Introduction
Prostate cancer is the most frequent malignancy in
men (1). Approximately 10–20% of
patients suffer from cancer invading into the lymph system at the
time of diagnosis (2–4). Finding tumor cells in a lymph node is
unequivocal proof of dissemination and is associated with poor
patient prognosis (5–9). Thus, novel therapies are needed to
effectively target metastatic cancer cells.
Approximately 50% of prostate cancers harbor a gene
fusion linking the androgen-regulated gene transmembrane protease,
serine 2 (TMPRSS2) with transcription factors of the
erythroblastosis virus E26 transforming sequence (ETS) family,
typically erythroblast transformation-specific-related gene (ERG)
(10). The TMPRSS2:ERG fusion protein
may be an optimal target for a novel therapy, as it is highly
specific for prostate cancer cells. In addition, potential
anti-TMPRSS2:ERG therapy is unlikely to have major side effects,
since this fusion protein is absent in normal tissues. Recent
advances in the delivery of inhibitory RNAs or peptides to human
cancer raise the possibility that anti-TMPRSS2:ERG therapy may
become available in the future (11–14).
It has been recently demonstrated that the
TMPRSS2:ERG fusion typically occurs early during tumor development
and is often homogeneously distributed across the cancer bulk
(15–18). However, we also observed that up to
60% of ERG-positive cancers may at the least have small areas
lacking ERG expression (18,19). This raises the question whether lymph
node metastasis arises from ERG-negative or -positive areas, and
whether the ERG status of the primary cancer represents the ERG
status of the lymph node metastasis. In a recent study, discrepant
ERG findings were observed in the lymph nodes of 30% of 84 prostate
cancers (20). Such differences in
the ERG status between primary and metastatic tumor sites would
challenge the concept of anti-ERG therapy. We herein performed a
thorough ERG-mapping study in 77 prostate cancers exhibiting lymph
node involvement at the time of diagnosis. We constructed a tissue
microarray (TMA) with 20 spots per primary cancer and all
tumor-containing lymph nodes for maximal representation of the
tumor bulk. Our findings demonstrated a high degree of concordance
of the ERG expression status between primary prostate cancers and
their lymph node metastases, and little intratumoral
heterogeneity.
Materials and methods
Tissue samples
The prostate cancer heterogeneity TMA consisted of
1,727 prostate cancer tissue spots and 80 control spots from normal
tissue (lung, liver, skin, lymph node and kidney) distributed
across 4 paraffin blocks, all derived from 77 patients who
underwent radical prostatectomy at the UKE between 2009 and 2010,
who were found to have lymph node metastases. A total of 24–61
primary tumor-containing blocks and 1–16 blocks from the
corresponding lymph node metastases were collected from each
patient. For the TMA construction, 20 0.6-mm punches were collected
from each primary tumor, with one additional punch from each of the
corresponding metastatic lymph nodes. If possible, all 20 primary
cancer tissue punches were collected from different paraffin
blocks. The number of sampled lymph nodes per patient was as
follows: 1 node in 4 patients, 2 nodes in 17 patients, 3 nodes in
30 patients, 4 nodes in 7 patients, 5 nodes in 5 patients, 6 nodes
in 4 patients, 7 nodes in 5 patients, 10 nodes in 1 patient, 11
nodes in 1 patient, 14 nodes in 2 patients, and 16 nodes in 1
patient. All cancers were unifocal tumors according to the criteria
of Wise et al (21): The tumor
areas were defined as part of a single focus if they were within 3
mm of each other in any section or within 4 mm on adjacent
sections.
