Introduction
Melanoma is the most common type of malignant tumor
that occurs in the conjunctiva, accounting for between 2 and 5%
cases of eye cancer, and between 5 and 7% cases of primary
malignant melanoma of the eye (1).
The incidence of melanoma is reported to have increased gradually,
which may be associated with increased ultraviolet exposure
(2). The high degree of tumor
malignancy is associated with high recurrence rate and high
incidence of adjacent lymph node and liver metastasis (3). Despite treatment, the local recurrence
rate is 62%, and the mortality rate ranges between 18 and 44%
(3). Current clinical treatment
includes wide excision, which aims to remove the total tumor tissue
and avoid recurrence caused by residual cancer cells (4). Common adjuvant treatments include freeze
therapy, radiotherapy, chemotherapy, coagulation therapy and
biological therapy (4). Gradual and
comprehensive treatment methods decrease the recurrence rate to
some extent; however, following treatment the 5-year local
recurrence rate is between 30 and 50%, the 10-year mortality rate
is <30% and the prognosis remains poor (3).
Tumor protein p53 (p53) is the tumor suppressor gene
which has been most widely associated with human tumors (5). Under normal circumstances, p53 functions
in DNA repair and replication, arresting cell proliferation
following DNA damage, which inhibits tumor growth (6). Mutant p53 is more stable than the
wild-type gene, and can be identified by immunohistochemistry
(7). High expression of p53 protein
indicates a mutation of p53. p53 has been demonstrated to serve an
important function in the occurrence and development of melanoma
(5).
Nuclear factor-κB (NF-κB) is an important nuclear
transcription factor, involved in the regulation of numerous
physiological and pathological events, including mediating
inflammation, cell survival, apoptosis and tumor invasion (5). NF-κB has also been implicated in the
association between inflammation and cancer occurrence (5).
Anthraquinone compounds have been identified in
Rubiaceae, Polygonaceae and leguminous plants, and their extraction
and separation, component analysis and pharmacological effects have
been investigated (8). The basic
quinone nuclei of the commonly used anticancer drugs, doxorubicin
and mitoxantrone, have an anthraquinone structure (8). However, the mechanism of the antitumor
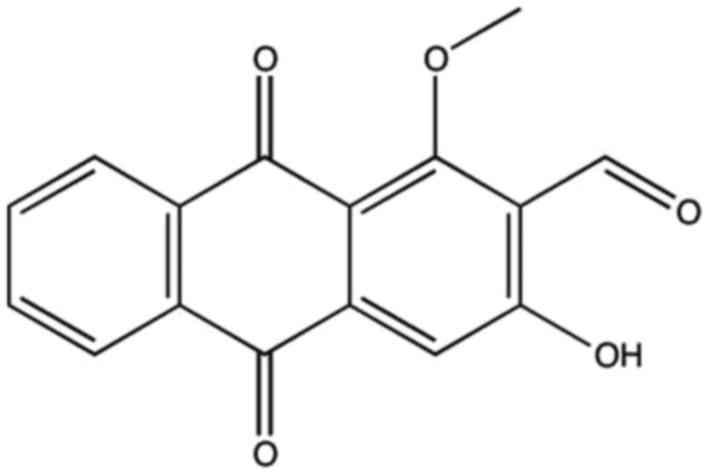
effect of anthraquinones remains unclear. Damnacanthal (Fig. 1) is a derivative of
Damnacanthus (a flowering plant of the Rubiaceae family
native to eastern Asia), and has been demonstrated to exhibit
potent anticancer properties (9).
Previous studies have demonstrated that the compound exhibits
cytotoxic activity and anticancer cell proliferation effects, which
may result in the apoptosis of cancer cells and an antitumor
function (8,10). The aim of the present study was to
investigate the underlying molecular mechanism of antitumorigenic
activity of damnacanthal on melanoma cells.
Materials and methods
Cell culture
The human melanoma cell line MUM-2B was obtained
from the Cell Bank of the Chinese Academy of Sciences (Beijing,
China) and cultured in Dulbecco's modified Eagle's medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Thermo Fisher Scientific, Inc.) in 5%
CO2 at 37°C.
