Introduction
The major challenge in improving the prognosis of
lung adenocarcinoma patients is the drug resistance to cisplatin,
in which the Kelch-like ECH-associated protein 1 (Keap1)/nuclear
factor, erythroid 2 like 2 (Nrf2)/antioxidant response element
(ARE) signaling pathway has a critical role (1–3). With the
stimulation of active oxygen, Nrf2 and Keap1 are uncoupled
(4). Subsequently, Nrf2 enters the
nucleus to form heterologous dimers with Maf to initiate the
transcription of ARE target genes (5)
and phase II detoxifying enzymes, including superoxide dismutase
(SOD), heme oxygenase-1 (HO-1) and glutathione S-transferase alpha
1 (GSTA1) (6). Activation of Nrf2
enhances cellular oxidative stress (7) and the growth of tumor cells (8), thus enhancing the drug resistance of
lung adenocarcinoma (9–11). However, treatment with short hairpin
RNA (shRNA) targeting Nrf2 may reverse the drug resistance of
certain types of cancer cell (12).
Multidrug resistance-associated proteins (MRPs) and
phase II detoxification enzymes have synergistic effects (13). Excessive activation of Nrf2 may cause
high expression of MRPs, which induces drug resistance in tumor
cells (14). Excessive activation of
Nrf2 also induces tumor cells to reach a state that is inert to
apoptosis and promotes the occurrence of tumors (15). However, after transfection of the
CaSki cell line with Nrf2 shRNA, the tumor drug resistance was
reversed (16). Nrf2 activation is
regulated by the mitogen-activated protein kinase (MAPK) pathway
(17), but direct phosphorylation of
Nrf2 by MAPKs does not induce Nrf2 activation. Activated Nrf2
enters the nucleus and forms heterologous dimers with
phosphorylated extracellular signal-regulated kinase (p-ERK), c-Jun
N-terminal kinase (JNK) and p38 MAPK (18). These Nrf2 dimer complexes then
activate the transcription of genes downstream of Nrf2 (19), which varies among different organs
(20). Nrf2 is known to interact with
phosphoinositide-3 kinase (PI3K) (21), and the PI3K/AKT/mammalian target of
rapamycin (mTOR) pathway is usually activated in various types of
human malignancy, including non-small cell lung cancer (8,22).
Metformin treatment reduces the risk of various
tumor types, including ovarian cancer and lung cancer, in diabetic
patients (23,24). It blocks different types of tumor cell
in the G0/G1 phase or inhibits the G1/S-phase transition in the
cell cycle by regulating the expression of cell cycle proteins and
their associated factors (25–27).
Metformin also exerts a dose-dependent inhibitory effect on the
proliferation of lung cancer cells of various pathological types
(28,29). The mechanism may include the
activation of the adenosine 5′monophosphate-activated protein
kinase (AMPK) pathway (30,31), which reduces the proliferation of
tumor cells by inhibiting epidermal growth factor receptor and
insulin-like growth factor 1 receptor pathways (26,32,33).
Metformin also inhibits the expression of tumor cell
apoptosis-associated proteins and prevents the oxidation of tumor
cells via an AMPK-independent pathway (34,35). In a
study on breast cancer, inhibition of the expression in AMPK by
AMPK inhibitors or shRNA abrogated the antineoplastic effect of
metformin (36).
To date, only few studies have examined the
mechanisms by which metformin inhibits tumor cells. It has been
indicated that metformin affects Nrf2 and regulates the Nrf2/ARE
pathway in lung adenocarcinoma cells (37). In the present study, the A549/DDP
cisplatin-resistant lung adenocarcinoma cell line was used. To the
best of our knowledge, the present study was the first to assess
whether metformin affects Nrf2 expression in native A549 and
A549/DDP cells, and whether it regulates the Nrf2 and MAPK pathways
to affect the expression of ATP-binding cassette subfamily C member
1 (ABCC1) and GSTA1.
Materials and methods
Reagents
Reverse transcription was performed using the
PrimeScript RT reagent kit with gDNA Eraser (cat. no. RR047A) and
Real-time polymerase chain reaction (PCR) was performed using the
SYBR PrimeScript (Perfect Real Time) kit (cat. no. RR086A; Takara,
Dalian, China). MAPK Family bodies Sampler kit (cat. no. 9926),
Phospho-MAPK Family Antibody Sampler kit (cat. no. 9910) and Human
GAPDH antibody (cat. no. 686613) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA), and anti-PCNA antibody (cat.
no. ab18197), anti-Nrf2 antibody (cat. no. ab62352), anti-ABCC1
antibody (cat. no. ab24102) and anti-GSTA1 antibody (cat. no.
ab111947) were purchased from Abcam (Cambridge, MA, USA). Primary
Antibody Dilution Buffer was purchased from Beyotime Institute of
Biotechnology (P0023A; Haimen, China). Nrf2-specific shRNA and
control shRNA was obtained from Shanghai GenePharma Co., Ltd.
(Shanghai, China) (Table I). The
Nuclear and cytoplasmic protein Extraction kit was purchased from
Beyotime Institute of Biotechnology (P0027). Metformin was obtained
from Sangon Biotech Co., Ltd (Shanghai, China). Primer synthesis
was performed by Biocolors Biological Technology Co., Ltd.
(Shanghai, China). PI3K-specific inhibitor LY294002, ERK-specific
inhibitor PD98059, JNK-specific inhibitor SP600125 and p38
kinase-specific inhibitor SB203580 were purchased from BioVision
Inc. (Milpitas, CA, USA).
 | Table I.shRNA sequences. |
Table I.
shRNA sequences.
| shRNA | Sequence |
|---|
| NRF2-shRNA-con |
5′-GGAGGCAAGAUAUAGAUCUTT-3′ |
|
|
5′-AGAUCUAUAUCUUGCCUCCTT-3′ |
| NRF2-shRNA-1 |
5′-CCAGAACACUCAGUGGAAUTT-3′ |
|
|
5′-AUUCCACUGAGUGUUCUGGTT-3′ |
| NRF2-shRNA-2 |
5′-GCCCAUUGAUGUUUCUGAUTT-3′ |
|
|
5′-AUCAGAAACAUCAAUGGGCTT-3′ |
| NRF2-shRNA-3 |
5′-GCACCUUAUAUCUCGAAGUTT-3′ |
|
|
5′-ACUUCGAGAUAUAAGGUGCTT-3′ |
Cell lines and culture
The A549 human lung adenocarcinoma cell line and
cisplatin-resistant A549/DDP cells were obtained from GAS Shanghai
Life Sciences Cell (Shanghai, China). Cells were cultured in
RPMI-1640 dry powder culture medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with fetal bovine serum (FBS;
Hyclone; GE Healthcare, Logan, UT, USA). Cell transfections were
performed with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) A549 and A549/DDP cells were cultured in
RPMI-1640 with 10% FBS, benzylpenicillin (100 U/ml) and
streptomycin (100 mg/ml), and cultured at 37°C in a humidified
atmosphere of air with 5% CO2.
