Introduction
Ginseng (Panax ginseng C. A. Meyer) is used
as general ‘tonic’ and therapeutic agent in Asian countries. Its
diverse pharmacological activities are largely attributed to
ginsenosides, which are the major active components found in
ginseng. Ginsenoside Rh2 (Rh2) is a key component of red ginseng
and reportedly has significant antitumor effects in numerous types
of cancers such as breast (1) ovarian
(2), prostate (3), leukemia (4), colorectal (5), and hepatocellular carcinoma (HCC)
(6). The specific effects of Rh2
include apoptosis promotion as well as inhibition of significant
growth, metastasis, and invasion (4,6–8). Although some signaling pathways such as
epidermal growth factor receptor (9),
tumor necrosis factor-α (10), Janus
kinase/signal transducer and activator of transcription 3 (11), and phosphoinositide 3-kinase/Akt
(7) have been implicated in the
regulatory process of Rh2 in cancer cells, the detailed mechanism
remains unclear, particularly the role of non-coding RNAs.
MicroRNAs (miRNAs) are small non-coding RNAs that
contain approximately 22 nucleotides. miRNAs play a key role in
numerous physiological processes such as cell metabolism, immune
function, cell proliferation, apoptosis, tissue development, and
differentiation (12). Furthermore,
miRNAs have been confirmed to play roles in cancer development,
epithelial-mesenchymal transition, and response to therapy
(13). Some miRNAs may be regulated
by Rh2 in human non-small cell lung cancer A549 and breast cancer
cells (14,15). In addition, Rh2 inhibits glioma cell
proliferation by targeting miR-128 (16). These studies suggested that miRNAs
play a key role in the regulatory effects of Rh2 in cancer cells.
However, additional studies are needed to confirm this
hypothesis.
Liver cancer, a highly fatal cancer, is much more
common in less developed countries, thus disproportionately
contributing to the overall cancer mortality rate in these
countries (17). Rh2 is known to
inhibit HCC cell growth in vivo and in vitro by
decreasing the number of cancer stem cell-like cells in a
dose-dependent manner (6). In the
present study, we investigated the effect of Rh2 on miRNA
expression and role of miRNAs in Rh2-mediated inhibition of liver
cancer cell growth and colony formation, as well as in the
promotion of liver cancer apoptosis. Our results showed that Rh2
treatment increased the expression levels of miR-200b-5p,
miR-224-3p, and miR-146a-5p and decreased those of miR-26b-3p and
miR-29a-5p. Further, we investigated the role of miR-146a-5p which
showed the greatest increase for the Rh2-mediated inhibitory effect
on liver cancer cell growth. In addition, we examined colony
formation and the promotion of liver cancer apoptosis following Rh2
treatment.
Materials and methods
Cell lines and culture
The liver cancer cell lines HepG2, Huh7, and
SMMC-7721 were obtained from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in minimum essential medium (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) with 10% fetal bovine serum (HyClone; GE
Healthcare Life Sciences) at 37°C in a humidified atmosphere
containing 5% CO2.
Lentivirus package and stable cell
construction
The primary sequence of miR-146a-5p
(NC_000005.10:160485152-160485650) was amplified by polymerase
chain reaction (PCR), using the primer pair
5′-CCGCTCGAGGGCTCAAGAGATCCACCCACATC-3′ and
5′-CGCGGATCCGAGATCATTCATTTAGCTACTTGG-3′ and then inserted into a
pLVX-IRES-Neo plasmid after digestion with XhoI and BamHI. Eight
repeated sequences of the miR-146a-5p inhibitor
(AACCCATGGAGACAGTTCTCA) were synthesized into a T vector and
inserted into the PLVX-SHRNA2 plasmid after digestion with BamHI
and EcoRI.
All recombinant pLVXs plus pHelper 1.0 and 2.0
plasmids were generated by transient transfection of 293T cells,
and the lentivirus was packaged in accordance with general
procedures. For infection, 2×105 HepG2 cells were
divided into three groups and subcultured in 6-well culture plates
for 24 h prior to transduction. The three cell groups were as
follows: Cells infected with empty lentivirus, lentivirus
expressing miR-146a-5p, and lentivirus expressing miR-146a-5p
inhibitor (designated as negative control, Lv-NC; Lv-miR-146a-5p,
and Lv-miR-146a-5p-inhibitor, respectively). Lentivirus
transduction and stable cell construction were carried out as
previously reported (18).
Cell treatment and groups
To detect the expression levels of miR-200b-5p,
miR-224-3p, miR-146a-5p, miR-26b-3p, and miR-29a-5p, the HepG2,
Huh7, and SMMC-7721 cells were treated with 20 µg/ml Rh2 or
dimethyl sulfoxide (DMSO) for 48 h and then harvested for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis. To detect the effect of miR-146a-5p on Rh2-induced cell
proliferation, stable Lv-NC, Lv-miR-146a-5p, and Lv-miR-146a-5p-I
cells were treated with 20 µg/ml Rh2 for 48 h, and stable Lv-NC
cells were also treated with DMSO as a negative control.
