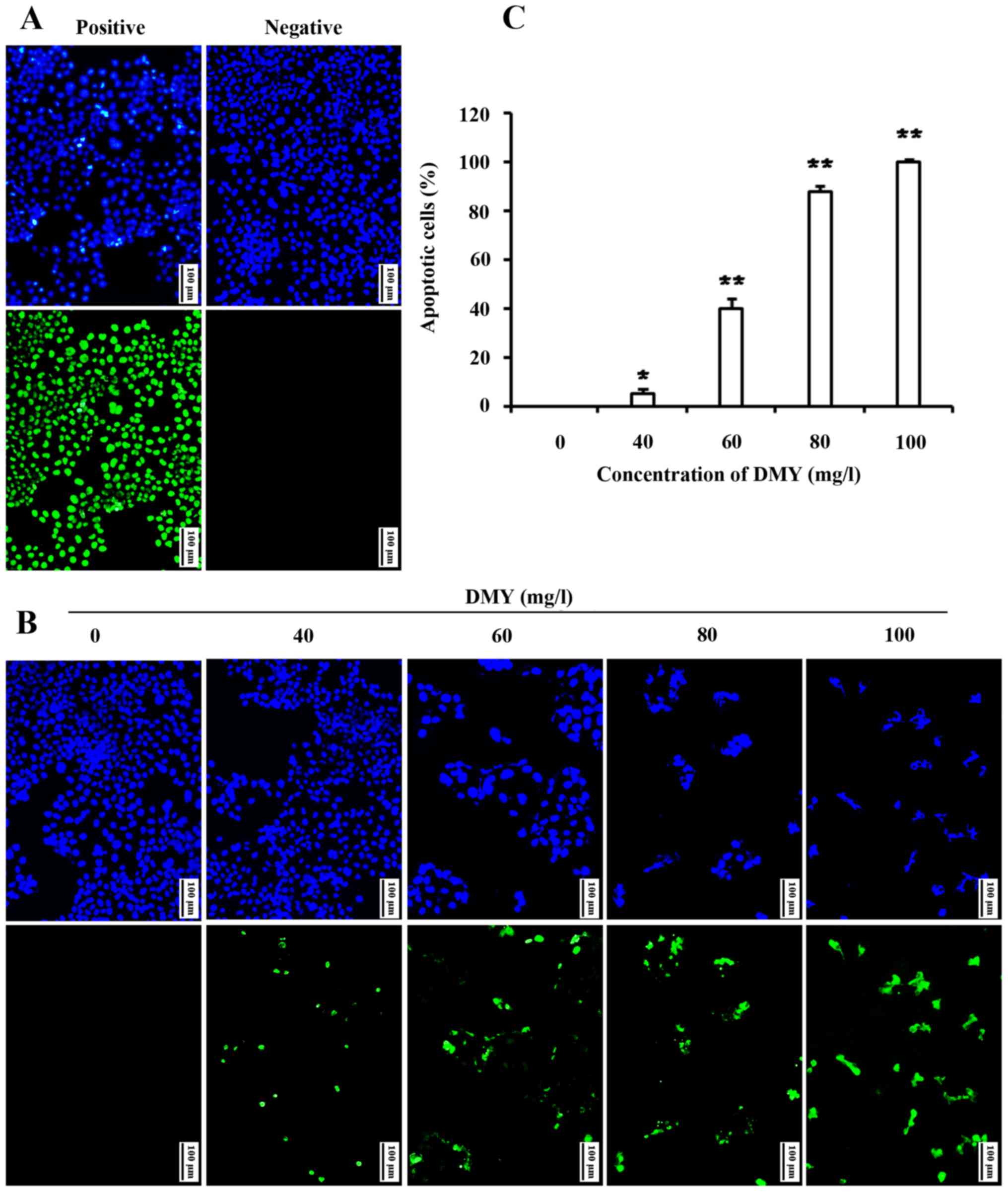
The DMY-induced apoptosis of JAr cells was also
confirmed by TUNEL staining. In the control group, few apoptotic
JAr cells were observed, but DMY treatment resulted in a
dose-dependent increase in TUNEL-positive JAr cells (P<0.05;
Fig. 3).