MTT cell viability assay
A total of 1×104 MUM-2B cells were seeded
in a 96-well plate for 24 h, then treated with 0, 1, 2.5, 5 10 or
20 µM damnacanthal for 12, 24 or 48 h. A total of 20 µl MTT (5
mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to
each well prior to incubation for another 4 h at 37°C. The
supernatants were aspirated and 150 µl dimethylsulfoxide
(Sigma-Aldrich; Merck KGaA) was added to each well to dissolve the
formazan crystals for 20 min at 37°C. The optical density (OD) at
490 nm was determined using a microplate reader (Molecular Devices,
Sunnyvale, CA, USA).
DAPI assay
Apoptosis was analyzed using a DAPI assay (Beyotime
Institute of Biotechnology, Haimen, China), according to the
manufacturer's protocol. MUM-2B cells were seeded in a 6-well plate
at 1×106 cells/well for 24 h. The cells were treated
with 2.5, 5 and 10 µM damnacanthal for 24 h, prior to being washed
twice in PBS. A concentration of 100 ng/ml DAPI assay reagent was
added for 10 min at room temperature in the dark. The MUM-2B cells
were then washed twice with PBS and observed using a fluorescence
microscope at ×400, magnification.
Flow cytometry
Apoptosis was also detected using flow cytometry.
MUM-2B cells were seeded in a 6-well plate at 1×106
cells/well for 24 h and treated with 2.5, 5 and 10 µM damnacanthal
for 24 h. The cells were then harvested, washed and resuspended in
ice-cold PBS. The cells were stained with Annexin V-fluorescein
isothiocyanate (50 µg/ml, BD Biosciences, Franklin Lakes, NJ, USA)
and propidium iodide (10 µg/ml; BD Biosciences) in the dark for 15
min at room temperature. Cell apoptosis was examined using flow
cytometry (FACScan; BD Biosciences), according to the
manufacturer's protocol.
Caspase activity
MUM-2B cells were seeded in a 6-well plate at
1×106 cells/well for 24 h, then treated with 2.5, 5 and
10 µM damnacanthal for 24 h. Cells were washed twice with PBS and
lysed in radioimmunoprecipitation assay buffer (Beyotime Institute
of Biotechnology) for 1 h at 37°C. Cells lysates were centrifuged
at 12,000 × g for 15 min at 4°C, prior to determination of the
protein concentration using a bicinchoninic acid (BCA) assay
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. A total of 10 ng protein lysate was
analyzed using the caspase-3
(acetyl-Asp-Glu-Val-Asp-p-nitroanilide) and caspase-9
(acetyl-Leu-Glu-His-Asp-p-nitroanilide) activity assay kits (both
Beyotime Institute of Biotechnology), according to the
manufacturer's protocols. The optical density (OD) was measured
using a microplate reader (Molecular Devices, Sunnyvale, CA, USA)
at 405 nm.
Western blot analysis
MUM-2B cells were seeded in a 6-well plate at
1×106 cells/well for 24 h and were treated with 2.5, 5
and 10 µM damnacanthal for 24 h. Cells were washed twice with PBS
and lysed using radioimmunoprecipitation assay buffer. Cells
extracts were centrifuged at 12,000 × g for 15 min at 4°C, and the
protein concentration was determined using a BCA assay. Protein
lysates (50 µg) were separated by SDS-PAGE (8–10% gel) and
transferred onto a nitrocellulose membrane (GE Healthcare, Chicago,
IL, USA). The membrane was blocked with 5% skimmed milk in 0.1%
TBS-Tween-20 prior to incubation with primary antibodies against
the following: B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax;
cat. no. 5023; dilution, 1:2,000), p53 (cat. no. 2527; dilution,
1:2,000), p21 (cat. no. 2947; dilution, 1:2,000), NF-κB (cat. no.