RNA extraction and RT-qPCR
After predetermined times, total RNA was extracted
from the treated cells of each group using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The RNA was reverse
transcribed into cDNA using M-MLV reverse transcriptase (Promega
Corporation, Madison, WI, USA) in a 20-µl reaction volume with
miRNA-specific stem-loop primers. Equal amounts of cDNA were used
as templates for RT-qPCR to detect the expression levels of
miR-200b-5p, miR-224-3p, miR-26b-3p, miR-29a-5p, and miR-146a-5p
relative to that of U6 (endogenous control). The detection was
followed by quantitation using an ABI PRISM 7500 sequence detection
system using SYBR Green qPCR SuperMix (Invitrogen; Thermo Fisher
Scientific, Inc.) with the primers shown in Table I. Experiments were performed in
duplicate and repeated three times and the fold-induction of gene
expression was calculated using the 2−ΔΔCq method.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Sequence (5′-3′) |
|---|
| miR-26b-3p-F |
ACACTCCAGCTGGGCCTGTTCTCCATTACTTG |
| miR-224-3p-F |
ACACTCCAGCTGGGAAAATGGTGCCCTAGTGAC |
| miR-29a-5p-F |
ACACTCCAGCTGGGACTGATTTCTTTTGGTG |
| miR-200b-5p-F |
ACACTCCAGCTGGGCATCTTACTGGGCAGCATTG |
| miR-146a-5p-F |
ACACTCCAGCTGGGTGAGAACTGAATTCCATG |
| Universal
miRNA-R |
CTCAACTGGTGTCGTGGA |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
Western blot analysis
After predetermined times, the treated cells from
each group were washed twice with ice-cold phosphate-buffered
saline (PBS), and total protein was extracted using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China). Briefly, cells were lysed with
approximately 400 µl lysis buffer on ice for 30 min. These samples
were centrifuged at 4°C for 15 min at 14,000 rpm, after which the
supernatants were recovered and subpackaged. Total proteins were
quantified using the bicinchoninic acid protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Equal amounts of protein were
loaded and separated using 10–12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and then transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked for 1 h at 37°C with 5% milk in
Tris-buffered saline (TBS) containing 0.05% Tween-20 (TBST) and
then incubated for 1 h with anti-myeloid cell leukemia 1 (MCL1,
ab32087) and anti-nuclear factor (erythroid-derived 2)-like 2
(Nrf2, ab62352) antibodies (both 1:1,000), which were purchased
from Abcam (Cambridge, UK). The membranes were washed three times
with TBST, incubated with the secondary antibody for 40 min, washed
three times with TBST, and then visualized using Immobilon western
chemiluminescent horseradish peroxidase (HRP) substrate (EMD
Millipore). Glyceraldehyde 3-phosphate dehydrogenase served as an
internal loading control.
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay. The MTS assay was conducted using the CellTiter 96
AQueous One Solution cell proliferation assay kit (Promega
Corporation) according to the manufacturer's instructions. Briefly,
stable Lv-NC, Lv-miR-146a-5p, and Lv-miR-146a-5p-I cells
(1×104 cells/100 µl) were seeded into 96-well plates.
After adhesion, the cells were treated with 20 µg/ml Rh2 or DMSO
for 1, 2, and 3 days. Next, 10 µl CellTiter 96 AQueous One Solution
reagent was added to each well, followed by incubation for 4 h at
37°C, and then the absorbance of the reaction solution was measured
at 490 nm using a microplate reader (Multiskan MK3; Thermo Fisher
Scientific, Inc.). The survival rate was calculated using the
following formula: Survival rate (%)=[optical density
(OD)]test/ODnegative control] ×100.
Flow cytometric analysis
After predetermined times, each group of treated
HepG2 cells was digested, collected, and washed twice with PBS.
Cell apoptosis was subsequently analyzed using an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit
according to the manufacturer's instructions (Nanjing KeyGen
Biotech., Co., Ltd., Jiangsu, China). Briefly, the cell pellet
(~1–5×105 cells) was resuspended in 500 µl Binding
Buffer. Next, 5 µl each of Annexin V-FITC and propidium iodide (PI)
were added and mixed at room temperature (protected from light) for
15 min. After 1 h, the cells were detected by flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA). The cell cycle was analyzed
using cell cycle detection kits according to the manufacturer's
instructions (Nanjing KeyGen Biotech., Co., Ltd.). Briefly, the
cells were fixed in 500 µl 70% precooled ethanol at 4°C overnight.
An equal amount of PBS was added twice for washing, and then up to
100 µl RNase A was added at 37°C for 30 min, followed by addition
of 100 µl PI at 4°C in the dark for 30 min. Next, the cell cycle
was evaluated using a flow cytometry system (BD Biosciences) and
each experiment was repeated three times.
Colony formation assay
The Lv-NC, Lv-miR-146a-5p, and Lv-miR-146a-5p
inhibitor HepG2 cells were plated at a density of 100 cells/well in
96-well plates pre-coated with Matrigel (BD Biosciences) according
to the manufacturer's instructions. Cells were treated with DMSO or
Rh2, incubated for 10 days at 37°C in a humidified atmosphere of 5%
CO2, and during the colony growth, the culture medium
containing DMSO or Rh2 was replaced every 3 days. Photographs were
captured from five fields of view for each well using a Leica CTR
MIC microscope (Leica Microsystems GmbH, Wetzlar, Germany). The
number and size of the colonies were determined using ImageJ 1.49v
software (National Institutes of Health, Bethesda, MD, USA) and two
independent experiments were performed, each including three
replicates. The colony formation rate was calculated using the
following equation: colony formation rate (%)=(number of
colonies/number of seeded cells) ×100.
Tumorigenicity assay in nude mice
Six-week-old male athymic nude mice were
subcutaneously injected with 4×106 cells in 0.2 ml of
PBS in the middle upper abdominal region. Six mice were injected
with Lv-NC stable cells while three mice were injected with stable
Lv-miR-146a-5p or Lv-miR-146a-5p inhibitor cells. Four weeks later,
Rh2 (1 mg/kg body weight) was injected via the tail vein of the
mice twice weekly for 4 weeks until the end of the experiment.
Tumor sizes were measured using calipers. The control group
consisted of three mice injected with stable Lv-NC cells and
administered injections of 1% DMSO at the same volume and
frequency. The tumor volume was calculated using the following
formula: (L × W2)/2, where L and W are the length and
width of the tumor, respectively. All experimental procedures
involving animals were in accordance with the Guide for the Care
and Use of Laboratory Animals (NIH Publication no. 80-23, revised
1996) and performed according to the institutional ethical
guidelines for animal experiments. Ethical approval was obtained
from Nanfang Hospital (Guangdong, China) on June 10, 2016.