Nrf2 shRNA transfection
A549/DDP cells were cultured in 24-well cell culture
microplates at 1×105 cells/well (0.5 ml medium per well,
for PCR) or 6-well cell culture microplates at 5×104
cells/well (2 ml medium per well, for western blot) for 24 h. The
shRNA transfection was performed with Lipofectamine 2000, according
to the manufacturer's protocol. The shRNA/medium mixture was
incubated at room temperature for 5 min, then evenly mixed with
Lipofectamine 2000 and placed at room temperature for 20 min for
shRNA reagent/Lipofectamine complex formation. The 100-µl mixture
was then added to the cells. After 12 h, the medium was replaced
complete RPMI-1640 medium and the cells were cultured for another
48 h. Cells were then harvested for analysis. To screen for the
most effective Nrf2 shRNA, the number of transfected cells was
counted under the fluorescence microscope, and The knockdown
efficiency was further detected by PCR and western blot analysis,
which indicated that >70% of the cells were successfully
transfected. The shRNA sequences are listed in Table I.
Measurement of gene expression
A549 and A549/DDP cells were cultured in six-well
plates at a concentration of 1×105 cells/well for 24 h.
Total RNA was extracted with TRIzol reagent and reverse transcribed
to complementary DNA with the PrimeScript RT reagent kit with gDNA
Eraser, according to the manufacturer's protocol. Quantitative PCR
was performed using the SYBR Green PCR system on the CFX96 PCR
instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
thermocycling conditions were as follows: 1 min at 95°C, followed
by 40 cycles of 5 sec at 95°C and 30 sec at 60°C. The relative
expression of each gene was used to perform relative fold change
between treated and control groups using the 2−∆∆Cq
method (38). The primer sequences
are listed in Table II.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| mRNA | Sequence |
|---|
| NRF2 |
5′-ACACGGTCCACAGCTCATC-3′ |
|
|
5′-TGTCAATCAAATCCATGTCCTG-3′ |
| ABCC1 |
5′-TGTGGGAAAACACATCTTTGA-3′ |
|
|
5′-CTGTGCGTGACCAAGATCC-3′ |
| GSTA1 |
5′-TCCCTCATCTACACCAACTATGAG-3′ |
|
|
5′-GGTCTTGCCTCCCTGGTT-3′ |
| GAPDH |
5′-CCACCCATGGCAAATTCCATGGCA-3′ |
|
|
5′-TCTACACGGCAGGTCAGGTCCACC-3′ |
Western blot analysis
Intracellular protein was extracted with
radioimmunoprecipitation assay buffer. The protein concentration of
the cell extract was determined by bicinchoninic acid protein
assay, and 25 µg loaded sample amounts of total protein were
separated by 10% SDS-PAGE. Proteins were then transferred to a
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA
USA), which was blocked in 5% dry milk for 2 h. Membranes were then
probed with the specific primary antibodies: Akt (pan) rabbit mAb
(cat. no. 4685; 1:1,000 dilution); phospho-Akt (Ser473) rabbit mAb
(cat. no. 4060; 1:1,000 dilution); p44/p42 MAPK(ERK1/2) rabbit mAb
(cat. no. 4695; 1:1,000 dilution); phospho-p44/p42 MAPK(ERK1/2)
(Thr202/Tyr204) rabbit mAb (cat. no. 4370; 1:1,000 dilution); p38
MAPK Rabbit mAb (cat. no. 8690; 1:1,000 dilution); phospho-p38 MAPK
(Thr180/Tyr182) rabbit mAb (cat. no. 4511; 1:1,000 dilution);
SAPK/JNK antibody (cat. no. 9252; 1:1,000 dilution);
phospho-SAPK/JNK (Thr183/Tyr185) rabbit mAb (cat. no. 4668, 1:1,000
dilution); The aforementioned antibodies were all obtained by Cell
Signaling Technology, Inc.; anti-PCNA antibody (1:1,000 dilution);
anti-Nrf2 antibody (1:2,000 dilution); anti-ABCC1 antibody (1:1,000
dilution); anti-GSTA1 antibody (1:2,000 dilution); and GAPDH
antibody (cat. no. 686613, 1:1,000 dilution; Cell Signaling
Technology, Inc. MA, USA) was used as a loading control at 4°C
overnight. The membrane was washed three times with Tris-buffered
saline containing 1% Tween-20 (TBST), followed by incubation with
peroxidase-labeled secondary antibodies (anti-rabbit IgG; cat. no.
7074; dilution, 1:5,000; Cell Signaling Technology, Inc.) for 2 h
at room temperature. After 24 h incubation with 5 mM Metformin and
the different inhibitors (10–40 µM PI3K-specific inhibitor
LY294002; 10–40 µM ERK-specific inhibitor PD98059; 1–20 µM
JNK-specific inhibitor SP600125; and 0.1–10 µM p38 kinase-specific
inhibitor SB203580), the Nrf2 protein expression was evaluated by
western blot analysis. The blots were visualized with the enhanced
chemiluminescence plus kit purchased from Engreen Biosystem
(Auckland, New Zealand) and the Bio-Rad ChemiDoc MP Gel imaging
analysis system (Bio-Rad Laboratories, Inc.), and protein levels
were analyzed with ImageJ software v1.8.0 (National Institutes of
Health, Bethesda, VA, USA).
Nuclear protein extracts were prepared using the
Nuclear and cytoplasmic protein Extraction kit, according to the
manufacturer's protocol. The protein concentration of the cell
extract was determined by BCA protein assay. A total of 25 µg
protein were electrophoretically separated and other steps were
identical to that aforementioned.
Cell Counting kit 8 (CCK-8) assay
Transfected cells were cultured in 96-well plate at
a density of 1×104 cells/well with various
concentrations of metformin (0, 1, 5 or 10 mM) for various
durations. Next, 10 µl CCK8 was added to each well, followed by
incubation for 4 h. The absorbance at the wavelength of 450 nm was
recorded for each well using a FlexStation 3 microplate reader
(Molecular Devices, Sunnyvale, CA, USA), and the cell viability was
then evaluated according to the manufacturer's protocol.
