Introduction
Bladder cancer (BCa) is one of the most common
genitourinary malignancies worldwide that causes an estimated
150,000 mortalities annually globally (1). The incidence of BCa is complex and
includes genetic and environmental factors (2). Smoking is an established risk factor
(3). Clinical data indicate that BCa
has a high mortality rate, due to its propensity to recur and
metastasize, and the absence of symptoms in the early stage of the
disease (3). Therefore, early
diagnosis and appropriate treatment is the key to improving
survival in patients with BCa (4,5). With the
lack of effective treatments and monitoring strategies, novel
diagnostic markers and effective treatment strategies for BCa are
imperative.
MicroRNAs (miRNAs) are small (22 nucleotide long)
noncoding RNA molecules that are highly conserved in eukaryotes
(6). Generally, miRNAs identify their
target gene by imperfectly base pairing with their 3′untranslated
region (UTR) to exert their biological function (7). In 2007, the expression profiles of miRNA
in BCa were examined, and 10 miRNAs were identified as being
upregulated (8). Subsequently, the
use of the Solexa sequencing technology identified 33 miRNAs that
were upregulated and 41 miRNAs that were downregulated in BCa
(9). These data indicate that the
dysregulation of miRNA is markedly associated with BCa
tumorigenesis. miR-124 is a miRNA highly expressed in brain tissue
that is the most extensively studied miRNA in the nervous system
(10). Previously, miR-124 was
identified as a tumor suppressor that downregulates a variety of
human tumor types, including gastric, breast and colorectal cancer,
and BCa (11–14). Furthermore, signal transducer and
activator of transcription 3 (STAT3) has been demonstrated to be an
miR-124-downregulated target gene in tumors (15). However, the mechanisms underlying
miR-124-mediated inhibition of STAT3 in BCa are unknown.
The present study demonstrated a downregulation of
miR-124 in T24 BCa cells that was inversely associated with STAT3
expression. Furthermore, miR-124 inhibited STAT3 expression by
directly targeting its 3′UTR. Knockdown of STAT3 significantly
promoted apoptosis and suppressed cell cycle progression,
migration, proliferation, and colony formation in T24 cells. The
collective results suggest that miR-124 may directly inhibit the
expression of STAT3 in BCa, and that miR-124 may suppress BCa
tumorigenesis by targeting STAT3.
Materials and methods
Cell culture and RNA extraction
Human BCa T24, UM-UC-3, SW780, HT1376, RT4 and J82
cell lines, immortalized human bladder epithelium SV-HUC-1 cells
and the 293 cell line were purchased from the American Type Culture
Collection (Manassas, VA, USA). The cells were cultured according
to the manufacturer's instructions at 37°C in an atmosphere of 5%
CO2. Total mRNA and miRNA were independently extracted
from the cells using RNAzol RT RNA Isolation Reagent (Molecular
Research Center, Inc., Cincinnati, OH, USA) and stored at −20°C for
subsequent analysis.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) for miR-124 and STAT3
The expression of miR-124 was quantified using an
All-in-one miRNA qPCR kit (GeneCopoeia, Inc., Rockville, USA) and
U6 was used as the control. The primers used for the RT-PCR were:
miR-124 (forward 5′-GCGGCCGTGTTCACAGCGGACC-3′ and reverse
5′-GTGCAGGGTCCGAGGT-3′) and U6 (forward
5′-CGCTTCGGCAGCACATATACTA-3′ and reverse
5′-CGCTTCACGAATTTGCGTGTCA-3′). To determine the mRNA expression of
STAT3, the total RNA in T24 cells was reverse transcribed into cDNA
at 37°C using Eastep RT Master Mix kit (Promega Corporation,
Madison, WI, USA), then STAT3 mRNA was quantified using SYBR Premix
Ex Taq (Takara Bio, Inc., Otsu, Japan) and GAPDH was used as the
control. The thermocycling conditions were as follows: 95°C for 30
sec, followed by 40 cycles at 95°C for 5 sec, 60°C for 34 sec, then
72°C for 45 sec. The primers used for qPCR were: STAT3 (forward
5′-ATCACGCCTTCTACAGACTGC-3′ and reverse
5′-CATCCTGGAGATTCTCTACCACT-3′) and GAPDH (forward
5′-CCACTCCTCCACCTTTGAC-3′ and reverse 5′-ACCCTGTTGCTGTAGCCA-3′).
Relative expression was calculated using the 2−ΔΔCq
method (16).
Western blot analysis
T24 cells were centrifuged in 1,000 × g for 5 min at
37°C subsequent to the addition of 1 ml ice-cold PBS and washed
three times with ice-cold PBS and lysed using a Nuclear and
Cytoplasmic Protein Extraction kit (Beyotime Institute of
Biotechnology, Haimen, China) for 30 min at 4°C. The cell lysate
protein was centrifuged at 15,000 × g at 4°C for 10 min and the
protein was quantified using a BCA Protein Assay kit (Beyotime
Institute of Biotechnology). A total of 20 µg protein/lane was
separated by 10% SDS-PAGE, and the resolved proteins were
transferred to a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA). The membrane was blocked using 5% skim milk
powder at 4°C overnight and then hybridized with the primary
antibodies targeting the proteins including GADPH (1:2,500; cat no.