Immunohistochemistry (IHC)
Paraffin-embedded specimens were cut into 4-µm-thick
sections, incubated at 60°C for 60 min, deparaffinized with xylene,
and rehydrated. These sections were immersed in
ethylenediaminetetraacetic acid antigenic retrieval buffer in a
pressure cooker for 5 min, cooled to room temperature, and treated
with 3% hydrogen peroxide in methanol to quench endogenous
peroxidase activity. After incubation with goat serum for 30 min,
the sections were incubated with anti-MCL1 and anti-Nrf2 primary
antibody (1:100) overnight at 4°C. After washing three times with
PBS, protein expression was visualized using a ChemMate™
DAKO Envision™ detection kit (Glostrup, Denmark)
according to the manufacturer's instructions. Briefly, tissue
sections were incubated with biotinylated secondary antibody for 30
min at room temperature, followed by incubation with
streptavidin-HRP for 5 min. After washing three times with PBS,
diaminobenzidine was added for visualization, and the sections were
counterstained with hematoxylin.
Statistical analysis
Statistical analysis was performed using the SPSS
v.19.0 software (IBM Corp., Armonk, NY, USA). The results are
presented as the mean ± standard deviation. Statistical comparisons
were performed by one-way analysis of variance, followed by
Scheffe's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of Rh2 treatment on miRNA
expression level
After treatment with Rh2 for 48 h, the cells were
harvested for RT-qPCR to detect the expression levels of
miR-200b-5p, miR-224-3p, miR-26b-3p, miR-29a-5p, and miR-146a-5p.
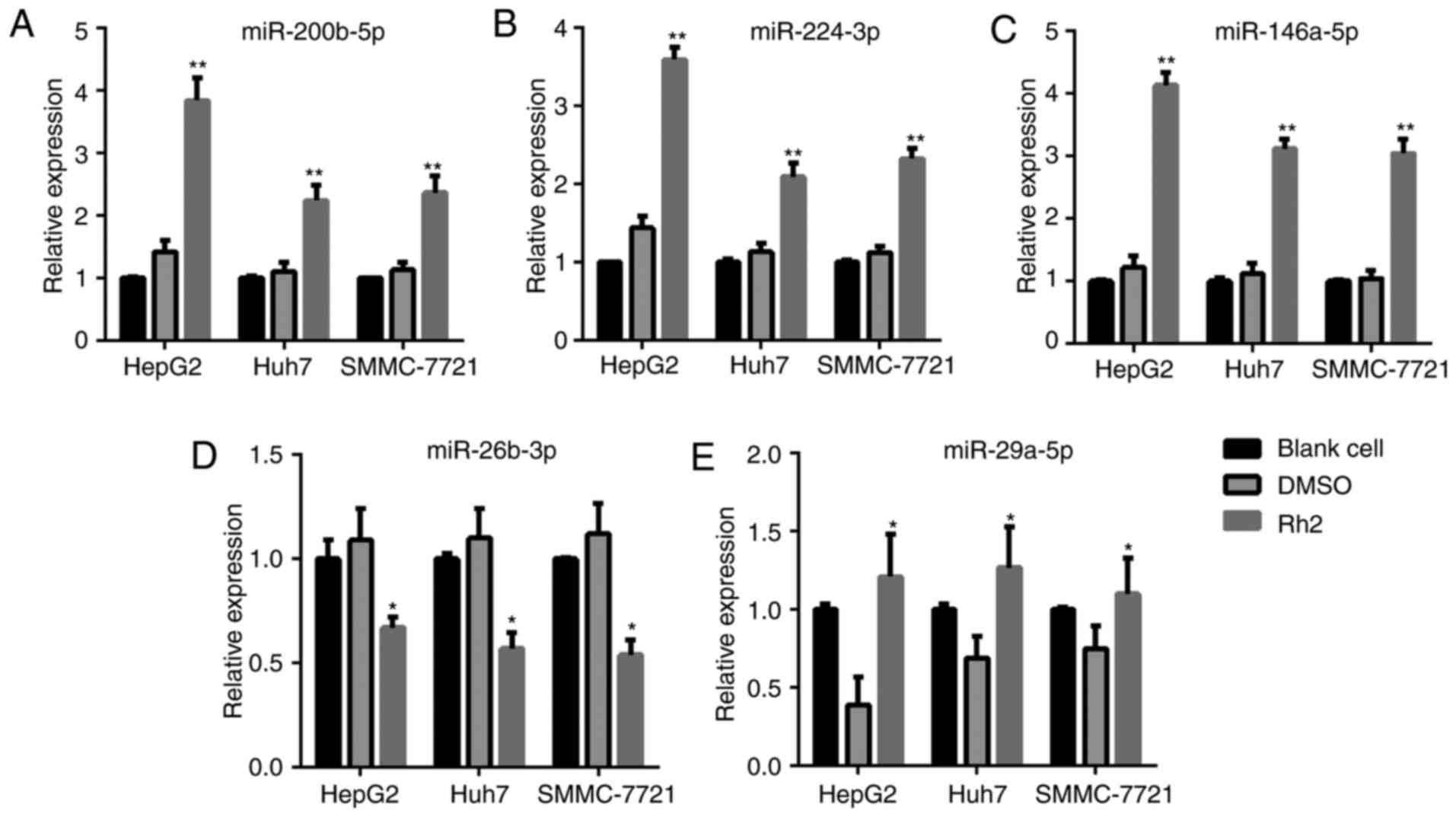
The results showed that Rh2 treatment increased the expression
level of miR-200b-5p, miR-224-3p, and miR-146a-5p compared to the
levels in DMSO-treated or blank cells in HepG2, Huh7, and SMMC-7721
cells (Fig. 1A-C). In addition, the
expression level of miR-26b-3p and miR-29a-5p decreased after Rh2
treatment in HepG2, Huh7, and SMMC-7721 cells (Fig. 1D and E). Among the three upregulated
miRNAs, miR-146a-5p exhibited the highest fold-increase in HepG2;
therefore, HepG2 cells and miR-146a-5p were used in subsequent
assays.
