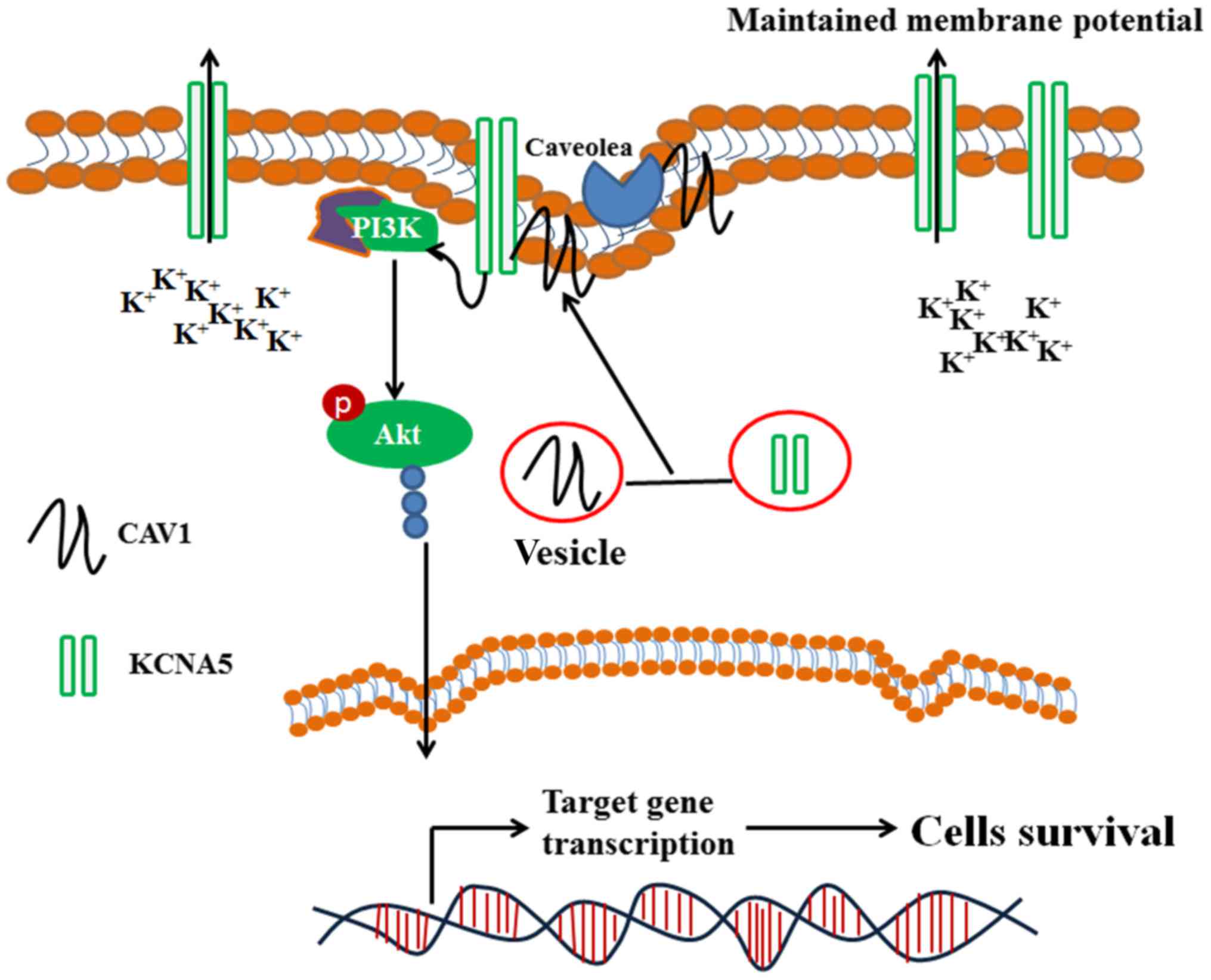
Introduction
Voltage-gated potassium (Kv) channels
play a key role in several physiological processes, such as the
membrane potential maintainance, Ca2+ signaling, and the
cell volume regulation. In addition, they are also involved in cell
survival and migration (1). Most
studies have focused on the function of Kv channels in
viability of tumor cells, particularly tumor cells of epithelial
origin, such as human mammary epithelial cell (2,3).
Accumulated evidence indicated that the expression of Kv
channels is related to tumor development (4). However, the mechanism of Kv
channels in these cells is still unknown. Thus, many drugs and
toxins that specifically block Kv channels have been
tested for their effect on cell proliferation. Among the
Kv channels subunits, potassium voltage-gated channel
subfamily A member 5 (KCNA5) specifically has been shown to be
involved in the viability and apoptosis of oligodendrocytes,
hippocampus microglia, macrophages and human mammary epithelial
cells (5–7). It has reported that most tumor cells had
increased expression of KCNA5 (8).
Furthermore, the transition of quiescent cells into the
G1 phase is accompanied by the over-expression of Kv1.3
and KCNA5 proteins in rat oligodendrocyte precursor cells. Blocking
KCNA5 sufficiently slowed the viability of astrocytes rather than
oligodendrocytes, indicating that this channel may play different
roles in different cells.
AKT phosphorylation is transduced and amplified
through downstream kinase cascades, inducing cell survival, growth,
differentiation as well as metabolic changes (2). In general, Kv channels are
modulated by mitogenic signals, such as growth factor-mediated
signaling (8,9). In HEK293 cells, IGF-1 induces the
expression of several Kv channels in response to
mitogenic signals (10). The
mitogenic stimulation of G0 phase activates
K+ channels, drives the cells into G1 phase,
and then initiates proliferation (3,11).
Kv channels play a major role in advancing the cell
cycle when activated by mitogenic factors (12,13).
Interestingly, Kv channels are upstream modulators of
growth factor-mediated MAPK and PI3K/Akt pathways (14,15). And
many current studies suggest that K+ channels involved
in the initial mitogenic signaling events occur at the membrane
level. Moreover, the activation of K+ channels may lead
to receptor clustering, thereby facilitating transmembrane
signaling (2).
Lipid rafts support numerous cellular events in
membrane trafficking and signal transduction mediated by multiple
membrane proteins (16–18). Caveolae are a type of lipid raft
containing specific scaffolding proteins, like caveolin (19). Various lipid rafts share similar lipid
proteins (20–22). The caveolin family has three members,
including caveolin-1 (Cav-1), Cav-2 and Cav-3, of which Cav-3 is
restricted to muscle cells (23,24). Many
signaling molecules are directly related to Cav-1 (25,26). Some
proteins have been well-characterized, including Kv channels that
interact with caveolin, which are to concentrate the cargo proteins
in the caveolae (27,28). It has been reported that the
expression and localization of Kv channels are important to play
their function fully in cells (29).
Numerous Kv channels localize to the lipid raft domains and/or
caveolae in the plasma membrane. KCNA5 has been found in raft
microdomains and their functions are influenced by lipid-protein
interactions. Martens et al showed that KCNA5 can localize
to caveolae microdomains, and KCNA5 was associated with caveolae
(27). However, we are unknown for
the mechanisms controlling their interactions and the physiological
functions of this localization. Recent research has found a role of
Cav-1 in transporting proteins to the cell membrane (30). And according to some recent studies,
Cav-1 regulates proteins that co-localize with it, such as estrogen
receptor (ER), KCNA5, and desmoglein 2 (Dsg2) (31–33).
However, the role of Cav-1 in mediating the membrane localization
of KCNA5 channel has not been elucidated.
Our previous study demonstrated that Kv
channels were required for the viability of the normal MCF-10A-neoT
cells (7). In this study, we
described that KCNA5 and Cav-1 co-localize in the cytoplasm of
MCF-7 human breast cancer cells. The study also found that the
knockdown KCNA5 inhibited the PI3K/AKT signaling pathway in
MCF-10A-neoT cells, and cells upregulated with Cav-1 and KCNA5
promoted survival in MCF-7 cells through PI3K/AKT signaling. In
addition, it was showed that the downregulation of Cav-1 decreased
the expression of KCNA5, indicating that Cav-1 was involved in the
KCNA5-promoted survival of human mammary cells.