8242; dilution, 1:2,000), cyclin D (cat. no. 2978; dilution,
1:2,000) and cyclin E (cat. no. 20808; dilution, 1:2,000) (all Cell
Signaing Technology, Inc., Danvers, MA, USA), and GAPDH (dilution,
1:5,000; Bioworld Technology, Inc., St. Louis Park, MN, USA) at 4°C
overnight. Following washing three times with TBS-0.1% Tween-20,
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (cat. no. 7074; dilution,
1:5,000; Cell Signaling Technology, Inc.) for 1 h at room
temperature. Protein bands were detected using a chemiluminescence
system (Beyotime Institute of Biotechnology) and quantified using
ImageLab software (version 3.0; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
All results are expressed as the mean ± standard
deviation. Differences between groups were identified by one-way
analysis of variance and Tukey's post-hoc test using SPSS software
(version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Damnacanthal inhibits proliferation of
melanoma cells
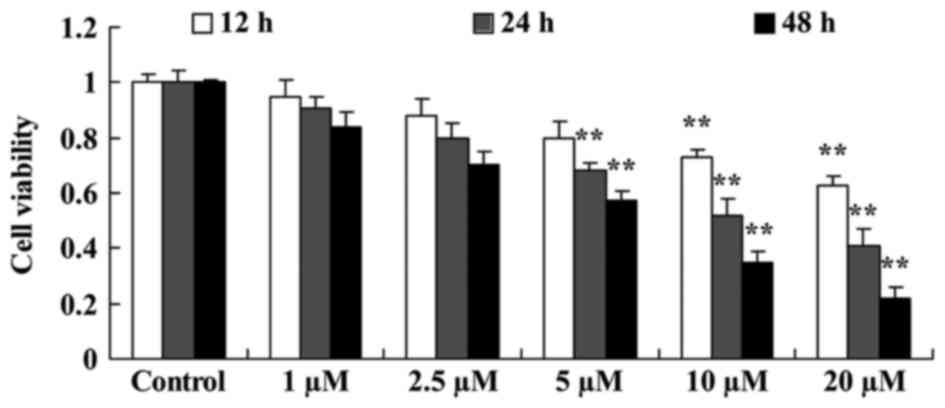
The effect of damnacanthal on proliferation of
melanoma cells was investigated using an MTT assay. Damnacanthal
treatment decreased the proliferation of MUM-2B cells time- and
dose-dependently. A treatment of 5–20 µM damnacanthal significantly
decreased cell proliferation after 24 and 48 h compared with
untreated cells. Treatments of 10 and 20 µM damnacanthal were able
to significantly decrease the proliferation of MUM-2B cells after
12 h compared with untreated cells (Fig.
2).
Damnacanthal induces melanoma cell
apoptosis
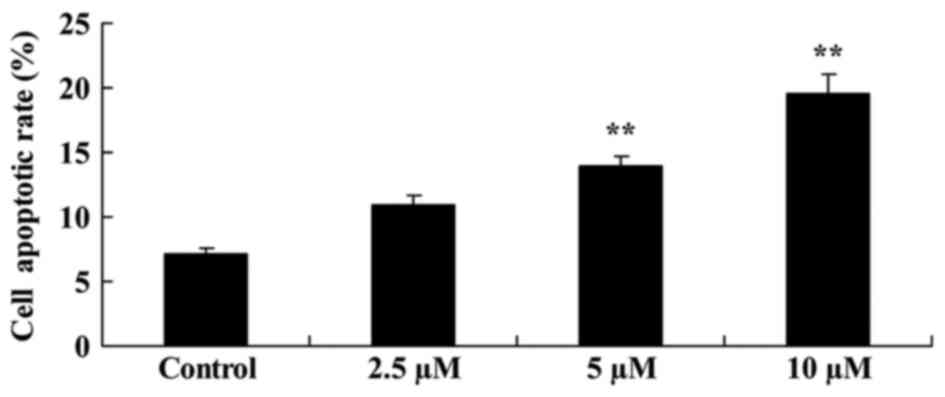
Flow cytometric analysis demonstrated that
damnacanthal treatment induced melanoma cell apoptosis. Following
treatment with 5 and 10 µM damnacanthal for 24 h, apoptotic rate of
MUM-2B cells was significantly increased compared with the
untreated control cells (Fig. 3). A
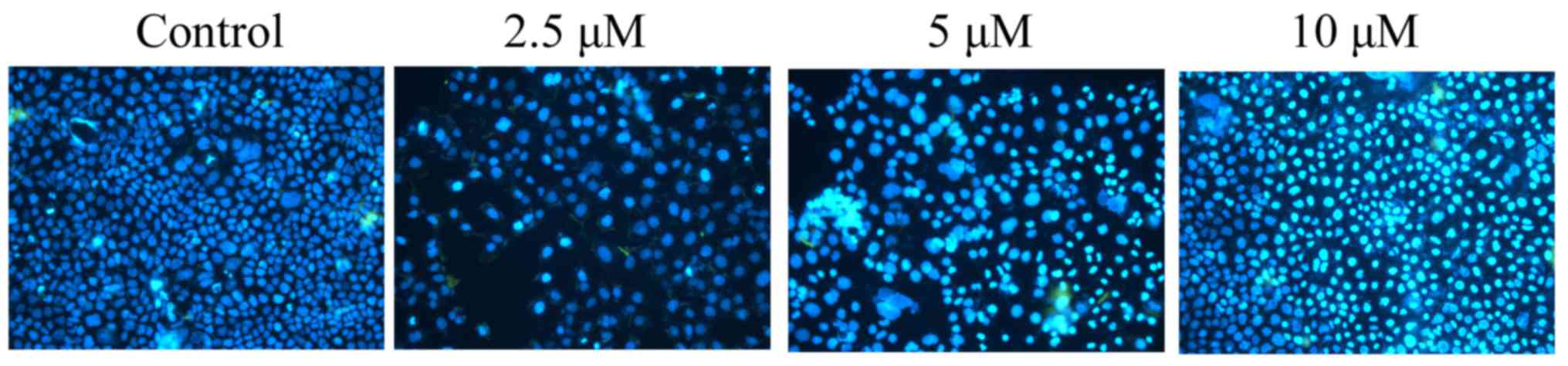
DAPI assay was used to stain apoptotic melanoma cells treated with
damnacanthal for 24 h. As presented in Fig. 4, 5 and
10 µM damnacanthal treatment observably increased the number of
apoptotic cells after 24 h, compared with untreated control
cells.
