Introduction
Ovarian cancer has the highest mortality rate of all
gynecological cancers worldwide and is frequently (in >75% of
cases) diagnosed at an advanced stage (1). Debulking surgery and platinum-based
chemotherapy are, at present, the cornerstones of treatment
(2). However, even with optimal
debulking surgery followed by aggressive front-line chemotherapy,
which results in an 80% initial cure rate, advanced-stage disease
in the majority of cases is incurable (3). This is due to the development of
chemoresistant disease, which results in recurrence within 16–22
months and a 5-year survival rate of only 27% (3). Therefore, the option of an effective
platinum-based chemotherapeutic regimen to improve the sensitivity
of primary platinum therapy to prevent tumor recurrence, extend the
platinum-free interval and improve ovarian cancer survival rates
has been the focus of clinical research.
The traditional approach to assess drug sensitivity
in cancer cells includes the MTT cell viability assay, Cell
Counting Kit-8 proliferation assay, flow cytometry and adenosine
triphosphate-based assays. However, these endpoint assays have
similar disadvantages, such as multiple steps in their protocols
that may lead to false-positive results, and steps performed at
multiple time points to gain information during treatment, which
may also introduce errors (4). To
obtain accurate and uninterrupted information about the efficacy of
drug combinations, the real-time cell analyzer system (RTCA) was
selected for the present study. This system has been widely used to
determine cell proliferation (4),
evaluate drug sensitivity (5), screen
drug profiles and reveal windows of drug response (6) in real time. The full list of references
to the RTCA device is available via the ACEA Biosciences, Inc.
website (aceabio.com/publications).
Metformin (MTF) has been used to treat type 2
diabetes mellitus for almost 60 years (7). In recent years, the effect of MTF on the
treatment and prevention of cancer has become an important focus of
research (8). MTF intake was
reportedly associated with improved survival times in patients with
ovarian cancer (9–11), and was also reported to increase the
sensitivity of cells to cisplatin (DDP) in primary ovarian cancer
(12). However, the mechanism of
action was unclear and the potential benefit of MTF for patients
without diabetes has not been confirmed.
In the present study, a patient that had received
primary surgery who had not received adjuvant chemotherapy was
selected to perform drug sensitivity testing. The aim was to
establish a rapid method to identify the most effective
chemotherapeutic combination and to determine whether MTF is
beneficial to patients. Omental metastatic (OM) cells were isolated
and cultured from a female patient with extensive invasive cancer.
The RTCA system was used to evaluate the efficiency of common
platinum-based chemotherapy regimens. In addition, this in
vitro analysis also evaluated whether MTF could increase the
sensitivity of platinum drugs.
Patients and methods
Patient
A 69-year-old female patient with ovarian cancer was
hospitalized in the Gynecological Oncology department of The
Affiliated Cancer Hospital of Guangxi Medical University (Nanning,
China) in October 2016. The patient received a diagnosis of stage
III ovarian cancer (FIGO staging system) (13) according to physical examination and
diagnostic imaging tests and was scheduled for cytoreductive
surgery. Written informed consent was obtained from the patient
prior to surgery. The patient received no additional treatment
prior to surgery, and was released from the hospital in December
2016. Postoperative pathology confirmed the specimen from the
primary lesion was high-grade serous papillary carcinoma. The
ethics review committee of The Affiliated Tumor Hospital of Guangxi
Medical University approved the present study.
Chemicals
DDP and paclitaxel (PTX) were obtained from Hospira
Australia Pty Ltd.; Pfizer Australia (West Ryde, New South Wales,
Australia). Carboplatin (CBP) was obtained from Qilu Pharmaceutical
Co., Ltd. (Shandong, China). Nedaplatin (NDP) was obtained from
Jiangsu Aosai Kang Pharmaceutical Co., Ltd. (Jiangsu, China).
Docetaxel (DTX) was obtained from Jiangsu Hengrui Medicine Co.,
Ltd. (Jiangsu, China). MTF hydrochloride was obtained from Hebei
Tiancheng Pharmaceutical Co., Ltd. (Hebei, China). RMPI-1640
culture medium, fetal bovine serum, glutamate and 0.05% trypsin
were obtained from Corning, Incorporated (Corning, NY, USA).
Recombinant human insulin was obtained from Novo Nordisk (Bagsværd,
Denmark).
Primary cell culture
Specimens from the transected primary lesions and OM
cells were collected, cut into pieces and gently digested with
0.025% trypsin (cat. no. 25-053-CI; Corning Incorporated) in
RMPI-1640 medium on a horizontal shaker for 15 min at 37°C.
Digested tissues were filtered with a 200-mesh filter. The
unfiltered digested tissues were further crushed and filtered again
through a 200-mesh filter. The filtrate was collected and
centrifuged at 300 × g for 5 min at room temperature. The cells
were resuspended in full culture medium consisting of RMPI-1640
medium, 20% fetal bovine serum, 1% glutamate, 0.01 mg/ml insulin,
100 U/ml penicillin and 100 U/ml streptomycin. The cells were then
cultured 37°C in an incubator containing 5% CO2. The
phase-contract morphology of cells was observed with a
magnification of ×100 or ×200 and recorded using an Olympus IX71
microscope (Olympus Corporation, Tokyo, Japan).
Cell viability and cytotoxicity assays
on the RCTA platform
The cell viability and drug toxicity analyses were
performed using the RTCA xCELLigence DP system (ACEA Biosciences,
Inc., San Diego, CA, USA), a real-time and label-free system used
to monitor cell viability, migration and invasion. For toxicity
analysis, cells were plated in an E-Plate 16 culture plate of the
RTCA system using full culture medium in 37°C overnight.
Subsequently, the cells were monitored until the exponential phase,
when they were treated with PTX (30 nM), DTX (50 nM), DDP (15 µM),
CBP (330 µM), NDP (95 µM), DDP (15 µM) + PTX (30 nM), CBP (330 µM)
+ DTX (50 nM), or NDP (95 µM) + DTX (50 nM), with or without 8 mM
MTF. For evaluation of effect of MTF, cells were treated with NDP
(95 µM) + DTX (50 nM) with 4, 8 and 16 mM MTF. The viability and
proliferation of the cells were monitored every 1 min in the first
2 h and monitored every 30 min for up to 200 h. Duplicate wells
were used for each concentration of drug. The results are presented
as the normalized cell index (CI), and were derived from the ratio
of CIs prior to and following the addition of the compounds.
AlamarBlue® cell viability
assay
OM cells were plated at 2,500 cells/well in 96-well
plates (Nalge Nunc International, Penfield, NY, USA). Detached and
attached cells were counted using a Vi-CELL XR Cell Viability
Analyzer (Beckman Coulter, Inc., Brea, CA, USA). For
chemosensitivity assessment, 24 h after plating, cells were treated
with PTX (30 nM), DTX (50 nM), DDP (15 µM), CBP (330 µM) and NDP
(95 µM) only. Viability was assessed after 24, 36 and 48 h using an
alamarBlue assay (Invitrogen; Thermo Fisher Scientific, Inc.;
Waltham, MA, USA) according to the manufacturer's protocol,
determined using Synergy microplate readers (BioTek Instruments,
Inc., Winooski, VT, USA) with the fluorescence intensity at 590
nm.