Immunohistochemistry (IHC)
IHC analysis of ERG was performed as previously
described (17). Rabbit recombinant
monoclonal ERG antibody (clone EPR3864, dilution 1:450; Epitomics)
was used and visualized with DAKO EnVision (Dako Diagnostics AG,
Zug, Switzerlan). Freshly cut TMA sections were analyzed in one day
and in one experiment. The sections were deparaffinized and exposed
to heat-induced antigen retrieval for 5 min at 121°C in citrate
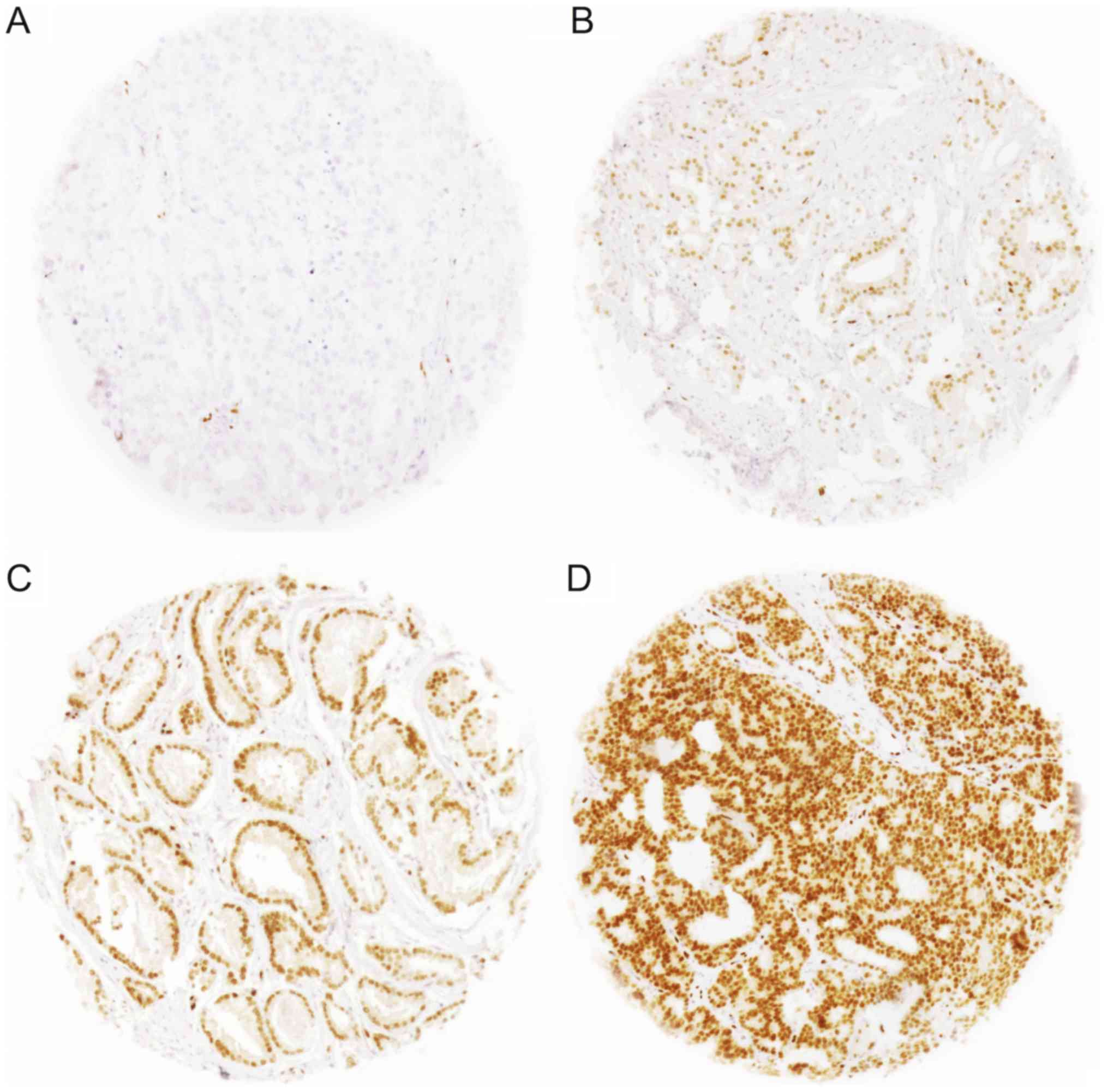
buffer (pH 7.8). Only nuclear ERG staining was scored. Staining
intensity was assessed on a scale of 0 (negative), 1 (weak), 2
(moderate), and 3 (strong) (Fig. 1).