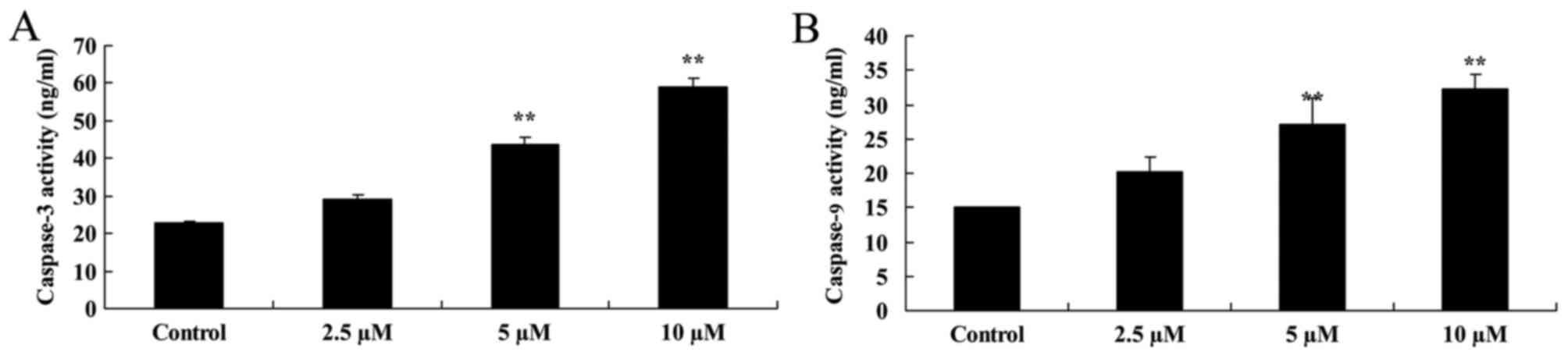
Damnacanthal induces caspase-3/9
activity in melanoma cells
To explore the underlying molecular mechanism of the
effect of damnacanthal on apoptosis of MUM-2B cells, caspase-3/9
activity was analyzed using caspase activity assay kits. As
presented in Fig. 5, caspase-3/9
activity was significantly increased by 5 and 10 µM damnacanthal
treatment for 24 h compared with untreated control cells.