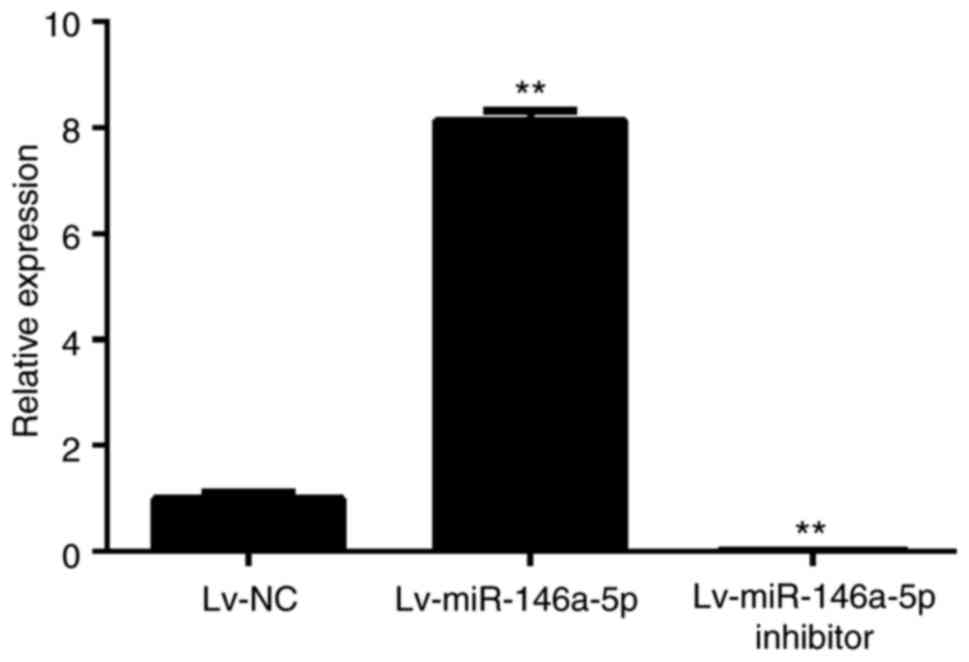
Construction of miR-146a-5p
overexpressing or knockdown stable HepG2 cells
To construct miR-146a-5p overexpressing or knockdown
stable HepG2 cells, the cells were infected with Lv-miR-146a-5p and
Lv-miR-146a-5p inhibitor and then harvested for RT-qPCR to detect
the miR-146a-5p expression level. As shown in Fig. 2, HepG2 cells infected with
Lv-miR-146a-5p and the Lv-miR-146a-5p inhibitor successfully
overexpressed and showed knockdown of miR-146a-5p, respectively,
compared to in Lv-NC transfected cells.
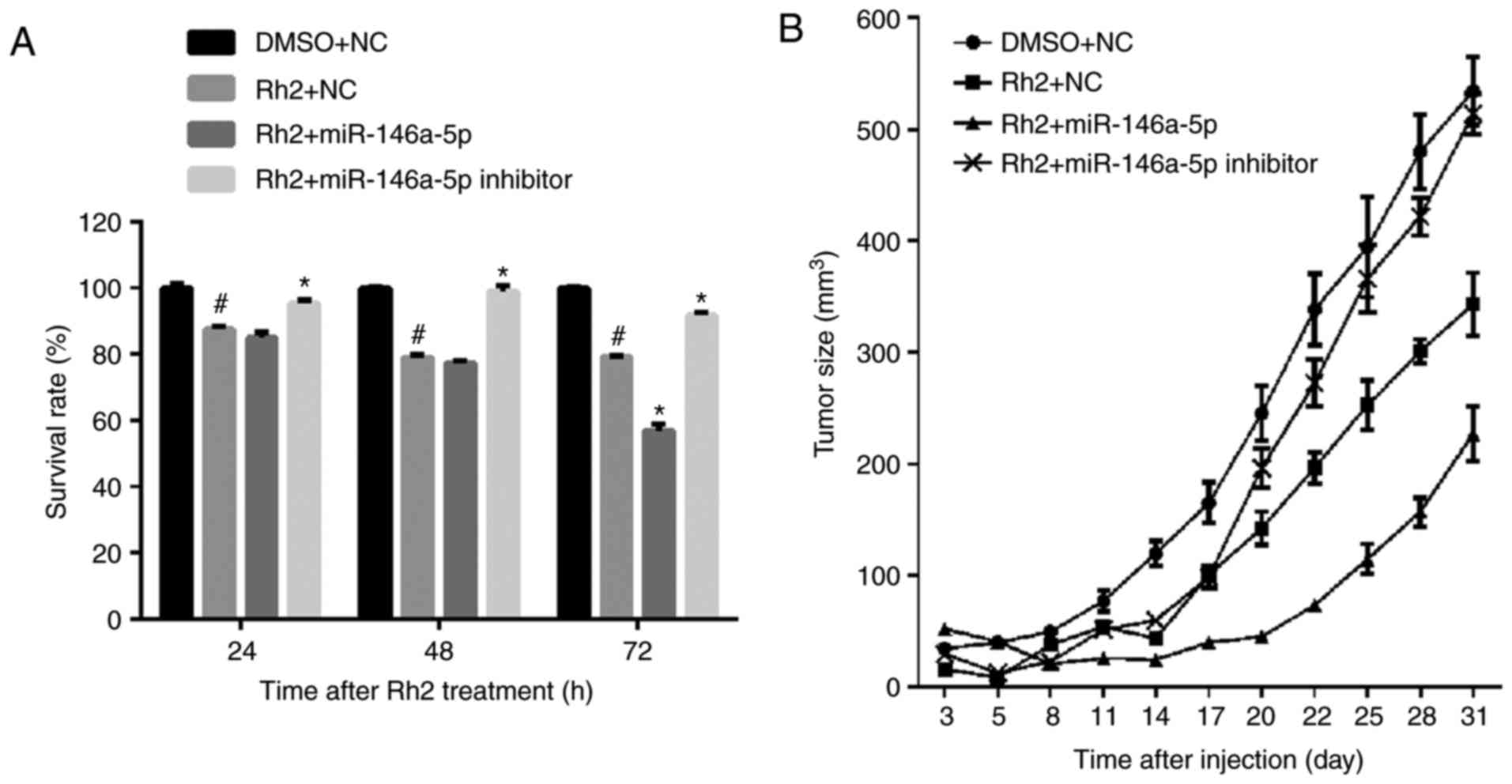
miR-146a-5p promoted inhibitory effect
of Rh2 on cell growth in vitro and in vivo
The results of the MTS assay showed that the
survival rate of Rh2-treated Lv-NC HepG2 cells (Rh2 + NC) was
clearly lower than those treated with DMSOs (DMSO + NC). This
result indicates that Rh2 inhibited the proliferation of HepG2
cells (Fig. 3A). To examine the
effect of miR-146a-5p on the cell survival of Rh2 treated HepG2
cells, stable cells expressing miR-146a-5p or the miR-146a-5p
inhibitor were treated with Rh2 for 24, 48, and 72 h. The results
of the MTS assay showed that miR-146a-5p overexpression promoted
the inhibitory effect of Rh2 on cell survival, while the
miR-146a-5p inhibitor weakened this effect (Fig. 3A). To further verify the role of
miR-146a-5p in HepG2 cell proliferation, stable cells expressing
miR-146a-5p or the miR-146a-5p inhibitor were injected into the
right armpit region of the mice, which were subsequently gavaged