Any detectable staining (≥1) was considered as ERG-positive.
Results
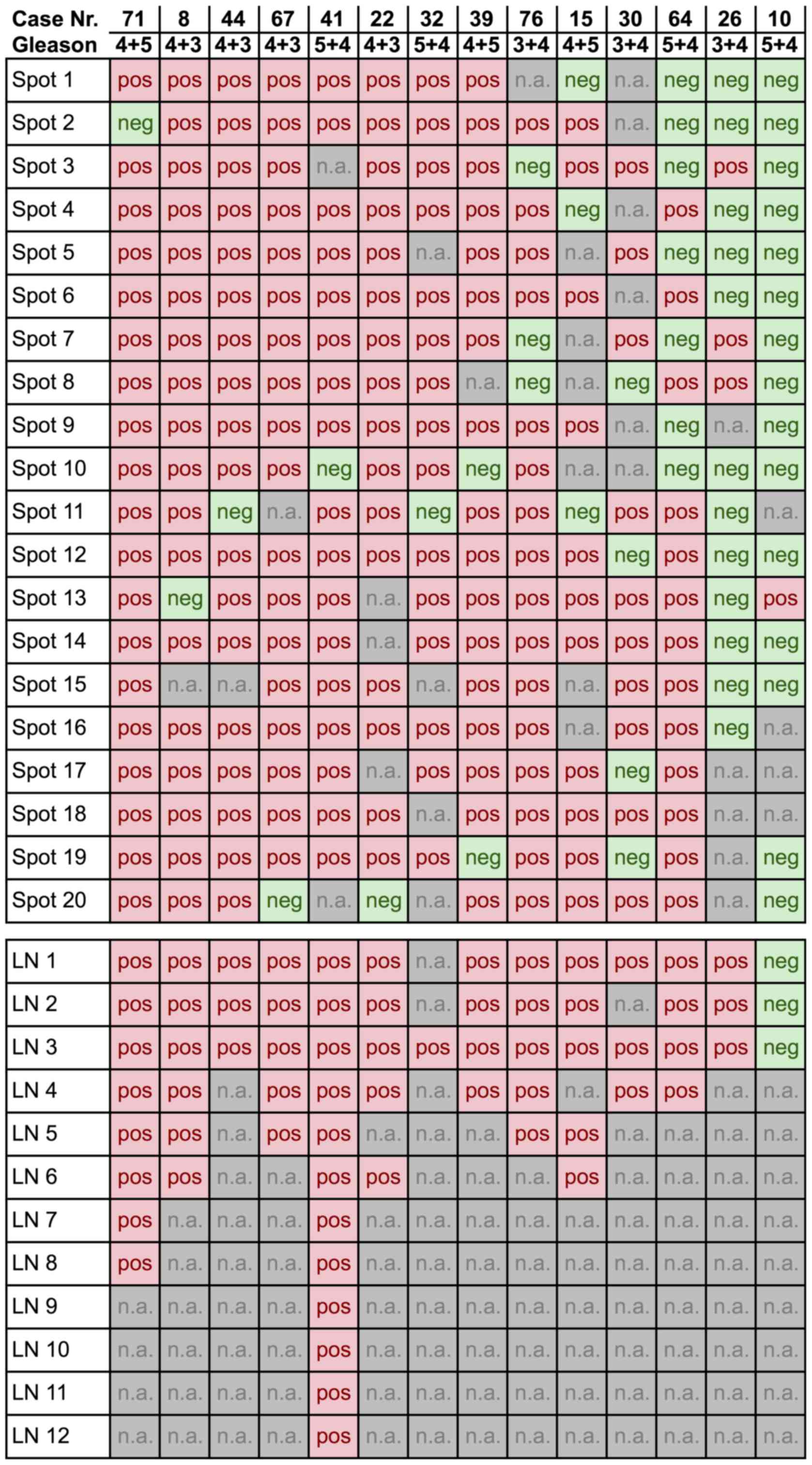
Of the 77 different cancers, 69 had at least 9
interpretable ERG results in the 20 tissue spots obtained from the
primary cancer: 12 cancers had 20 interpretable spots, 21 cancers
had 19 interpretable spots, 35 cancers had 10–18 interpretable
spots, and 1 cancer had 9 interpretable spots. At least one lymph
node metastasis was analyzable from 70 cancers, whereas 54 cancers
had at least three analyzable lymph node metastases.