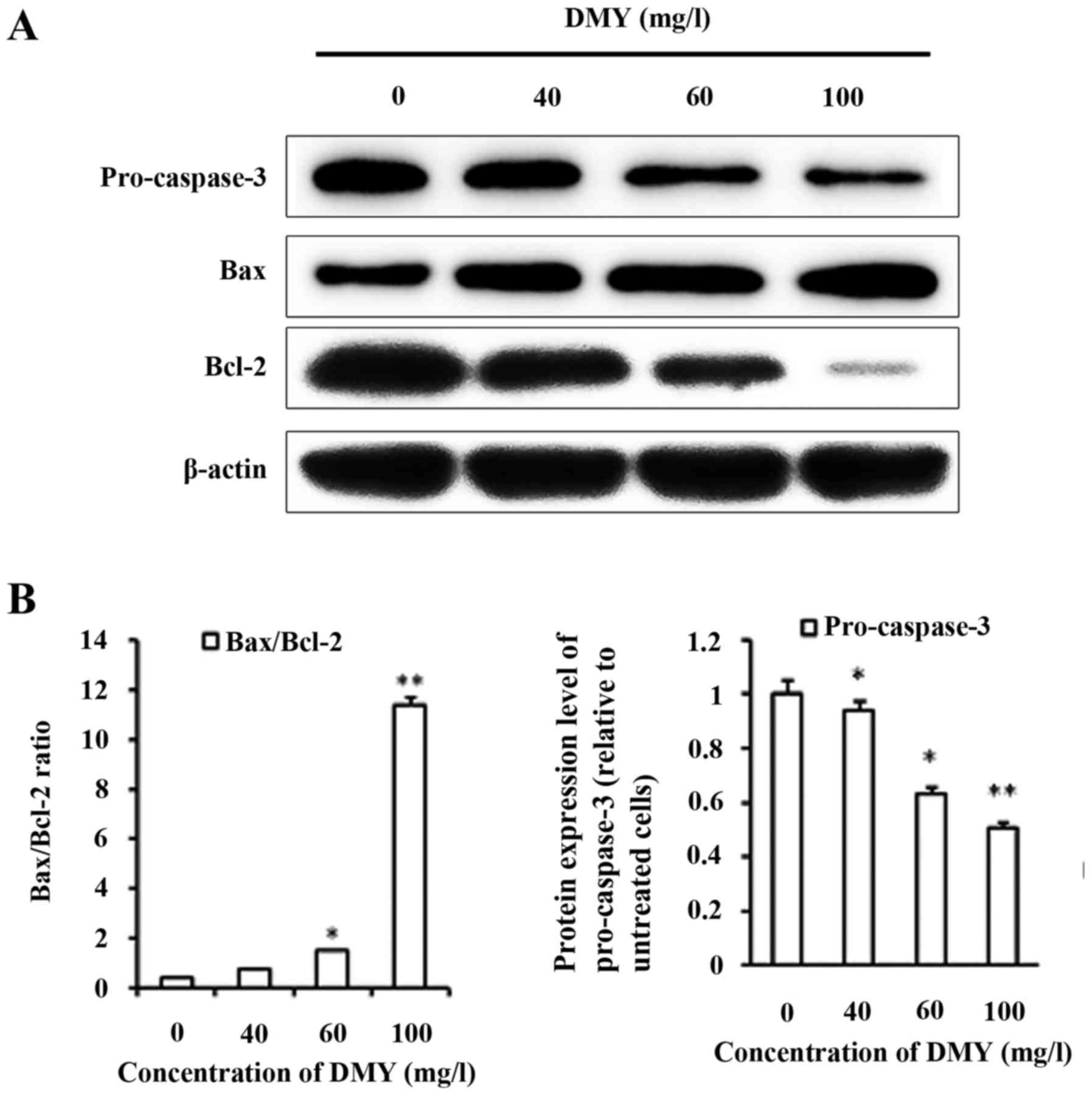
DMY induced apoptosis increases the
Bax/Bcl-2 ratio and decreases pro-caspase-3 expression
With DMY treatment, the protein expression level of
Bax increased, whereas the expression level of Bcl-2 decreased in a
dose-dependent manner (Fig. 4A). The
Bax/Bcl-2 ratio significantly increased with DMY treatment in
comparison with that of the control group (P<0.05), particularly
in the 100 mg/l group (P<0.01; Fig.
4B). The expression of pro-caspase-3 decreased with DMY
treatment, (P<0.05) and cleaved caspase-3 was not detected
(Fig. 4B).
Discussion
In the current study, an MTT proliferation assay was
performed to determine the effect of DMY treatments at different
concentrations and for different time periods. The results revealed
that DMY reduced JAr cell viability in a time- and dose-dependent
manner. Two distinct modes of cell death, apoptosis and necrosis,
can be distinguished from the differences in the morphological,
biochemical and molecular changes of cells. Flow cytometry was used
to detect phosphatidylserine ectropion and the TUNEL assay was used
to detect DNA fragmentation of apoptosis. The two methods detect
the morphological and the biochemical processes of apoptosis and
were performed at 48 h of DMY treatment, the time point at which
the inhibitory effect was most significant. The results revealed
that DMY induced the apoptosis of JAr cells in a dose-dependent
manner, suggesting that DMY reduced proliferation through the
induction of apoptosis.