Apoptosis detection
For apoptosis detection, transfected cells were
seeded in a 24-well plate at 5×104 cells/ml. After
treatment with metformin at different concentrations for 24 or 48
h, the cells were collected by trypsin digestion and re-suspended
in binding buffer. Cells were incubated with 5 µl Annexin
V-fluorescein isothiocyanate (20 µg/ml) and 5 µl propidium iodide
(5 µg/ml) in 100 µl volume. Apoptosis was detected using a BD FACS
Aria II flow cytometer (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. All analyses were performed with SPSS software version
13.0 (SPSS Inc., Chicago, IL, USA). Student's t-test was used for
comparison of data between two groups. When multiple groups were
compared, one-way analysis of variance followed by the
least-significant differences post-hoc test was used for data with
a normal distribution, as assessed by the Shapiro-Wilk test. A
two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of metformin on gene expression
in cisplatin-resistant lung adenocarcinoma cells
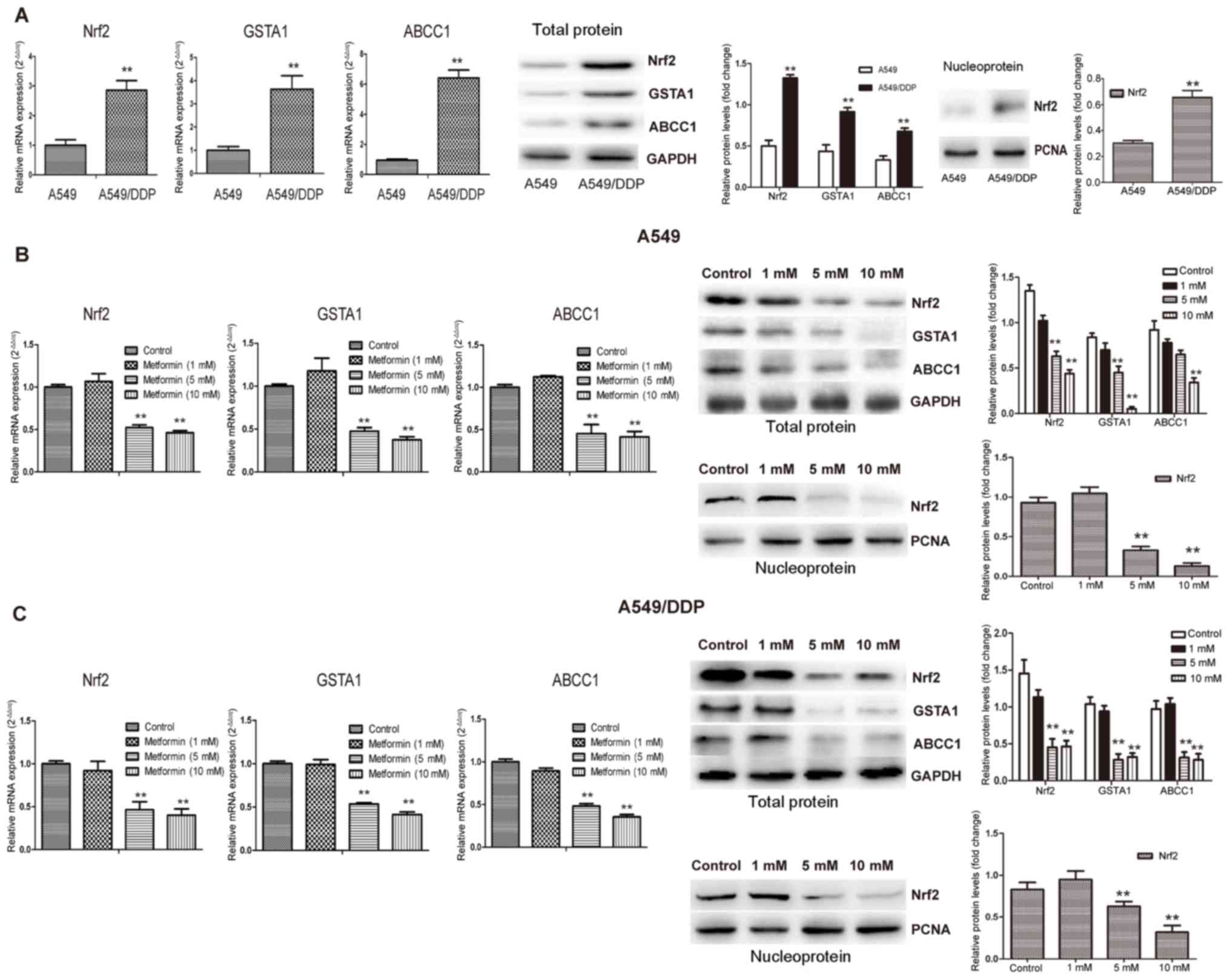
First, the mRNA and protein expression of GSTA1,
ABCC1 and Nrf2 was compared between A459 and A549/DDP cells
(Fig. 1). The mRNA and protein
expression of Nrf2, ABCC1 and GSTA1 in A549/DDP cells were
significantly higher than those in A549 cells (P<0.01) (Fig. 1A). Furthermore, the response to
different concentrations of metformin (1, 5 and 10 mM) was
examined. In A549 and A549/DDP cells, the mRNA and protein
expression of Nrf2, GSTA1 and ABCC1 were decreased by metformin in
a concentration-dependent manner, with a significant reduction in
mRNA expression achieved with metformin concentrations of 5 and 10
mM (P<0.01) (Fig. 1B).
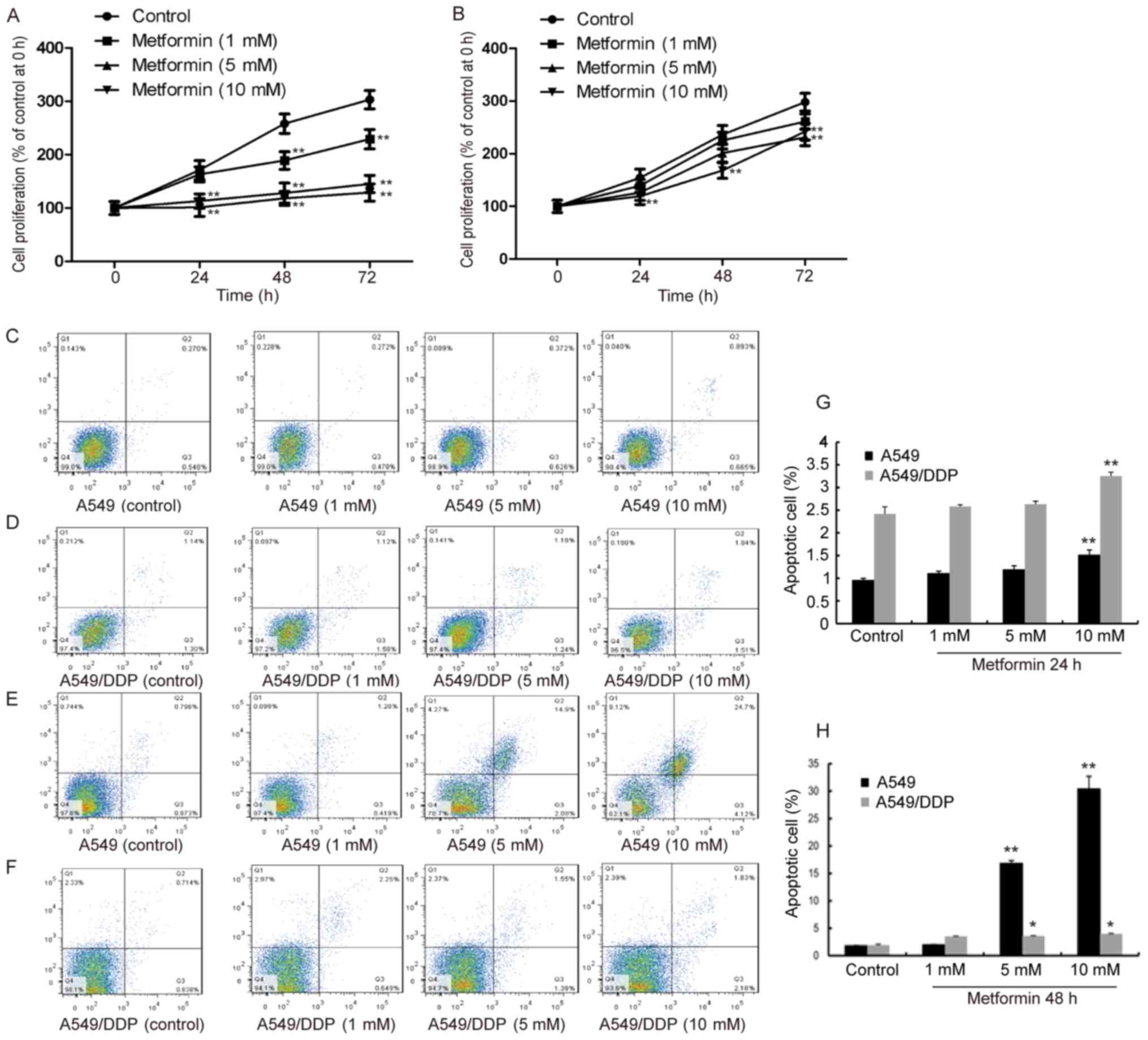
Effect of metformin on the
proliferation and apoptosis of cisplatin-resistant lung
adenocarcinoma cells
Cell proliferation and apoptotic rates were then
assessed using a CCK-8 assay and flow cytometry, respectively
(Fig. 2). A549 cells treated with 5
and 10 mM metformin exhibited decreased proliferation from 24–72 h,
and most prominently by 10 mM metformin (Fig. 2A), while A549/DDP cells only displayed
a significant decrease in proliferation ability in the presence of
10 mM metformin at 48 h (P<0.01) (Fig.