Materials and methods
Plasmids and antibodies
The KCNA5 plasmid was from Dr Jie Zheng (University
of California, Davis). The Cav-1 plasmid and siRNA plasmid specific
for Cav-1 (target sequence Oligo 1,
5′-ACCTCATTAAGAGCTTCCTGATTGAGTCAAGAGCTCAATCAGGAAGCTCTTAATTT-3′,
Oligo 2,
5′-CAAAAAATTAAGAGCTTCCTGATTGAGCTCTTGACTCAATCAGGAAGCTCTTAATG-3′)
were obtained from the Cancer Center at Creighton University.
Anti-KCNA5 (rabbit polyclonal, 1:500; EMD Millipore,
Billerica, MA, USA), anti-Cav-1 (mouse monoclonal, 1:1,000, Santa
cruz biotechnology), anti-p-MAPK (mouse monoclonal, 1:1,000),
anti-MAPK (rabbit polyclonal, 1:1,000), anti-p-AKT (rabbit
monoclonal, 1:1,000) (all from Cell Signaling Technology, Danvers,
MA, USA), anti-AKT (goat polyclonal, 1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), 1:500), anti-PCNA (mouse
monoclonal, 1:500) and anti-β-actin (mouse monoclonal, 1:1,000)
(all from Wuhan Boster Biological Technology, Ltd., Wuhan, China).
HRP-conjugated goat anti-rabbit, anti-mouse or anti-goat specific
secondary antibody (1:6,000; Zhongshan Golden Bridge Biotechnology,
Beijing, China).
Patients
A total of 23 breast cancer tissues were obtained
from patients in the First Affiliated Hospital of Dalian Medical
University. All the patients were females aged 29–83 with
infiltrative non-specific breast cancer. The selected tissue
samples express both ERα and ER-α36 under immunofluorescence
observation, and without any radiation, chemotherapy, or
endocrinotherapy treatment before surgical resection. We got the
patients' relatives written informed consent for the procedures,
which were also approved by the Ethics Committee on the Use of
Human Subjects (the First Affiliated Hospital of Dalian Medical
University).
Cell culture and transfection
The MCF-10A-neoT, MCF-7 and MDA-MB-231 cells were
purchased from ATCC (Rockville, MD, USA). Stable clones (designated
as MCF-10A-neoTCE) were established as described in our previous
study (34,35). The MCF-10A-neoT and MCF-10A-neoTCE
cells were cultured in DMEM/F12 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 5% horse
serum (HyClone, Logan, UT, USA), penicillin (100 U/ml),
streptomycin (100 µg/ml) (both from Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), hydrocortisone (1.4×10-6 M; HyClone), insulin
(10 µg/ml), cholera toxin (100 ng/ml) and EGF (20 ng/ml) (both from
Sigma-Aldrich, Merck KGaA). MCF7, MDA-MB-231 were cultured in
RPMI-1640 supplemented with 10% fetal bovine serum (both from
Gibco; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml), and
streptomycin (100 µg/ml). All cells were maintained in a humidified
atmosphere at 37°C in 5% CO2. Lipofectamine 2000TM (Invitrogen;
Thermo Fisher Scientific, Inc.) was used for cell transfection
according to the manufacturer's instructions. After 24–48 h of
transfection and subsequent culture in 1 µM wortmannin or 50 µM
Ly294002 (Sigma-Aldrich, Tokyo, Japan), cells were harvested for
western blot analysis or
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Methyl-β-cyclodextrin (MβCD) was used to disrupt caveolae.
MCF-10A-neoT cells pretreated with MβCD (2 mM) for 90 min were used
for immunofluorescent microscopy analysis.
Western blotting
Cells were harvested and then lysed in a cold lysis
buffer (20 mmol L−1 Tris-HCl, pH 7.5, 70 mmol
L−1 NaCl, 0.1% SDS, 1% sodium deoxycholate, 1% Triton
X-100 and 1% PMSF) to extract protein (35). The concentration of total protein was
determined by the Bradford method. The protein samples were then
subjected to 10% SDS-PAGE. After electrophoresis, protein bands
were transferred to a polyvinylidene fluoride (PVDF) membrane, and
then blocked in PBS-T (pH 7.4) containing 5% dried skim milk. Then
the PVDF membrane was probed with the specified primary antibody,
followed by the appropriate secondary antibody, and finally
visualized using the ECL™ which is a Western blotting
chemiluminescent reagent kit (Amersham Biosciences, Piscataway, NJ,
USA) according to the manufacturer's instructions. Immunoblot data
were quantified using ImageJ software (NIH, Bethesda, MD, USA). The
region of interest was marked and measured in every lane, and the
background was subtracted to give the final band intensity.
RNA interference
The small interfering RNAs (siRNA) against the KCNA5
mRNA sequence (GenBank accession number NM_002234) were predesigned
and synthesized in Takara (Dalian, China), meanwhile, an unrelated
siRNA serving as a negative control was randomly designed. The
sequences of the KCNA5 siRNA vector plasmid (target sequence Oligo
1,
5′-GATCCACCAGGGAACCCATTTCTCTCTGTGAAGCCACAGATGGGAGAGAAATGGGTTCCCTGGTTTTTTTAT-3′,
target sequence Oligo 2,
5′-CGATAAAAAAACCAGGGAACCCATTTCTCTCCCATCTGTGGCTTCACAGAGAGAAATGGGTTCCCTGGTG-3′),
and the negative control siRNA plasmid (target sequence Oligo 1,
5′-GATCCAGATCCTCACGATACCGTCTCTGTGAAGCCACAGATGGGAGACGGTATCGTGAGGATCTTTTTTTAT-3′,
target sequence Oligo 2,
5′-CGATAAAAAAAGATCCTCACGATACCGTCTCCCATCTGTGGCTTCACAGAGACGGTATCGTGAGGATCTG-3′).
Knocking down the basal expression levels of KCNA5
or Cav-1 in MCF-10A-neoT or MDA-MB-231 cells were performed using
the respective siRNAs, as well as scrambled control siRNA. The
cells were transfected with Lipofectamine™ 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) and 4 µg of Cav-1-siRNA according to the
manufacturer's recommendation. After 48 h of transfection, culture
medium with G418 (Amresco, Solon, OH, USA) was added to the cells.
The cells were harvested when the monoclone formed and were
processed for further analysis.
Survival assay
For the MTT assay, 20 µl MTT reagent was added to
each well and incubated for 4 h. The supernatant was discarded and
replaced with DMSO to dissolve the formazan product, which was
measured by a spectrophotometric plate reader at 490 nm.
Hematoxylin and eosin staining
Human breast cancer specimens were fixed for 1 week
in 4% (w/v) PBS-buffered formaldehyde solution at room temperature,
dehydrated using graded ethanol, and embedded in Paraplast
(Sherwood Medical, Mahwah, NJ, USA). Sections were stained with
hematoxylin and eosin, then deparaffinized with xylene. All
sections were studied by using a AxioVision zeiss (Olympus, Tokyo,
Japan) microscope.
Immunofluorescent staining
Cells were grown on glass coverslips coated with
poly-L-lysine, and cultured for 24 h. The cells were then fixed in
4% ice cold paraformaldehyde for 15–20 min. Cells were
permeabilized with PBS containing 0.1% Triton X-100 for 10 min.
After blocking with 5% bovine serum albumin (BSA) for 1 h, the
cells were then incubated with appropriate antibodies at 4°C
overnight, followed by further incubation with
fluorescein-conjugated affinipure goat anti-mouse antibodies
(1:300) and Rhodamine-conjugated affinipure goat anti-rabbit
antibodies (1:300) (both from Zhongshan Golden Bridge
Biotechnology). Nucleus was stained using DAPI (0.2 µg/ml;
Sigma-Aldrich, Merck KGaA). Fluorescence was imaged with a Bio-Rad
MRC 600 confocal imaging system. Images were taken with a Leica TCS
SP2 multiphoton confocal microscope.