Western blot assay
Cells were pretreated with NDP at the indicated
concentration alone or with MTF for 24 h. Protein extracts were
obtained using radioimmunoprecipitation lysis buffer (CW Biotech,
Beijing, China) and protein concentration was quantified using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Proteins (40 µg)
were separated by SDS-PAGE (10% gels) and transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were blocked with 5% bovine serum albumin in
Tris-buffered saline containing 0.1% Tween-20 for 2 h at room
temperature, and probed with antibodies against poly (ADP-ribose)
polymerase, Rabbit anti-caspase 3 and GAPDH at 4°C overnight.
Rabbit anti-PARP (1:1,000; cat. no. 9542), Rabbit anti-caspase-3
(1:1,000; cat. no. 9665) and mouse anti-GAPDH monoclonal antibodies
(1:1000; cat. no. 5174) were from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Then membranes were probed with secondary
antibody for 2 h at room temperature. Secondary rabbit anti-mouse
(1:3,000; cat. no. ab 6728) and goat anti-rabbit antibodies
(1:3,000; cat. no. ab 6721) were from Abcam (Cambridge, MA, USA).
Immunoreactive bands were visualized using the Pierce ECL Western
Blotting Substrate (cat. no. 32106; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Bands were scanned and analyzed using the
FluorChem M system with AlphaView (version 3.4.0.0; ProteinSimple,
San Jose, CA, USA). All experiments were performed three times.
In-cell ELISA assay
In total, 5,000 cells/well were seeded into a
96-well plate. The plates were incubated overnight at 37°C in 5%
CO2. Cells were treated with 24 or 48 mM NDP with or
without 8 mM MTF. The expression of cleaved PARP or cleaved
caspase-3 was analyzed using a human PARP (Cleaved) Multispecies
In-Cell ELISA kit (cat. no. 62219; Thermo Fisher Scientific, Inc.)
or human caspase-3 (cleaved) Multispecies In-Cell ELISA kit (cat.
no. 62218; Thermo Fisher Scientific, Inc.) at 24 and 36 h after
drug exposure. The results were normalized with the expression of
α-tubulin according to the manufacturer's instruction.
Statistical analysis
The CI readouts provided by the RTCA system are
expressed as the mean ± standard deviation from the duplicated
results. Every result was from two repeats and in duplicated wells.
Representative results are presented. A one-way analysis of
variance with Bonferroni's post hoc test was used to compare the
differences between groups. Statistical analyses were performed
using SPSS for Windows (version 20.0; IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Primary ovarian tumor cells generated
from the primary lesion and OM
In the present study, cancer cells were generated
from the primary lesion and OM lesion successfully. The morphology
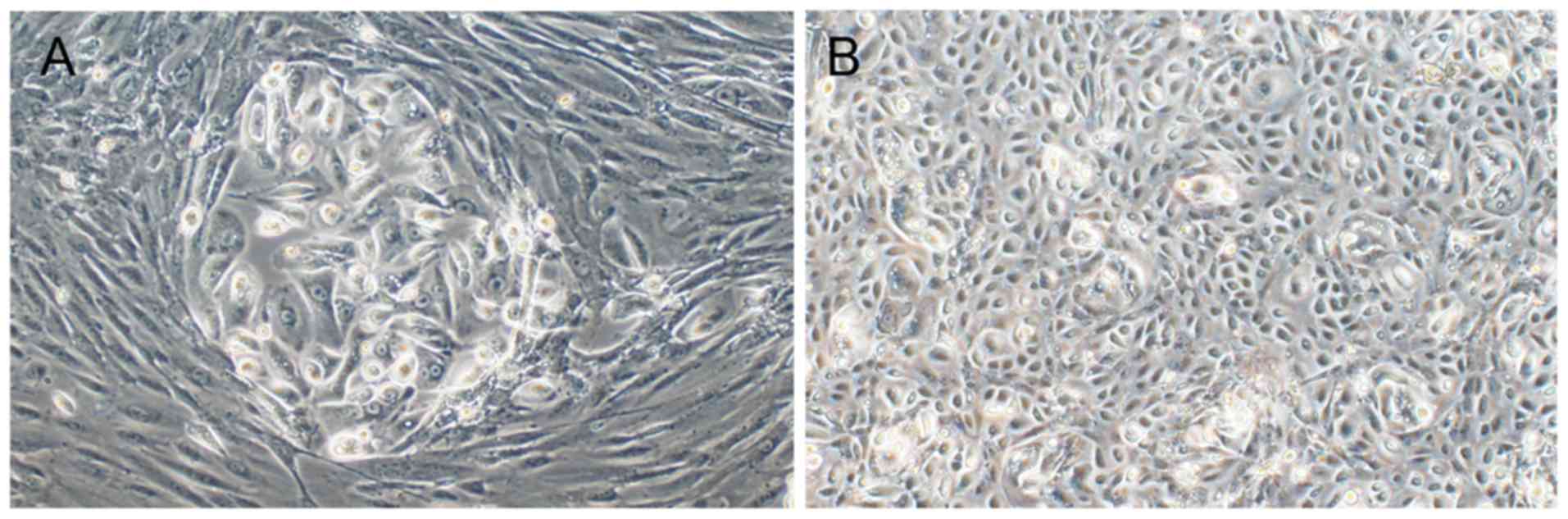
of cells at day 4 is presented in Fig.
1. However, cells of different orientation had dissimilar
morphology and biological characteristics. Cancer cells derived
from the primary lesion were larger, polygonal, clustered and
surrounded by fibroblasts (Fig. 1A).
Following a number of passages, cells from the primary lesion lost
the ability to proliferate and soon underwent apoptosis. The OM
cells had an improved proliferative ability and were round and
spaced apart from fibroblasts, and non-tumor cells were almost
invisible (Fig. 1B). As the primary
lesion had been removed, there was no requirement to further test
the drug sensitivity in these cells. Thus, all cytotoxicity assays
were conducted on Passage 3-Passage 5 OM cells.
Dynamic assessment of the cytotoxicity
of commonly used chemotherapy drug combinations using RTCA
To avoid the drift of biological characteristics,
cells used in the present study were limited to a maximum of five
passages. At the time of experimentation, the cell morphology had
not changed when observed under a microscope. The commonly used
chemotherapy drug combinations tested in the present study were
selected as advised by the National Comprehensive Cancer Network
2014 clinical practice guidelines for ovarian cancer (https://www.nccn.org/patients/guidelines/quick_guides/ovarian/treatment_planning/index.html)
and the evaluation of the physician in charge of treatment.