ab9485), STAT3 (1:1,000; cat no. ab68153), vascular endothelial
growth factor receptor (VEGFR; 1:1,000; cat no. ab11939), B-cell
lymphoma 2 (Bcl-2; 1:1,000; cat no. ab32124), B-cell lymphoma-extra
large (Bcl-xl; 1:1,000; cat no. ab32370), MCL1, Bcl2 family
apoptosis regulator (Mcl-1; 1:2,000; cat no. ab32087), Cyclin D1
(1:10,000; cat no. ab134175) and MYC proto-oncogene, BHLH
transcription factor (cMyc; 1:1,000; cat no. ab32072) (all from
Abcam, Cambridge, UK) at 4°C overnight. Subsequent to washing using
PBS with 0.05% Tween-20 three times at 5 min each time, the
membrane was incubated with the horseradish peroxidase-conjugated
secondary antibody Goat Anti-Rabbit immunoglobulin G H&L
(1:2,000; cat no. ab6721; Abcam) conjugated to horseradish
peroxidase at 4°C overnight. Results were quantified by the
Molecular Imager ChemiDoc XRS+ System 2.0 (Bio-Rad Laboratories,
Hercules, CA, USA). GAPDH was used as the control.
Luciferase assay
The whole 3′UTR of the STAT3 gene was cloned and
amplified in 293 cells. TargetScan 6.2 bioinformatics prediction
software (http://www.targetscan.org/; date of
access, 20 February 2017) was used to predict their binding sites
in 20 February, 2017. The conserved sites of miR-124 were selected
and then target gene STAT3 was selected for sites in its 3′UTR. A
mutation in the 3′UTR of the STAT3 gene at the putative binding
site of miR-124 was generated with the QuickChange Site-Directed
Mutagenesis kit (Stratagene; Agilent Technologies, Inc. Santa
Clara, CA, USA). The wild and mutant STAT3 genes were cloned into
the pmirGlO-vector (Promega Corporation) immediately downstream of
the Renilla luciferase gene. Cells were co-transfected with
pmirGlO-3′UTR-STAT3 (50 ng), miR-124 (50 nM), or scramble mimic (50
nM) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Cell lysates were prepared
using Passive Lysis Buffer (Promega Corporation) 48 h after
transfection. The luciferase activity was measured using the
Dual-Luciferase Reporter Assay (Promega Corporation) and normalized
to Renilla luciferase activity.
Transfection
T24 (1×105 cells/plate) were plated in
6-well plates at 37°C overnight and transfected with miR-124 mimics
or NC mimics (20 nM; GeneCopoeia, Inc.) using
Lipofectamine® 2000. The miR-124 mimic sequence used
was: Forward,
5′-GCTCTAGAGGCCTCTCTCTCCGTGTTCCACAGCGGACCTTGATTTAAATGTCCATACAATTAAGGCACGCGGTTGAATGCCAAGAATGGGGCTG-3′
and reverse,
5′-CGGGATCCCAGCCCCATTCTTGGCATTCACCGCGTGCCTTAATTGTATGGACATTTAAATCAAGGTCCGCTGTGAACACGGAGAGAGAGGCCT-3′.
Following transfection for 6 h at 25°C, the Opti-MEM medium (Gibco;
Thermo Fisher Scientific, Inc.) without serum was changed with the
fresh medium. Then cells were assayed by RT-qPCR and western blot
analysis for each group according to the aforementioned protocols
following culture for 48 h.
Construction of plasmids
Small interfering RNA Target Finder online design
software (Ambion; Thermo Fisher Scientific, Inc.) was used to
select a segment (5′-AAGAGTCAAGGAGACATGCAA-3′, 670–690 bp) in the
coding region of STAT3 (GenBank serial no. NM139276) as a target
sequence. Then, the corresponding DNA template strands containing
restriction sites for HindIII and BamH I were
designed for the target sequences. The template strand sequences
were as follows: Sense,
5′-GATCCGAGTCAAGGAGACCATGCAATTCAAGAGATTGCATGTCTCCTTGACTCTTTTTTGG-3′;
and anti-sense,
5′-AGCTTTTCCAAAAAAGAGTCAAGGAGACATGCAATCTCTTGAATTGCATGTCTCCTTGACTCG-3′.
The shRNA sequences were obtained by PCR amplification, and vector
plasmid pGENESIL (Wuhan Genesil Biotechnology Co., Ltd., Wuhan,
China) were digested and connected by HindIII and
BamH I enzymes (Thermo Fisher Scientific, Inc.). The
recombinant plasmid (pGENESIL-shRNA-STAT3) was amplified and
infected (0.8 µg plasmid, at 25°C for 20 min) into the T24 cells.
After 6 weeks of G418 (800 µg/ml; Invitrogen; Thermo Fisher
Scientific, Inc.) selection, stably-transfected cells (STAT3-shRNA)
were used for subsequent experiments.
Cell cycle and apoptosis analysis by
flow cytometry
The adherent cells were digested at 37°C with 0.25%
trypsin (Bioswamp; Wuhan Myhalic Biotechnological Co., Ltd., Wuhan,
China) and centrifuged at 1,000 × g at 25°C for 5 min. The
supernatant was discarded and the cells were washed three times
with PBS. The cells (1–5×105) were collected and
resuspended in 200 µl binding buffer (cat no. C1052-1; Beyotime
Institute of Biotechnology) for analysis using the Cell Cycle and
Apoptosis Analysis kit (Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. Analyses were performed
using a LSRII Flow Cytometry System equipped with FACSDiva software
4.1 (BD Biosciences, Franklin Lakes, NJ, USA). Data was analyzed
with the ModFit LT software package version 4.0 (Verity Software
House, Inc., Topsham, ME, USA).