Statistical analysis
All data were expressed as the mean ± SE Unpaired
Student's t-test was used to test for statistical significance
between the control and test groups. Comparisons of multiple groups
were analyzed using a one- or two-way ANOVA followed by post hoc
Tukey's test. P-value <0.05 was considered significance.
Results
Cav-1 and KCNA5 are co-expression in
human breast cancer cell lines
Cav-1 and KCNA5 have been shown to be related to
epithelial cell growth and survival, and are abnormally expressed
in malignant tumors including breast cancer. The study demonstrated
that Kv channels activate MAPK and AKT pathways, and
Cav-1 and KCNA5 were co-localized in the membrane of MCF-10A-neoT
non-tumorigenic epithelial cell line (36). The mechanism by which KCNA5 activates
AKT signaling has not been fully determined, but it has been
speculated to be activated through Cav-1. To examine the expression
of KCNA5 and Cav-1 in breast cancer tissues and cells, hematoxylin
and eosin staining was used to determine the region of cancer
(Fig. 1A). Next, the study tried to
determine whether there was co-expression between Cav-1 and KCNA5
in breast cancer tissues and cells. Cav-1 and KCNA5 both expressed
and co-expressed in breast cancer tissues (Fig. 1A) and cells (Fig. 1B). After enlarging MCF-7 fluorescence
images, it could be found KCNA5 and Cav-1 are co-expression in
cytoplasm and cell membrane, but Cav-1 mainly expressed in
cytoplasm of MCF-7 (Fig. 1B). Here,
we examined KCNA5 and Cav-1 expression in MCF-10A-neoT, MCF-7 and
MDA-MB-231 cells. Western blot analysis showed that both KCNA5 and
Cav-1 expression are lower in MCF-7 breast cancer cell compared
with MCF-10A-neoT non-tumorigenic epithelial cell and KCNA5 and
Cav-1 expressions are significantly higher in MDA-MB-231 breast
cancer cell than MCF-7 (Fig. 1C).
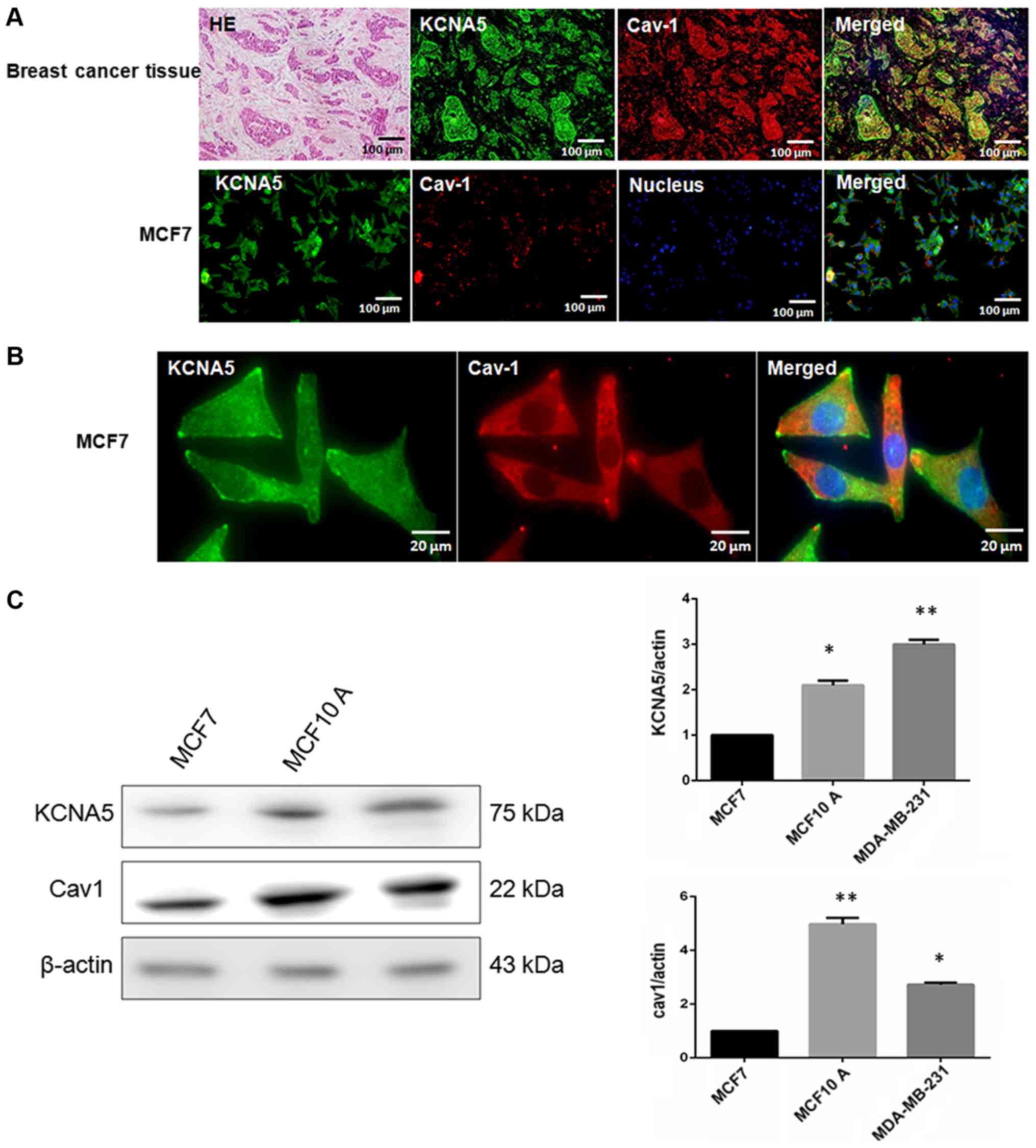 | Figure 1.The co-expression of KCNA5 and Cav-1
in human breast cancer cells. Hematoxylin and eosin staining for
human breast cancer tissue. (A) Immunofluorescence staining with
KCNA5 and Cav-1 antibody in breast cancer tissue (KCNA5 for green,
Cav-1 for red, Hoechst 33324 to label nuclear DNA). Bar, 100 µm.
(B) MCF-7 human breast cancer cells (KCNA5 for green, Cav-1 for
red, Hoechst 33324 to label nuclear DNA), bar, 100 µm or bar, 20
µm. Representative images were obtained by a Leica TCS SP 2
multiphoton confocal microscope. (C) Western blot analyses of Cav-1
and KCNA5 expression in MCF-7, MDA-MB-231 and MCF-10A-neoT cells.