ERG heterogeneity in the primary
cancer
Of the 69 primary cancers with at least 9
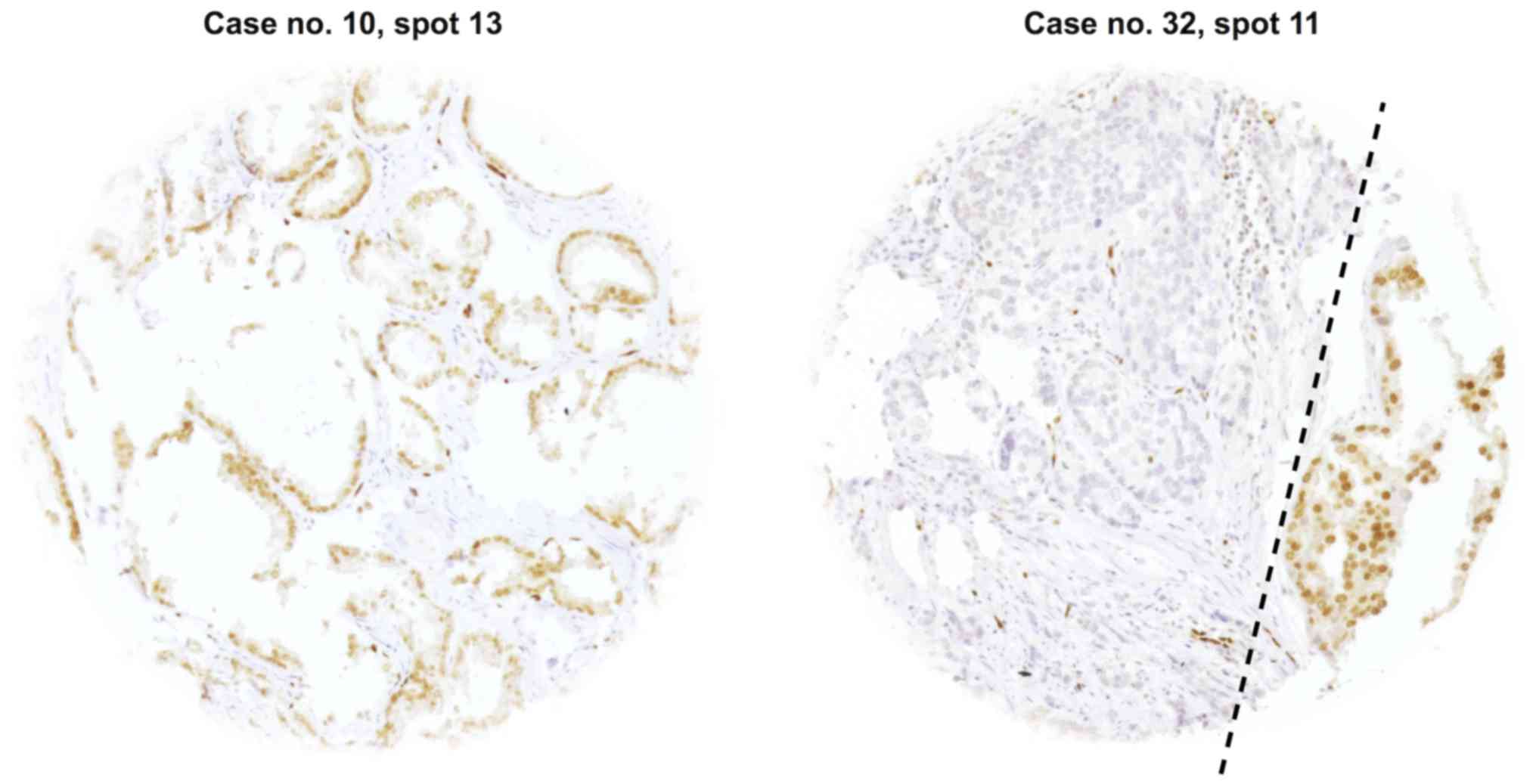
interpretable tissue spots, 14 (20%) were heterogeneous (Fig. 2). Heterogeneity was found in every
Gleason score. Representative images of heterogeneous cases with
single discrepant ERG-positive or ERG-negative spots are shown in
Fig. 3. An identical ERG result was
found in all tissue spots of 55/69 (80%) tumors, of which 26/69
(38%) primary cancers were homogeneously ERG-positive and 29/69
(42%) homogeneously ERG-negative.
