Introduction
Dermatomyositis (DM) is a rare idiopathic
inflammatory myopathy (IIM) (1). In
1916, Stertz (2) reported for the
first time that IIMs are associated with malignancy. The overall
malignancy risk is high in patients with IIM, particularly DM,
compared with age- and sex-matched controls (3,4).
Malignancy is usually diagnosed within 1 year after the diagnosis
of DM and is a major cause of mortality among patients with DM
(5). This implies that cancer may be
dormant at the time of DM diagnosis, therefore, extensive
evaluation of cancer-associated symptoms is recommended for
patients with DM (6). However,
numerous patients do not undergo a thorough cancer screening owing
to financial constraints and the risk of iatrogenic impairment (for
example, exposure to large doses of X-ray radiation) (1). Furthermore, the optimal frequency and
intensity of cancer screening in DM patients remain undefined
(4).
The majority of early epidemiological studies that
describe the clinical characteristics of IIMs and their association
with cancer were conducted in Western countries (7–9). These
studies suggest that older age, male sex, elevated
erythrocyte-sedimentation rate (ESR), dysphagia and cutaneous
necrosis, among others, are risk factors for malignancy in patients
with DM. The type of malignancy also varies among patients, and
adenocarcinoma of the ovary, lung, or gastrointestinal tract,
melanoma and non-Hodgkins lymphoma are the most common types of
malignancy among patients with DM in Western countries (10,11). In
contrast, nasopharyngeal cancer is the predominant cancer
associated with DM in Asian regions, including Hong Kong, Taiwan,
southern China, and southeast Asia, as well as in north Africa;
other types of cancer that are common in these regions are cancers
of the lung, breast, stomach, ovary, liver and lymph nodes
(5,12,13). Thus,
the distribution of malignancy varies with geographic region and
ethnicity. Recently, myositis-specific autoantibodies (MSAs) have
been investigated as potential predictors of malignancy in patients
with DM. DM patients who test positive for anti-translation
initiation factor (TIF)1γ and negative for anti-melanoma
differentiation-associated (MDA)5 have been demonstrated to have an
increased risk of malignancy (14).
However, few large case studies have been conducted on this topic
because DM is a rare disease. Furthermore, early studies have
achieved conflicting results (4), and
data from China are relatively limited. Therefore, in the present
study, long-term follow-up clinical data of 239 patients with DM
from a single institution in northern China were analyzed to
identify predictors of malignancy in DM.
Materials and methods
Patients
Data was collected from 239 patients with DM who had
been admitted to Yuhuangding Hospital affiliated to Qingdao
University (Yantai, China) between 1997 and 2016. DM was diagnosed
according to the Bohan and Peter criteria (15). Electromyography was used for 105
patients, and muscle biopsy had been performed in 163 patients. The
patients were categorized into two groups according to the presence
or absence of malignancy. As the criteria for DM in young and adult
patients are different, we excluded patients with juvenile DM
(<17 years old). The study was approved by the ethics committee
of Yantai Yuhuangding Hospital, Qingdao University (Yantai, China;
approval number: 2016-176).
Data collection
All data, including demographic, clinical and
laboratory data, reported in this retrospective inception cohort
study were obtained from hospital records. The following parameters
were assessed: age at onset, sex, clinical features [hypertension,
diabetes, smoking, interstitial lung disease (ILD), myalgia,
proximal muscle weakness, dysphagia, dyslalia, skin changes,
periungual erythematosus, nail cuticle hypertrophy, mechanic's
hand, Raynaud phenomenon, arthralgia and lymphadenectasis],
laboratory data at the time of DM diagnosis [lactate dehydrogenase
(LDH), creatinine phosphokinase (CPK), creatine kinase-MB (CK-MB),
aspartate aminotransferase (AST), ESR, C-reactive protein (CRP),
immunoglobulin G (IgG), anti-Jo1 antibody, ferroprotein, cancer
antigen (CA)153, CA125, CA199, carcinoembryonic antigen (CEA), and
neuron-specific enolase (NSE)], mortality rate, cause of mortality,
timing of tumor diagnosis, tumor type and pathological
classification. ILD was diagnosed using both chest radiography and
high-resolution computed tomography of the lung, and manifested as
a ground-glass opacity, a reticular shadow, an irregular linear
opacity, traction bronchiectasis, a cyst or a subpleural
curvilinear shadow (16). All
patients were followed up until mortality, loss to follow-up or 1
October 2017.
Statistical analysis
Between-group comparisons of normally distributed
measurement data were conducted using Student's t-test or the
Mann-Whitney U-test. The χ2 test was used to analyze
differences baseline characteristics data. To identify independent
risk factors, the odds ratio (OR) and 95% confidence intervals (CI)
were analyzed using multivariate Cox proportional hazards
regression analysis. Variables with P<0.05 in univariate
analysis were analyzed by multivariate analysis. Patient survival
was analyzed using the Kaplan-Meier curve and the log-rank test.
All statistical analyses were carried out using SPSS version 19.0
(IBM Corp., Armonk, NY, USA). The results are reported as the
median [interquartile range (IQR)]. P<0.05 was considered to
indicate a statistically significant difference.
Results
Demographic data
Of the 239 patients with DM, 161 (67.36%) were women
and 78 (32.64%) were men. The median age of the patients at the
time of DM onset was 58 years (IQR, 53.00–67.75 years). There were
42 smokers (17.57%), 55 patients with hypertension (23.01%), 26
with diabetes mellitus (10.88%), and 115 with ILD (48.12%). A total
of 48 fatalities occurred during the study period. The median
follow-up duration was 38.00 months (16.50–75.00 months). The
patients were divided into two groups: 43 patients with
malignancies (17.99%), and 196 patients without malignancies
(82.01%). The patients with malignancies were significantly older
than those without malignancies [59.00 years (53.50–73.00) vs.
57.50 (48.00–62.50 years); P=0.003)]. The demographic
characteristics of the two groups are presented in Table I.
 | Table I.Baseline demographic characteristics
of patients with dermatomyositis. |
Table I.