with Rh2 once per day. The results showed that miR-146a-5p
overexpression promoted the inhibitory effect of Rh2 on tumor size,
while the miR-146a-5p inhibitor weakened this effect (Fig. 3B). These results indicate that Rh2
inhibited cell growth and miR-146a-5p enhanced this inhibitory
effect in vitro and in vivo.
miR-146a-5p increased Rh2-induced cell
apoptosis of HepG2 cells
The results of flow cytometry analysis showed that
the number of early apoptotic Rh2 + NC cells was clearly higher
than that of the DMSO + NC cells, indicating that Rh2 promoted the
apoptosis of HepG2 cells (Fig. 4A and
B). To examine the role of miR-146a-5p on cell apoptosis of
Rh2-treated HepG2, stable cells expressing miR-146a-5p or the
miR-146a-5p inhibitor were treated with Rh2 for 48 h. The results
showed that miR-146a-5p overexpression enhanced the cell apoptosis
effect of Rh2, while the miR-146a-5p inhibitor weakened this effect
(Fig. 4A and B).
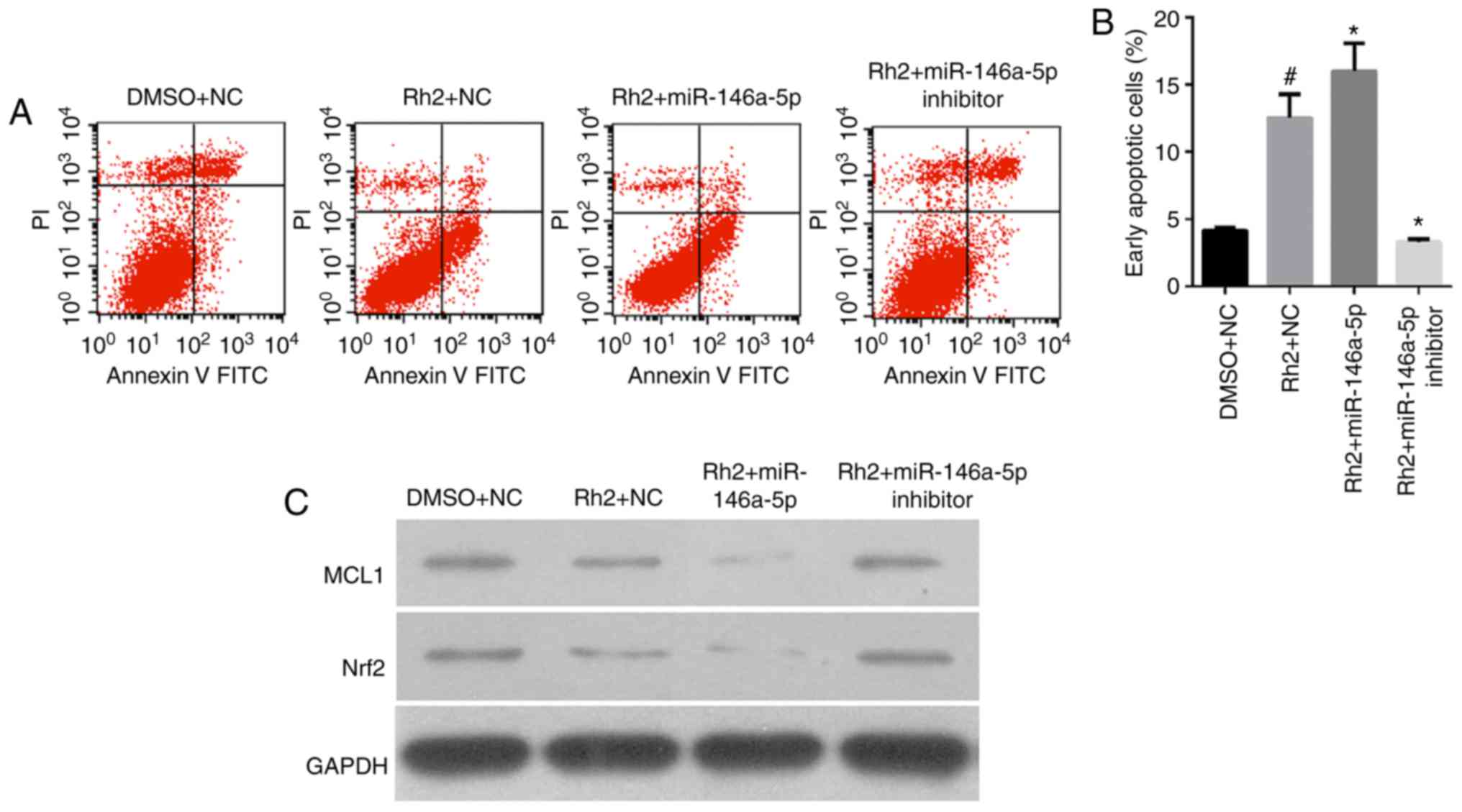 | Figure 4.miR-146a-5p enhances the promoting
effect of Rh2 on cell apoptosis in vitro. Stable cells
expressing miR-146a-5p, miR-146a-5p inhibitor or NC cells were
treated with Rh2 or DMSO for 48 h. (A) Cell apoptotic rate was then
analyzed by flow cytometry, and representative flow cytometry
analysis graphs are presented. (B) Statistical analysis of early
apoptotic cells. #P<0.05 vs. DMSO+NC; *P<0.05 vs.
Rh2+NC. (C) MCL1 and Nrf2 protein expression was detected by
western blotting. miR, microRNA; Rh2, ginsenoside Rh2; NC, negative