Comparison between primary cancers and
their lymph node metastasis
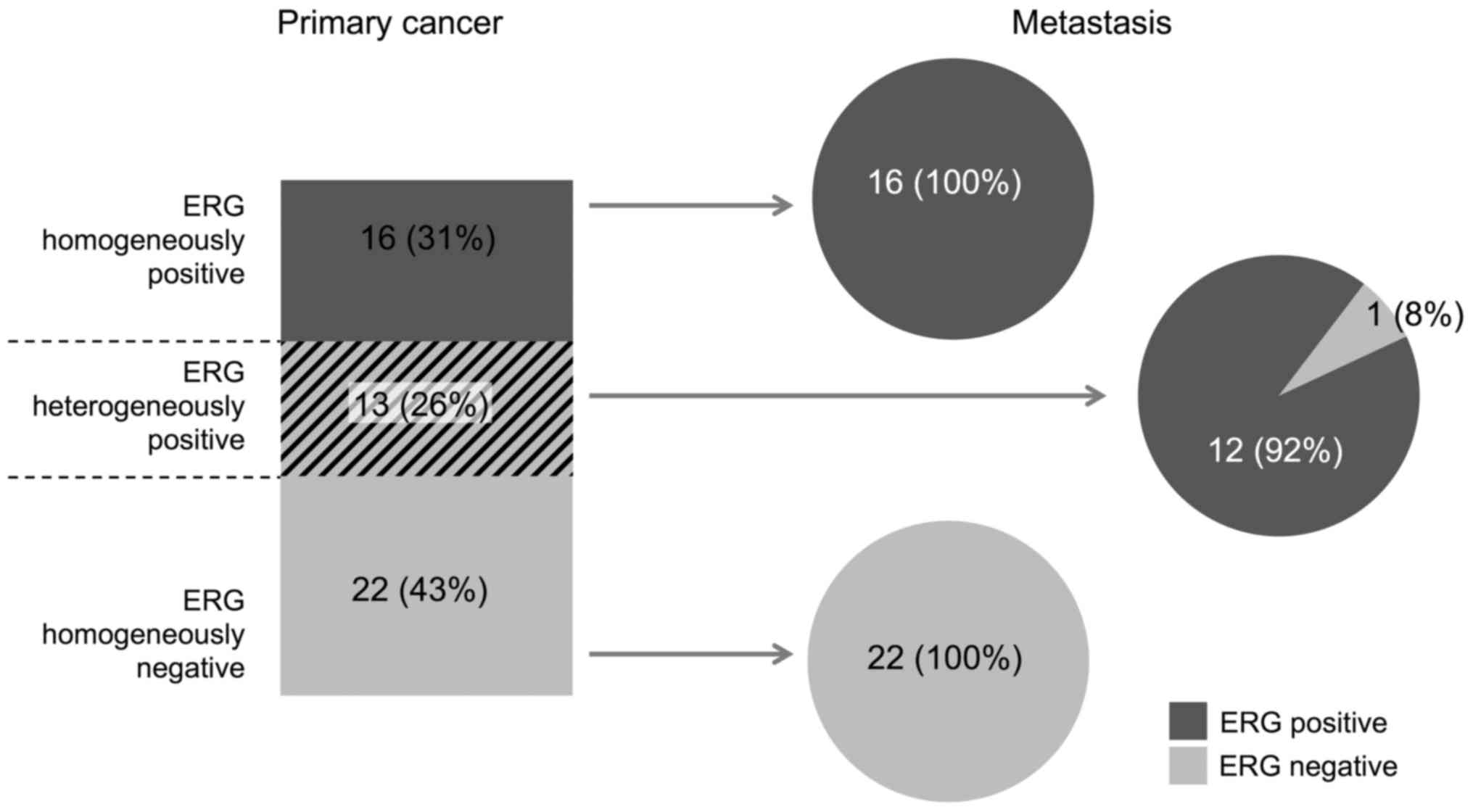
This analysis was restricted to the subset of 49
cancers with at least 9 interpretable tumor spots in the primary
cancer and with at least 3 interpretable lymph node metastases
(Fig. 4). In the subset of primary
cancers with a homogeneous ERG staining result, 38/38 (100%) were
identical with their metastasis. In the subset of primary cancers
with a heterogeneous ERG staining result, 12/13 (92%) cancers were
ERG-positive and 1/13 (8%) was ERG-negative.