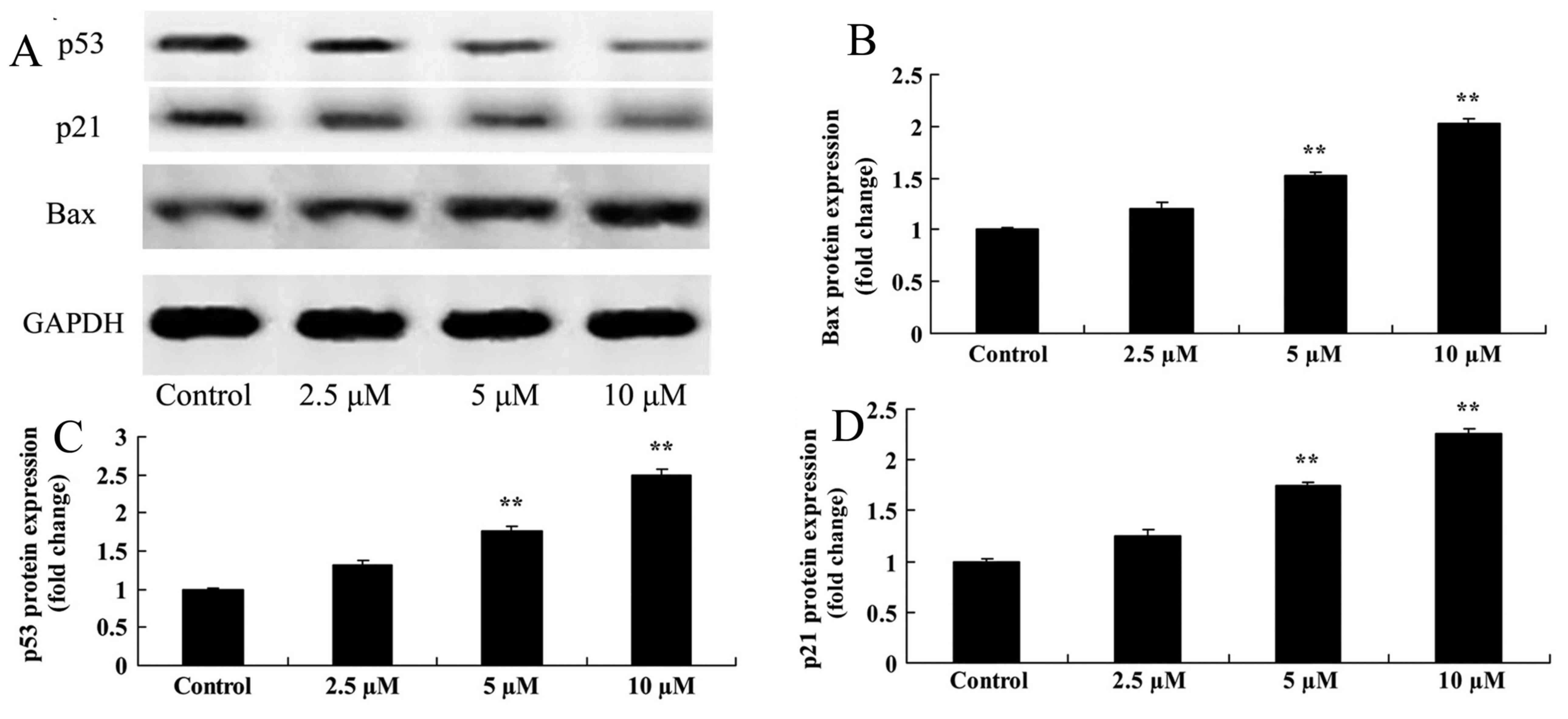
Damnacanthal treatment upregulates
Bax, p53 and p21 protein expression levels in melanoma cells
Bax protein expression was analyzed by western
blotting after 24 h treatment with damnacanthal. The assay revealed
that 5 and 10 µM damnacanthal significantly upregulated the Bax
protein expression level in MUM-2B cells, compared with the
untreated control cells (Fig. 6A, B).
It was also investigated whether p53/p21 signaling may function in
the anticancer effects of damnacanthal on melanoma cells. As
presented in Fig. 6A, 6C-6D, p53 and
p21 protein expression levels in MUM-2B cells were significantly
increased following treatment with 5 and 10 µM damnacanthal,
compared with untreated control cells.