control; DMSO, dimethyl sulfoxide; MCL1, myeloid cell leukemia 1;
Nrf2, nuclear factor (erythroid-derived 2)-like 2; FITC,
fluorescein isothiocyanate; PI, propidium iodide. |
In addition, we detected the expression of cell
apoptosis-related proteins, MCL1, B-cell lymphoma-2 (Bcl2), and
Nrf2 by western blotting. The results showed that MCL1 and Nrf2
expression levels in Rh2 + NC cells were clearly lower than those
in DMSO + NC cells were. This result indicates that Rh2 suppressed
MCL1 and Nrf2 expression in HepG2 cells (Fig. 4C).
To examine the effect of miR-146a-5p on MCL1, Bcl2,
and Nrf2 expression in Rh2-treated HepG2 cells, stable cells
expressing miR-146a-5p or the miR-146a-5p inhibitor were treated
with Rh2 for 48 h. The results showed that miR-146a-5p
overexpression enhanced the inhibitory effect of Rh2 on MCL1 and
Nrf2 and increased its effect on Bcl2 expression. Furthermore, the
miR-146a-5p inhibitor weakened the effect of Rh2 on MCL1 and Nrf2
expression (Fig. 4C). In addition,
the role of miR-146a-5p on MCL1, and Nrf2 expression in Rh2-treated
HepG2 cells was further verified in tumor samples in tumorigenicity
assays of nude mice using IHC and the results are consistent with
those of the in vitro experiments (Fig. 5).
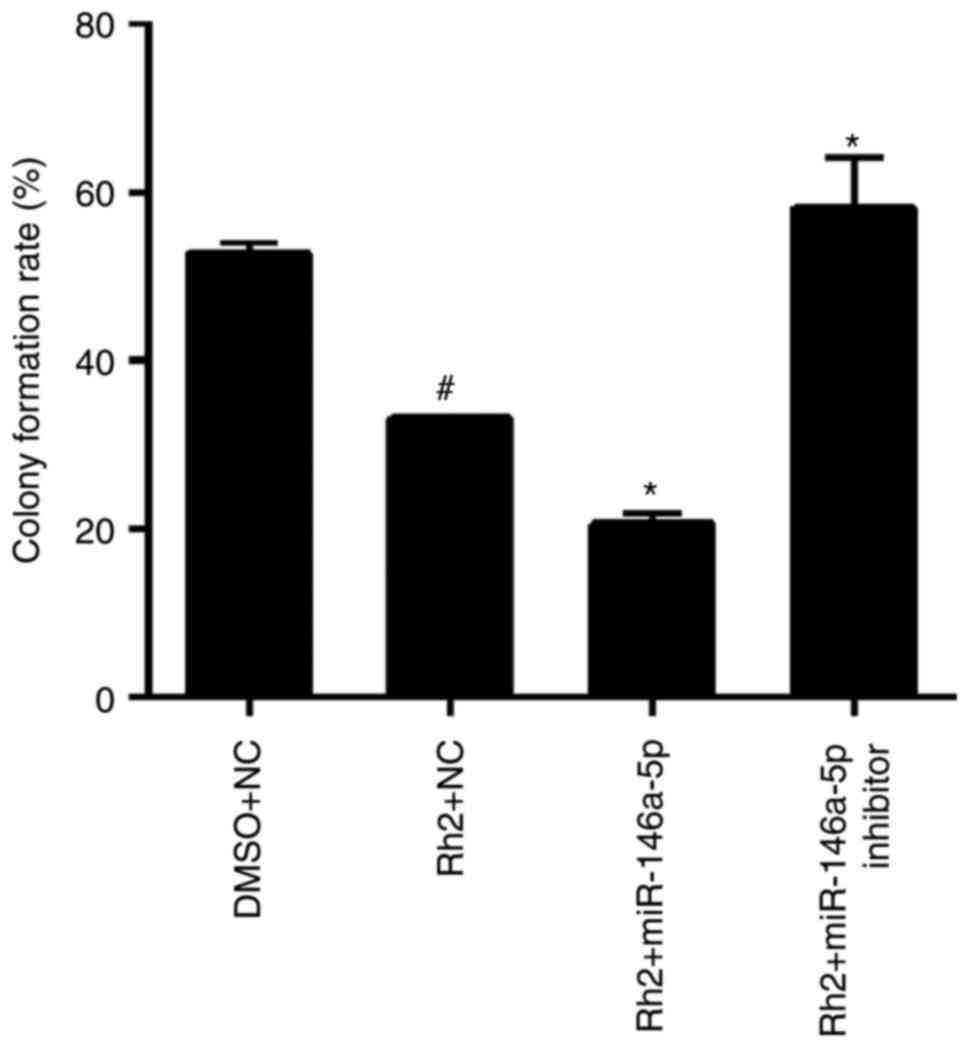
miR-146a-5p promoted effect of Rh2 on
colony formation in vitro
The results of the colony formation assay showed
that the colony formation rate of Rh2 + NC cells was clearly lower
than that of DMSO + NC cells, indicating that Rh2 suppressed the
colony formation of HepG2 cells (Fig.