DDP, CBP and NDP were the most commonly used
platinum-based drugs for the treatment of ovarian cancer (14). The sensitivity of OM cells to these
drugs alone was initially analyzed. Platinum drugs combined with
PTX or DTX are standard treatments for ovarian cancer (14). Thus, the cytotoxicity of the
chemotherapy drug combinations, including DDP+PTX, CBP+DTX, and
NDP+DTX, were also assessed (Fig.
2).
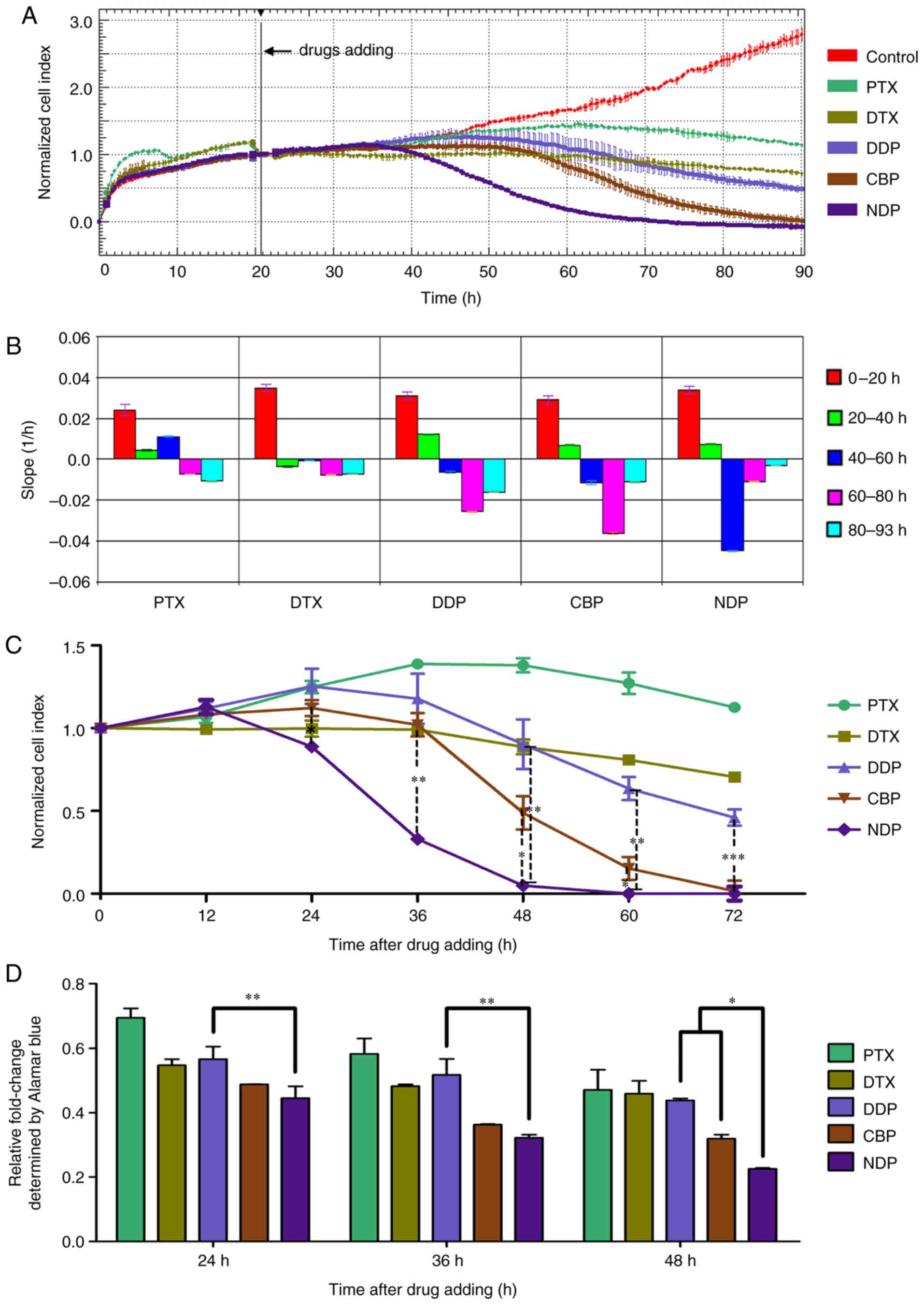 | Figure 2.Real-time cell analysis of the
cytotoxic effect of single chemotherapy drugs on OM cells. OM cells
were seeded into the E-Plate and the CI was determined. (A) The
normalized CI of OM cells treated with PTX (30 nM), DTX (50 nM),
DDP (15 µM), CBP (330 µM) or NDP (95 µM). The drugs were added at
~20 h as indicated by the black line. The normalized CI was
generated by normalizing CIs to the CI at the time of drug
addition. (B) Slopes of CI curves in (A) at 0–20 (pretreatment),
20–40, 40–60 and 60–80 h. (C) The normalized CI was calculated at
various time points. The results are expressed as the mean ± SD.
*P<0.05, **P<0.01 and ***P<0.001, compared with DDP; (D)
The inhibitory rate of single chemotherapy drugs on OM cells was
calculated on the basis of cell viability analyzed using an
alamarBlue assay. The results are expressed as the mean ± SD.
*P<0.05, **P<0.01. OM, omental metastatic; CI, cell index;
CBP, carboplatin; PTX, paclitaxel; DDP, cisplatin; DTX, docetaxel;
NDP, nedaplatin; SD, standard deviation. |
Initially, to rapidly screen the most effective
drug, single and combination regimens were tested at certain
concentrations calculated according to the dosage based on body
surface area. The biosensors detected the attachment of OM cells
and began to produce impedance-based readout values of CI at 4–5 h
after plating. Cells entered the exponential phase of proliferation
at ~5 h after plating. The cells were then exposed PTX (30 nM), DTX
(50 nM), DDP (15 µM), CBP (330 µM) or NDP (95 µM) when the CI value
reached 0.9 to 10 (Fig. 2A).
As presented in Fig.
2, non-treated OM cells (control) maintained aggressive
proliferation. Neither PTX or DTX was able to induce apoptosis of
OM cells as indicated by their CI curves, which became flat
following exposure to PTX or DTX (Fig.
2A); the slope was close to zero (Fig. 2B), suggesting that those drugs only
inhibited the proliferation of OM cells.
The cells exposed to DDP, CBP, and NDP all exhibited
a typical downward shift in their proliferation curves (Fig. 2A). The earliest and sharpest decline
was observed following NDP treatment, indicating the start of
apoptosis at 20 h after the addition of drugs, whereas those
treated with CBP and DDP exhibited a more gradual decline in their
proliferation curves and apoptosis commenced at 35 h after the
addition of drugs (Fig. 2A). The most
extensive apoptosis occurred at ~30 h following exposure to NDP,
and at ~50 h following exposure to CBP or DDP. The slope of the CI
value exhibited the same trend (Fig.