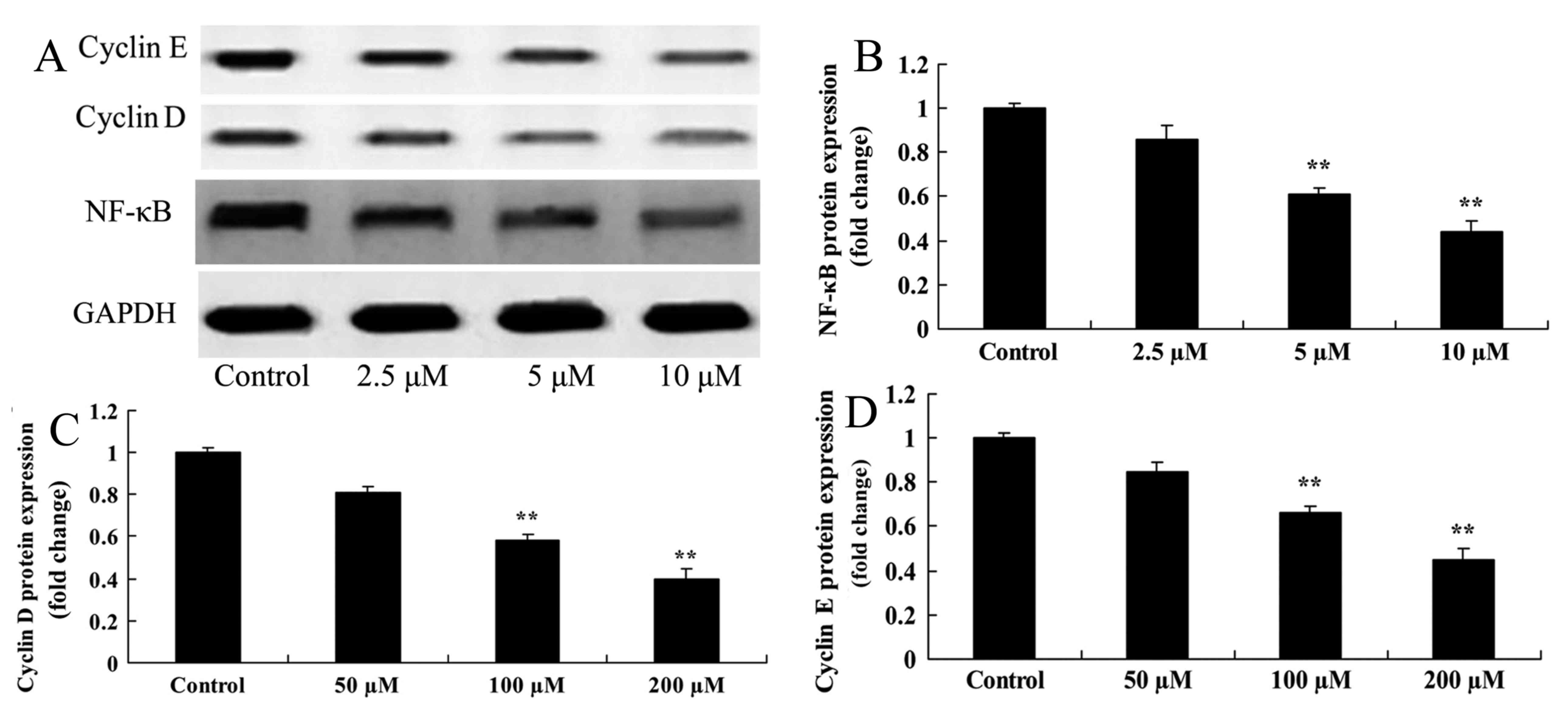
Damnacanthal treatment inhibits the
protein expression level of NF-κB, cyclin D and cyclin E in
melanoma cells
Western blotting was used to determine the effect of
damnacanthal on NF-κB, cyclin D and cyclin E protein expression
levels in melanoma cells. A significant inhibition of NF-κB, cyclin
D and cyclin E protein expression was observed in MUM-2B cells
after 24 h treatment with 5 and 10 µM damnacanthal compared with
untreated control cells (Fig. 7).
Discussion
Melanoma is a highly malignant tumor originating in
melanocytes and mainly identified in the skin; it is often caused
by heritable genetic variation or environmental factors (3). The exogenous factor of greatest risk is
exposure to ultraviolet irradiation. Malignant melanoma may also be
derived from nerve sheath cells, which are able to generate melanin
due to a mutation in nerve sheath cells, and the abnormalities of
pigment generation and tyrosine metabolism (11). In the present study, damnacanthal
treatment significantly decreased proliferation and increased the
apoptotic rate of MUM-2B cells. Sukamporn et al (12) suggested that damnacanthal exhibits
anticancer activity via downregulation of cyclin D1 expression.
p53 is a transcription factor which monitors the
integrity of genomic cell DNA (13).
p53 activates the transcription of the p21WAF1 gene,
causing cell cycle arrest in G1/S phase, whereas
activation of the 14-3-3 gene leads to cell cycle arrest in the
G2/M phase, thus inhibiting tumor cell viability through
cell cycle regulation (13). It also
activates the expression of downstream genes, including Bax and
NADPH oxidase activator, launching the apoptotic program and
inhibiting the generation of cells with cancerous tendencies,
thereby preventing malignant cell proliferation (14). Therefore, p53 serves an important
function in the maintenance of normal cell viability and function.