Baseline demographic characteristics
of patients with dermatomyositis.
| Variables | Overall | Malignancy | No malignancy | P-value |
|---|
| Cases, n (%) | 239 (100.00) | 43 (17.99) | 196 (82.01) | – |
| Age (years) | 58
(53.00–67.75) | 59.00
(53.50–73.00) | 57.50
(48.00–62.50) | 0.003a |
| Women, n (%) | 161 (67.36) | 25 (58.14) | 136 (69.39) | 0.109 |
| Smoking, n (%) | 42 (17.57) | 12 (27.91) | 30 (15.31) | 0.059 |
| Hypertension, n
(%) | 55 (23.01) | 13 (30.23) | 42 (21.43) | 0.247 |
| Diabetes, n
(%) | 26 (10.88) | 10 (23.26) | 16 (8.16) | 0.005a |
| ILD, n (%) | 115 (48.12) | 8 (18.60) | 107 (54.59) | 0.000a |
| Mortality, n
(%) | 48 (20.08) | 18 (41.86) | 30 (15.31) | 0.003a |
Clinical data of patients with
malignancies
Of the 43 DM patients with malignancies, 18 were
men, and 25 were women. A total of 15 of these suffered fatality.
Lung cancer was the most common type of malignancy, present in 6/43
(13.59%) patients. Other malignances included breast cancer (n=5,
11.63%), gastric cancer (n=5, 11.63%), colorectal cancer (n=5,
11.63%), ovarian cancer (n=4, 9.30%), nasopharyngeal cancer (n=4,
9.30%), thyroid cancer (n=3, 6.98%), cervical cancer (n=2, 4.65%),
and non-Hodgkins lymphoma, hepatocellular carcinoma, leukemia,
spinal tumor, laryngocarcinoma, melanoma, esophageal cancer,
fallopian tube carcinoma and esophageal cancer (n=1 for each type,
2.33%). The most common type of malignancy among women was breast
cancer (n=5, 20.00%), followed by ovarian cancer (n=4, 16.00%) and
lung cancer (n=4, 16.00%). Nasopharyngeal cancer, gastric cancer
and colorectal cancer were the most common types of cancer among
men (n=3 for each type, 15.79%), followed by lung cancer (n=2,
10.52%). The incidence of cancers detected before or after DM
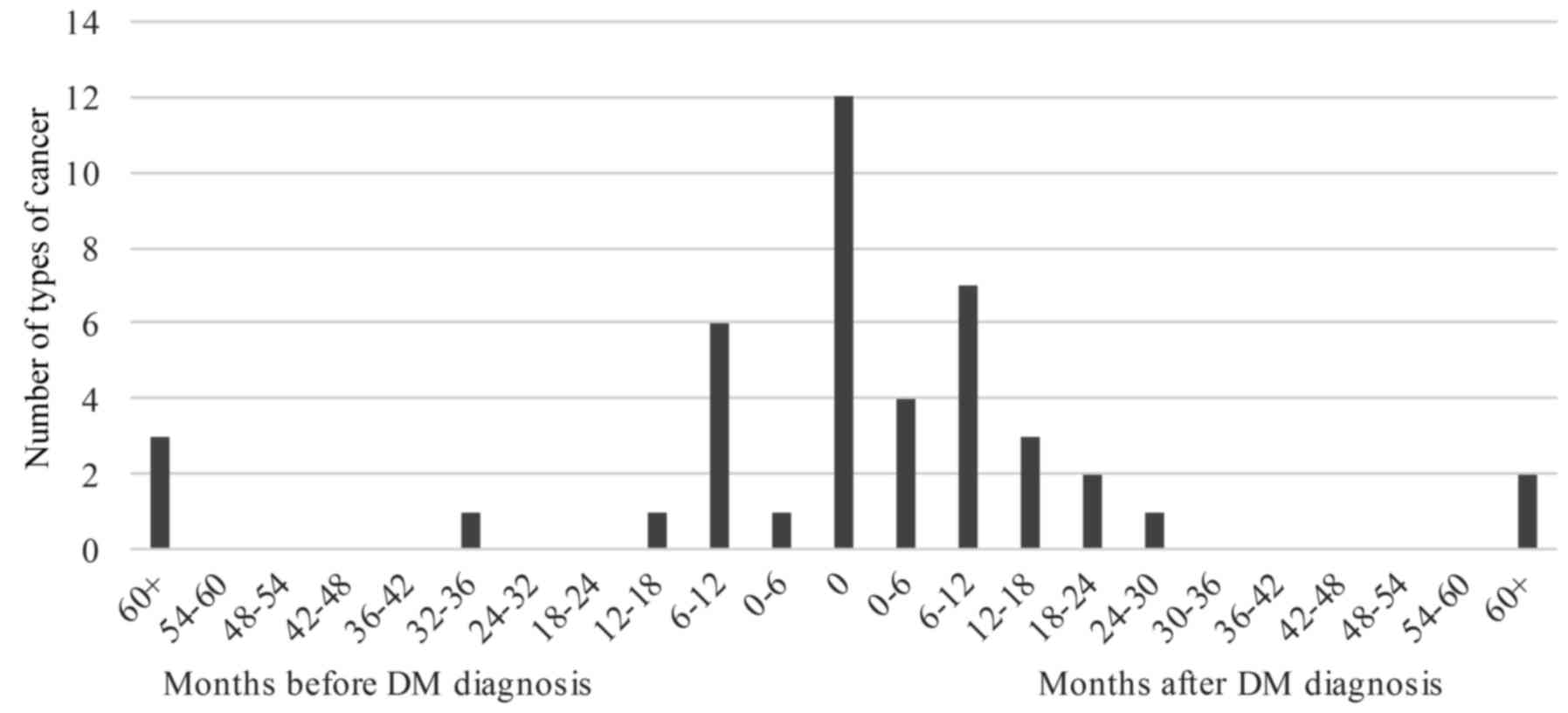
diagnosis is illustrated in Fig. 1.
Overall, 30 cases of malignancy (69.77%) were detected within 1
year before or after DM diagnosis. Malignancy was detected at the
time of the DM diagnosis in 12 (27.91%) patients, after DM
diagnosis in 19 (44.19%) patients, and before DM diagnosis in 12
(27.91%) patients.
Factors associated with
malignancy
Univariate analysis of risk factors in patients with
DM with and without malignancy are presented in Tables I and II. The results demonstrated that the age of
onset was significantly higher in patients with malignancy than in
those without malignancy [59.00 years (53.50–73.00 years) vs. 57.50
years 48.00–62.50 years); P=0.003)]. Furthermore, diabetes mellitus
(23.26% vs. 8.16%; P=0.005) and the Gottron sign (69.77% vs.
63.78%; P=0.049) were significantly more common in the malignancy
group than in the non-malignancy group. In contrast, ILD (18.6% vs.