2B). The CI declined to almost 0 at 40 and 70 h after exposure
to NDP or CBP. However, the CI curve for the OM cells treated with
DDP reached a plateau 70 h after treatment, and the CI value only
declined to ~60%, which may indicate that these OM cells were less
sensitive to DDP. The cell viability normalized from CI indicated
that, from 24 h, NDP had a statistically significant killing effect
compared with DDP and CBP (P<0.05). This difference existed up
to 72 h (Fig. 2C). To confirm this
result, a chemosensitivity assay was conducted using alamarBlue.
The results revealed similar trends, in that NDP exhibited the
strongest toxicity towards OM cells, followed by CBP and DDP
(Fig. 2D).
The present study demonstrated that combinations of
platinum with taxanes were more toxic to OM cells compared with
platinum alone. The CI curves began to gradually decrease following
the addition of the chemotherapeutic drugs except DDP+PTX, compared
with the slight increase in cells treated with platinum only
(Fig. 3A). The slopes of each drug
combination between 0 and 20 h exposure (Fig. 3B) were similar to those of PTX or DTX
alone (Fig. 2B), and indicated that
this result may be the effect of the taxanes. The normalized CI
curves revealed that NDP in combination with DTX exhibited the most
cytotoxicity among drug combinations without MTF in OM cells
(Fig. 3A). The slopes also indicated
that the steepest decline occurred at ~20 to 40 h after exposure to
NDP+DTX. The steepest decrease occurred between 40 and 60 h after
treatment with CBP+DTX or DDP+PTX (Fig.
3B and C, C-1). However, the CI curve of cells treated with
DDP+PTX, as well as DDP alone, entered a plateau 70 h after
treatment (Fig. 3A). The combination
of NDP and DTX exhibited the most marked killing ability in the
three combinations between 24 and 72 h (P<0.01; Fig. 3C, C-1).
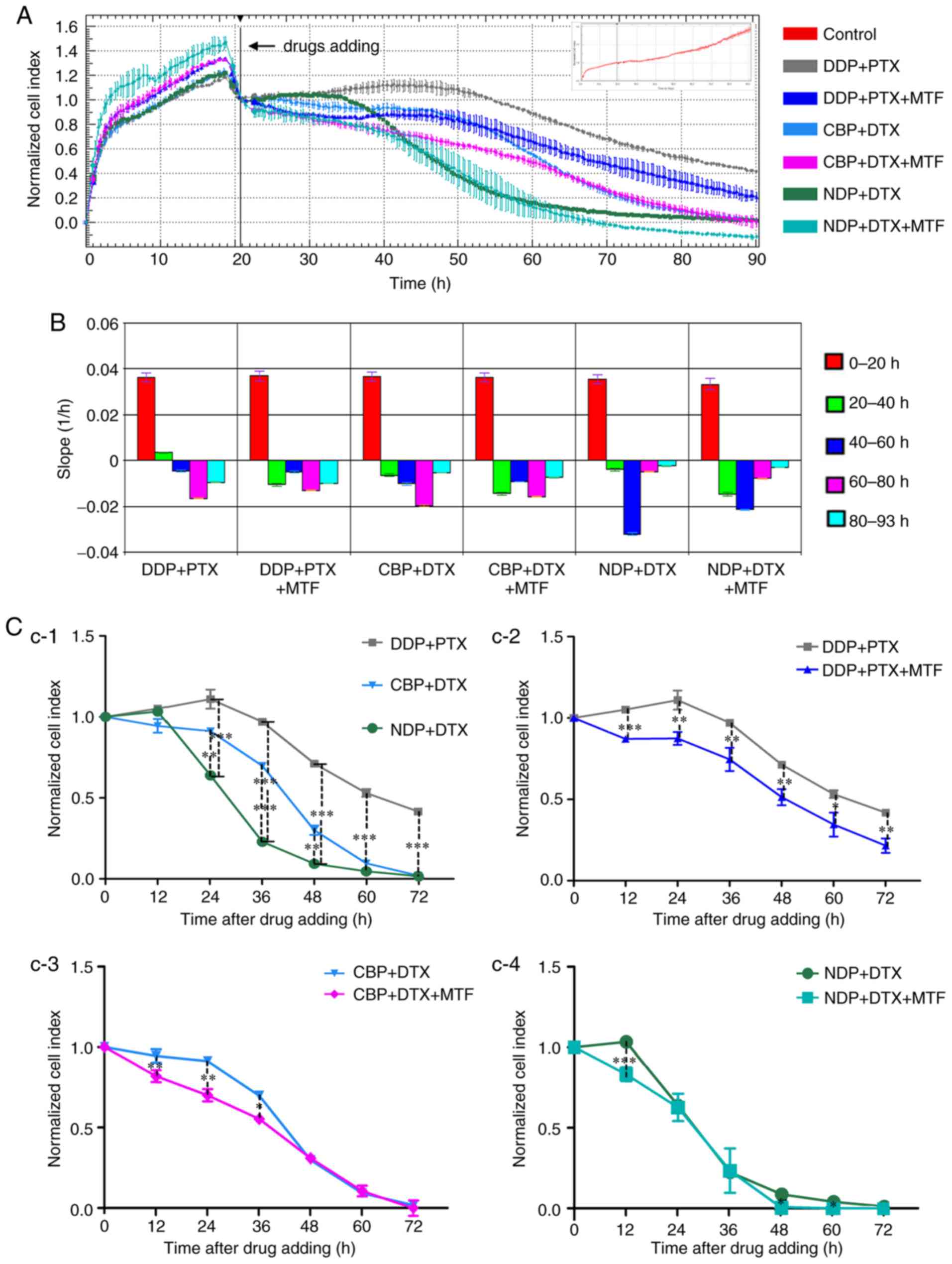 | Figure 3.Real-time cell analysis of the
cytotoxic effect of chemotherapy drug combinations on the OM cells.