The experiment was repeated four times; SE. *P<0.05 and
**P<0.01 for MCF-10A-neoT and MDA-MB-231 cells vs. MCF-7 cells.
KCNA5, potassium voltage-gated channel subfamily A member 5; Cav-1,
caveolin-1. |
KCNA5 and Cav-1 are coupled in
caveolae of human breast cancer cells
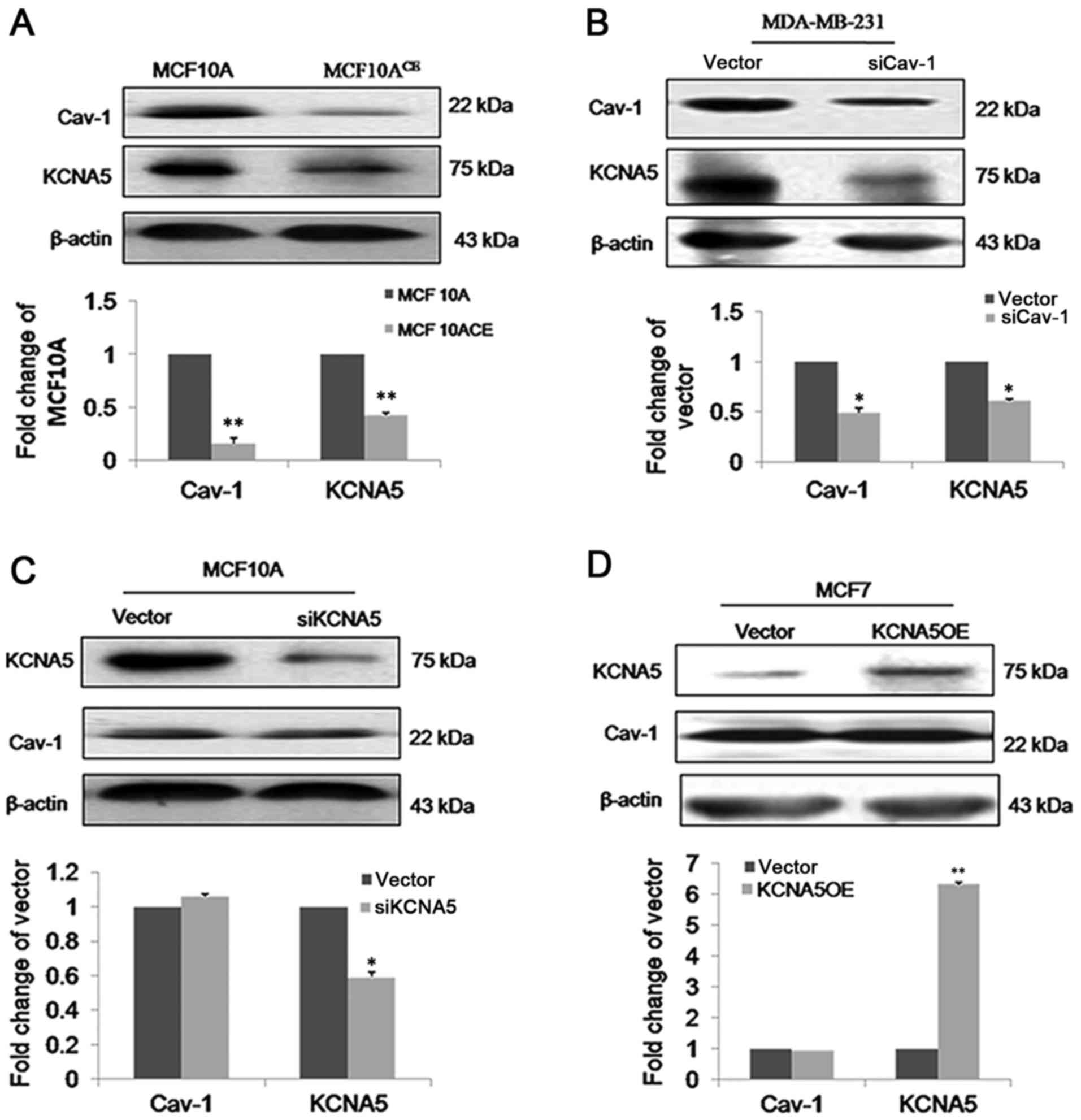
Our lab already has generated stable
MCF-10A-neoTCE, which is a Cav-1 knockdown cell line
(34). Compared with control cells,
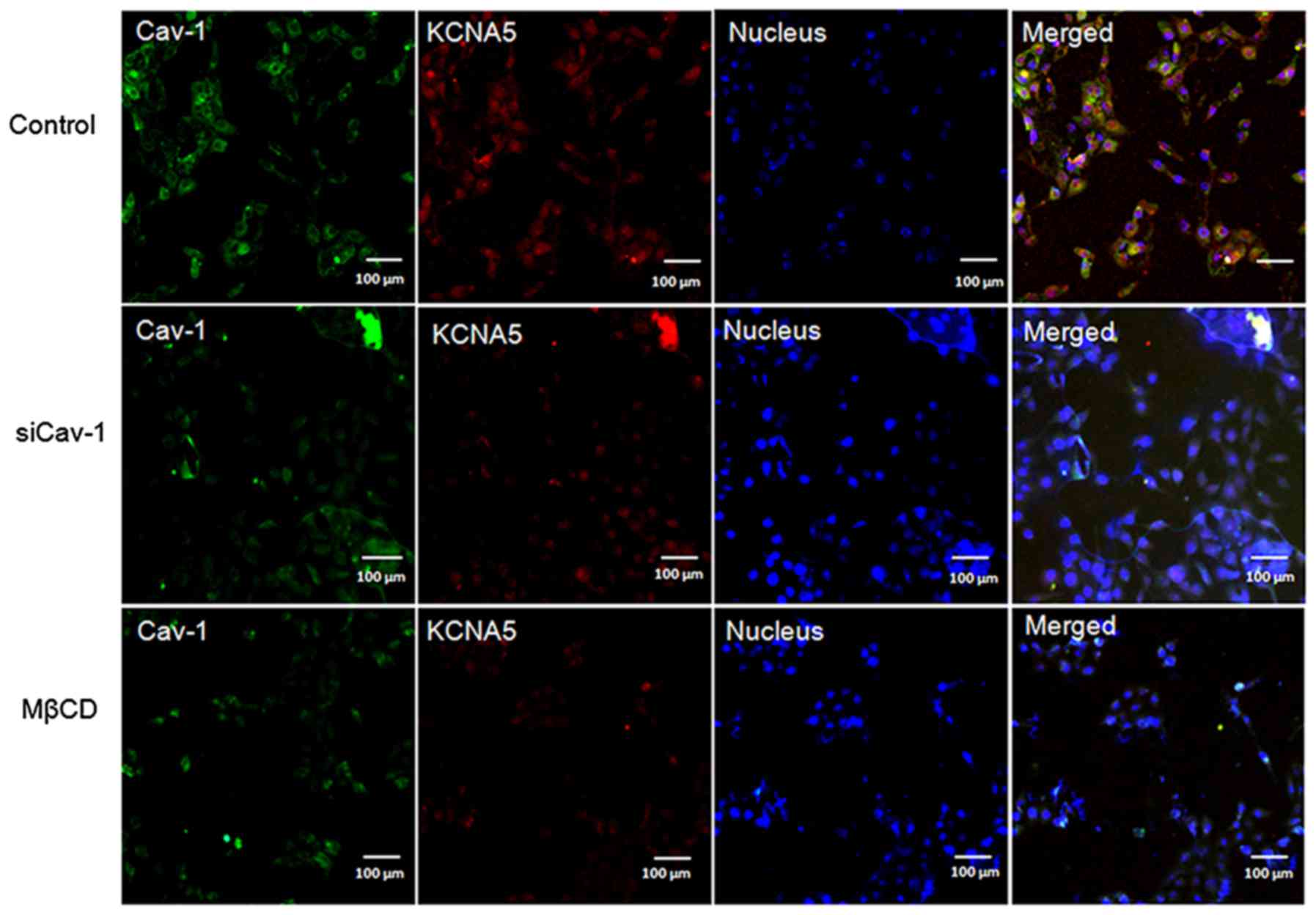
immunofluorescence (Fig. 2) and
immunoblot (Fig. 3A) both showed
reduced expression of KCNA5 in MCF-10A-neoTCE cells.
Quantification of the immunoblots demonstrated that the siRNA
reduced Cav-1 by ~80% and KCNA5 by ~50% in
MCF-10A-neoTCE. To further confirm the association of
Cav-1 with KCNA5, MCF-10A-neoT was treated with 2 mM MβCD to
disrupt caveolae and lipid rafts, and it showed that the
expressions of Cav-1 and KCNA5 both significantly decreased in the
cell membrane of MCF-10A-neoT (Fig.
2). Collectively, our data demonstrated that the knockdown of
Cav-1 reduced KCNA5 levels in MCF-10A-neoT. Similar results were
also obtained in MDA-MB-231 (Fig.