6). To examine the role of miR-146a-5p in colony formation of
Rh2-treated hepG2 cells, stable cells expressing miR-146a-5p or the
miR-146a-5p inhibitor were treated with Rh2 for 10 days. The
results showed that miR-146a-5p overexpression enhanced the
inhibitory effect of Rh2 on colony formation, while the miR-146a-5p
inhibitor weakened this effect (Fig.
6).
Discussion
Liver cancer is one of the leading causes of
malignancy-related deaths worldwide (17), and its clinical therapy is very
challenging. Rh2 is a compound isolated from P. ginseng,
which is popular in China for its nourishing and protective effects
on the human body. Rh2 has been demonstrated to suppress tumor
growth without causing severe side effects in both H22 cells and a
hepatoma-bearing mouse model (19).
In addition, Rh2 reduced HCC cell viability and the number of
cancer stem cell-like cells (6).
Therefore, Rh2 likely has antitumor activity against liver cancer
cells. However, its regulatory mechanism is not clear, although Rh2
is known to increase autophagy and inhibit β-catenin signaling
(6). As an important regulator, the
effect of miRNAs on the activity of Rh2 in liver cancer cells is
unclear. In the present study, we detected the expression levels of
miR-200b-5p, miR-224-3p, miR-26b-3p, miR-29a-5p, and miR-146a-5p.
Of the three upregulated miRNAs (miR-200b-5p, miR-224-3p, and
miR-146a-5p), the fold-increase in miR-146a-5p was the highest;
therefore, we further determined its role in Rh2-induced
proliferation suppression and apoptosis promotion in the liver
cancer cell line HepG2. HepG2 is a hepatoblastoma-derived cell line
(20). For in vitro studies,
the HepG2 cell line is frequently employed as experimental model
because it is not only widely available, but also a
well-characterized liver cancer cell line (21). Thus, HepG2 cells are suitable for
studying the effect of Rh2 and miR-146a-5p on liver cancer.
The effect of Rh2 on cell apoptosis and colony
formation of liver cancer cells is unknown. In the present study,
we found that Rh2 increased the number of early apoptotic HepG2
cells. In addition, Rh2 decreased MCL1 and Nrf2 expression levels
and increased Bcl2 expression. MCL1, a member of the Bcl-2 family,
is an anti-apoptotic protein. Nrf2, a member of a small family of
basic leucine zipper proteins, is also an anti-apoptotic protein
(22,23). These results revealed that Rh2
promoted liver cancer cell apoptosis. In addition, our results
indicated that Rh2 suppressed colony formation. Collectively, our
results demonstrated the antitumor effects of Rh2 in liver cancer
cells.
In accordance with a previous study (6), we found that Rh2 inhibited liver cancer
cell growth in vitro and in vivo, and this inhibitory
effect was enhanced by miR-146a-5p overexpression and weakened by
the miR-146a-5p inhibitor. Our results were further supported by
examining the role of miR-146a-5p in liver cancer cells.
miR-146a-5p expression was decreased in liver cancer tissues
compared to in corresponding adjacent tissues, while miR-146a
overexpression suppressed the proliferation of the liver cancer
cell lines HepG2 and SMMC7721 (24).
This observation suggests an antitumor role of miR-146a-5p. In
addition, the promoting effect of Rh2 on cell apoptosis and its
inhibitory effect on colony formation were enhanced by miR-146a-5p
overexpression and weakened by the miR-146a-5p inhibitor. Further,
miR-146a-5p expression was upregulated by Rh2 treatment. Thus, the
antitumor effect of Rh2 may be mediated through the regulation of
miR-146a-5p expression.
In conclusion, our study provides new in
vitro evidence that Rh2 promoted liver cancer cell apoptosis
and inhibited colony formation. However, further studies using
animal models are required to verify our findings. In addition, Rh2
upregulated miR-146a-5p expression. Collectively, these results
indicate that miR-146a-5p overexpression enhanced the effect of Rh2
on liver cancer cell growth, apoptosis, and colony formation.
Therefore, the Rh2-induced regulation of liver cancer cell growth,
apoptosis, and colony formation was mediated by miR-146a-5p.
However, several issues remain unclear, such as determining the
target of miR-146a-5p, elucidating the mechanisms underlying the
regulatory effect of miR-146a-5p on the activity of Rh2 in liver
cancer cells, and evaluating the relationship between miR-338-3p
and other miRNAs in Rh2-induced actions in liver cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Science and
Technology Program of Guangzhou (grant no. 201607010015) and
Natural Science Foundation of Guangdong (grant no.