54.59%; P<0.001), arthralgia (18.60% vs. 42.86%; P=0.002), and
anti-Jo-1 antibody (0.00% vs. 9.18%; P=0.041) were significantly
less common in the malignancy group than in the non-malignancy
group. In addition, the levels of CA125 [14.65 U/ml (11.18–32.42
U/ml) vs. 12.95 U/ml (8.83–20.09 U/ml); P=0.003)] and NSE [17.07
ng/ml (14.18–38.52 ng/ml) vs. 18.53 ng/ml (15.00–29.14 ng/ml);
P=0.021)] were significantly higher in the malignancy group than in
the non-malignancy group. The above factors were analyzed by Cox
proportional hazards regression analysis to identify independent
factors associated with malignancy. The multivariate analysis
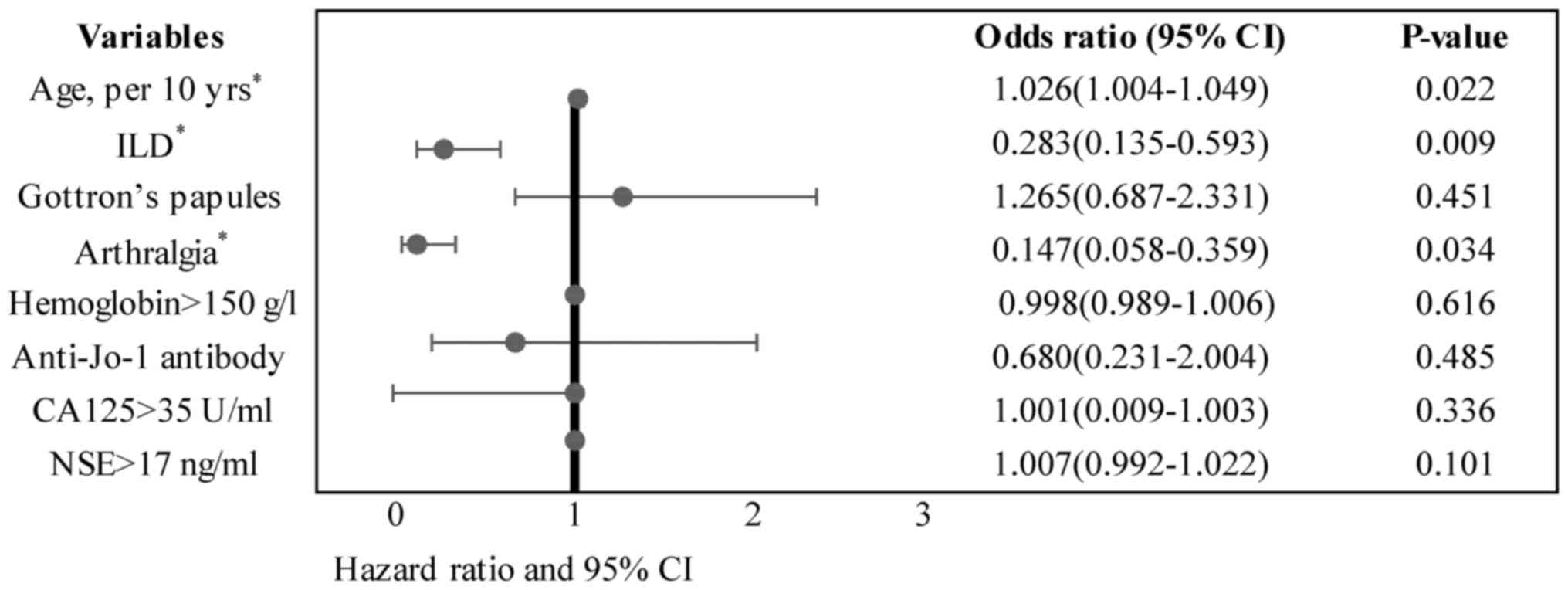
revealed that older age at onset (P=0.022), absence of ILD
(P=0.009), and absence of arthralgia (P=0.034) were independently
associated with malignancy in patients with DM (Fig. 2 and Table
III).
 | Table II.Univariate analysis of factors
potentially associated with malignancy in patients with
dermatomyositis. |
Table II.
Univariate analysis of factors
potentially associated with malignancy in patients with
dermatomyositis.
| Variables | Malignancy
(n=43) | No malignancy
(n=196) | P-value |
|---|
| Muscle pain, n
(%) | 26 (60.47) | 114 (58.16) | 0.91 |
| Proximal muscle
weakness, n (%) | 23 (53.49) | 129 (65.82) | 0.141 |
| Dysphagia, n
(%) | 16 (37.21) | 46 (23.47) | 0.077 |
| Dysphonia, n
(%) | 7 (16.28) | 28 (14.29) | 0.783 |
| Heliotrope rash, n
(%) | 21 (48.84) | 109 (55.61) | 0.343 |
| Shawl rash, n
(%) | 19 (44.19) | 60 (30.61) | 0.109 |
| V-shaped rash, n
(%) | 21 (48.84) | 65 (33.16) | 0.069 |
| Gottron papules, n
(%) | 30 (69.77) | 125 (63.78) | 0.049a |
| Pruritus, n
(%) | 25 (58.14) | 83 (42.35) | 0.081 |
| Poikiloderma, n
(%) | 9 (20.93) | 32 (16.33) | 0.511 |
| Periungual
erythema, n (%) | 6 (13.95) | 30 (15.31) | 0.779 |
| Nail cuticle
hypertrophy, n (%) | 2 (4.65) | 9 (4.59) | 0.989 |
| Mechanic's hand, n
(%) | 12 (27.91) | 67 (34.18) | 0.378 |
| Raynaud phenomenon,
n (%) | 1 (2.33) | 15 (7.65) | 0.196 |
| Arthralgia, n
(%) | 8 (18.60) | 84 (42.86) | 0.002a |
| Lymphadenectasis, n
(%) | 5 (11.63) | 26 (13.27) | 0.734 |
| CK-MB, ng/ml
(0–4.94) | 13.57
(3.95–47.86) | 6.77
(2.21–32.00) | 0.612 |
| AST, IU/l
(15–40) | 61.00
(31.5–144.5) | 58.00
(32.25–118.00) | 0.945 |
| CPK, IU/l
(40–200) | 444.00
(99.00–2810.00) | 209.00
(74.75–1401.75) | 0.084 |
| LDH, IU/l
(120–250) | 437.90
(334.00–680.00) | 368.50
(262.50–514.50) | 0.76 |
| ESR, mm/h
(0–15) | 22.00
(12.75–28.50) | 27.00
(16.00–46.00) | 0.06 |
| CRP, mg/l
(0–5.0) | 6.05
(3.45–13.50) | 6.55
(3.45–18.05) | 0.955 |
| IgG, g/l
(7.0–16.0) | 10.90
(9.09–14.80) | 13.50
(10.38–16.83) | 0.075 |
| Anti-Jo-1 antibody,
n (%) | 0 (0.00) | 18 (9.18) | 0.041a |
| CEA, ng/ml
(0–5.0) | 1.53
(1.11–2.78) | 2.04
(1.09–3.75) | 0.936 |
| CA125, U/ml
(0–35.0) | 14.65
(11.18–32.42) | 12.95
(8.83–20.09) | 0.003a |
| CA153, U/ml
(0–25.0) | 11.37
(8.78–16.79) | 15.54
(11.51–22.65) | 0.423 |
| CA199, U/ml
(0–39.0) | 9.57
(5.71–18.70) | 9.57
(5.22–18.93) | 0.209 |
| NSE, ng/ml
(0–17.0) | 17.07
(14.18–38.52) | 18.53
(15.00–29.14) | 0.021a |
| Ferroprotein, ng/ml
(13–150) | 196.30
(137.90–502.60) | 300.30
(156.85–719.15) | 0.929 |
 | Table III.Multivariate analysis of factors
associated with malignancy in patients with dermatomyositis. |
Table III.