3B), which indicated that Cav-1 increased KCNA5 expression in
human breast cancer cells. However, the expression of Cav-1 in
MCF-10A-neoT-siKCNA5 and MCF-10A-neoT-vector were not changed
(Fig. 3C). We over-expressed KCNA5 in
MCF7 cell that Cav-1 is low expression, however, we found that
overexpression of KCNA5 does not affect Cav-1 expression in MCF7
cells (Fig. 3D).
KCNA5 promotes human breast cancer
cells survival through Cav-1
The PI3K/AKT and MAPK signaling pathways are the two
major pathways involved in the regulation of cell viability
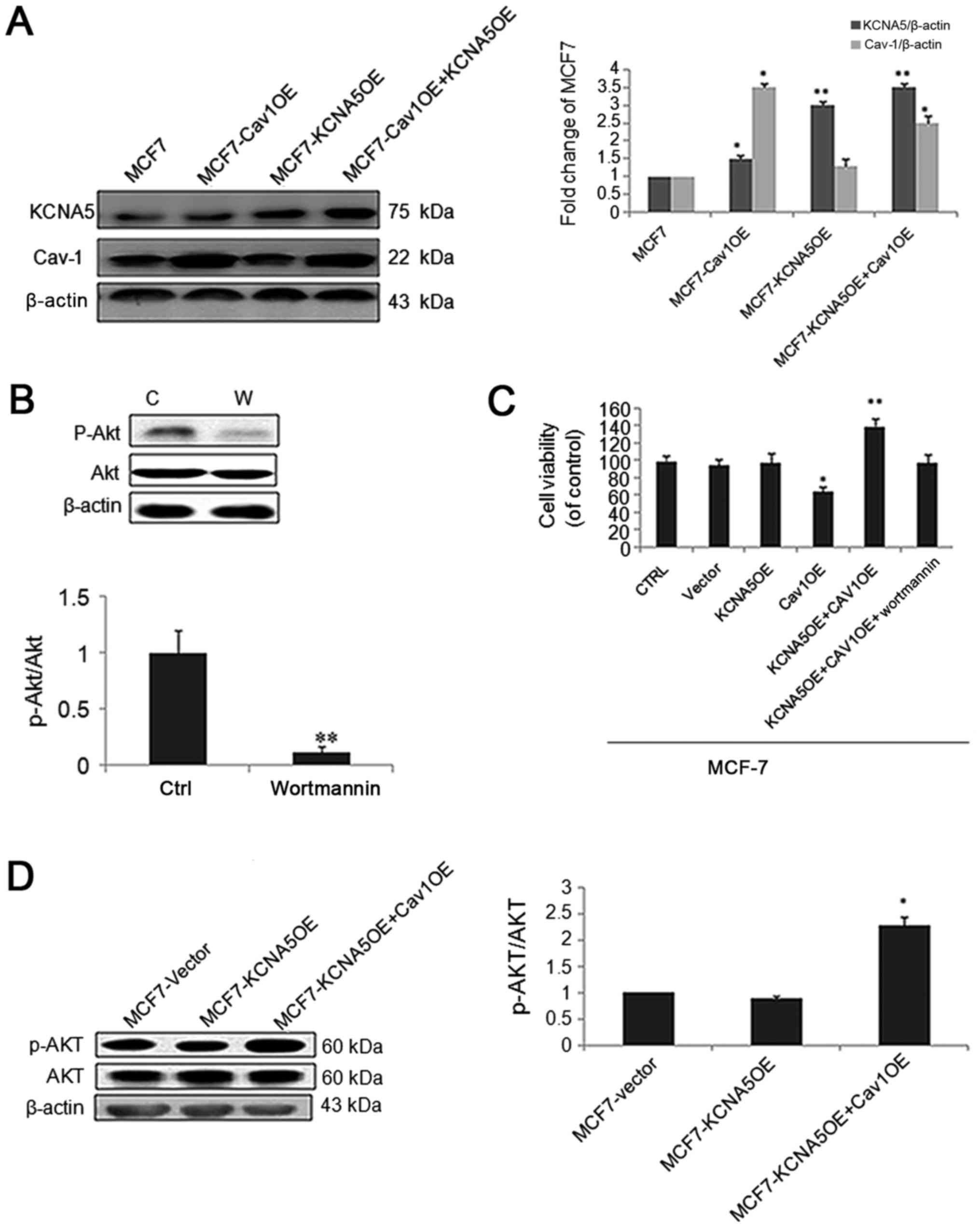
(37). To test the function of Cav-1
and KCNA5 in MCF-7 cells, we made a Cav-1 and KCNA5 vectors
co-transfection. Intriguingly, cells with increased Cav-1 and KCNA5
promoted cell survival, and the AKT signaling pathway inhibitor,
Wortmanin significantly inhibited cell survival in MCF-7 (Fig. 4A-C). What's more, cells with both
upregulated Cav-1 and KCNA5 had increased AKT activation (Fig. 4D). Therefore, we believe KCNA5
involves in cells survival, only when Cav-1 is overexpression. And
PI3K/AKT signaling pathway involves in KCNA5-dependent cell
survival.
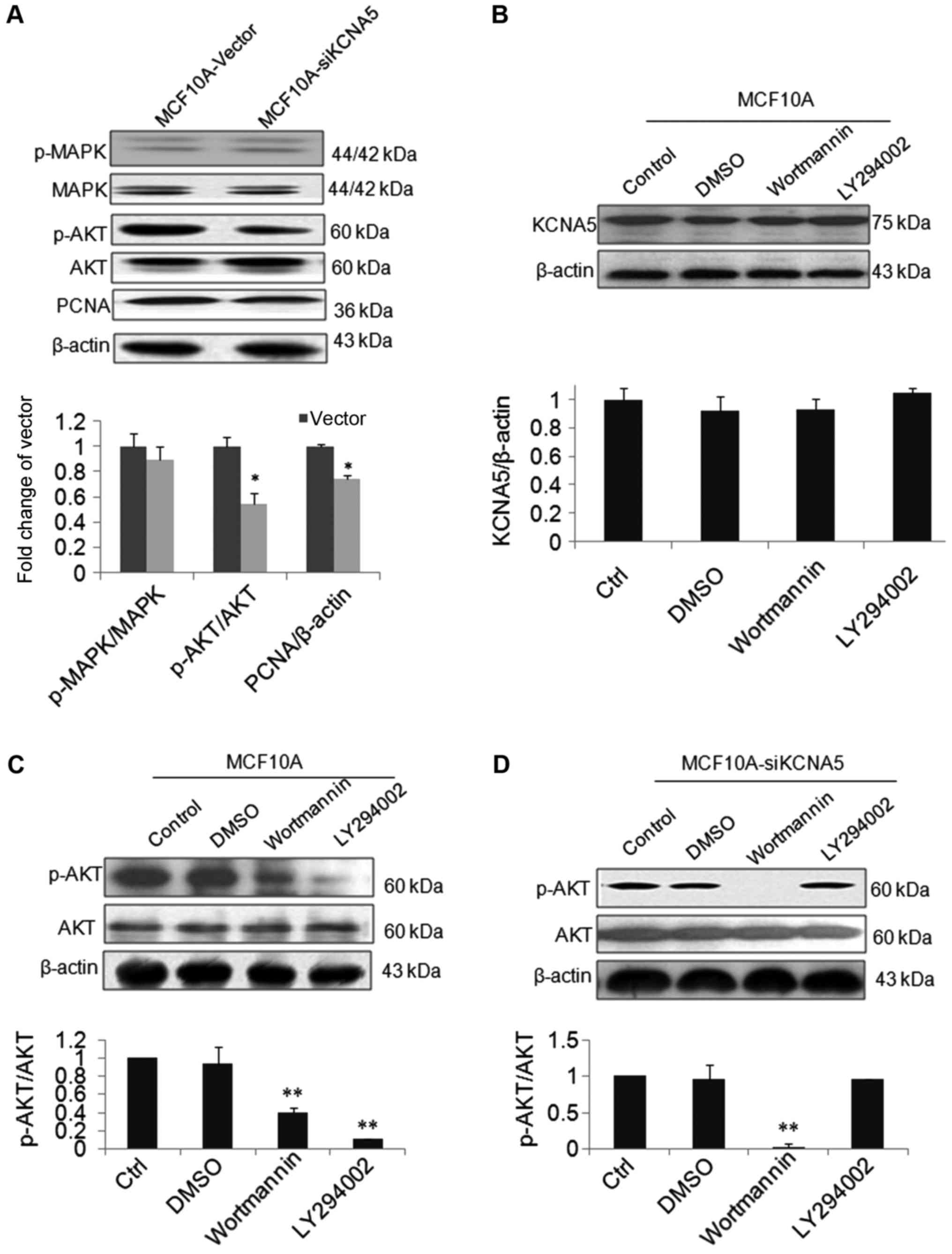
To determine whether KCNA5 survival through Cav-1 in
human breast cancer cell lines, we used MCF-10A-neoT as a cell
model, which has an overexpression Cav-1 showing in Fig. 1C. Besides, by knocking down KCNA5 in
MCF-10A-neoT, we detected whether or not the reduced expression of
KCNA5 inhibited cell survival through the PI3K/AKT and/or MAPK
signaling pathways in MCF-10A-neoT. As shown in Fig. 5A, there was no obvious change of
p-MAPK in the two cell lines, but the level of p-AKT was
decreased.