However, wild-type p53 has poor stability, a short intracellular
half-life, often <20 min, and cannot be reliably identified
using immunohistochemistry (15). The
intracellular level and activity of p53 are finely regulated at the
transcriptional, translational and post-translational levels, and
by subcellular localization, among other processes (7). In the present study, it was demonstrated
that damnacanthal significantly suppressed cyclin D and cyclin E
protein expression, promoted caspase-3/9 activity, and induced Bax,
p53 and p21 protein expression in melanoma cells. Aziz et al
(16) reported that damnacanthal
induced apoptosis by stimulating p53 and p21 expression in the
breast cancer cell line MCF-7 (16).
NF-κB is a transcription factor widely distributed
in eukaryotic cells, which servers a key function in tumor cell
proliferation, apoptosis, invasion, metastasis and angiogenesis
(17). In recent years, it has been
reported that tumor resistance to chemotherapeutic drugs is
associated with NF-κB (18). NF-κB is
able to be activated by a variety of factors, including
pro-inflammatory cytokines, growth factors and cell stress; it may
also be activated by chemotherapeutic drugs, including
daunorubicin, doxorubicin and cisplatin and other chemotherapy
drugs (19). NF-κB is also a key
regulator of apoptosis, and may induce the expression of
anti-apoptotic factors, including survivin and Bcl-2 (20). NF-κB activation is strictly regulated
by inhibitor of NF-κB, therefore conventional chemotherapy drugs
are often accompanied by NF-κB inhibitors, to inhibit the NF-κB
signaling pathway, decrease local recurrence and improve patient
survival rate (21). The results of
the present study demonstrated that damnacanthal significantly
suppressed NF-κB protein expression in MUM-2B cells. Kim et
al (22) suggested that
damnacanthal inhibits the NF-κB signaling pathway in mast cells
(22).
In conclusion, the results of the present study
demonstrated that damnacanthal treatment inhibits cell
proliferation, induces cell apoptosis, increases caspase-3/8/9
activity, upregulates the protein expression level of Bax, and
downregulates the protein expression levels of cyclin D and cyclin
E in melanoma cells through the p53/p21 and NF-κB/cyclin/caspase-3
signaling pathways. These results suggest that damnacanthal may
serve as a potential novel drug in patients with melanoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Clinical
Science Fund of People's Liberation Army General Hospital (grant
no. 2013FC-ZHCG-1007).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
CL designed the experiment; XZ, PF, ZZ, XD, FX, YW
performed the experiment; CL and XZ analyzed the data; CL wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McMasters KM, Egger ME, Edwards MJ, Ross
MI, Reintgen DS, Noyes RD, Martin RC II, Goydos JS, Beitsch PD,
Urist MM, et al: Final results of the sunbelt melanoma trial: A
multi-institutional prospective randomized phase III study
evaluating the role of adjuvant high-dose interferon Alfa-2b and
completion lymph node dissection for patients staged by sentinel
lymph node biopsy. J Clin Oncol. 34:1079–1086. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamid O, Ilaria R Jr, Garbe C, Wolter P,
Maio M, Hutson TE, Arance A, Lorigan P, Lee J, Hauschild A, et al:
A randomized, open-label clinical trial of tasisulam sodium versus
paclitaxel as second-line treatment in patients with metastatic
melanoma. Cancer. 120:2016–2024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zimmer L, Vaubel J, Mohr P, Hauschild A,
Utikal J, Simon J, Garbe C, Herbst R, Enk A, Kämpgen E, et al:
Phase II DeCOG-study of ipilimumab in pretreated and
treatment-naive patients with metastatic uveal melanoma. PLoS One.
10:e01185642015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrucci PF, Minchella I, Mosconi M,
Gandini S, Verrecchia F, Cocorocchio E, Passoni C, Pari C, Testori
A, Coco P and Munzone E: Dacarbazine in combination with
bevacizumab for the treatment of unresectable/metastatic melanoma:
A phase II study. Melanoma Res. 25:239–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Genov M, Kreiseder B, Nagl M, Drucker E,
Wiederstein M, Muellauer B, Krebs J, Grohmann T, Pretsch D, Baumann
K, et al: Tetrahydroanthraquinone derivative
(+/−)-4-deoxyaustrocortilutein induces cell cycle arrest and
apoptosis in melanoma cells via upregulation of p21 and p53 and
downregulation of NF-kappaB. J Cancer. 7:555–568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vilgelm A and Richmond A: Combined
therapies that induce senescence and stabilize p53 block melanoma
growth and prompt antitumor immune responses. Oncoimmunology.