Multivariate analysis of factors
associated with malignancy in patients with dermatomyositis.
| Variables | Odds ratio (95%
CI) | P-value |
|---|
| Age, per 10
yrs | 1.026
(1.004–1.049) | 0.022a |
| Diabetes | 1.370
(0.527–3563) | 0.773 |
| ILD | 0.283
(0.135–0.593) | 0.009a |
| Gottron
papules | 1.265
(0.687–2.331) | 0.451 |
| Arthralgia | 0.147
(0.058–0.359) | 0.034a |
| Hemoglobin >150
g/l | 0.998
(0.989–1.006) | 0.616 |
| Anti-Jo-1
antibody | 0.680
(0.231–2.004) | 0.485 |
| CA125 >35
U/ml | 1.001
(0.009–1.003) | 0.336 |
| NSE >17
ng/ml | 1.007
(0.992–1.022) | 0.101 |
MSAs associated with malignancy
MSA detection was first performed at the study
hospital in 2016; therefore, only 17 patients underwent MSA
detection. A total of 5 patients tested positive for anti-TIF1γ
antibody, 3 of whom had cancer (sigmoid colon adenocarcinoma,
esophageal cancer and nasopharyngeal carcinoma). A total of 4
patients were positive for anti-MDA5 antibody; none of whom had
tumors, but all had ILD. A total of 3 patients were positive for
anti-NXP2, and none of them had tumors. Among the 4 patients who
underwent MSA testing and had cancer, 3 (75%) were positive for
anti-TIF1γ, and 1 (with breast cancer) was positive for anti-RO-52.
One patient with cancer was positive for both anti-TIF1γ and
anti-SRP. Among the 13 patients with DM without tumors, 6 were
positive for anti-RO-52, 2 for anti-SRP, and 2 for both anti-Mi-2α
and anti-Mi-2β. In addition, the patients without tumors were
positive for anti-Ku or anti-PL-7. The detailed results of the MSA
tests are provided in Table IV.
 | Table IV.Clinical data of 17 patients who
underwent tests for MSAs. |
Table IV.
Clinical data of 17 patients who
underwent tests for MSAs.
| Patient no. | Sex | Age | Diagnosis | ILD | MSA |
|---|
| 1 | F | 44 | Breast carcinoma,
DM | Y | Anti-R0-52+ |
| 2 | F | 74 | Sigmoid colon
adenocarcinoma, DM | N | Anti-TIF1γ+,
anti-SRP+ |
| 3 | M | 79 | Esophageal
carcinoma, DM | N | Anti-TIF1γ++ |
| 4 | M | 60 | Nasopharyngeal
carcinoma, DM | N | Anti-TIF1γ+ |
| 5 | F | 64 | DM | Y | Anti-MDA5+,
anti-Ku+, anti-RO-52 +++ |
| 6 | F | 45 | DM | Y | Anti-MDA5++ |
| 7 | F | 45 | DM | Y | Anti-MDA5++,
anti-R0-52+ |
| 8 | M | 70 | DM | Y | Anti-MDA5+++,
anti-R0-52++ |
| 9 | M | 51 | DM | N | Anti-Mi-2α+,
anti-Mi-2β+ |
| 10 | F | 52 | DM | N | Anti-Mi-2α+,
anti-Mi-2β++ |
| 11 | M | 82 | DM | Y | Anti-PL-7±,
anti-R0-52+ |
| 12 | F | 56 | DM | N | Anti-SRP+++,
anti-R0-52++ |
| 13 | F | 71 | DM | N | Anti-TIF1γ+,
anti-R0-52+++ |
| 14 | F | 47 | DM | N | Anti-TIF1γ ++,
anti-SRP+ |
| 15 | F | 27 | DM | N | Anti-NXP2+ |
| 16 | F | 49 | DM | N | Anti-NXP2+ |
| 17 | F | 20 | DM | N | Anti-NXP2+ |
Survival analysis
Of the 239 patients with DM, 48 mortalities occurred
during the follow-up period (20.08%). The mortality rate was
significantly higher in patients with malignancy (n=18, 41.86%)
than in those without malignancy (n=30, 15.31%; P=0.003). The
presence of malignancy was inversely associated with the survival
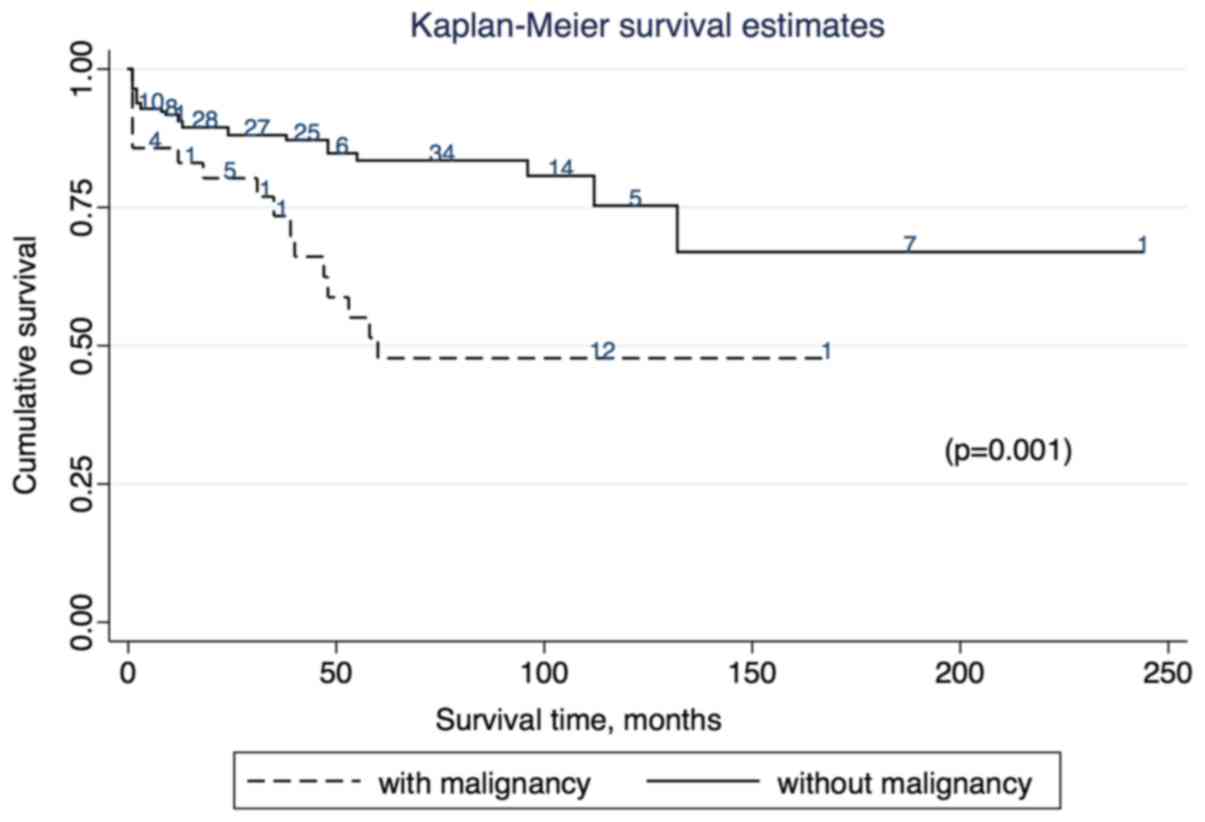
rate. Fig. 3 depicts the survival
curves for patients with DM with or without malignancy.
Kaplan-Meier survival curves showed that the survival rate was
significantly lower in patients with malignancy than in patients
without malignancy (P=0.001; Fig. 3).
The 1-, 5- and 10-year overall survival rates were 88.70, 85.36 and
80.33%, respectively. The cumulative survival rates in the
malignancy group were 81.40% at 1 year and 58.14% at 5 years.
Discussion
Our study demonstrated that DM was associated with a
high risk of malignancy, which frequently developed within 1 year
of DM diagnosis. The association between DM and malignancy is well
documented; DM can occur at the same time as, before, or after
cancer diagnosis (3,4,16,17). The precise association between DM and
malignancy is unclear, but may include the following: i) DM may
occur in the setting of an immunological response to an internal
malignancy; ii) there may be an increased risk of cancer in the
setting of immunosuppressive therapy for DM, and iii) detection