To study whether KCNA5 directly promote the cells
survival through PI3K/AKT pathway, we observed the effects of
wortmannin, LY 294002, inhibitors of AKT and PI3K on expression of
KCNA5 and phospho-AKT protein in both MCF-10A-neoT and
MCF-10A-neoT-siKCNA5 cell lines. As shown in Fig. 5B, the level of KCNA5 was not changed
after treatment with two inhibitors in the MCF-10A-neoT.
MCF-10A-neoT treated with both inhibitors, AKT activation was
inhibited compared with control (Fig.
5C). In addition, compared with control, the level of p-AKT was
significantly inhibited by wortmannin, however, LY29002 had no
effect on p-AKT in the MCF-10A-neoT-siKCNA5 cells (Fig. 5D). Finally, we summarized the possible
mechanism of how KCNA5 enhances breast cancer cells viability in
Fig. 6.
Discussion
The study demonstrated that KCNA5 and Cav-1
co-expression in breast cancer and normal mammary tissue, and in
MCF-10A-neoT and MCF-7 cells. And it was expounded that KCNA5 and
Cav-1 upregulation in human breast cancer cells promoted cell
survival and activated AKT. Furthermore, KCNA5 knockdown with siRNA
resulted in a decreased phosphorylation of AKT, but not inhibit
MAPK signaling in MCF-10A-neoT. Additionally, Cav-1 knockdown led
to decreased KCNA5 expression in MCF-10A-neoT and MDA-MB-231 cells.
Overall, these data suggested that the expression and function of
KCNA5 are related to Cav-1, and that KCNA5 facilitates the
activation of AKT signaling with lipid rafts.
Kv channels are often observed in the MCF-7 human
breast cancer cells. Abdul et al, found that the potassium
channel activator, minoxidil promoted the survival of MCF-7 cells
(38). Moreover, the use of specific
and non-specific K+ channels blocker on MCF-7 cells
resulted in apoptosis, which implicated the involvement of ATP
sensitive channels, SK channels, and Kv channels were related to
cells survival in the MCF-7 cells (39). KCNA5 was involved in the survival of
many mammalian cells. Wonderlin and Strobl found that KCNA5 was
involved in survival of human breast cancer cells (40). From our previous study, Kv channels
increased the survival of MCF-10A-neoT non-tumorigenic epithelial
cell line (7), but the relevant
mechanism is still unclear. In our present study, MCF-10A-neoT
normal cells expressed high levels of KCNA5 and Cav-1 while MCF-7
human breast cancer cells expressed low levels of KCNA5 and Cav-1.
We established a KCNA5 knockdown cell line with MCF-10A-neoT cells.
Interestingly, we observed that KCNA5 knockdown inhibited AKT
phosphorylation. And wortmannin and LY294002 PI3-kinase inhibitors
have differential effects on p-AKT in MCF-10A-neoT-siKCNA5 cells.
According to some studies, LY294002 potently block Kv
currents and promote increase in [Ca2+]i operates
independently of PI3K (35,41). Our result showed that LY294002 failed
to activate AKT in MCF-10A-neoT-siKCNA5 cells, which is related to
cell membrane depolarization and Ca2+ decrease. In MCF-7
cells, KCNA5 had no distinct effect on cell survival, but the
overexpression of Cav-1 and KCNA5 promoted cell survival. It has
been reported that the suppression of Cav-1 increased AKT
phosphorylation facilitated the survival of
MCF-10A-neoTCE cells, which downregulated Cav-1
expression, indicating that Cav-1 may be involved in KCNA5-mediated
AKT phosphorylation (42). Therefore,
KCNA5 is related to survival of human breast cancer cells via Cav-1
that has not been reported so far.
Cav-1, a signature protein in the integrated
membrane component of caveolae, plays a scaffolding role in
molecular signaling and endocytic trafficking (41). Various membrane proteins are
endocytosed through a caveolin-dependent pathway, such as
fibronectin, estrogen receptors, and ion channels proteins
(43,44). Previous evidence suggested that lipid
rafts regulate channels function in different ways (45,46). The
interplay of ion channels and proteins, which regulates channel
function, can impact the channel behavior. One important finding of
our study is that Cav-1 associates with KCNA5 in order to promote
cell survival, which is consistent with previous studies that Cav-1
regulates the trafficking of KCNA5 to lipid raft microdomains in
cardiac cells stably transfected with human vectors of KCNA5 and
caveolin (47). In our study, we
found Cav-1 is mainly expressed in the cytoplasm, but there is low
level of Cav-1 expressed in the cell membrane in MCF-7 cells. And
immunofluorescence suggested that a mass of Cav-1 and KCNA5 cannot
co-express in membrane. Therefore, a large amount of KCNA5 cannot
be located in caveolae. At the same time, it was showed that Cav-1
and KCNA5 were co-localized in cell membrane of MCF-10A-neoT cells,
and the immunoprecipitation was also used to determine the
interaction between KCNA5 and Cav-1 in MCF-10A-neoT cells (34). The MCF7 cells with KCNA5
overexpression had no effect on cell survival; but cells with both
KCNA5 and Cav-1 overexpression had increased cell survival. Our
study speculated that KCNA5 regulated cell survival when located in
the caveolae. Moreover, cholesterol-depleting experiment showed the
decreased expression of KCNA5 and Cav-1 in the plasma membrane,
which indicated that channel function, is related to the
cholesterol in the membrane microenvironment. Our results were
consistent with ones obtained from previous studies in different
cell lines (48,49). Some studies have focused on the
multiple function of Cav-1 in different cells and tissues,
different stages of development, different physiological and
pathological processes. Since 2000, our lab has been forces on the
multiple function of Cav-1 in different cells and tissues,
different stages of development, different physiological and
pathological processes (50). To date
interestingly, as functional marker, it plays a role either as a
tumor suppressor or an oncogene depending on the tumor type and
context of tumor progression. Disruption of Caveolae integrity or
downregulation of Cav-1 appears to be a common theme in oncogenic
transformation in breast cancer cells.