2B). The apoptotic rate of A549 treated with 5 and 10 mM
metformin was elevated at 24 h (Fig.
2C) and further increased at 48 h (P<0.01) (Fig. 2E). However, in A549/DDP cells, the
apoptotic rate was low at 24 h and had slightly risen at 48 h
(P<0.05) (Fig. 2D and F). These
results indicate that metformin induced the largest amount of
apoptosis in drug-resistant cells at 48 h. Thus, the subsequent
experiments were performed using an incubation time of 48 h.
Metformin inhibits cisplatin-resistant
lung adenocarcinoma cells via Nrf2
The Nrf2 shRNA sequence no. 2 produced a more
pronounced decrease of Nrf2 mRNA and protein expression than the
other two shRNAs (P<0.01) (Fig.
3A); thus, this shRNA was selected for the subsequent
experiments. After transfection of A549/DDP cells with Nrf2 shRNA,
the gene expression of GST and ABCC1 was decreased (Fig. 3B). It is likely that this
downregulation of GSTA1 and ABCC1 may in part influence biological
functions including cell proliferation and apoptosis. In A549/DDP
cells without transfection, the proliferation was only inhibited by
10 mM metformin; however, the sensitivity of the cells to metformin
was enhanced by transfection of Nrf2 shRNA, and a significant
reduction in proliferation was achieved by metformin for 48 h at
only 5 mM (Fig. 3C). Additionally, at
72 h, 10 mM metformin had a significant inhibition effect, compared
with the control (P<0.01). Furthermore, after transfection with
Nrf2 shRNA and culture with metformin for 48 h, the apoptotic rate
of A549/DDP cells increased from 4.24 to 27.47% (P<0.01)
(Fig. 3C). The apoptotic rate was
similar to that of A549 cells treated with metformin for 48 h,
which indicated that knockdown of Nrf2 sensitized A549/DDP cells to
metformin and abrogates their acquired drug resistance.
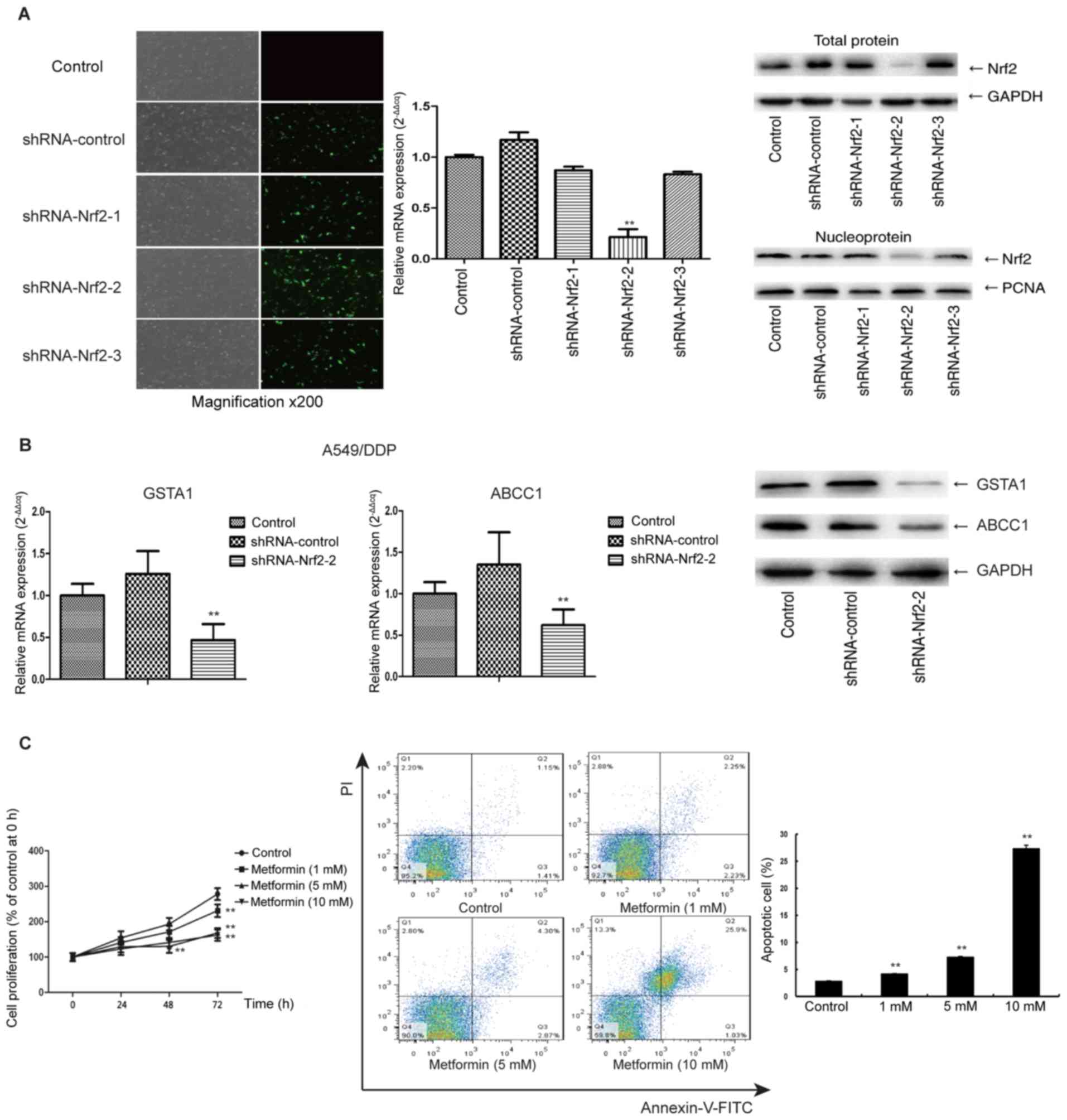 | Figure 3.(A) Nrf2 shRNA sequence 2 produced
more pronounced decrease of Nrf2 mRNA and protein expression than
the other ones (magnification, ×200). (B) After Nrf2 knockdown in
A549/DDP cells, the mRNA levels of GSTA1 and ABCC1 were decreased.
(C) After Nrf2 knockdown, A549/DDP cell proliferation was reduced
by metformin at a concentration of 5 mM only. (C) Nrf2 shRNA
increased the apoptotic rate of A549/DDP from 4.24 to 27.47%.