rates may be increased in the setting of heightened surveillance
after either diagnosis (18). It is
difficult, in most cases, to determine whether a malignancy has
contributed to the development of DM or whether DM has contributed
to the development of a malignant tumor.
Older age was the only independent risk factor for
malignant tumors in patients with DM, while arthralgia and ILD were
protective factors. In our study, the incidence of malignancy among
DM patients was 17.99%, which is consistent with previous reports
from Western (8.6–32%) (1,5,17,19,20) and
Asian countries (3.8–24.4%) (3,21–23). The most common malignancy in our study
was lung cancer (n=6, 13.95%), followed by breast (n=5, 11.63%),
gastric (n=5, 11.63%), colorectal (n=4, 9.30%), nasopharyngeal
(n=4, 9.30%) and ovarian cancer (n=4, 9.30%). The risk of different
types of cancer differs with geographic region and ethnicity.
Within China, the incidence and mortality of nasopharyngeal
carcinoma is higher in south China than in north China (24). In Western countries, lung, breast, and
colorectal cancers are the most common types of cancer among DM
patients (2,17,25). In a
retrospective analysis from Scotland (17), the rates of cervical, ovarian and lung
cancer were 12,10 and 5 times higher in DM patients than in the
general population, respectively. After analyzing data from Sweden,
Denmark and Finland, Hill et al (25) found that 32.04% patients with DM
developed cancer, and that the most common types of malignancies
were ovarian, lung, pancreatic, gastric and colorectal cancer, and
non-Hodgkin lymphoma. The most common pathological type was
adenocarcinoma. In a Tunisian study of 130 DM patients, 20 patients
developed cancer, the majority of cases being breast (35%) and
nasopharyngeal cancer (25%) (26). In
a Portuguese study, prostate and colorectal cancers were the most
prevalent (27). In a Japanese study,
gastric, colorectal and ovarian cancers were the most common
(28). Nasopharyngeal carcinoma is
one of the most common types of cancer in southern China (22), Hong Kong (29), Taiwan (5), and Southeast Asia (12,30), and
the most common DM-associated cancer in Asia (21).
The type of cancer also varied with sex, with breast
cancer (n=5, 20.00%) being the most common type of cancer among
women and nasopharyngeal, gastric and colorectal cancers (n=3 for
each type, 15.79%) being the most common type among men.
Adenocarcinoma was the most common pathological type in the present
study. Therefore, we recommend that during cancer screening of DM
patients, special attention must be paid to examinations of the
lung, breast (in women) and digestive system (in men). The nose and
throat should also be examined, and tests for the Epstein-Barr
virus should be performed.
The incidence of cancer among patients with DM
varied with the duration of DM. Cancer was detected within the
first year after DM diagnosis in 53.49% patients, within 2–5 years
in 17.28% patients, and after 5 years in 4.65% patients. The
majority of cases of cancer (30/43, 69.77%) occurred within 1 year
before or after the diagnosis of DM, which is consistent with
previous reports (2,31,32).
Recently, a large meta-analysis from Canada revealed that the
standard incidence ratio of malignancy among DM patients was 17.29
(95% CI, 11.08–26.99) in the first year, 2.7 (95% CI, 1.96–3.72)
from 2–5 years, and 1.37 (95% CI, 1.27–1.48) after 5 years
(33). Some authors have reported
that DM symptoms improved after successful tumor treatment and
worsened with tumor recurrence (34,35). This
parallel course of DM and malignancy suggests a paraneoplastic
phenomenon (10). The findings of the
present study emphasize the importance of tumor screening for DM
patients, particularly in the first 5 years after diagnosis. The
tumor risk remains high during the first 5 years, and, although it
declines thereafter, it remains higher than the risk in the general
population. Thus, we recommend that tumor screening in DM patients
be continued for life.