Disruption of Caveolae integrity or downregulation
of Cav-1 appears to be a common theme in oncogenic transformation
of breast cancer cells. In addition, it has been shown that Cav-1
can inhibit the activity of several caveolae-associated signaling
molecules, including Src, H-Ras and G-proteins. On the other hand,
evidence in support of an oncogenic role for Cav-1 was provided by
studies showing that Cav-1 promotes cell survival in metastatic
prostate cancer cells. Indeed, Cav-1 expression is upregulated in
numerous cancer cell lines and tumor specimens. Our lab also
reported that knockdown Cav-1 increased cell proliferation, and
colony formation in mammary epithelial cells, which expression of
KCNA5 is more than that in MDA-MB-231 than MCF-7 cells (34,51,52). In
this study, it has been shown that expression of Cav-1 was more in
MDA-MB-231 metastatic breast cancer cell than MCF-7. In this study,
the important interaction between KCNA5 and Cav-1 in mammary cells
was showed, and it was proved that Cav-1 is required for expression
of KCNA5 channel for cell survival. In addition, our data showed a
strong co-expression of Cav-1 and KCNA5 in MCF-10A-neoT
non-tumorigenic epithelial cell line. And it is reported that the
interaction also exists in both heart and vascular smooth muscle
(28,53). Our results strongly suggest that the
KCNA5 channel increased survival of human breast cancer cells
through the PI3K/AKT signaling pathway rather than the MAPK
pathway.
Acknowledgements
The authors would like to thank Dr Jie Zheng
(Department of Physiology and Membrane Biology, School of Medicine,
UC Davis) for providing the KCNA5 vector.
Funding
This study was supported by grants (grant nos.
30570225 and 30970353) from the National natural science foundation
of China. And Science and technology plan projects in Liaoning
Province, China (grant nos. L2015020568 and L201783647).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CQ, JS, and YL were involved in conceptualization of
the study, data acquisition, formal analysis, and writing of the
original draft. XW, CH, LW and QC were involved in methodology
planning and data analysis. TG, YZ, HL and YW analyzed data. WZ and
JL designed the project, and acquired funding and resources.
Ethics approval and consent to
participate
Patients' relatives provided written informed
consent for the procedures. The present study was approved by the
Ethics Committee of The First Affiliated Hospital of Dalian Medical
University for the use of human subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Kv
|
voltage-gated K+
channel
|
|
MCF-10A-neoT
|
non-tumorigenic epithelial cell
line
|
|
MCF7 and MDA-MB-231
|
human breast cancer cell lines
|
|
MAPK
|
ras/mitogen-activated protein
kinase
|
|
AKT
|
serine/threonine protein kinase B
|
|
Cav-1
|
caveolin-1
|
|
PCNA
|
proliferation cell nuclear antigen
|
|
MβCD
|
methyl-β-cyclodextrin
|
References
|
1
|
Wulff H, Castle NA and Pardo LA:
Voltage-gated potassium channels as therapeutic targets. Nat Rev
Drug Discov. 8:982–1001. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kunzelmann K: Ion channels and cancer. J
Membr Biol. 205:159–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JH, Park JW, Byun JK, Kim HK, Ryu PD,
Lee SY and Kim DY: Silencing of voltage-gated potassium channel
KV9.3 inhibits proliferation in human colon and lung carcinoma
cells. Oncotarget. 6:8132–8143. 2015.PubMed/NCBI
|
|
4
|
Delgado-Ramírez M, Morán-Zendejas R,
Aréchiga-Figueroa IA, Toro-Castillo C, Ramírez-Martínez JF and
Rodríguez-Menchaca AA: Modulation of the voltage-gated potassium
channel Kv2.1 by the anti-tumor alkylphospholipid perifosine.
Pharmacol Rep. 68:457–461. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tegla CA, Cudrici C, Rozycka M, Soloviova
K, Ito T, Singh AK, Khan A, Azimzadeh P, Andrian-Albescu M, Khan A,
et al: C5b-9-activated, K(v)1.3 channels mediate oligodendrocyte
cell cycle activation and dedifferentiation. Exp Mol Pathol.
91:335–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kazama I, Baba A, Matsubara M, Endo Y,
Toyama H and Ejima Y: Benidipine suppresses in situ proliferation
of leukocytes and slows the progression of renal fibrosis in rat
kidneys with advanced chronic renal failure. Nephron Exp Nephrol.
128:67–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Feng S, Zhang L, Wu Z, Chen Q,
Cheng W, Wang SQ and Zou W: Expression and properties of potassium
channels in human mammary epithelial cell line MCF10A and its
possible role in proliferation. Sheng Li Xue Bao. 62:203–209.
2010.(In Chinese). PubMed/NCBI
|
|
8
|
Ru Q, Tian X, Wu YX, Wu RH, Pi MS and Li
CY: Voltage-gated and ATP-sensitive K+ channels are associated with
cell proliferation and tumorigenesis of human glioma. Oncol Rep.
31:842–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pincus DW, DiCicco-Bloom E and Black IB:
Role of voltage-sensitive calcium channels in mitogenic stimulation
of neuroblasts. Brain Res. 553:211–214. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei T, Liang Z, Jin Y and Zhang L: Effect
of berberine, liensinine and neferine on HERG channel expression.
Zhongguo Zhong Yao Za Zhi. 38:239–244. 2013.(In Chinese).
PubMed/NCBI
|
|
11
|
Zhu YX, Yin H, Bruins LA, Shi CX,
Jedlowski P, Aziz M, Sereduk C, Kortuem KM, Schmidt JE, Champion M,
et al: RNA interference screening identifies lenalidomide
sensitizers in multiple myeloma, including RSK2. Blood.
125:483–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo TB, Lu J, Li T, Lu Z, Xu G, Xu M, Lu L
and Dai W: Insulin-activated, K+-channel-sensitive Akt
pathway is primary mediator of ML-1 cell proliferation. Am J
Physiol Cell Physiol. 289:C257–C263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roderick C, Reinach PS, Wang L and Lu L:
Modulation of rabbit corneal epithelial cell proliferation by
growth factor-regulated K(+) channel activity. J Membr Biol.
196:41–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ballou LM, Lin RZ and Cohen IS: Control of
cardiac repolarization by phosphoinositide 3-kinase signaling to
ion channels. Circ Res. 116:127–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shepherd AJ, Loo L and Mohapatra DP:
Chemokine co-receptor CCR5/CXCR4-dependent modulation of Kv2.1
channel confers acute neuroprotection to HIV-1 glycoprotein gp120
exposure. PLoS One. 8:e766982013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harder T and Simons K: Caveolae, DIGs, and
the dynamics of sphingolipid-cholesterol microdomains. Curr Opin
Cell Biol. 9:534–542. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takaguri A, Kamato M, Satoh Y, Ohtsuki K
and Satoh K: Effect of alteration of caveolin-1 expression on
doxorubicin-induced apoptosis in H9c2 cardiac cells. Cell Biol Int.
39:1053–1060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Banadakoppa M, Goluszko P, Liebenthal D
and Yallampalli C: Nitric oxide induces segregation of decay
accelerating factor (DAF or CD55) from the membrane lipid-rafts and
its internalization in human endometrial cells. Cell Biol Int.
36:901–907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takaguri A, Kamato M, Satoh Y, Ohtsuki K
and Satoh K: Effect of alteration of caveolin-1 expression on
doxorubicin-induced apoptosis in H9c2 cardiac cells. Cell Biol Int.
39:1053–1060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng ZJ, Singh RD, Marks DL and Pagano
RE: Membrane microdomains, caveolae, and caveolar endocytosis of
sphingolipids. Mol Membr Biol. 23:101–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Parton RG and Simons K: The multiple faces
of caveolae. Nat Rev Mol Cell Biol. 8:185–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lapierre LA, Ducharme NA, Drake KR,
Goldenring JR and Kenworthy AK: Coordinated regulation of
caveolin-1 and Rab11a in apical recycling compartments of polarized
epithelial cells. Exp Cell Res. 318:103–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shvets E, Ludwig A and Nichols BJ: News
from the caves: Update on the structure and function of caveolae.