A549/DDP, cisplatin-resistant A549 lung adenocarcinoma cell line;
PCNA, proliferating cell nuclear antigen; shRNA, short hairpin RNA;
Q, quadrant; Nrf2, nuclear factor, erythroid 2 like 2; GSTA1,
glutathione S-transferase α 1; ABCC1, ATP-binding cassette
subfamily C member 1. **P<0.01, compared with control. |
The PI3K/Akt and ERK1/2 signaling
pathways are involved upstream of Nrf2 in the inhibitory effects of
metformin on A549 cells
A549/DDP cells are the cells pretreated by
cisplatin, which is a interference factor that may activate some
downstream signalling pathways to regulate Nrf2. In addition, the
results (Fig. 2) identified that the
sensitivity of A549/DDP cells to metformin is not as good as that
of A549 cells. Therefore the native A549 cell line was selected to
study the signaling pathway involved in the process. In A549 cells
cultured with 5 and 10 mM metformin, the phosphorylation of Akt and
ERK1/2 was decreased, while the phosphorylation of p38 MAPK and
phospho-p46 subunit of p-JNK was sharply increased (Fig. 4A) (39).
These results indicated that metformin inhibits the Akt and ERK1/2
pathways in A549 and activates the p38 MAPK and JNK pathways.
Furthermore, in the presence of metformin, inhibitors of the p38
MAPK and JNK signaling pathway at different concentrations did not
affect the levels of Nrf2 (Fig. 4B).
However, inhibitors of the Akt and ERK1/2 pathway reduced the
expression of Nrf2 (P<0.01, Fig.
4B). This suggests that metformin reduces the mRNA and protein
expression of Nrf2 by inhibiting the PI3K/Akt and ERK1/2 signaling
pathways to affect proliferation and apoptosis.
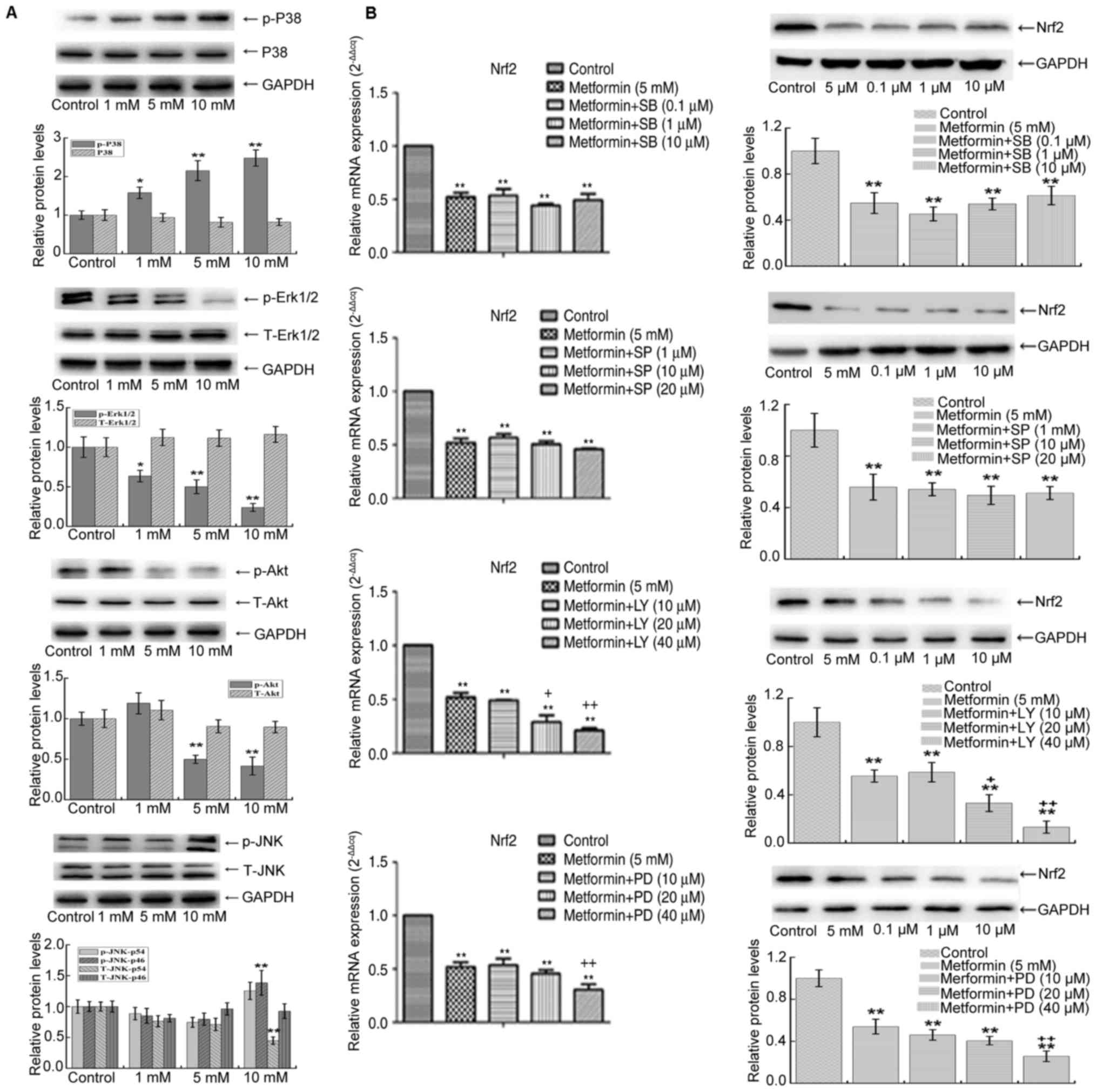 | Figure 4.(A) The phosphorylation of Akt and
ERK1/2 was decreased, while the phosphorylation of p38 MAPK and JNK
was increased after metformin treatment in A549 cells. (B)
Treatment with 5 mM metformin and different concentrations of
inhibitors of the p38 MAPK and JNK pathway were added, Nrf2 was not
affected in the presence of 5 mM metformin. However, when
inhibitors of the Akt and ERK1/2 pathway were added, the inhibitory
effect of metformin on Nrf2 was further enhanced. **P<0.01 vs.
control; +P<0.05 and ++P<0.01 vs. 5 mM
metformin group; +P<0.05 vs. 5 mM metformin group.
Nrf2, nuclear factor, erythroid 2 like 2; T-JNK, total c-Jun
N-terminal kinase; MAPK, mitogen-activated protein kinase; p-ERK,
phosphorylated extracellular signal-regulated kinase; LY,
phosphoinositide-3 kinase-specific inhibitor LY294002; PD,
ERK-specific inhibitor PD98059; SP, JNK-specific inhibitor
SP600125; SB, p38 kinase-specific inhibitor SB203580. |
Discussion
In the present study, statistically significant
differences in the expression of Nrf2, GSTA1 and ABCC1 genes were
detected between A549/DDP and A549 cells (P<0.01). These results
were consistent with those of previous studies (5,11). As a
possible mechanism, it has been proposed that Nrf2 functions in
tumor drug resistance. In a previous study, knockdown of Nrf2
expression by shRNA reversed the drug resistance of tumor cells to
tamoxifen (40). Nrf2 increases the
expression of Mrp2 by binding to the ARE locus of Mrp2 genes under
the stimulation of Nrf2 inducer (41). Previous studies have also indicated
that inhibition of Nrf2 is likely to decrease the expression of GSH
(42,43). Certain drugs promote Nrf2 nuclear
translocation and also stimulate GSH biosynthesis (44), which has an important role in
anti-cancer and anti-oxidant activities. Through efflux from the
cells, the intracellular concentration of anti-cancer drugs is
reduced and the combination of intracellular anticancer drugs and
target sites is prevented, leading to the drug resistance of tumor
cells (45,46). After reduction of the expression of
Mrps in human lung adenocarcinoma, the drug resistance to cisplatin
was reduced (47). GSH may promote
the repair of DNA (45). GSTA1
inhibitors have been reported to enhance the cytotoxicity of
cisplatin in drug-resistant cell lines (48). GSTA1 is a phase II conjugated enzyme
that detoxifies active electrophilic metabolites. The effects of
GSTA1 depend on the level of GSH, which in turn depends on
glutamate cysteine ligase and GSH synthase (49).