OM cells were seeded into the E-Plate and the CI was recorded. (A)
The normalized CI of OM cells treated with DDP (15 µM) + PTX (30
nM), CBP (330 µM) + DTX, (50 nM), or NDP (95 µM) + DTX (50 nM),
with or without 8 mM MTF. The drugs were added at ~20 h as
indicated by the black line. The normalized CI was generated by
normalizing CIs to the CI at the time of drug addition. The CI of
non-treated cells is presented in the inset as the CI reached 3.0
at the end of experiment. (B) The slope of CI curves in (A) at 0–20
(pretreatment), 20–40, 40–60, 60–80 and 80–93 h. (C) The normalized
CI following the addition of drugs were calculated (C-1: DDP+PTX
vs. CBP+DTX vs. NDP+DTX; C-2: DDP+PTX vs. DDP+PTX+MTF; C-3: CBP+DTX
vs. CBP+DTX+MTF; C-4: NDP+DTX vs. NDP+DTX+MTF). The results are
expressed as the mean ± standard deviation. *P<0.05, **P<0.01
and ***P<0.001; C-1, compared with DDP+PTX; C-2 to C-4, compared
with drug combinations without MTF respectively. OM, omental
metastatic; CI, cell index; CBP, carboplatin; PTX, paclitaxel; DDP,
cisplatin; DTX, docetaxel; NDP, nedaplatin; MTF, metformin. |
MTF increases drug sensitivity in
cells in a dose-dependent manner
It has been reported previously that MTF improves
ovarian cancer cell sensitivity to platinum-based drugs (10). In the present study, the effect of MTF
on OM cells was further examined. As hypothesized, the CI curve
declined more and the slopes were steeper during the first 20 h of
exposure, and the time it took for CI to approach zero was
decreased in every combination with MTF compared with the
treatments without MTF (Fig. 3A and C,
C2-C4). In DDP+PTX+MTF-treated cells, the CI fell below 0.2
with MTF, compared with 0.4 without MTF, at 70 h after treatment.
Between 24 and 72 h, in different combinations, adding MTF in all
groups led to an increased cytotoxic effect (P<0.01; Fig. 3C, C2-C4). Furthermore, the combination
of NDP+DTX with MTF was examined at 4, 8 and 16 mM. The results
demonstrated that MTF improved the cell sensitivity at 12, 24 and
36 h (P<0.05; Fig. 4).
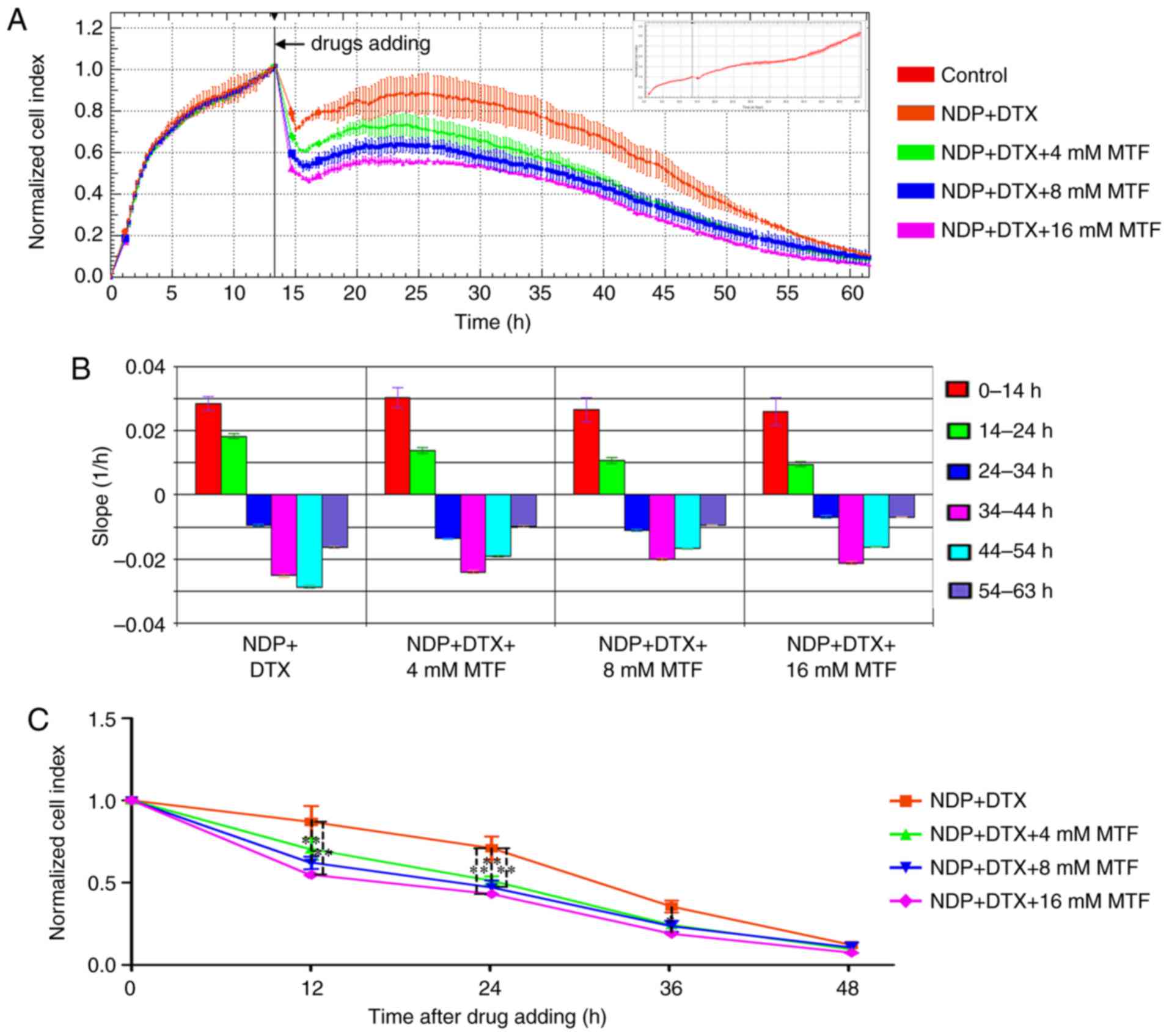 | Figure 4.Real-time cell analysis demonstrates
that the chemotherapy sensitization effect of MTF was
dose-dependent. In total, 5,000 OM cells were seeded into the
E-Plate and the CI was recorded. (A) The normalized CI of OM cells
treated with NDP (95 µM) + DTX (50 nM) with 4, 8 and 16 mM MTF. The
drugs were added at ~14 h as indicated by the black line. The
normalized CI was generated by normalizing CIs to the CI at the
time of drug addition. The CI of non-treated cells is presented in
the inset as the CI reached 3.5 at the end of the experiment. (B)
The slope of CI curves in (A) at 0–14 (pre-treatment), 14–24,
24–34, 34–44, 44–54 and 54–63 h. (C) The normalized CI was
calculated at various time points. The results are expressed as the
mean ± standard deviation. *P<0.05 and **P<0.01. OM, omental
metastatic; CI, cell index; DTX, docetaxel; NDP, nedaplatin; MTF,
metformin. |
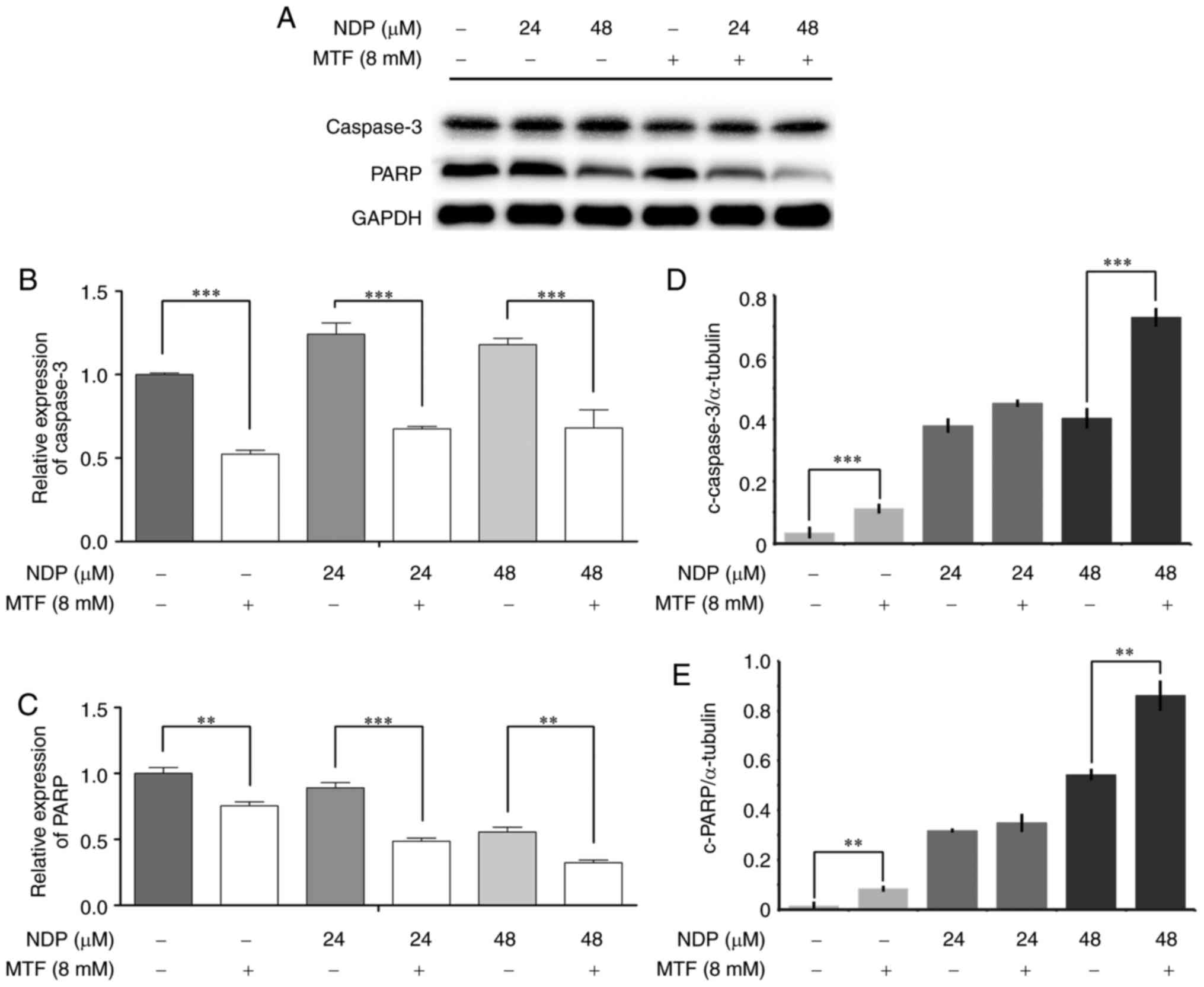
MTF increases platinum drug-induced
apoptosis
To further confirm that the decrease in the CI value
was the result of apoptosis, PARP expression was analyzed by
western blotting. MTF alone was not able to induce apoptosis;
however, the apoptosis induced by NDP was enhanced as the PARP
expression decreased along with the dosage of NDP when MTF was used
(Fig. 5A-C). The expression of
caspase-3 was also increased (Fig.
5A-C). To establish whether PARP or caspase-3 were activated,
an in-cell ELISA was performed and it confirmed that MTF at
concentrations as low as 8 mM was able to significantly (P<0.05)