Cell proliferation and colony-forming
assays
The WST-1 Cell Proliferation and Cytotoxicity Assay
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol was used to analyze cell viability and cell
proliferation at 0, 24, 48, 72 and 96 h. All experiments were
performed in triplicate. For the colony formation assay, cells were
plated at a density of 200 cells/well in standard culture
conditions (5% CO2 and 37°C) for 14 days. The colonies
were fixed with 5 ml 4% absolute methanol for 15 min at 25°C,
stained at 37°C with 0.1% crystal violet for 20 min and washed
three times. Then a light microscope (TS-100F; Nikon, Tokyo, Japan)
was used to visualize the results at a magnification of ×40.
Wound healing assay
T24 cells were seeded (1×106 cells/plate)
in a 6-well plate. Wounding of the confluent cell growth was
performed by scratching with a 10 µl pipette tip 24 h after cell
plating. The cells were then cultured for 24 h and wound closure
was detected by inverted microscopy (magnification, ×200; Olympus,
Tokyo, Japan). The experiment was repeated three times.
Statistical analysis
Statistical analysis was conducted using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Unpaired Student's t-tests
were used to analyze the results. Pearson's linear correlation
analysis was used to analyze the association between the expression
of STAT3 and miR-124. All experiments were performed in triplicate
and the data are expressed as mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
miR-124 and STAT3 are inversely
expressed in BCa cell lines
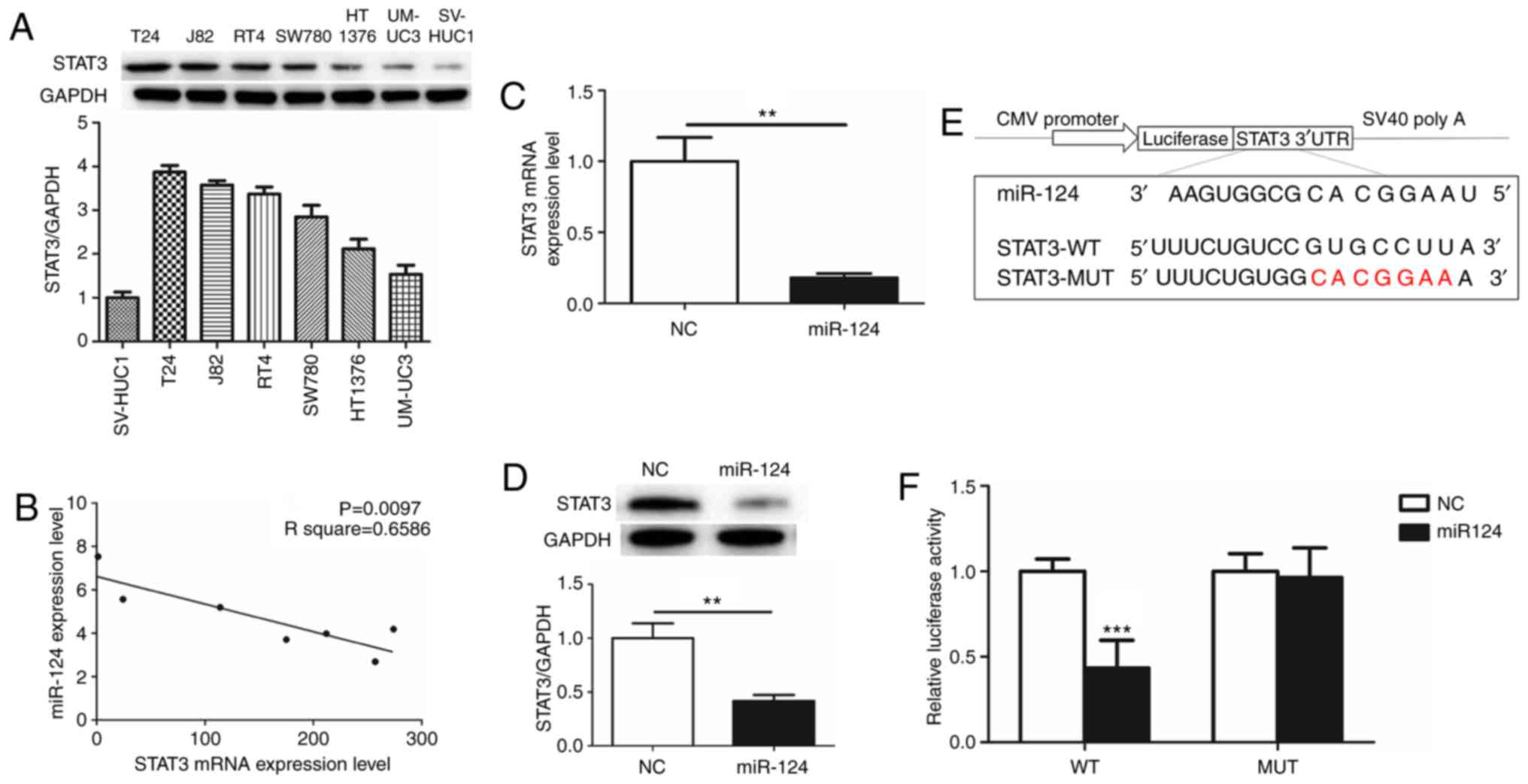
To evaluate the function of STAT3 in human BCa, the
expression of STAT3 was detected in 6 human BCa cell lines (T24,
UM-UC-3, SW780, HT1376, RT4 and J82) and immortalized human bladder
epithelium SV-HUC-1 cells. STAT3 was overexpressed as an oncogene
in the BCa cell lines compared with SV-HUC-1 cells. The expression
of STAT3 was highest in T24 cells (Fig.