The risk factors for malignancy in DM patients was
also analyzed. Univariate analysis showed that older age, diabetes,
Gottron sign, hemoglobin, CA125 level and NSE were positively
associated with malignancy, whereas joint pain, ILD, and anti-Jo-1
antibody were negatively associated with malignancy. In
multivariate analysis, older age was the only independent risk
factor for malignant tumors, and joint pain and ILD were protective
factors. Older age has been proven to predict malignancy in
patients with DM (1–5,17,19,22,23,27,33).
Other reported risk factors include male sex, itching, skin
necrosis, skin heterochromia, surrounding erythema, Gottron sign,
heliotrope rash, dysphagia, low albumin, elevated ALT, AST, LDH,
ESR, CRP and CA125 levels, decreased complement C4 expression,
elevated lymphocytes numbers, no response to cortisol therapy and
rapid progression of skin and/or muscle changes (1,3–5,22,23,27,33).
Protective factors include ILD, arthralgia, Raynaud phenomenon,
anti-extractable nuclear antigen and anti-Jo-1 antibody (2,4,22). The association between CPK levels and
risk of malignancy is controversial. Some authors (31,36) have
reported that decreased CPK levels are associated with a higher
risk of cancer, while others (1,2) have
stated that increased CPK levels are associated with a higher risk
of cancer. In the present study, CPK levels appeared to be
increased in patients with malignancy than in those without
malignancy, but the difference was not significant. In the present
study, patients with DM with diabetes had a high risk of
malignancy. To the best of our knowledge, this finding has not been
previously reported. Epidemiological evidence suggests an
association between diabetes and increased cancer risk (37,38),
although the underlying pathogenesis remains unclear. Possible
factors include insulin resistance, hyperglycemia, impaired immune
function and increased insulin levels due to hypoglycemic agents
(39,40). The association between diabetes and
risk of malignancy in DM patients will be investigated in future
studies.
In recent years, MSAs have been evaluated in
patients with DM, as these autoantibodies are often associated with
distinct clinical phenotypes (41,42). In
the present study, however, too few patients underwent MSA testing
for us to be able to conduct statistical analyses. One patient with
sigmoid colon adenocarcinoma was positive for anti-TIF1-γ antibody.
DM patients positive for anti-TIF1-γ and negative for anti-MDA5
antibodies may have an increased risk of malignancy. Between 50 and
100% DM patients with cancer have anti-TIF1-γ antibodies (14,43–48).
Dupont et al (49) revealed
that TIF1-γ is overexpressed in colonic adenocarcinoma. Anti-TIF1-γ
and anti-MDA5 antibodies are specific autoantibodies, which react
with 155/140- and 140-kDa proteins, respectively, in patients with
DM (43–46,50).
Hoshino et al (14) found that
anti-TIF1-γ and anti-MDA5 antibodies do not co-exist in the same
serum sample, and that anti-MDA5 antibody-negative patients have a
higher risk of developing malignancies than anti-MDA5
antibody-positive patients. Furthermore, the study showed that
anti-MDA5 antibodies were associated with intractable ILD,
particularly in patients with amyopathic DM. In the present study,
there were 3 anti-NXP2-positive patients, none of whom had cancer.
In a Japanese study (51), 3/7
anti-NXP2 antibody-positive patients with DM had malignancies
(prostate, pancreatic and lung cancer). All patients were male with
advanced cancer (stage IIIb-IV), but the study was insufficiently
powered to definitively demonstrate a statistical association.
Another study revealed that anti-NXP2 antibody was robustly
associated with cancer, and with male sex (52). However, two more recent studies did
not confirm this association (47,53). The
controversy regarding this antibody highlights the importance of
studying larger cohorts of patients to accurately delineate the
association. Performance of MSA detection is limited at present,
due to its unavailability in hospitals and its high cost. In a
follow up study, we expect to include a large number of cases for
which MSA detection is performed.
In the present study, the overall 5- and 10-year
survival rates were 85.36 and 80.33%, respectively. The 5-year
survival rate of DM patients with malignancy was 58.14%. The
overall mortality rate was 20.08%, while the mortality rate in the
malignancy group was 41.86%. These rates are consistent with
previous findings. The reported 5- and 10-year survival rates of DM
patients are 60–90.1 and 50–86.4% (20,54,55),
respectively, while the 5-year survival rate of patients with DM
and cancer is only 10–56% (26,31,32). The
prognosis of patients with DM and cancer is worse than that of
those without cancer, mostly because cancer is typically diagnosed
at a late stage. The reported mortality rates for DM patients
ranges from 16–75% (26).
In summary, patients with DM have a high incidence
of malignancy, which reduces their survival rate. Thus, prompt
screening for malignancies is required to enable early diagnosis
and treatment, particularly within 1 year of diagnosis of DM. Older
age was demonstrated to be a risk factor for malignancy, and the
type of malignancy differed with sex and ethnicity. In the present
study of patients from northern China, the risk of lung, breast,
gastric, colorectal, ovarian and nasopharyngeal cancer was
increased. Therefore, special attention must be paid to the lung,
ear, nose, throat, breast, ovary and digestive system during cancer
screening. In addition, expression of particular MSAs may predict
cancer in patients with DM, thus, MSA testing should be performed
in patients with DM.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YLiu and HW conceived and designed the research. LX
and NZ collected the data. XL and YLia analyzed the data. YLiu and
YT performed the registration of follow-up data for all patients
and contributed reagents, materials, and analysis tools. YT was
responsible for assessing the disease and determining complications
for all patients. YLiu wrote the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was requested and obtained from the
Ethics Committee of the Affiliated Yantai Yuhuangding Hospital of
Qingdao University (Yantai, China). Written informed consent was
obtained from all participants.
Patient consent for publication
All participants provided written informed consent
for the publication of this data and any associated images.
Conflict of interest
The authors declare that they have no conflicts of
interest.
References
|
1
|
Fardet L, Dupuy A, Gain M, Kettaneh A,
Chérin P, Bachelez H, Dubertret L, Lebbe C, Morel P and Rybojad M:
Factors associated with underlying malignancy in a retrospective
cohort of 121 patients with dermatomyositis. Medicine (Baltimore).