Metformin mainly inhibits mitochondrial respiratory
chain complex I (50). Inhibition of
AMPK expression by shRNA has been reported to reverse the
anti-tumor proliferation effect of metformin (51–53). In
the present study, metformin inhibited A549 cell proliferation and
promoted their apoptosis in a dose- and time-dependent manner
(P<0.01). However, in A549/DDP cells, only the highest metformin
concentration produced a significant effect after 48 h (P<0.05).
This observation was consistent with those of a previous study
(28). After stimulation with
metformin, differences in the mRNA and protein expression of GSTA1,
ABCC1 and Nrf2 were detected between A549 and A549/DDP (P<0.05),
particularly the Nrf2 levels of total protein and nuclear protein,
were reduced with increasing concentrations of metformin, which was
expected and indicated potential tumor inhibition mechanisms of
metformin. These results further confirmed the anti-tumor effect of
metformin.
The association between MAPKs and Nrf2 may be
associated with the presence of MAPK protein phosphorylation sites
in the trans-activation domain of Nrf2 (19). A large number of studies reported that
MAPKs have an effect on the activity of Nrf2 (17,54), but
there were discrepancies regarding the function of ERK, JNK and p38
in regulating the activity of Nrf2 between different tumor cell
types. The present study revealed that the levels of GSTA1 and
ABCC1 were decreased after knockdown of Nrf2. These results suggest
that GSTA1 and ABCC1 may be involved in the regulation of Nrf2 and
the biological consequences of Nrf2 perturbation, including
proliferation and apoptosis. The changes in growth, apoptosis and
gene expression in A549/DDP cells after knockdown of Nrf2 were
consistent with those reported in a previous study (16). Therefore, these results suggest that
metformin affects the expression of Nrf2 and its downstream genes
via the ERK, JNK and p38 MAPK pathways. Subsequent experiments
indicated that treatment with 5 and 10 mM metformin reduced the
phosphorylation levels of Akt and ERK1/2, while the phosphorylation
levels of p38 MAPK and JNK were increased compared with those in
the control group. This confirmed that metformin inhibited the Akt
and ERK1/2 pathway and activated the MAPKs p38 and JNK. The
PI3K/Akt pathway is involved in the regulation of the
proliferation, movement and metabolism of normal cells (55), and mutation and overexpression of
these genes frequently occur in cancer (56). The regulatory mechanisms of ERK/MAPK
and PI3K/Akt/mTOR pathways are complex, and proteins are currently
known to be associated with the PI3K pathway (57), while proteins are known to be
associated with MAPK signaling (58).
These pathways share interactions between multiple protein nodes,
and these interactions are affected by various factors, including
cell type, cell differentiation stage and receptor expression
levels (59–66). The roles of the PI3K/Akt/mTOR and
ERK/MAPK pathways in tumor cell growth, proliferation,
differentiation, metastasis and drug resistance are well
established (67).
The results of the present study indicated that
pharmacological inhibitors of the p38 MAPK and JNK signaling
pathways at different concentrations had no impact on Nrf2.
However, in response to treatment with Akt and ERK1/2 pathway
inhibitors, the level of Nrf2 was reduced. These results suggest
that metformin inhibits PI3K/Akt and ERK1/2 to further reduce the
expression of Nrf2 gene and protein, thus affecting cell
proliferation and apoptosis. These results are consistent with
those of previous studies (34,35).
Epigenetic regulation of kelch-like ECH associated protein 1
(Keap1) also affects the response of Nrf2 gene expression to
cisplatin (68,69), and Nrf2 then increases the expression
of multidrug resistance genes (70,71).
Whether Nrf2 is affected by Keap1 in the experiments of the present
study will be further explored in a subsequent study.
In conclusion, the present study suggest that
metformin reduces the expression of Nrf2 and its downstream
resistance genes GSTA1 and ABCC1 by inhibiting PI3K/Akt and ERK1/2
signaling. Knockdown of Nrf2 abrogated the acquired drug resistance
of A549/DDP cells and sensitized them to metformin to a similar
level to that of native A549 cells These results may provide a
theoretical basis and therapeutic targets for the clinical
treatment of tumors. The signaling pathways of MAPK/AKT/ERK/JNK may
be downstream effects of the primary effects of metformin, and the
upstream mechanisms should be assessed in a future study.
Acknowledgements
No applicable.
Funding
The present study was supported by the Regional
Science Foundation Project of the National Natural Science
Foundation of China (grant no. 81560093) and the Health Industry
Research Plan Project of Gansu Province (grant no.
GSWSKY-2015-02).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JZ conducted the experiment and wrote the
manuscript. KJ collected the data. JL conceived the paper and
revised the manuscript. YX collected and analyzed the data.
Furthermore, the final version of the manuscript has been read and
approved by all authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sporn MB and Liby KT: Nrf2 and cancer: The
good, the bad and the importance of context. Nat Rev Cancer.
12:564–571. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao Q, Mao A, Yan J, Sun C, Di C, Zhou X,
Li H, Guo R and Zhang H: Downregulation of Nrf2 promotes
radiation-induced apoptosis through Nrf2 mediated Notch signaling
in non-small cell lung cancer cells. Int J Oncol. 48:765–773. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z1, Ye X, Tang N, Shen S, Li Z, Niu
X, Lu S and Xu L: The histone acetylranseferase hMOF acetylates
Nrf2 and regulates anti-drug responses in human non-small cell lung
cancer. Br J Pharmacol. 171:3196–3211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Biswas M and Chan JY: Role of Nrf1 in
antioxidant response element-mediated gene expression and beyond.
Toxicol Appl Pharmacol. 244:16–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li SS, Chen ZY, Li J, Xu Z and Yang X:
Research progress of Keap1/Nrf2/ARE signaling pathway in central
nervous system diseases. Chinese Gen Pract. 3641–3644. 2014.
|
|
6
|
Namani A, Li Y, Wang XJ and Tang X:
Modulation of NRF2 signaling pathway by nuclear receptors:
Implications for cancer. Biochim Biophys Acta. 1843:1875–1885.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arnold P, Mojumder D, Detoledo J, Lucius R
and Wilms H: Pathophys-iological processes in multiplesclerosis:
Focus on nu-clear factor erythroid-2-related factor 2 and emerging
pathways. Clin Pharmacol. 6:35–42. 2014.PubMed/NCBI
|
|
8
|
Mitsuishi Y, Taguchi K, Kawatani Y,
Shibata T, Nukiwa T, Aburatani H, Yamamoto M and Motohashi H: Nrf2
redirects glucose and glutamine into anabolic pathways in metabolic
reprogramming. Cancer Cell. 22:66–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar H, Kim IS, More SV, Kim BW and Choi
DK: Natural product-derived pharmacological modulators of Nrf2/ARE
pathway for chronic diseases. Nat Prod Rep. 31:109–139. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Homma S, Ishii Y, Morishima Y, Yamadori T,
Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N,
et al: Nrf2 enhances cell proliferation and resistance to
anticancer drugs in human lung cancer. Clin Cancer Res.