1A). The association between miR-124 and STAT3 in the BCa cell
lines was additionally evaluated by RT-qPCR. Notably, there was a
significant inverse correlation between miR-124 and STAT3 (Fig. 1B). From these results, it was
hypothesized that miR-124 is a potent miRNA regulator of STAT3
expression. T24 cells, which overexpressed STAT3 and exhibited low
expression of miR-124, were selected for subsequent
experiments.
miR-124 directly targets STAT3
3′UTR
The effects of the transfection of miR-124 mimics on
STAT3 mRNA and protein expression levels were determined by RT-qPCR
and western blot analysis. There was an ~80% decrease in STAT3 mRNA
expression and a 60% decrease in STAT3 protein expression in T24
cells transfected with miR-124 mimics (Fig. 1C and D). This suggested that STAT3 may
be downregulated by miR-124. Post-transcriptional regulation by
miRNAs involves binding to the 3′UTR of the relevant downstream
genes (17). To additionally define
the association of miR-124 with STAT3, Target Scan 6.2
bioinformatics prediction software (http://www.targetscan.org/) was used to predict their
binding sites. The results revealed that the miR-124 seed-matching
sequence in STAT3 3′UTR was ‘CACGGAA’ (Fig. 1E). Luciferase reporter assays
demonstrated that the presence of miR-124 resulted in a marked
decrease in luciferase activity when the STAT3 plasmid containing
wild type 3′UTR was present, while luciferase activity did not
change significantly when the mutant 3′UTR was present (Fig. 1F). These data suggested that miR-124
may target STAT3 transcription, potentially contributing to the
downregulation of STAT3 expression.
Knockdown of STAT3 inhibits cell cycle
progression, proliferation and colony formation of T24 BCa cells in