88:91–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stertz G: Polymyositis. Berl Klin
Wochenschr. 53:4891916.
|
|
3
|
So MW, Koo BS, Kim YG, Lee CK and Yoo B:
Idiopathic inflammatory myopathy associated with malignancy: A
retrospective cohort of 151 Korean patients with dermatomyositis
and polymyositis. J Rheumatol. 38:2432–2435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu X, Yang H, Shu X, Chen F, Zhang Y,
Zhang S, Peng Q, Tian X and Wang G: Factors predicting malignancy
in patients with polymyositis and dermatomyostis: A systematic
review and meta-analysis. PLoS One. 9:e941282014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang YF, Wu YJ, Kuo CF, Luo SF and Yu KH:
Malignancy in dermatomyositis and polymyositis: analysis of 192
patients. Clin Rheumatol. 35:1977–1984. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zampieri S, Valente M, Adami N, Biral D,
Ghirardello A, Rampudda ME, Vecchiato M, Sarzo G, Corbianco S, Kern
H, et al: Polymyositis, dermatomyositis and malignancy: A further
intriguing link. Autoimmun Rev. 9:449–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zantos D, Zhang Y and Felson D: The
overall and temporal association of cancer with polymyositis and
dermatomyositis. J Rheumatol. 21:1855–1859. 1994.PubMed/NCBI
|
|
8
|
Buchbinder R and Hill CL: Malignancy in
patients with inflammatory myopathy. Curr Rheumatol Rep. 4:415–426.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madan V, Chinoy H, Griffiths CE and Cooper
RG: Defining cancer risk in dermatomyositis. Part I. Clin Exp
Dermatol. 34:451–455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zahr ZA and Baer AN: Malignancy in
myositis. Curr Rheumatol Rep. 13:208–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jouary T, Gracia C, Lalanne N, Vital A,
Taieb A and Delaunay M: Rapidly lethal dermatomyositis associated
with metastatic melanoma. J Eur Acad Dermatol Venereol. 22:399–401.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teoh JW, Yunus RM, Hassan F, Ghazali N and
Abidin ZA: Nasopharyngeal carcinoma in dermatomyositis patients: A
10-year retrospective review in Hospital Selayang, Malaysia. Rep
Pract Oncol Radiother. 19:332–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Noda T, Iijima M, Noda S, Maeshima S,
Nakanishi H, Kimura S, Koike H, Ishigaki S, Iguchi Y, Katsuno M and
Sobue G: Gene expression profile of inflammatory myopathy with
malignancy is similar to that of dermatomyositis rather than
polymyositis. Intern Med. 55:2571–2580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoshino K, Muro Y, Sugiura K, Tomita Y,
Nakashima R and Mimori T: Anti-MDA5 and anti-TIF1-gamma antibodies
have clinical significance for patients with dermatomyositis.
Rheumatology (Oxford). 49:1726–1733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bohan A and Peter JB: Polymyositis and
dermatomyositis (second of two parts). N Engl J Med. 292:403–407.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ikeda S, Arita M, Misaki K, Mishima S,
Takaiwa T, Nishiyama A, Ito A, Furuta K, Yokoyama T, Tokioka F, et
al: Incidence and impact of interstitial lung disease and
malignancy in patients with polymyositis, dermatomyositis, and
clinically amyopathic dermatomyositis: A retrospective cohort
study. SpringerPlus. 4:2402015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stockton D, Doherty VR and Brewster DH:
Risk of cancer in patients with dermatomyositis or polymyositis,
and follow-up implications: A Scottish population-based cohort
study. Br J Cancer. 85:41–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leatham H, Schadt C, Chisolm S, Fretwell
D, Chung L, Callen JP and Fiorentino D: Evidence supports blind
screening for internal malignancy in dermatomyositis: Data from 2
large US dermatology cohorts. Medicine (Baltimore). 97:e96392018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Souza FH and Shinjo SK: Newly diagnosed
dermatomyositis in the elderly as predictor of malignancy. Rev Bras
Reumatol. 52:713–721. 2012.PubMed/NCBI
|
|
20
|
Sigurgeirsson B, Lindelof B, Edhag O and
Allander E: Risk of cancer in patients with dermatomyositis or
polymyositis. A population-based study. N Engl J Med. 326:363–367.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ungprasert P, Leeaphorn N, Hosiriluck N,
Chaiwatcharayut W, Ammannagari N and Raddatz DA: Clinical features
of inflammatory myopathies and their association with malignancy: A
systematic review in Asian population. ISRN Rheumatology.
2013:5093542013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen D, Yuan S, Wu X, Li H, Qiu Q, Zhan Z,
Ye Y, Lian F, Liang L, Xu H and Yang X: Incidence and predictive
factors for malignancies with dermatomyositis: A cohort from
southern China Clin Exp Rheumatol. 32:615–621. 2014.
|
|
23
|
Zhang W, Jiang SP and Huang L:
Dermatomyositis and malignancy: A retrospective study of 115 cases.
Eur Rev Med Pharmacol Sci. 13:77–80. 2009.PubMed/NCBI
|
|
24
|
Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou
ZX and Chen WQ: Nasopharyngeal carcinoma incidence and mortality in
China in 2010. Chin J Cancer. 33:381–387. 2014.PubMed/NCBI
|
|
25
|
Hill CL, Zhang Y, Sigurgeirsson B, Pukkala
E, Mellemkjaer L, Airio A, Evans SR and Felson DT: Frequency of
specific cancer types in dermatomyositis and polymyositis: A
population-based study. Lancet. 357:96–100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mebazaa A, Boussen H, Nouira R, Rokbani L,
Ben Osman-Dhahri A, Bouaouina N, Laouani-Kechrid C, Louzir B, Zahaf
A and Kamoun MR: Dermatomyositis and malignancy in Tunisia: A
multicenter national retrospective study of 20 cases. J Am Acad
Dermatol. 48:530–534. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Travassos AR, Borges-Costa J, Filipe P and
Marques MS: Malignancy associated with dermatomyositis-A
retrospective single-center study with 33 patients. Acta Reumatol
Port. 38:92–97. 2013.PubMed/NCBI
|
|
28
|
Azuma K, Yamada H, Ohkubo M, Yamasaki Y,
Yamasaki M, Mizushima M and Ozaki S: Incidence and predictive
factors for malignancies in 136 Japanese patients with
dermatomyositis, polymyositis and clinically amyopathic
dermatomyositis. Mod Rheumatol. 21:178–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fung WK, Chan HL and Lam WM: Amyopathic
dermatomyositis in Hong Kong-Association with nasopharyngeal
carcinoma. Int J Dermatol. 37:659–663. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yosipovitch G, Tan A, LoSicco K, Manabat
CG, Kannagra A, Carroll C, Chan YH, Ng P and Jorizzo J: A
comparative study of clinical characteristics, work-up, treatment,
and association to malignancy in dermatomyositis between two
tertiary skin centers in the USA and Singapore. Int J Dermatol.