2016A030313525).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WC and SC performed the cell culture, stable cell
construction, flow cytometric analysis, MTS assay and colony
formation assay. WC performed immunohistochemistry and the
tumorigenicity assay in nude mice. HL performed RNA extraction,
reverse transcription-quantitative polymerase chain reaction and
western blot analysis. YQ performed statistical analysis and
designed study. WC wrote the manuscript and SC helped to draft the
manuscript. WC and SC read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oh M, Choi YH, Choi S, Chung H, Kim K, Kim
SI, Kim DK and Kim ND: Anti-proliferating effects of ginsenoside
Rh2 on MCF-7 human breast cancer cells. Int J Oncol. 14:869–875.
1999.PubMed/NCBI
|
|
2
|
Nakata H, Kikuchi Y, Tode T, Hirata J,
Kita T, Ishii K, Kudoh K, Nagata I and Shinomiya N: Inhibitory
effects of ginsenoside Rh2 on tumor growth in nude mice bearing
human ovarian cancer cells. Jpn J Cancer Res. 89:733–740. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie X, Eberding A, Madera C, Fazli L, Jia
W, Goldenberg L, Gleave M and Guns ES: Rh2 synergistically enhances
paclitaxel or mitoxantrone in prostate cancer models. J Urol.
175:1926–1931. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia T, Wang YN, Zhou CX, Wu LM, Liu Y,
Zeng QH, Zhang XL, Yao JH, Wang M and Fang JP: Ginsenoside Rh2 and
Rg3 inhibit cell proliferation and induce apoptosis by increasing
mitochondrial reactive oxygen species in human leukemia Jurkat
cells. Mol Med Rep. 15:3591–3598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang J, Yuan D, Xing T, Su H, Zhang S, Wen
J, Bai Q and Dang D: Ginsenoside Rh2 inhibiting HCT116 colon cancer
cell proliferation through blocking PDZ-binding kinase/T-LAK
cell-originated protein kinase. J Ginseng Res. 40:400–408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Z, Zhao T, Liu H and Zhang L:
Ginsenoside Rh2 inhibits hepatocellular carcinoma through β-catenin
and autophagy. Sci Rep. 6:193832016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guan N, Huo X, Zhang Z, Zhang S, Luo J and
Guo W: Ginsenoside Rh2 inhibits metastasis of glioblastoma
multiforme through Akt-regulated MMP13. Tumour Biol. 36:6789–6795.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Gao Y, Ma W, Cheng T and Liu Y:
Ginsenoside Rh2 inhibits invasiveness of glioblastoma through
modulation of VEGF-A. Tumour Biol. 2015.(Epub ahead of print).
|
|
9
|
Li S, Gao Y, Ma W, Guo W, Zhou G, Cheng T
and Liu Y: EGFR signaling-dependent inhibition of glioblastoma
growth by ginsenoside Rh2. Tumour Biol. 35:5593–5598. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang J, Peng K, Wang L, Wen B, Zhou L,
Luo T, Su M, Li J and Luo Z: Ginsenoside Rh2 inhibits proliferation
and induces apoptosis in human leukemia cells via TNF-α signaling
pathway. Acta Biochim Biophys Sin (Shanghai). 48:750–755. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han S, Jeong AJ, Yang H, Kang Bin K, Lee
H, Yi EH, Kim BH, Cho CH, Chung JW, Sung SH and Ye SK: Ginsenoside
20(S)-Rh2 exerts anti-cancer activity through targeting
IL-6-induced JAK2/STAT3 pathway in human colorectal cancer cells. J
Ethnopharmacol. 194:83–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joshi P, Middleton J, Jeon YJ and Garofalo
M: MicroRNAs in lung cancer. World J Methodol. 4:59–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen X, Zhang HD, Zhao L, Yao YF, Zhao JH
and Tang JH: Ginsenoside Rh2 differentially mediates microRNA
expression to prevent chemoresistance of breast cancer. Asian Pac J
Cancer Prev. 16:1105–1109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An IS, An S, Kwon KJ, Kim YJ and Bae S:
Ginsenoside Rh2 mediates changes in the microRNA expression profile
of human non-small cell lung cancer A549 cells. Oncol Rep.
29:523–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu N, Wu GC, Hu R, Li M and Feng H:
Ginsenoside Rh2 inhibits glioma cell proliferation by targeting
microRNA-128. Acta Pharmacol Sin. 32:345–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo H and Xia B: Collapsin response
mediator protein 4 isoforms (CRMP4a and CRMP4b) have opposite
effects on cell proliferation, migration, and invasion in gastric
cancer. BMC Cancer. 16:5652016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lv Q, Rong N, Liu LJ, Xu XL, Liu JT, Jin
FX and Wang CM: Antitumoral activity of (20R)- and
(20S)-Ginsenoside Rh2 on transplanted hepatocellular carcinoma in
mice. Planta Med. 82:705–711. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
21
|
Qiu GH, Xie X, Xu F, Shi X, Wang Y and
Deng L: Distinctive pharmacological differences between liver
cancer cell lines HepG2 and Hep3B. Cytotechnology. 67:1–12. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moi P, Chan K, Asunis I, Cao A and Kan YW:
Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic
leucine zipper transcriptional activator that binds to the tandem
NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl
Acad Sci USA. 91:9926–9930. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Milkovic L, Zarkovic N and Saso L:
Controversy about pharmacological modulation of Nrf2 for cancer
therapy. Redox Biol. 12:727–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zu Y, Yang Y, Zhu J, Bo X, Hou S, Zhang B,
Qiu J and Zheng J: MiR-146a suppresses hepatocellular carcinoma by
downregulating TRAF6. Am J Cancer Res. 6:2502–2513. 2016.PubMed/NCBI
|