vitro
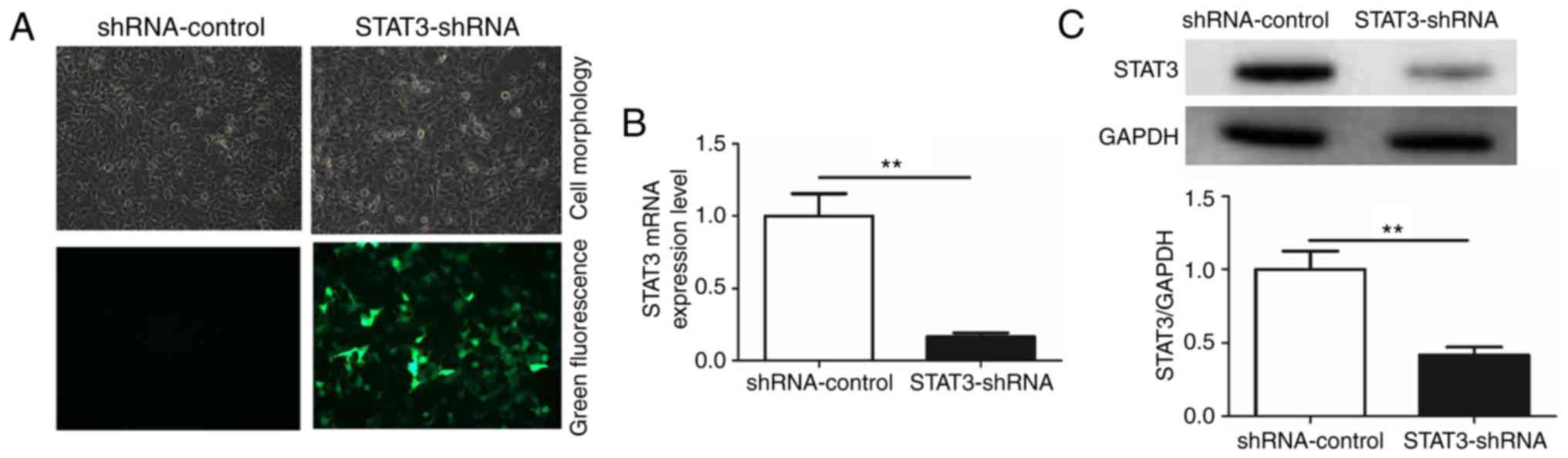
To additionally investigate the role of
miR-124/STAT3 signaling pathway in BCa, a stable cell line with low
STAT3 expression (STAT3-shRNA-T24) and a control cell line
(shRNA-control-T24) were constructed (Fig. 2A). The result demonstrated that STAT3
mRNA and protein levels in the STAT3-shRNA-T24 were significantly
inhibited (Fig. 2B and C). Flow
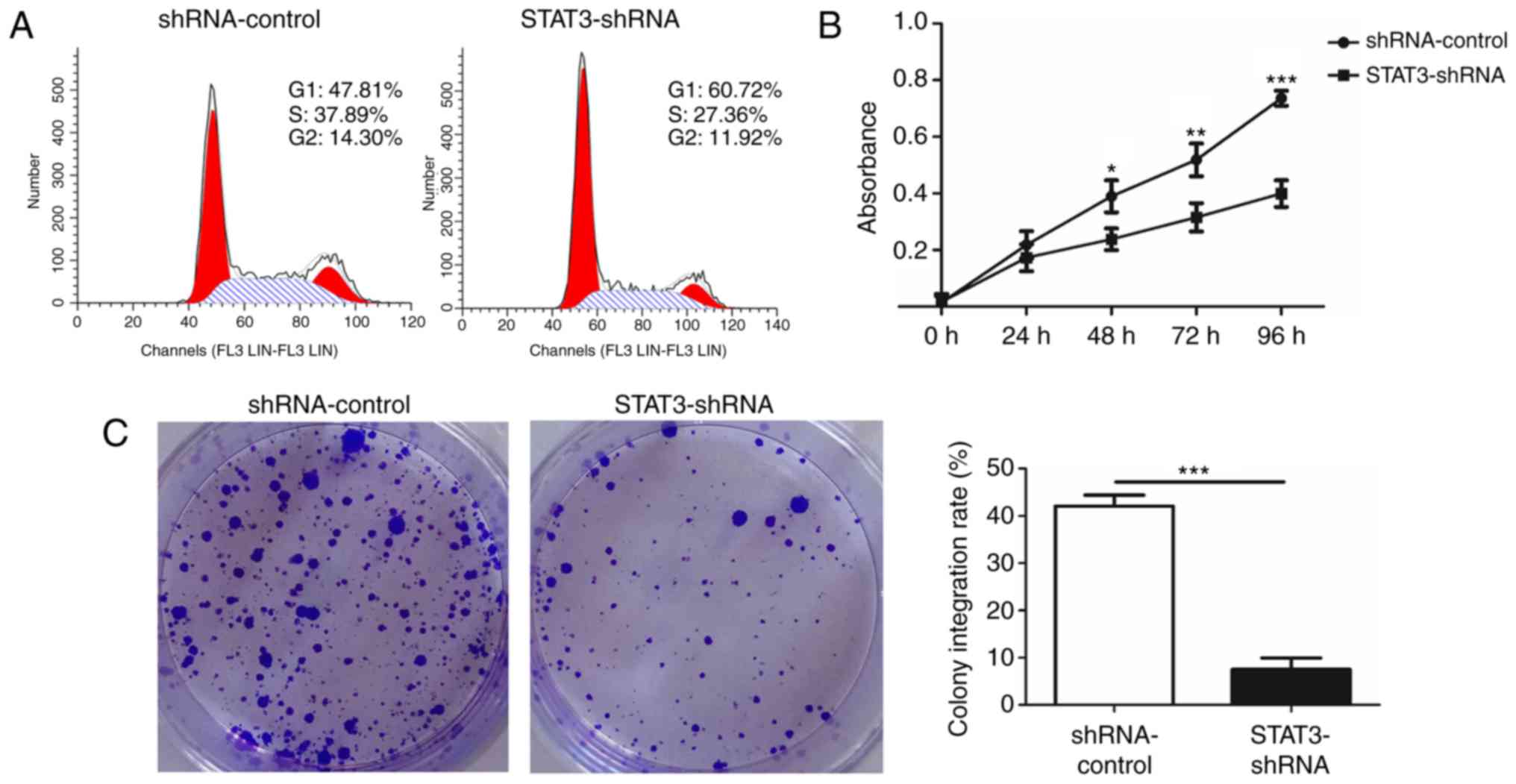
cytometry was conducted to confirm that the knockdown of STAT3
suppressed the growth of T24 BCa cells. A significant increase in
numbers of cells in the G1 phase was observed in STAT3-shRNA-T24
cells compared with that in shRNA-control-T24 cells. This increase
in G0/G1 cell population was accompanied with a concomitant
decrease in cell numbers in S and G2-M phases in the
STAT3-shRNA-T24 cells (Fig. 3A). A
WST-1 assay was then performed to investigate whether STAT3
exhibited a biological function in the proliferation of T24 cells.
A significant inhibition in STAT3-shRNA-T24 was evident (Fig. 3B). Consistent with the result from the
WST-1 assay, the colony formation assay revealed markedly fewer and
smaller STAT3-shRNA-T24 colonies compared with the
shRNA-control-T24 cell line (Fig.
3C).
Knockdown of STAT3 promotes apoptosis
and suppresses cell migration and STAT3 downstream target genes of
T24 BCa cells in vitro
To investigate the effect of STAT3 on the migration
of T24 BCa cells, the capacity of STAT3-shRNA-T24 cells to migrate
was measured using a wound healing assay. STAT3-shRNA-T24 cells
exhibited slower rates of wound closure and decreased numbers of
stained cells in the wound healing assay compared with the
shRNA-control-T24 cells (Fig. 4A).