Discussion
The results of the present study suggest a high
degree of concordance (50/51, 98%) of ERG expression between
primary cancer and lymph node metastasis. We found 40/69 (58%) of
the cancers to be ERG-positive, in accordance with earlier reports
of 40–60% ERG positivity in studies with 13–317 prostate cancers,
which were analyzed as conventional large sections (15,17,18,22,23)
or as TMA spots (19,24,25). For
example, we found 45% ERG-positive cases in a recent study of all
tumor-containing conventional large sections of 317 prostate
cancers obtained from 125 patients (18), and 55% ERG-positive cancers in a TMA
study with one 0.6-mm tissue spot per cancer using the same ERG
antibody and IHC protocol (19).
Thus, the fraction of ERG-positive cancer is independent from the
amount of tissue analyzed.
Whether ERG activation is associated with prostate
cancer aggressiveness is a matter of debate. For example, certain
studies have suggested an association between ERG expression and
advanced tumor stage (26,27), high Gleason grade (26), or poor patient prognosis (26,28), while
other studies, including ours, could not confirm such findings
(19,29,30). In
our earlier TMA study on 2,800 cancers, ERG expression was
identified in 55% non-metastatic and 54% metastatic cancers
(19). The similar rate of ERG
positivity (58%) in the present high-risk cancer series selected
for lymph node metastasis provides strong additional evidence
against a relevant role of ERG in tumor aggressiveness.
While 80% of the primary cancers were homogeneous in
terms of ERG expression, this percentage increased to 98% for the
metastases. These data suggest that the ERG expression status is
typically preserved during metastatic spread. Similar findings were
reported by an earlier heterogeneity study on 86 primary prostate
cancers with matched lymph node metastases (20), where 71% of the cancers had identical
ERG findings in at least 2 samples, each obtained from the primary
and metastatic tumor sites. Smaller studies on 13 (31) and 26 (32) cancers reported a concordance rate
between 77 and 100%.
In our study, discrepant findings between the
primary and metastatic sites were yielded from cases with
heterogeneous ERG expression in the primary cancer. However, it
should be noted that 8 of 14 primary cancers with heterogeneous ERG
staining were rated as discrepant only because one individual TMA
spot yielded a different ERG result. It remains uncertain whether
such cases represent true heterogeneity. Although we strictly
followed the guidelines to identify individual foci, it cannot be
excluded that some discrepant findings are due to collision of
cancer foci that cannot be distinguished histologically, or that
local variations of staining account for rare false-negative
findings.
Although some heterogeneous findings may be
associated with technical issues, this is not always the case. In
order to ensure a high representativeness of the analyzed cancers,
we limited our study to tumors with at least 9 analyzable primary
tissue spots and at least 3 analyzable metastases. Identifying ≥2
discrepant spots in ~7% of primary cancers suggests that a fraction
of cancers may harbor true intrafocal heterogeneity. Such findings
support our hypothesis that ERG fusion may also develop at a later
time after the cancer has been established. We have already made
this observation in our earlier study on conventional large
sections, where we found 42% of ERG-positive tumors to have at
least small ERG-negative areas (17).