Apoptosis is a form of programmed cell death that
serves an important role in the development and treatment of tumors
(21). The regulatory pathways of
apoptosis, which include a number of gene families, orchestrate the
specific morphological and biochemical changes that occur during
the apoptotic process (22). In the
mitochondrial pathway to cell death, the apoptotic threshold is set
by interactions on the mitochondrial outer membrane between three
functionally and structurally distinct subgroups of the Bcl-2
protein family: Bcl-2 homology 3 (BH3)-only proteins, which convey
signals to initiate apoptosis, pro-survival cell guardians,
including Bcl-2 itself, and pro-apoptotic effector proteins,
including Bax and BCl-2 antagonist/killer (23). Bax forms oligomers, which permeabilize
the mitochondrial outer membrane, releasing cytochrome c into the
cytosol and activating the effector caspases (24–26). Bcl-2
binds to Bax and inhibits its pro-apoptotic activity (27). To investigate the mechanism by which
DMY triggers apoptosis, western blot analysis was performed to
analyze the potential proteins involved. The results revealed that
DMY treatment increased the protein expression level of Bax and
decreased that of Bcl-2. Although cleaved caspase-3 was not
detected, the expression level of pro-caspase-3 decreased, which
indicated that it was cleaved and activated. Pro-caspase-3 is
processed by autoproteolytic cleavage (or by one or more other
proteases in transit) that leads to the assembly of the active
heterotetrameric enzyme (28). The
activation of caspase-3 raises the question of whether this
protease is required for cell death (28).
It was concluded that DMY induced apoptosis in the
JAr cell line and it was confirmed that the apoptosis was
mitochondrially mediated by changes in the expression level of the
Bax/Bcl-2 protein ratio and decreased protein expression of
pro-caspase-3. To determine whether DMY could be a novel
therapeutic drug for choriocarcinoma, further studies are being
performed, in which the promising prospects of the present study
will be reported in detail.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Key Subjects
in Universities and Colleges of Hebei Province of China [Pathology
and Pathophysiology; grant no. JiJiaoGao(2013)4], the Excellent
Innovation Talent Support Plan of Hebei Education Department (grant
no. SLRC2017018) and the Project in Hebei province Department of
Education (grant no. QN2016012).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZZ designed the study, performed the experiment,
analyzed and interpreted the data and prepared the first draft of
the manuscript. QX, YJL and DYS contributed to the design, data
analysis and revision of the manuscript. KW and YTL performed the
experiment. XJL contributed to flow cytometry assay. YHL developed
the concept of the study, performed the data analysis and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu Q, Tan Y, Zhang K and Li Y: Crosstalk
between p38 and Smad3 through TGF-β1 in JEG-3 choriocarcinoma
cells. Int J Oncol. 43:1187–1193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan Y, Xu Q, Li Y, Mao X and Zhang K:
Crosstalk between the p38 and TGF-β signaling pathways through
TβRI, TβRII and Smad3 expression in plancental choriocarcinoma
JEG-3 cells. Oncol Lett. 8:1307–1311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto E, Ino K, Yamamoto T, Sumigama S,
Nawa A, Nomura S and Kikkawa F: A pure nongestational
choriocarcinoma of the ovary diagnosed with short tandem repeat
analysis: Case report and review of the literature. Int J Gynecol
Cancer. 17:254–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobayashi Y, Shimizu T, Naoe H, Ueki A,
Ishizawa J, Chiyoda T, Onishi N, Sugihara E, Nagano O, Banno K, et
al: Establishment of a choriocarcinoma model from immortalized
normal extravillous trophoblast cells transduced with HRASV12. Am J
Pathol. 179:1471–1482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang
C, Li M, Bao S and Zhu R: Dihydromyricetin reduced Bcl-2 expression
via p53 in human hepatoma HepG2 cells. PLoS One. 8:e768862013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye J, Guan Y, Zeng S and Liu D: Ampelopsin
prevents apoptosis induced by H2O2 in MT-4 lymphocytes. Planta Med.