Furthermore, a marked induction of apoptosis was confirmed in
STAT3-shRNA-T24 cells (Fig. 4B). The
results demonstrated that the silencing of STAT3 significantly
suppressed the migratory capability and induced apoptosis of T24
cells. Finally, the downstream target genes of STAT3 following the
knockdown of STAT3 were examined using western blot analysis
(Fig. 4C). Notably, cyclin D1, cMyc,
Bcl-2, Bcl-xl, Mcl-1 and VEGFR were decreased in the
STAT3-shRNA-T24 cells. Taken together, these results demonstrate
that STAT3 knockdown repressed cell proliferation, migration,
invasion and vasculogenic mimicry, indicating the oncogenic role of
STAT3 in BCa.
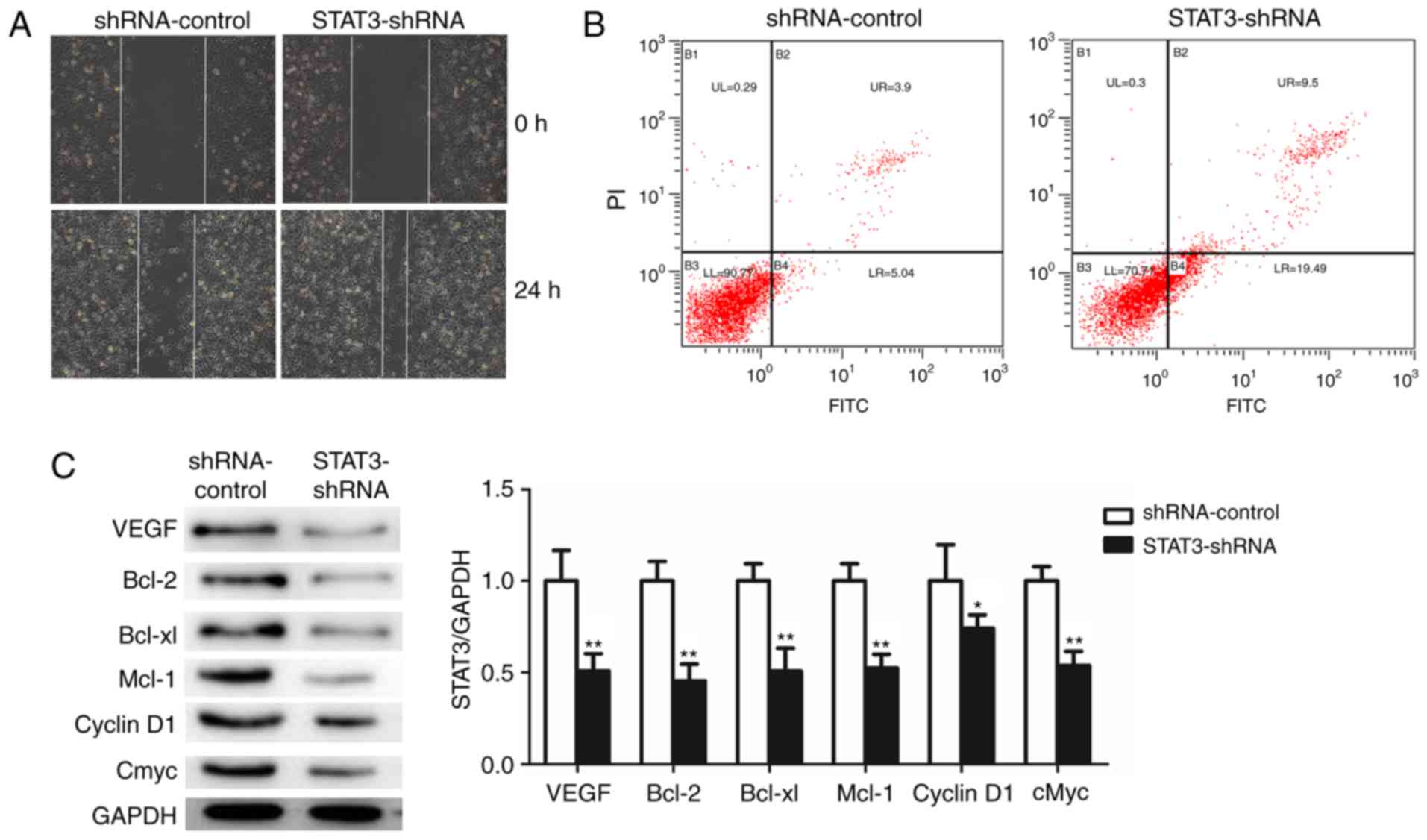 | Figure 4.Knockdown of STAT3 significantly
promotes apoptosis and suppresses cell migration and STAT3
downstream target genes in T24 cells. (A) The effects of knockdown
of STAT3 on migration in T24 were analyzed using wound healing
assays with a 24 h recovery period. (B) Apoptosis was determined by
fluorescence-activated cell sorting. Cells in early apoptosis are
in the bottom right quadrant, while cells in late apoptosis are in
the top right quadrant. (C) Western blot analysis was used to
detect the expression of STAT3 downstream target proteins. All data
are presented as the mean ± standard deviation of the mean.