Curr Opin Cell Biol. 29:99–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas CM and Smart EJ: Caveolae structure
and function. J Cell Mol Med. 12:796–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thompson MA, Prakash YS and Pabelick CM:
The role of caveolae in the pathophysiology of lung diseases.
Expert Rev Respir Med. 8:111–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chaudhary KR, Cho WJ, Yang F, Samokhvalov
V, El-Sikhry HE, Daniel EE and Seubert JM: Effect of ischemia
reperfusion injury and epoxyeicosatrienoic acids on caveolin
expression in mouse myocardium. J Cardiovasc Pharmacol. 61:258–263.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martens JR, Sakamoto N, Sullivan SA,
Grobaski TD and Tamkun MM: Isoform-specific localization of
voltage-gated K+ channels to distinct lipid raft
populations. Targeting of Kv1.5 to caveolae. J Biol Chem.
276:8409–8414. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cogolludo A, Moreno L, Lodi F, Frazziano
G, Cobeño L, Tamargo J and Perez-Vizcaino F: Serotonin inhibits
voltage-gated K+ currents in pulmonary artery smooth
muscle cells: Role of 5-HT2A receptors, caveolin-1, and KV1.5
channel internalization. Circ Res. 98:931–938. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brignell JL, Perry MD, Nelson CP, Willets
JM, Challiss RA and Davies NW: Steady-state modulation of
voltage-gated K+ channels in rat arterial smooth muscle
by cyclic AMP-dependent protein kinase and protein phosphatase 2B.
PLoS One. 10:e01212852015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brazer SC, Singh BB, Liu X, Swaim W and
Ambudkar IS: Caveolin-1 contributes to assembly of store-operated
Ca2+ influx channels by regulating plasma membrane
localization of TRPC1. J Biol Chem. 278:27208–27215. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anderson RG: The caveolae membrane system.
Annu Rev Biochem. 67:199–225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okamoto T, Schlegel A, Scherer PE and
Lisanti MP: Caveolins, a family of scaffolding proteins for
organizing ‘preassembled signaling complexes’ at the plasma
membrane. J Biol Chem. 273:5419–5422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Overmiller AM, McGuinn KP, Roberts BJ,
Cooper F, Brennan-Crispi DM, Deguchi T, Peltonen S, Wahl JK II and
Mahoney MG: c-Src/Cav-1-dependent activation of the EGFR by Dsg2.
Oncotarget. 7:37536–37555. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng S, Wang Y, Wang X, Wang Z, Cui Y, Liu
J, Zhao C, Jin M and Zou W: Caveolin-1 gene silencing promotes the
activation of PI3K/AKT dependent on Eralpha36 and the
transformation of MCF10ACE. Sci China Life Sci. 53:598–605. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Feng S, Zhang H, Wang Y, Cui Y,
Wang Z, Liu J and Zou W: RNA inference-mediated caveolin-1
downregulation decrease estrogen receptor alpha (ERα) signaling in
human mammary epithelial cells. Mol Biol Rep. 38:761–768. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Qu C, Li H, Zhang Y, Sun J, Yang S,
Liu J, An L and Zou W: Expression of KCNA5 protein in human mammary
epithelial cell line associated with caveolin-1. J Membr Biol.
249:449–457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qu C, Fu XZ, Han C, Chen Q, Liu Y, Wang
Xb, Xi RG, Liu J and Zou W: Voltage-gated potassium channel KV1.5
protects against MPP+ mediated neurotoxicity in PC12
cells. Adv Biochem. 2:103–108. 2014. View Article : Google Scholar
|
|
38
|
Abdul M, Santo A and Hoosein N: Activity
of potassium channel-blockers in breast cancer. Anticancer Res.
23:3347–3351. 2003.PubMed/NCBI
|
|
39
|
Zou W, Zhang L, Wang X and Zhou SS: Effect
of potassium channel antagonist on proliferation in the human
mammary epithelial cells MCF10A. J Liao Ning Norm Univ. 31:23–26.
2008.
|
|
40
|
Wonderlin WF and Strobl JS: Potassium
channels, proliferation and G1 progression. J Membr Biol.
154:91–107. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
El-Kholy W, Macdonald PE, Lin JH, Wang J,
Fox JM, Light PE, Wang Q, Tsushima RG and Wheeler MB: The
phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks
K(V) currents via a direct mechanism. FASEB J. 17:720–722. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nauc V, De Lamirande E, Leclerc P and
Gagnon C: Inhibitors of phosphoinositide 3-kinase, LY294002 and
wortmannin, affect sperm capacitation and associated
phosphorylation of proteins differently: Ca2+-dependent
divergences. J Androl. 25:573–585. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cipriani P, Di Benedetto P, Capece D,
Zazzeroni F, Liakouli V, Ruscitti P, Pantano I, Berardicurti O,
Carubbi F, Alesse E and Giacomelli R: Impaired Cav-1 expression in
SSc mesenchymal cells upregulates VEGF signaling: A link between
vascular involvement and fibrosis. Fibrogenesis Tissue Repair.
7:132014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sottile J and Chandler J: Fibronectin
matrix turnover occurs through a caveolin-1-dependent process. Mol
Biol Cell. 16:757–768. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Martens JR, Navarro-Polanco R, Coppock EA,
Nishiyama A, Parshley L, Grobaski TD and Tamkun MM: Differential
targeting of Shaker-like potassium channels to lipid rafts. J Biol
Chem. 275:7443–7446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Markandeya YS, Phelan LJ, Woon MT, Keefe
AM, Reynolds CR, August BK, Hacker TA, Roth DM, Patel HH and
Balijepalli RC: Caveolin-3 overexpression attenuates cardiac
hypertrophy via inhibition of T-type Ca2+ current
modulated by protein kinase cα in cardiomyocytes. J Biol Chem.
290:22085–22100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schilling JM, Horikawa YT, Zemljic-Harpf
AE, Vincent KP, Tyan L, Yu JK, McCulloch AD, Balijepalli RC, Patel
HH and Roth DM: Electrophysiology and metabolism of
caveolin-3-overexpressing mice. Basic Res Cardiol. 111:282016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vicente R, Villalonga N, Calvo M, Escalada
A, Solsona C, Soler C, Tamkun MM and Felipe A: Kv1.5 association
modifies Kv1.3 traffic and membrane localization. J Biol Chem.
283:8756–8764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pérez-Verdaguer M, Capera J,
Martínez-Mármol R, Camps M, Comes N, Tamkun MM and Felipe A:
Caveolin interaction governs Kv1.3 lipid raft targeting. Sci Rep.
6:224532016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zou W, Mcdaneld L and Smith LM: Caveolin 1
haploinsufficiency leads to partial transformation of human breast
epithelial cells. Anticancer Res. 23:4581–4586. 2003.PubMed/NCBI
|
|
51
|
Méndez-Bolaina E, Sánchez-González J,
Ramírez-Sánchez I, Ocharán-Hernández E, Núñez-Sánchez M,
Meaney-Mendiolea E, Meaney A, Asbun-Bojalil J, Miliar-García A,
Olivares-Corichi I and Ceballos-Reyes G: Effect of caveolin-1
scaffolding peptide and 17beta-estradiol on intracellular
Ca2+ kinetics evoked by angiotensin II in human vascular
smooth muscle cells. Am J Physiol Cell Physiol. 293:C1953–C1961.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ishikawa T, Yuhanna IS, Umetani J, Lee WR,
Korach KS, Shaul PW and Umetani M: LXRβ/estrogen receptor-α
signaling in lipid rafts preserves endothelial integrity. J Clin
Invest. 123:3488–3497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Abi-Char J, Maguy A, Coulombe A, Balse E,
Ratajczak P, Samuel JL, Nattel S and Hatem SN: Membrane cholesterol
modulates Kv1.5 potassium channel distribution and function in rat
cardiomyocytes. J Physiol. 582:1205–1217. 2007. View Article : Google Scholar : PubMed/NCBI
|