15:3423–3432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Singh A, Yegnasubramanian S,
Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG and Biswal S: Loss of
Kelch-like ECH-associated protein 1 function in prostate cancer
cells causes chemoresistance and radioresistance and promotes tumor
growth. Mol Cancer Ther. 9:336–346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang X, Wang D, Ma Y, Xu X, Zhu Z, Wang X,
Deng H, Li C, Chen M, Tong J, et al: Continuous activation of Nrf2
and its target antioxidant enzymes leads to arsenite-induced
malignant transformation of human bronchial epithelial cells.
Toxicol Appl Pharmacol. 289:231–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lubelska K, Milczarek M, Modzelewska K,
Krzysztoń-Russjan J, Fronczyk K and Wiktorska K: Interactions
between drugs and sulforaphane modulate the drug metabolism
enzymatic system. Pharmacol Rep. 64:1243–1252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji L, Li H, Gao P, Shang G, Zhang DD,
Zhang N and Jiang T: Nrf2 pathway regulates
multidrug-resistance-associated protein 1 in small cell lung
cancer. PLoS One. 8:e634042013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Son YO, Pratheeshkumar P, Roy RV, Hitron
JA, Wang L, Zhang Z and Shi X: Nrf2/p62 signaling in apoptosis
resistance and its Role in cadmium-induced carcinogenesis. J Biol
Chem. 289:28660–28675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma X, Zhang J, Liu S, Huang Y, Chen B and
Wang D: Nrf2 knockdown by shRNA inhibits tumor growth and increases
efficacy of chemotherapy in cervical cancer. Cancer Chemother
Pharmacol. 69:485–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee D, Bae J, Kim YK, Gil M, Lee JY, Park
CS and Lee KJ: Inhibitory effects of berberine on
lipopolysaccharide-induced inducible nitric oxide synthase and the
high-mobility group box 1 release in macrophages. Biochem Biophys
Res Commun. 431:506–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cong ZX, Wang HD, Wang JW, Zhou Y, Pan H,
Zhang DD and Zhu L: ERK and PI3K signaling cascades induce Nrf2
activation and regulate cell viability partly through Nrf2 in human
glioblastoma cells. Oncol Rep. 30:715–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Z, Huang Z and Zhang DD:
Phosphorylation of Nrf2 at multiple sites by MAP kinases has a
limited contribution in modulating the Nrf2-dependent antioxidant
response. PLoS One. 4:e65882009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levy S, Jaiswal AK and Forman HJ: The role
of c-Jun phosphorylation in EpRE activation of phase II genes. Free
Radic Biol Med. 47:1172–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abazeed ME, Adams DJ, Hurov KE, Tamayo P,
Creighton CJ, Sonkin D, Giacomelli AO, Du C, Fries DF, Wong KK, et
al: Integrative radiogenomic profiling of squamous cell lung
cancer. Cancer Res. 73:6289–6298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yip PY: Phosphatidylinositol
3-kinase-AKT-mammalian target of rapamycin (PI3K-Akt-mTOR)
signaling pathway in non-small cell lung cancer. Transl Lung Cancer
Res. 4:165–176. 2015.PubMed/NCBI
|
|
23
|
Romero IL, McCormick A, McEwen KA, Park S,
Karrison T, Yamada SD, Pannain S and Lengyel E: Relationship of
type II diabetes and metformin use to ovarian cancer progression,
survival, and chemosensitivity. Obstet Gynecol. 119:61–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh
DP and Chen CC: Antidiabetes drugs correlate with decreased risk of
lung cancer: A population-based observation in Taiwan. Clin Lung
Cancer. 13:143–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi M, Kato K, Iwama H, Fujihara S,
Nishiyama N, Mimura S, Toyota Y, Nomura T, Nomura K, Tani J, et al:
Antitumor effect of metformin in esophageal cancer: In vitro study.
Int J Oncol. 42:517–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kato K, Gong J, Iwama H, Kitanaka A, Tani
J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, et al:
The antidiabetic drug metformin inhibits gastric cancer cell
proliferation in vitro and in vivo. Mol Cancer Ther. 11:549–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Colquhoun AJ, Venier NA, Vandersluis AD,
Besla R, Sugar LM, Kiss A, Fleshner NE, Pollak M, Klotz LH and
Venkateswaran V: Metformin enhances the antiproliferative and
apoptotic effect of bicalutamide in prostate cancer. Prostate
Cancer Prostatic Dis. 15:346–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ashinuma H, Takiguchi Y, Kitazono S,
Kitazono-Saitoh M, Kitamura A, Chiba T, Tada Y, Kurosu K, Sakaida
E, Sekine I, et al: Antiproliferative action of metformin in human
lung cancer cell lines. Oncol Rep. 28:8–14. 2012.PubMed/NCBI
|
|
29
|
Koeck S, Amann A, Huber JM, Gamerith G,
Hilbe W and Zwierzina H: The impact of metformin and salinomycin on
transforming growth factor β-induced epithelial-to-mesenchymal
transition in non-small cell lung cancer cell lines. Oncol Lett.
11:2946–2952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yue W, Yang CS, DiPaola RS and Tan XL:
Repurposing of metformin and aspirin by targeting AMPK-mTOR and
inflammation for pancreatic cancer prevention and treatment. Cancer
Prev Res (Phila). 7:388–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferla R, Haspinger E and Surmacz E:
Metformin inhibits leptin-induced growth and migration of
glioblastoma cells. Oncol Lett. 4:1077–1081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu B, Fan Z, Edgerton SM, Deng XS,
Alimova IN, Lind SE and Thor AD: Metformin induces unique
biological and molecular responses in triple negative breast cancer
cells. Cell Cycle. 8:2031–2040. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vazquez-Martin A, Oliveras-Ferraros C and
Menendez JA: The antidiabetic drug metformin suppresses HER2
(erbB-2) oncoprotein overexpression via inhibition of the mTOR
effector p70S6K1 in human breast carcinoma cells. Cell Cycle.
8:88–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hanna RK, Zhou C, Malloy KM, Sun L, Zhong
Y, Gehrig PA and Bae-Jump VL: Metformin potentiates the effects of
paclitaxel in endometrial cancer cells through inhibition of cell
proliferation and modulation of the mTOR pathway. Gynecol Oncol.
125:458–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rocha GZ, Dias MM, Ropelle ER,
Osório-Costa F, Rossato FA, Vercesi AE, Saad MJ and Carvalheira JB:
Metformin amplifies chemotherapy-induced AMPK activation and
antitumoral growth. Clin Cancer Res. 17:3993–4005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gotlieb WH, Saumet J, Beauchamp MC, Gu J,
Lau S, Pollak MN and Bruchim I: In vitro metformin anti-neoplastic
activity in epithelial ovarian cancer. Gynecol Oncol. 110:246–250.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu C, Jiao Y, Xue J, Zhang Q, Yang H, Xing
L, Chen G, Wu J, Zhang S, Zhu W and Cao J: Metformin sensitizes
non-small cell lung cancer cells to an epigallocatechin-3-gallate
(EGCG) treatment by suppressing the Nrf2/HO-1 signaling pathway.