4:e10092992015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He T, Wu J, Chen Y and Zhang J: TP 53
polymorphisms and melanoma: A meta-analysis. J Cancer Res Ther.
11:409–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohashi K, Sampei K, Nakagawa M, Uchiumi N,
Amanuma T, Aiba S, Oikawa M and Mizuno K: Damnacanthal, an
effective inhibitor of LIM-kinase, inhibits cell migration and
invasion. Mol Biol Cell. 25:828–840. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abu N, Ali NM, Ho WY, Yeap SK, Aziz MY and
Alitheen NB: Damnacanthal: A promising compound as a medicinal
anthraquinone. Anticancer Agents Med Chem. 14:750–755. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaghayegh G, Alabsi AM, Ali-Saeed R, Ali
AM, Vincent-Chong VK and Zain RB: Cell cycle arrest and mechanism
of apoptosis induction in H400 oral cancer cells in response to
Damnacanthal and Nordamnacanthal isolated from Morinda citrifolia.
Cytotechnology. 68:1999–2013. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lian B, Si L, Cui C, Chi Z, Sheng X, Mao
L, Li S, Kong Y, Tang B and Guo J: Phase II randomized trial
comparing high-dose IFN-α2b with temozolomide plus cisplatin as
systemic adjuvant therapy for resected mucosal melanoma. Clin
Cancer Res. 19:4488–4498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sukamporn P, Rojanapanthu P, Silva G,
Zhang X, Gritsanapan W and Baek SJ: Damnacanthal and its
nanoformulation exhibit anti-cancer activity via cyclin D1
down-regulation. Life Sci. 152:60–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaluzki I, Hrgovic I, Hailemariam-Jahn T,
Doll M, Kleemann J, Valesky EM, Kippenberger S, Kaufmann R, Zoeller
N and Meissner M: Dimethylfumarate inhibits melanoma cell
proliferation via p21 and p53 induction and bcl-2 and cyclin B1
downregulation. Tumour Biol. 37:13627–13635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasad ML, Patel SG, Shah JP,
Hoshaw-Woodard S and Busam KJ: Prognostic significance of
regulators of cell cycle and apoptosis, p16(INK4a), p53, and bcl-2
in primary mucosal melanomas of the head and neck. Head Neck
Pathol. 6:184–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nylund C, Rappu P, Pakula E, Heino A,
Laato L, Elo LL, Vihinen P, Pyrhönen S, Owen GR, Larjava H, et al:
Melanoma-associated cancer-testis antigen 16 (CT16) regulates the
expression of apoptotic and antiapoptotic genes and promotes cell
survival. PLoS One. 7:e453822012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aziz MY, Omar AR, Subramani T, Yeap SK, Ho
WY, Ismail NH, Ahmad S and Alitheen NB: Damnacanthal is a potent
inducer of apoptosis with anticancer activity by stimulating p53
and p21 genes in MCF-7 breast cancer cells. Oncol Lett.
7:1479–1484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aggarwal BB and Sung B: NF-κB in cancer: A
matter of life and death. Cancer Discov. 1:469–471. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rabbie R and Adams DJ: Desmoplastic
melanoma: C>Ts and NF-κB. Pigment Cell Melanoma Res. 29:120–121.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shan X, Tian LL, Zhang YM, Wang XQ, Yan Q
and Liu JW: Ginsenoside Rg3 suppresses FUT4 expression through
inhibiting NF-κB/p65 signaling pathway to promote melanoma cell
death. Int J Oncol. 47:701–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thu YM and Richmond A: NF-κB inducing
kinase: A key regulator in the immune system and in cancer.
Cytokine Growth Factor Rev. 21:213–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu FH, Yuan Y, Li D, Lei Z, Song CW, Liu
YY, Li B, Huang B, Feng ZH and Zhang GM: Endothelial cell-expressed
Tim-3 facilitates metastasis of melanoma cells by activating the
NF-kappaB pathway. Oncol Rep. 24:693–699. 2010.PubMed/NCBI
|
|
22
|
Kim MH and Jeong HJ: Damnacanthal inhibits
the NF-κB/RIP-2/caspase-1 signal pathway by inhibiting p56lck
tyrosine kinase. Immunopharmacol Immunotoxicol. 36:355–363. 2014.
View Article : Google Scholar : PubMed/NCBI
|