*P<0.05 and **P<0.01. shRNA, short hairpin RNA; STAT3, signal
transducer and activator of transcription; PI, propidium iodide;
FITC, fluorescein isothiocyanate; VEGFR, vascular endothelial
growth factor receptor; Bcl-2, B-cell lymphoma 2; Bcl-xl, B-cell
lymphoma-extra large; Mcl-1, MCL1, Bcl2 family apoptosis regulator;
cMyc, MYC proto-oncogene, BHLH transcription factor. |
Discussion
miRNA has been studied intensively since it was
suggest to be associated with cancer in 2002 (18). In previous years, a number of miRNAs,
including miR-124, have also been identified to be dysregulated in
BCa (19). miR-124 inhibits the
expression of its downstream target genes, which include ubiquitin
like with PHD and ring finger domains 1, cyclin dependent kinase 4
and Rho-associated protein kinase, to suppress BCa tumorigenesis by
competitive binding to their 3′UTRs (20–22). The
STAT3 oncogene has been demonstrated as a downstream target gene of
miR-124 in other types of cancer (23). In the present study, a significant
downregulation of miR-124 was evident in BCa cell lines. The
results are consistent with the majority of other similar studies.
Next, STAT3 expression was detected in BCa cell lines, and it was
determined that it was negatively correlated with miR-124. The
ectopic overexpression of miR-124 inhibited STAT3 expression. In
addition, miR-124 directly bound to the 3′UTR of STAT3. These data
suggest a targeted association between STAT3 and miR-124 in
BCa.
STAT3 is a recognized oncogene that belongs to the
STAT family. In tumors, it is generally always stimulated by a
variety of extracellular signals that include growth factors and
cytokines (24–26). Its aberrant activation causes abnormal
cell proliferation and malignant transformation (27). Previous studies (28,29) have
indicated that increased STAT3 activity is closely associated with
BCa tumor growth and survival (30).
The use of small interfering RNA technology in the present study
revealed that the knockdown of STAT3 expression in T24 BCa cells
substantially inhibited tumor cell cycle progression, migration,
proliferation, colony formation and in vitro invasiveness.
This is consistent with one previous study (28). The STAT3 protein ranges between 750
and 795 amino acids in length and contains 6 functional domains:
The amino-terminal domain (SH2), coiled-coil domain, DNA binding
domain, linker domain, SH2 domain and transactivation domain
(31). Under the effects of external
stimuli, STAT3 is activated by tyrosine phosphorylation. The
activated STAT3 monomer forms a homodimer with the tyrosine
phosphorylated SH2 domain of STAT3 (32). The homodimer translocates into the
nucleus and binds to the specific DNA response element to regulate
the transcriptional activity of downstream target genes involved in
the regulation of cell cycle, proliferation and apoptosis (33–35). In
human cancer, 7 downstream target genes of STAT3 have been
identified: Cell cycle-associated genes (cyclin D1, cMyc);
apoptosis-associated genes (Bcl-2, Bcl-xl and Mcl-1) and an
angiogenesis-associated gene (VEGFR). The present study
demonstrated that knockdown of STAT3 expression significantly
suppressed the protein expression of these genes. These
observations suggest that STAT3 is a key regulatory factor
regulated by miR-124 in BCa, and that targeting the inhibition of
its signaling pathway will effectively suppress tumorigenesis
through a number of mechanisms.
In conclusion, the data of the present study
indicate a novel role of the miR-124/STAT3 signaling pathway in BCa
and demonstrate the potential to use miR-124 or STAT3 as a
diagnostic marker or therapeutic tool for human BCa. However, there
were several limitations in the present study. The study was
validated in only one bladder cancer cell line¸ T24, therefore the
results of this study suggest that this may be cell-type specific.
Therefore, the association between miR-124 and STAT3 in
vitro requires additional exploration.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
Project of Hainan Natural Science Foundation of China (grant no.
20168304) and Project capital of Hainan Provincial Department of
health (grant no. 2013 self raising-10).
Availability of data and materials
All datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
SW was responsible for the project and performed the
experiments. PL lead experimental work and conducted
immunohistochemistry work; GW actualized the fluorescence
detection. YH conducted the fluorescence detection and specimen
treatment. The immunohistochemistry experiments were performed by
PS, JC and YW. JY implemented autofluorescence acquisition and
software system processing.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of urothelial bladder
carcinoma. Nature. 507:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malats N and Real FX: Epidemiology of
bladder cancer. Hematol Oncol Clin North Am. 29:177–189, vii. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Käsmann L, Manig L, Janssen S and Rades D:
Chemoradiation including paclitaxel for locally recurrent
muscle-invasive bladder cancer in elderly patients. In Vivo.
31:239–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ide H, Inoue S and Miyamoto H:
Histopathological and prognostic significance of the expression of
sex hormone receptors in bladder cancer: A meta-analysis of
immunohistochemical studies. PLoS One. 12:e01747462017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilczynska A and Bushell M: The complexity
of miRNA-mediated repression. Cell Death Differ. 22:22–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gottardo F, Liu CG, Ferracin M, Calin GA,
Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, et
al: Micro-RNA profiling in kidney and bladder cancers. Urol Oncol.
25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YH, Wang SQ, Wu XL, Shen M, Chen ZG,
Chen XG, Liu YX, Zhu XL, Guo F, Duan XZ, et al: Characterization of
microRNAs expression profiling in one group of Chinese urothelial
cell carcinoma identified by Solexa sequencing. Urol Oncol.