The higher heterogeneity rate in the latter study is most likely
attributed to the higher amount of cancer tissue that can be
analyzed in conventional large sections. Our findings raise the
possibility that a relevant fraction of ERG-positive prostate
tumors may initially develop as ERG-negative. This is of interest
in the light of earlier discussions on whether ERG fusion is
sufficient to initiate prostate cancer (33,34). Based
on our data, it may be hypothesized that ERG activation represents
a very early progression event rather than a cancer-initiating
alteration in several cases. This view is further supported by
studies reporting that mouse prostate with forced ERG expression
displayed only subtle morphological changes (34), and that additional alterations, such
as loss of the PTEN tumor suppressor, was required to develop
invasive cancer (34).
The limitation of the present study is that it is
purely descriptive and does not involve any comparisons between
molecular groups that would allow for statistical testing. The same
is the case for the comparison of the ERG status in primary cancers
and their metastases: All cancers are metastatic so that there is
no comparison between subsets that are defined by specific
molecular or histologic features.
The TMPRSS2:ERG fusion oncogene is unique to 50% of
prostate cancers, making it an attractive target for highly
specific anticancer therapy. To date, no TMPRSS2:ERG-specific drug
has been developed, but recent advances suggest that gene silencing
with small interfering RNAs (siRNAs) or peptides may become an
option in the future (11,13,14). For
example, in a xenograft tumor mouse model, knockdown of ERG
overexpression strongly inhibited tumor growth already in the first
week of treatment, and partially restored tumor cell
differentiation without any signs of toxicity (11). The typically homogeneous ERG
expression across all primary and metastatic tumor cells in
ERG-positive prostate cancer suggests that anti-TMPRSS2:ERG therapy
may prove to be highly effective in the future. In addition, the
homogenous staining makes it highly likely that ERG-positive
cancers will be reliably identified through analysis of prostate
biopsies.
In summary, the results of our study demonstrated a
high degree of concordance of ERG expression between primary
prostate cancers and their lymph node metastases, with some
intratumoral heterogeneity. Analysis of small samples of the
cancer, such as needle biopsies, may be sufficient to select
patients for putative anti-TMPRSS2:ERG therapy, should it become
available in the future.
Acknowledgements
The authors would like to thank Mrs. Janett Lütgens,
Mrs. Sünje Seekamp and Mrs. Inge Brandt for their technical
assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FB, KG, RS and TS designed the study and drafted the
manuscript. HHe, HHu and MG participated in study design. SM, MCT,
CW, FJ, CF and SS performed immunohistochemical analysis and
scoring. MK and RS participated in pathology data analysis. CHM and
RS performed statistical analysis. DL, CMK, AH and PL participated
in data interpretation and helped to draft the manuscript. WW
participated in data interpretation. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Ärztekammer Hamburg
approved the study protocol (approval no. WF-049/09). According to
local laws (HmbKHG §12a), patient informed consent was not
required. Patient records/information were anonymized and
de-identified prior to analysis. All procedures have been performed
in compliance with the principles outlined in the Helsinki
Declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ERG
|
erythroblast
transformation-specific-related gene
|
|
ICH
|
immunohistochemistry
|
|
TMPRSS2:ERG
|
transmembrane protease, serine 2:
erythroblast transformation-specific-related gene fusion
|
|
TMA
|
tissue microarray
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cai T, Nesi G, Tinacci G, Giubilei G,
Gavazzi A, Mondaini N, Zini E and Bartoletti R: Clinical importance
of lymph node density in predicting outcome of prostate cancer
patients. J Surg Res. 167:267–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bader P, Burkhard FC, Markwalder R and
Studer UE: Disease progression and survival of patients with
positive lymph nodes after radical prostatectomy. Is there a chance
of cure? J Urol. 169:849–854. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weckermann D, Dorn R, Trefz M, Wagner T,
Wawroschek F and Harzmann R: Sentinel lymph node dissection for
prostate cancer: Experience with more than 1,000 patients. J Urol.