Int J Biol Sci. 13:1560–1569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rodenak-Kladniew B, Castro A, Stärkel P,
De Saeger C, de Bravo García M and Crespo R: Linalool induces cell
cycle arrest and apoptosis in HepG2 cells through oxidative stress
generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life
Sci. 199:48–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SK, Yang JW, Kim MR, Roh SH, Kim HG,
Lee KY, Jeong HG and Kang KW: Increased expression of
Nrf2/ARE-dependent anti-oxidant proteins in tamoxifen-resistant
breast cancer cells. Free Radic Biol Med. 45:537–546. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vollrath V, Wielandt AM, Iruretagoyena M
and Chianale J: Role of NrO in the regulation of the Mrp2 (ABCC2)
gene. Biochem J. 395:599–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang X, Wang H, Fan L, Wu X, Xin A, Ren H
and Wang XJ: Luteolin inhibits Nrf2 leading to negative regulation
of the Nrf2/ARE pathway and sensitization of human lung carcinoma
A549 cells to therapeutic drugs. Free Radic Biol Med. 50:1599–1609.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hwang YP, Choi JH, Choi JM, Chung YC and
Jeong HG: Protective mechanisms of anthocyanins from purple sweet
potato against tert-butyl hydroperoxide-induced hepatotoxicity.
Food Chem Toxicol. 49:2081–2089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Soeur J, Eilstein J, Léreaux G, Jones C
and Marrot L: Skin resistance to oxidative stress induced by
resveratrol: From Nrf2 activation to GSH biosynthesis. Free Radic
Biol Med. 78:213–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Meyer Zu, Schwabedissen HE, Grube M,
Heydrich B, Linnemann K, Fusch C, Kroemer HK and Jedlitschky G:
Expression, localization, and function of MRP5 (ABCC5), a
transporter for cyclic nucleotides, in human placenta and cultured
human trophoblasts: Effects of gestational age and cellular
differentiation. Am J Pathol. 166:39–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu BC, Li J, Yu WF, Zhang GZ, Wang HM and
Ma HM: Elevated expression of Nrf2 mediates multidrug resistance in
CD133+ head and neck squamous cell carcinoma stem cells.
Oncol Lett. 12:4333–4338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang J, Liu X and Jiang W: Co-transfection
of MRP and bcl-2 antisense S-oligodeoxynucleotides reduces drug
resistance in cisplatin-resistant lung cancer cells. Chin Med J
(Engl). 113:957–960. 2000.PubMed/NCBI
|
|
48
|
Neubauer H, Stefanova M, Solomayer E,
Meisner C, Zwirner M, Wallwiener D and Fehm T: Predicting
resistance to platinum-containing chemotherapy with the ATP tumor
chemosensitivity assay in primary ovarian cancer. Anticancer Res.
28:949–955. 2008.PubMed/NCBI
|
|
49
|
Meijerman I, Beijnen JH and Schellens JH:
Combined action and regulation of phase II enzymes and multidrug
resistance proteins in multidrug resistance in cancer. Cancer Treat
Rev. 34:505–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Stein SC, Woods A, Jones NA, Davison MD
and Carling D: The regulation of AMP-activated protein kinase by
phosphorylation. Biochem J. 345:437–443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tsuji K, Kisu I, Banno K, Yanokura M, Ueki
A, Masuda K, Kobayashi Y, Yamagami W, Nomura H, Susumu N and Aoki
D: Metformin: A possible drug for treatment of endometrial cancer.
Open J Obstet Gynecol. 2:1–6. 2012. View Article : Google Scholar
|
|
52
|
Kato K, Ogura T, Kishimoto A, Minegishi Y,
Nakajima N, Miyazaki M and Esumi H: Critical roles of AMP-activated
protein kinase in constitutive tolerance of cancer cells to
nutrient deprivation and tumor formation. Oncogene. 21:6082–6090.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rattan R, Graham RP, Maguire JL, Giri S
and Shridhar V: Metformin suppresses ovarian cancer growth and
metastasis with enhancement of cisplatin cytotoxicity in vivo.
Neoplasia. 13:483–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Krall EB, Wang B, Munoz DM, Ilic N,
Raghavan S, Niederst MJ, Yu K, Ruddy DA, Aguirre AJ, Kim JW, et al:
KEAP1 loss modulates sensitivity to kinase targeted therapy in lung
cancer. Elife. 6:pii: e18970. 2017. View Article : Google Scholar
|
|
55
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Forbes SA, Bindal N, Bamford S, Cole C,
Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al:
COSMIC: Mining complete cancer genomes in the catalogue of somatic
mutations in cancer. Nucleic Acids Res. 39:(Database Issue).
D945–D950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Samatar AA and Poulikakos PI: Targeting
RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug
Discov. 13:928–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pilot-Storck F, Chopin E, Rual JF, Baudot
A, Dobrokhotov P, Robinson-Rechavi M, Brun C, Cusick ME, Hill DE,
Schaeffer L, et al: Interactome mapping of the phosphatidylinositol
3-kinase-mammalian target of rapamycin pathway identifies deformed
epidermal autoregulatory factor-1 as a new glycogen synthase
kinase-3 interactor. Mol Cell Proteomics. 9:1578–1593. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Aksamitiene E, Kiyatkin A and Kholodenko
BN: Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt
pathways: A fine balance. Biochem Soc Trans. 40:139–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fruman DA and Rommel C: P13K and cancer:
Lessons, challenges and opportunities. Nat Rev Drug Discov.
13:140–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Steelman LS, Chappell WH, Abrams SL, Kempf
RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F,
Mazzarino MC, et al: Roles of the Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity
to therapy-implications for cancer and aging. Aging (Albany NY).
3:192–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Will M, Qin AC, Toy W, Yao Z,
Rodrik-Outmezguine V, Schneider C, Huang X, Monian P, Jiang X, de
Stanchina E, et al: Rapid induction of apoptosis by PI3K inhibitors
is dependent upon their transient inhibition of RAS-ERK signaling.
Cancer Discov. 4:334–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chandarlapaty S, Sawai A, Scaltriti M,
Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK,
Baselga J and Rosen N: AKT inhibition relieves feedback suppression
of receptor tyrosine kinase expression and activity. Cancer Cell.
19:58–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S,
Malaponte G, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade
inhibitors: How mutations can result in therapy resistance and how
to overcome resistance. Oncotarget. 3:1068–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fritsch R, de Krijger I, Fritsch K, George
R, Reason B, Kumar MS, Diefenbacher M, Stamp G and Downward J: RAS
and RHO families of GTPases directly regulate distinct
phosphoinositide 3-kinase isoforms. Cell. 153:1050–1063. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang HK, Xu TJ, Ju YH and Yu AM: PI3K/AKT
and MAPK/ERK pathways induce cell cycle arrest and apoptosis in
A549 cell through the regulation of FOXO1 transcription factor. J
China Med Univ. 908–911. 2012.
|
|
68
|
Tian Y, Wu K, Liu Q, Han N, Zhang L, Chu Q
and Chen Y: Modification of platinum sensitivity by KEAP1/NRF2
signals in non-small cell lung cancer. J Hematol Oncol. 9:832016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tian Y, Liu Q, He X, Yuan X, Chen Y, Chu Q
and Wu K: Emerging roles of Nrf2 signal in non-small cell lung
cancer. J Hematol Oncol. 9:142016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Singer E, Judkins J, Salomonis N, Matlaf
L, Soteropoulos P, McAllister S and Soroceanu L: Reactive oxygen
species-mediated therapeutic response and resistance in
glioblastoma. Cell Death Dis. 6:e16012015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Del Vecchio CA, Feng Y, Sokol ES, Tillman
EJ, Sanduja S, Reinhardt F and Gupta PB: De-differentiation confers
multidrug resistance via noncanonical PERK-Nrf2 signaling. PLoS
Biol. 12:e10019452014. View Article : Google Scholar : PubMed/NCBI
|