increase the amount of cleaved PARP and cleaved caspase-3 (Fig. 5D and E).
Discussion
Ovarian cancer is the most lethal gynecological
malignancy affecting women worldwide and the fifth most common
cause of cancer-associated mortality among women in the USA
(15). According to statistics, only
45% of patients with ovarian cancer have a survival time of 5 years
following their diagnosis (16). In
recent decades, despite the development of novel therapeutic
strategies, surgery and platinum-based chemotherapy remain the
standard treatments for ovarian cancer (17). The clinical chemotherapy options are
limited, as patient reactions to the chemotherapy vary and
chemoresistance develops rapidly, particularly for patients at the
middle and advanced stage of ovarian cancer. The majority of
patients with ovarian cancer have their primary ovarian tumors
removed and subsequently succumb to metastatic disease rather than
their primary tumor (18). In the
present study, the patient OM-derived primary cells were
investigated for chemotherapy drug sensitivity using the
xCELLigence system. The results demonstrated that the metastatic
cell sensitivity to the commonly used clinical chemotherapy drugs
varied considerably. The cell responses to certain drugs, such as
NDP and DTX, were positive; however, for other drugs, such as CBP
and PTX, the cytotoxic effects were not evident. The results of the
present study also revealed that MTF exhibited a promising
sensitization effect and may assist in reversing drug
resistance.
Pelvic metastases are common in ovarian cancer
(19). Currently, the treatment of
pelvic metastatic ovarian cancer relies on platinum-based
chemotherapy regimens (1,2,19). The
clinical selection of drugs for these patients often relies on
doctors' experience and lacks clear drug markers (2,19).
However, the majority of patients eventually become resistant to
chemotherapeutic drugs and this causes the prognosis of these
patients to be unsatisfactory (20).
Previous studies for ovarian cancer were primarily based on cells
generated from primary lesions (21,22), and
few of these studies focused on the omentum (23). It has been suggested that metastatic
cancer is a markedly heterogeneous disease at the genetic,
transcriptomic and microenvironment levels (24). Therefore, the results generated from
primary lesions could not fully reflect the properties of
metastasis. In addition, the heterogeneity between tumors indicated
that drug sensitivity may also vary for each patient. A rapid
method to determine the sensitivity to the drug was beneficial for
effective drug regimen selection. In the present study, primary
tumor cells were successfully isolated and cultured from the
patient's omentum, and the platinum sensitivity was evaluated by
the xCELLigence system within 10 days of surgery.
The response of the cells to different chemical
drugs was varied, which could not have been predicted by clinical
experience. Certain first-line chemotherapeutics, such as CBP, did
not produce a satisfactory effect even at a high concentration (330
µM). Compared with other primary cultured cell lines, primary
omentum ovarian cancer cells in this case were resistant to DDP.
This indicated that this tumor cell type was not effectively killed
by this widely used chemotherapy regimen, and drug-resistant cells
may become the principal reason for tumor recurrence. In clinical
practice, if it were possible to tailor individualized treatment
based on the specific patient response to different treatments, the
curative effect would be improved.