31:219–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia H, Cheung WK, Ng SS, Jiang X, Jiang S,
Sze J, Leung GK, Lu G, Chan DT, Bian XW, et al: Loss of
brain-enriched miR-124 microRNA enhances stem-like traits and
invasiveness of glioma cells. J Biol Chem. 287:9962–9971. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimizu T, Suzuki H, Nojima M, Kitamura H,
Yamamoto E, Maruyama R, Ashida M, Hatahira T, Kai M, Masumori N, et
al: Methylation of a panel of microRNA genes is a novel biomarker
for detection of bladder cancer. Eur Urol. 63:1091–1100. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu CB, Li QL, Hu JF, Zhang Q, Xie JP and
Deng L: miR-124 inhibits growth and invasion of gastric cancer by
targeting ROCK1. Asian Pac J Cancer Prev. 15:6543–6546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng T, Xu D, Tu C, Li W, Ning Y, Ding J,
Wang S, Yuan L, Xu N, Qian K, et al: MiR-124 inhibits cell
proliferation in breast cancer through downregulation of CDK4.
Tumour Biol. 36:5987–5997. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou L, Xu Z, Ren X, Chen K and Xin S:
MicroRNA-124 (MiR-124) inhibits cell proliferation, metastasis and
invasion in colorectal cancer by downregulating Rho-associated
protein kinase 1(ROCK1). Cell Physiol Biochem. 38:1785–1795. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Yu Z, Li Y, Liu S, Gao C, Hou X, Yao
R and Cui L: The tumor suppressor miR-124 inhibits cell
proliferation by targeting STAT3 and functions as a prognostic
marker for postoperative NSCLC patients. Int J Oncol. 46:798–808.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Banaei-Esfahani A, Moazzeni H, Nosar PN,
Amin S, Arefian E, Soleimani M, Yazdani S and Elahi E: MicroRNAs
that target RGS5. Iran J Basic Med Sci. 18:108–114. 2015.PubMed/NCBI
|
|
18
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:pp. 15524–15529. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong F, Xu T, Shen Y, Zhong S, Chen S,
Ding Q and Shen Z: Dysregulation of miRNAs in bladder cancer:
Altered expression with aberrant biogenesis procedure. Oncotarget.
8:27547–27568. 2017.PubMed/NCBI
|
|
20
|
Zhang T, Wang J, Zhai X, Li H, Li C and
Chang J: MiR-124 retards bladder cancer growth by directly
targeting CDK4. Acta Biochim Biophys Sin (Shanghai). 46:1072–1079.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Wu Q, Xu B, Wang P, Fan W, Cai Y,
Gu X and Meng F: MiR-124 exerts tumor suppressive functions on the
cell proliferation, motility and angiogenesis of bladder cancer by
fine-tuning UHRF1. FEBS J. 282:4376–4388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu X, Li S, Lin Y, Chen H, Hu Z, Mao Y, Xu
X, Wu J, Zhu Y, Zheng X, et al: MicroRNA-124-3p inhibits cell
migration and invasion in bladder cancer cells by targeting ROCK1.
J Transl Med. 11:2762013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng Y, Li Y, Nian Y, Liu D, Dai F and
Zhang J: STAT3 is involved in miR-124-mediated suppressive effects
on esophageal cancer cells. BMC Cancer. 15:3062015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wan J, Zhao XF, Vojtek A and Goldman D:
Retinal injury, growth factors, and cytokines converge on β-catenin
and pStat3 signaling to stimulate retina regeneration. Cell Rep.
9:285–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Simone V, Franzè E, Ronchetti G,
Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald
TT, Pallone F, et al: Th17-type cytokines, IL-6 and TNF-α
synergistically activate STAT3 and NF-kB to promote colorectal
cancer cell growth. Oncogene. 34:3493–3503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Reilly S, Ciechomska M, Cant R and van
Laar JM: Interleukin-6 (IL-6) trans signaling drives a
STAT3-dependent pathway that leads to hyperactive transforming
growth factor-β (TGF-β) signaling promoting SMAD3 activation and
fibrosis via Gremlin protein. J Biol Chem. 289:9952–9960. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
28
|
Huang SY, Chang SF, Liao KF and Chiu SC:
Tanshinone IIA inhibits epithelial-mesenchymal transition in
bladder cancer cells via modulation of STAT3-CCL2 signaling. Int J
Mol Sci. 18(pii): E16162017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang J, Ren Y, Lou ZG, Wan X, Weng GB and
Cen D: Paeoniflorin inhibits the growth of bladder carcinoma via
deactivation of STAT3. Acta Pharm. 68:211–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho PL, Lay EJ, Jian W, Parra D and Chan
KS: Stat3 activation in urothelial stem cells leads to direct
progression to invasive bladder cancer. Cancer Res. 72:3135–3142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang T, Kee WH, Seow KT, Fung W and Cao
X: The coiled-coil domain of Stat3 is essential for its SH2
domain-mediated receptor binding and subsequent activation induced
by epidermal growth factor and interleukin-6. Mol Cell Biol.
20:7132–7139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang TT, Su JC, Liu CY, Shiau CW and Chen
KF: Alteration of SHP-1/p-STAT3 signaling: A potential target for
anticancer therapy. Int J Mol Sci. 18:2017. View Article : Google Scholar
|
|
33
|
Kamran MZ, Patil P and Gude RP: Role of
STAT3 in cancer metastasis and translational advances. Biomed Res
Int. 2013:4218212013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carpenter RL and Lo HW: STAT3 target genes
relevant to human cancers. Cancers (Basel). 6:897–925. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|