74:252–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Y, Bai J, Zhong K, Huang Y and Gao H: A
dual antibacterial mechanism involved in membrane disruption and
DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus
deodara against Staphylococcus aureus. Food Chem. 218:463–470.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu B, Huang S, Wang C, Zhang H, Fang S and
Zhang Y: Anti-inflammatory effects of dihydromyricetin in a mouse
model of asthma. Mol Med Rep. 15:3674–3680. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu B, Zhou W, Chen X, Xu F, Chen Y, Liu
J, Zhang Q, Bao S, Chen N, Li M and Zhu R: Dihydromyricetin induces
mouse hepatoma Hepal-6 cell apoptosis via the transforming growth
factor-β pathway. Mol Med Rep. 11:1609–1614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JT, Jiao P, Zhou Y and Liu Q:
Protective effect of dihydromyricetin against
lipopolysaccharide-induced acute kidney injury in a rat model. Med
Sci Monit. 22:454–459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan TF, Wu TF, Bu LL, Ma SR, Li YC, Mao L,
Sun ZJ and Zhang WF: Dihydromyricetin promotes autophagy and
apoptosis through ROS-STAT3 signaling in head and neck squamous
cell carcinoma. Oncotarget. 7:59691–59703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Zhang H, Chen S, Xu Y, Yao A,
Liao Q, Han L, Zou Z and Zhang X: Dihydromyricetin induces
mitochondria-mediated apoptosis in HepG2 cells through
down-regulation of the Akt/Bad pathway. Nutr Res. 38:27–33. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji FJ, Tian XF, Liu XW, Fu LB, Wu YY, Fang
XD and Jin HY: Dihydromyricetin induces cell apoptosis via a
p53-related pathway in AGS human gastric cancer cells. Genet Mol
Res. 14:15564–15571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kao SJ, Lee WJ, Chang JH, Chow JM, Chung
CL, Hung WY and Chien MH: Suppression of reactive oxygen
species-mediated ERK and JNK activation sensitizes
dihydromyricetin-induced mitochondrial apoptosis in human non-small
cell lung cancer. Environ Toxicol. 32:1426–1438. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng S, Liu D, Ye Y, Wang L and Wang W:
Anti-tumor effects of ampelopsin on human lung cancer GLC-82
implanted in nude mice. Zhong Yao Cai. 27:842–845. 2004.(In
Chinese). PubMed/NCBI
|
|
16
|
Luo GQ, Zeng S and Liu DY: Inhibitory
effects of ampelopsin on angiogenesis. Zhong Yao Cai. 29:146–150.
2006.(In Chinese). PubMed/NCBI
|
|
17
|
Zhou FZ and Zhang XF: Suppression of
distant pulmonary metastasis of 4T1 mice breast carcinoma by
dihydromyricetin administration. Chin J Clinicians(Electronic Ed).
8:1674–1678. 2014.(In Chinese).
|
|
18
|
Jiang L, Zhang Q, Ren H, Ma S, Lu C, Liu
B, Liu J, Liang J, Li M and Zhu R: Dihydromyricetin enhances the
chemo-sensitivity of nedaplatin via regulation of the p53/Bcl-2
pathway in hepatocellular carcinoma cells. PLoS One.
10:e01249942015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong IL, Wang BC, Yuan J, Duan LX, Liu Z,
Liu T, Li XM, Hu X, Zhang XY, Jiang T, et al: Potent and nontoxic
chemosensitizer of P-Glycoprotein-mediated multidrug resistance in
cancer: Synthesis and evaluation of methylated epigallocatechin,
gallocatechin, and dihydromyricetin derivatives. J Med Chem.
58:4529–4549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su DL, Huang JH and Yao MJ: The acute
toxicological evaluation of dihydromyricetin and its control effect
for alcoholic hepatic injury. Hun Agricultural Sci. 90–93. 2009.(In
Chinese).
|
|
21
|
Biaglow JE and Miller RA: The thioredoxin
reductase/thioredoxin system: Novel redox targets for cancer
therapy. Cancer Biol Ther. 4:6–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kiraz Y, Adan A, Yandim Kartal M and Baran
Y: Major apoptotic mechanisms and genes involved in apoptosis.
Tumour Biol. 37:8471–8486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghoneum M, Matsuura M, Braga M and
Gollapudi S: S. cerevisiae induces apoptosis in human metastatic
breast cancer cells by altering intracellular Ca2+ and the ratio of
Bax and Bcl-2. Int J Oncol. 33:533–539. 2008.PubMed/NCBI
|
|
25
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: A
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bar-Am O, Weinreb O, Amit T and Youdim MB:
Regulation of Bcl-2 family proteins, neurotrophic factors, and APP
processing in the neurorescue activity of propargylamine. FASEB J.
19:1899–1901. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prenek L, Boldizsár F, Kugyelka R, Ugor E,
Berta G, Németh P and Berki T: The regulation of the mitochondrial
apoptotic pathway by glucocorticoid receptor in collaboration with
Bcl-2 family proteins in developing T cells. Apoptosis. 22:239–253.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|