The resistance of ovarian cancer to platinum-based
treatment may be intrinsic or acquired, and is brought about
through a wide array of mechanisms (25). This includes pumps that eject the drug
from the cell (26), to promoting the
expression of genes that enable alternative growth pathways
(27,28), as cancer cells explore all avenues in
their bid to survive and proliferate (25). Further complicating matters is the
time spent understanding which mechanism or mechanisms are active
in any particular individual (29).
It is hoped that the results of drug susceptibility testing are
useful in platinum-based chemotherapy to help achieve optimal
chemotherapeutic effects, or at least to increase treatment
intervals and improve the quality of life of the patient.
There were limitations to the present study, such as
the primary cells used were a mixture of fibroblasts or other cell
types, even though fibroblasts almost disappeared within a few
passages. Conversely, fibroblasts were able to supply cytokines and
maintain the in vivo microenvironments for tumor cells. In
the present study, only cells between P3 and P5 were used when the
fibroblasts were almost invisible. Furthermore, the fetal bovine
serum used for cell culture may be replaced by an improved
serum-free formula to minimize the influence of the cytokines in
serum.
MTF, the most widely used drug for type 2 diabetes,
has advantages including fewer side effects and low cost (7). It has been demonstrated that MTF
effectively inhibits the proliferation of numerous ovarian cancer
cell lines, including DDP and taxol chemoresistant cell lines
(30). Previous clinical studies and
in vitro analysis indicated a promising application of MTF
to improve the effect of chemotherapy for ovarian cancer (9,10). In the
present study, the enhanced sensitivity of MTF was confirmed in OM
cells from one patient without diabetes. Western blot analysis
demonstrated that MTF could accelerate apoptosis of OM cells caused
by NDP.
The main adverse effect of platinum is
myelosuppression (20), so if MTF can
improve chemotherapy sensitivity, it will help to decrease the
dosage of NDP to achieve a similar killing effect to the regimens
without MTF (12). However, it is not
certain whether all patients with ovarian cancer may benefit from
this drug at present, as cases of MTF resistance have been reported
(30,31). For example, in vitro cell
experiments, in the ovarian cancer cell line SKOV3ip were reported
to be not as sensitive to MTF as other ovarian cancer cells
(30).
In clinical practice, identifying patients who would
benefit the most from the use of MTF in combination chemotherapy is
the top priority. However, the lack of clinical indicators and
tests for rapid prediction has become a major constraint to the
clinical application of MTF in the treatment of ovarian cancer
(32,33). Great efforts have been made to
elucidate the mechanism of action of MTF. A previous study
indicated that MTF downregulated the expression of the
anti-apoptotic proteins B-cell lymphoma 2 (Bcl-2) and myeloid cell
leukaemia 1, and upregulated the expression of the pro-apoptotic
protein Bcl-2-associated X protein (Bax) in cancer cells (34,35). It
has been suggested that MTF may serve an antitumor role directly
via an AMP-dependent protein kinase (AMPK)-dependent or
AMPK-independent pathway (31). In
addition, MTF may increase the transcriptional expression of p53
and its downstream targets Bax and p21, thus promoting apoptosis
and inhibiting tumor growth (36). A
previous study demonstrated that MTF blocked cancer cells in the G1
stage (37) and induced cell cycle
stagnation or apoptosis. Cancer stem cells appear to be key to
chemoresistance and tumor relapse. A previous study implied that
MTF at a low dose selectively inhibited
CD44+CD117+ ovarian cancer stem cells through
inhibition of epithelial-mesenchymal transition and potentiated the
effect of DDP (38). Although in
numerous studies, the dosages of MTF were higher compared with the
dosage in clinical patients, the use of MTF has increased the
understanding of the mechanisms of tumor cell chemoresistance,
metastasis and recurrence (39–41).
However, there was no consensus in these studies concerning the
markers to identify patients that would benefit from MTF. MTF was
traditionally applied in patients with type 2 diabetes mellitus;
however, there is not enough evidence to support its use in all
patients without type 2 diabetes mellitus, and MTF-resistant cells
have also been identified (30,31). The
method developed in the present study may help to identify patients
sensitive to MTF. However, as the present study only concerned one
case, an increased number of cases including patients with type 2
diabetes mellitus will be recruited for further study. In
conclusion, the sensitivity of OM cells to different platinum-based
regimens varied considerably. The real-time cell analyzer assay may
assist in selecting personalized chemotherapy regimens in clinical
practice. Investigating and clarifying the underlying molecular
mechanism of MTF, a drug with a chemotherapy sensitization effect,
may shed some light on the treatment of ovarian cancers,
particularly those with extensive metastatic tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81360341), National
Natural Science Foundation of China (grant no. 81560428), National
High Technology Research and Development Program (863 Program)
(grant no. 2014AA020605), Guangxi Zhuang Autonomous Region Health
and Family Planning Commission Self-Financing Project (grant no.
Z2015578) and Guangxi Nanning Qingxiu District Science and
Technology Development Project (grant no. 2015S14).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT, QW and YF contributed to the conception and
design of the work. YL, YF, JW and HL contributed to the
acquisition, analysis and interpretation of data. YF and YL drafted
the manuscript. QW and YT revised the manuscript and gave final
approval of the version to be published.
Ethics approval and consent to
participate
Written informed consent for this research was
obtained from the patient prior to surgery. The ethics review
committee of The Affiliated Tumor Hospital of Guangxi Medical
University approved the present study.
Patient consent for publication
The patient has reviewed the material to be
published and provided written permission for the publication.
Competing interests
The authors declared that they have no competing
interests.
References
|
1
|
Coleman RL: Ovarian cancer in 2015:
Insights into strategies for optimizing ovarian cancer care. Nat
Rev Clin Oncol. 13:71–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Metzger-Filho O, Moulin C and D'Hondt V:
First-line systemic treatment of ovarian cancer: A critical review
of available evidence and expectations for future directions. Curr
Opin Oncol. 22:513–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kipps E, Tan DS and Kaye SB: Meeting the
challenge of ascites in ovarian cancer: New avenues for therapy and
research. Nat Rev Cancer. 13:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roshan Moniri M, Young A, Reinheimer K,
Rayat J, Dai LJ and Warnock GL: Dynamic assessment of cell
viability, proliferation and migration using real time cell
analyzer system (RTCA). Cytotechnology. 67:379–386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan T, Huang B, Zhang W, Gabos S, Huang DY
and Devendran V: Cytotoxicity assessment based on the AUC50 using
multi-concentration time-dependent cellular response curves. Anal
Chim Acta. 764:44–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kho D, MacDonald C, Johnson R, Unsworth
CP, O'Carroll SJ, du Mez E, Angel CE and Graham ES: Application of
xCELLigence RTCA biosensor technology for revealing the profile and
window of drug responsiveness in real time. Biosensors (Basel).