52:813–819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andras C, Ponyi A, Constantin T, Csiki Z,
Szekanecz E, Szodoray P and Dankó K: Dermatomyositis and
polymyositis associated with malignancy: A 21-year retrospective
study. J Rheumatol. 35:438–444. 2008.PubMed/NCBI
|
|
32
|
Wakata N, Kurihara T, Saito E and
Kinoshita M: Polymyositis and dermatomyositis associated with
malignancy: A 30-year retrospective study. Int J Dermatol.
41:729–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiang JK, Kim WB, Baibergenova A and
Alhusayen R: Risk of malignancy in dermatomyositis and
polymyositis: A systematic review and meta-analysis. J Cutan Med
Surg. 21:131–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Masuda H, Urushibara M and Kihara K:
Successful treatment of dermatomyositis associated with
adenocarcinoma of the prostate after radical prostatectomy. J Urol.
169:10842003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshinaga A, Hayashi T, Ishii N, Ohno R,
Watanabe T and Yamada T: Successful cure of dermatomyositis after
treatment of nonseminomatous testicular cancer. Int J Urol.
12:593–595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mahe E, Descamps V, Burnouf M and Crickx
B: A helpful clinical sign predictive of cancer in adult
dermatomyositis: Cutaneous necrosis. Arch Dermatol.
139:5392003.PubMed/NCBI
|
|
37
|
Giovannucci E, Harlan DM, Archer MC,
Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M,
Regensteiner JG and Yee D: Diabetes and cancer: A consensus report.
Diabetes Care. 33:1674–1685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vigneri P, Frasca F, Sciacca L, Pandini G
and Vigneri R: Diabetes and cancer. Endocr Relat Cancer.
16:1103–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lukanova A, Zeleniuch-Jacquotte A, Lundin
E, Micheli A, Arslan AA, Rinaldi S, Muti P, Lenner P, Koenig KL,
Biessy C, et al: Prediagnostic levels of C-peptide, IGF-I, IGFBP
−1, −2 and −3 and risk of endometrial cancer. Int J Cancer.
108:262–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang J, Nishihara R, Zhang X, Ogino S and
Qian ZR: Energy sensing pathways: Bridging type 2 diabetes and
colorectal cancer? J Diabetes Complications. 31:1228–1236. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Betteridge ZE, Gunawardena H and McHugh
NJ: Novel autoantibodies and clinical phenotypes in adult and
juvenile myositis. Arthritis Res Ther. 13:2092011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Casciola-Rosen L and Mammen AL: Myositis
autoantibodies. Curr Opin Rheumatol. 24:602–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Targoff IN, Mamyrova G, Trieu EP, Perurena
O, Koneru B, O'Hanlon TP, Miller FW and Rider LG; and Childhood
Myositis Heterogeneity Study Group, : International Myositis
Collaborative Study Group: A novel autoantibody to a 155-kd protein
is associated with dermatomyositis. Arthritis Rheum. 54:3682–3689.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kaji K, Fujimoto M, Hasegawa M, Kondo M,
Saito Y, Komura K, Matsushita T, Orito H, Hamaguchi Y, Yanaba K, et
al: Identification of a novel autoantibody reactive with 155 and
140 kDa nuclear proteins in patients with dermatomyositis: An
association with malignancy. Rheumatology (Oxford). 46:25–28. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chinoy H, Fertig N, Oddis CV, Ollier WE
and Cooper RG: The diagnostic utility of myositis autoantibody
testing for predicting the risk of cancer-associated myositis. Ann
Rheum Dis. 66:1345–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gunawardena H, Wedderburn LR, North J,
Betteridge Z, Dunphy J, Chinoy H, Davidson JE, Cooper RG and McHugh
NJ; and Juvenile Dermatomyositis Research Group UK, : Clinical
associations of autoantibodies to a p155/140 kDa doublet protein in
juvenile dermatomyositis. Rheumatology (Oxford). 47:324–328. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ceribelli A, Isailovic N, De Santis M,
Generali E, Fredi M, Cavazzana I, Franceschini F, Cantarini L,
Satoh M and Selmi C: Myositis-specific autoantibodies and their
association with malignancy in Italian patients with polymyositis
and dermatomyositis. Clin Rheumatol. 36:469–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Trallero-Araguas E, Rodrigo-Pendas JA,
Selva-O'Callaghan A, Martínez-Gómez X, Bosch X, Labrador-Horrillo
M, Grau-Junyent JM and Vilardell-Tarrés M: Usefulness of anti-p155
autoantibody for diagnosing cancer-associated dermatomyositis: A
systematic review and meta-analysis. Arthritis Rheum. 64:523–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dupont S, Zacchigna L, Cordenonsi M,
Soligo S, Adorno M, Rugge M and Piccolo S: Germ-layer specification
and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase.
Cell. 121:87–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sato S, Hirakata M, Kuwana M, Suwa A,
Inada S, Mimori T, Nishikawa T, Oddis CV and Ikeda Y:
Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese
patients with clinically amyopathic dermatomyositis. Arthritis
Rheum. 52:1571–1576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ichimura Y, Matsushita T, Hamaguchi Y,
Kaji K, Hasegawa M, Tanino Y, Inokoshi Y, Kawai K, Kanekura T,
Habuchi M, et al: Anti-NXP2 autoantibodies in adult patients with
idiopathic inflammatory myopathies: Possible association with
malignancy. Ann Rheum Dis. 71:710–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fiorentino DF, Chung LS, Christopher-Stine
L, Zaba L, Li S, Mammen AL, Rosen A and Casciola-Rosen L: Most
patients with cancer-associated dermatomyositis have antibodies to
nuclear matrix protein NXP-2 or transcription intermediary factor
1gamma. Arthritis Rheum. 65:2954–2962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Albayda J, Pinal-Fernandez I, Huang W,
Parks C, Paik J, Casciola-Rosen L, Danoff SK, Johnson C,
Christopher-Stine L and Mammen AL: Antinuclear matrix protein 2
autoantibodies and edema, muscle disease, and malignancy risk in
dermatomyositis patients. Arthritis Care Res. (Hoboken).
69:1771–1776. 2017.
|
|
54
|
Airio A, Kautiainen H and Hakala M:
Prognosis and mortality of polymyositis and dermatomyositis
patients. Clin Rheumatol. 25:234–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Danko K, Ponyi A, Constantin T, Borgulya G
and Szegedi G: Long-term survival of patients with idiopathic
inflammatory myopathies according to clinical features: A
longitudinal study of 162 cases. Medicine (Baltimore). 83:35–42.
2004. View Article : Google Scholar : PubMed/NCBI
|