5:199–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bailey CJ: Metformin: Historical overview.
Diabetologia. 60:1566–1576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Decensi A, Puntoni M, Goodwin P, Cazzaniga
M, Gennari A, Bonanni B and Gandini S: Metformin and cancer risk in
diabetic patients: A systematic review and meta-analysis. Cancer
Prev Res (Phila). 3:1451–1461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Romero IL, McCormick A, McEwen KA, Park S,
Karrison T, Yamada SD, Pannain S and Lengyel E: Relationship of
type II diabetes and metformin use to ovarian cancer progression,
survival, and chemosensitivity. Obstet Gynecol. 119:61–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar S, Meuter A, Thapa P, Langstraat C,
Giri S, Chien J, Rattan R, Cliby W and Shridhar V: Metformin intake
is associated with better survival in ovarian cancer: A
case-control study. Cancer. 119:555–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bar D, Lavie O, Stein N, Feferkorn I and
Shai A: The effect of metabolic comorbidities and commonly used
drugs on the prognosis of patients with ovarian cancer. Eur J
Obstet Gynecol Reprod Biol. 207:227–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel S, Singh N and Kumar L: Evaluation
of effects of metformin in primary ovarian cancer cells. Asian Pac
J Cancer Prev. 16:6973–6979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mutch DG and Prat J: 2014 FIGO staging for
ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol.
133:401–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Metzgerfilho O, Moulin C and D'hondt V:
First-line systemic treatment of ovarian cancer: A critical review
of available evidence and expectations for future directions. Curr
Opin Oncol. 22:513–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, et al:
SEER cancer statistics review, 1975–2014. National Cancer
Institute; Bethesda MD: https://seer.cancer.gov/csr/1975_2014/based on
November 2016 SEER data submission, posted to the SEER web site.
April. 2017
|
|
17
|
Mei L, Chen H, Wei DM, Fang F, Liu GJ, Xie
HY, Wang X, Zou J, Han X and Feng D: Maintenance chemotherapy for
ovarian cancer. Cochrane Database Syst Rev. 29:CD0074142013.
|
|
18
|
Davidson B and Tropé CG: Ovarian cancer:
Diagnostic, biological and prognostic aspects. Womens Health
(Lond). 10:519–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holmes D: Ovarian cancer: Beyond
resistance. Nature. 527:S2172015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kischkel FC, Meyer C, Eich J, Nassir M,
Mentze M, Braicu I, Kopp-Schneider A and Sehouli J: Prediction of
clinical response to drugs in ovarian cancer using the chemotherapy
resistance test (CTR-test). J Ovarian Res. 10:722017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O Donnell RL, Mccormick A, Mukhopadhyay A,
Woodhouse LC, Moat M, Grundy A, Dixon M, Kaufman A, Soohoo S,
Elattar A, et al: The use of ovarian cancer cells from patients
undergoing surgery to generate primary cultures capable of
undergoing functional analysis. PLoS One. 9:e906042014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kar R, Chawla D, Gupta B, Mehndiratta M,
Wadhwa N and Agarwal R: Establishment of primary cell culture from
ascitic fluid and solid tumor obtained from epithelial ovarian
carcinoma patients. Int J Gynecol Cancer. 27:2000–2005. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robinson DR, Wu YM, Lonigro RJ, Vats P,
Cobain E, Everett J, Cao X, Rabban E, Kumar-Sinha C, Raymond V, et
al: Integrative clinical genomics of metastatic cancer. Nature.
548:297–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gillet JP and Gottesman MM: Mechanisms of
multidrug resistance in cancer. Methods Mol Biol. 596:47–76. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sherman-Baust CA, Becker KG, Wood Iii WH,
Zhang Y and Morin PJ: Gene expression and pathway analysis of
ovarian cancer cells selected for resistance to cisplatin,
paclitaxel, or doxorubicin. J Ovarian Res. 4:212011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: The role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen D, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato S and Itamochi H: Ovarian cancer and
drug resistance. Curr Obstet Gynecol Rep. 4:18–25. 2015. View Article : Google Scholar
|
|
30
|
Rattan R, Giri S, Hartmann LC and Shridhar
V: Metformin attenuates ovarian cancer cell growth in an AMP-kinase
dispensable manner. J Cell Mol Med. 15:166–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Do MT, Kim HG, Choi JH and Jeong HG:
Metformin induces microRNA-34a to downregulate the
Sirt1/Pgc-1α/Nrf2 pathway, leading to increased susceptibility of
wild-type p53 cancer cells to oxidative stress and therapeutic
agents. Free Radic Biol Med. 74:21–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shank JJ, Yang K, Ghannam J, Cabrera L,
Johnston CJ, Reynolds RK and Buckanovich RJ: Metformin targets
ovarian cancer stem cells in vitro and in vivo. Gynecol Oncol.
127:390–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Romero IL, McCormick A, McEwen KA, Park S,
Karrison T, Yamada SD, Pannain S and Lengyel E: Relationship of
type II diabetes and metformin use to ovarian cancer progression,
survival, and chemosensitivity. Obstet Gynecol. 119:61–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gwak H, Kim Y, An H, Dhanasekaran DN and
Song YS: Metformin induces degradation of cyclin D1 via AMPK/GSK3β
axis in ovarian cancer. Mol Carcinog. 56:349–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang H, Zhang Z, Wei X and Dai R:
Small-molecule inhibitor of Bcl-2 (TW-37) suppresses growth and
enhances cisplatin-induced apoptosis in ovarian cancer cells. J
Ovarian Res. 8:32015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li P, Zhao M, Parris AB, Feng X and Yang
X: p53 is required for metformin-induced growth inhibition,
senescence and apoptosis in breast cancer cells. Biochem Biophys
Res Commun. 464:1267–1274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao ZY, Liu Z, Bi MH, Zhang JJ, Han ZQ,
Han X, Wang HY, Sun GP and Liu H: Metformin induces apoptosis via a
mitochondria-mediated pathway in human breast cancer cells in
vitro. Exp Ther Med. 11:1700–1706. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang R, Zhang P, Wang H, Hou D, Li W,
Xiao G and Li C: Inhibitory effects of metformin at low
concentration on epithelial-mesenchymal transition of
CD44(+)CD117(+) ovarian cancer stem cells. Stem Cell Res Ther.
6:2622015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ling S, Song L, Fan N, Feng T, Liu L, Yang
X, Wang M, Li Y, Tian Y, Zhao F, et al: Combination of metformin
and sorafenib suppresses proliferation and induces autophagy of
hepatocellular carcinoma via targeting the mTOR pathway. Int J
Oncol. 50:297–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chou CC, Lee KH, Lai IL, Wang D, Mo X,
Kulp SK, Shapiro CL and Chen CS: AMPK reverses the mesenchymal
phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a
signaling axis. Cancer research. 74:1–4795. 2014. View Article : Google Scholar
|