177:916–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gervasi LA, Mata J, Easley JD, Wilbanks
JH, Seale-Hawkins C, Carlton CE Jr and Scardino PT: Prognostic
significance of lymph nodal metastases in prostate cancer. J Urol.
142:332–336. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng L, Zincke H, Blute ML, Bergstralh
EJ, Scherer B and Bostwick DG: Risk of prostate carcinoma death in
patients with lymph node metastasis. Cancer. 91:66–73. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zwergel U, Lehmann J, Wullich B, Schreier
U, Remberger K, Zwergel T and Stoeckle M: Lymph node positive
prostate cancer: Long-term survival data after radical
prostatectomy. J Urol. 171:1128–1131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fleischmann A, Rocha C, Schobinger S,
Seiler R, Wiese B and Thalmann GN: Androgen receptors are
differentially expressed in Gleason patterns of prostate cancer and
down-regulated in matched lymph node metastases. Prostate.
71:453–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ko K, Jeong IG, Choi WS, Lim JH, Suh JH,
Ku JH, Park Y, Moon KC, Kim HH, Kim CS and Kwak C: Effect of
Gleason scores of lymph node metastases on prognosis of patients
with prostate cancer. Int J Clin Exp Pathol. 7:6141–6148.
2014.PubMed/NCBI
|
|
10
|
Tomlins SA, Rhodes DR, Perner S,
Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J,
Kuefer R, et al: Recurrent fusion of TMPRSS2 and ETS transcription
factor genes in prostate cancer. Science. 310:644–648. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Urbinati G, Ali HM, Rousseau Q, Chapuis H,
Desmaële D, Couvreur P and Massaad-Massade L: Antineoplastic
effects of siRNA against TMPRSS2-ERG junction oncogene in prostate
cancer. PLoS One. 10:e01252772015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urbinati G, de Waziers I, Slamiç M,
Foussignière T, Ali HM, Desmaële D, Couvreur P and Massaad-Massade
L: Knocking down TMPRSS2-ERG fusion oncogene by siRNA could be an
alternative treatment to flutamide. Mol Ther Nucleic Acids.
5:e3012016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shao L, Tekedereli I, Wang J, Yuca E,
Tsang S, Sood A, Lopez-Berestein G, Ozpolat B and Ittmann M: Highly
specific targeting of the TMPRSS2/ERG fusion gene using liposomal
nanovectors. Clin Cancer Res. 18:6648–6657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Qiao Y, Asangani IA, Ateeq B,
Poliakov A, Cieślik M, Pitchiaya S, Chakravarthi BVSK, Cao X, Jing
X, et al: Development of peptidomimetic inhibitors of the ERG gene
fusion product in prostate cancer. Cancer Cell. 31(532–548):
e72017.
|
|
15
|
Furusato B, Tan SH, Young D, Dobi A, Sun
C, Mohamed AA, Thangapazham R, Chen Y, McMaster G, Sreenath T, et
al: ERG oncoprotein expression in prostate cancer: Clonal
progression of ERG-positive tumor cells and potential for ERG-based
stratification. Prostate Cancer Prostatic Dis. 13:228–237. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehra R, Tomlins SA, Shen R, Nadeem O,
Wang L, Wei JT, Pienta KJ, Ghosh D, Rubin MA, Chinnaiyan AM and
Shah RB: Comprehensive assessment of TMPRSS2 and ETS family gene
aberrations in clinically localized prostate cancer. Mod Pathol.
20:538–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Minner S, Gärtner M, Freudenthaler F,
Bauer M, Kluth M, Salomon G, Heinzer H, Graefen M, Bokemeyer C,
Simon R, et al: Marked heterogeneity of ERG expression in large
primary prostate cancers. Mod Pathol. 26:106–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsourlakis MC, Stender A, Quaas A, Kluth
M, Wittmer C, Haese A, Graefen M, Steurer S, Simon R, Korbel J, et
al: Heterogeneity of ERG expression in prostate cancer: A large
section mapping study of entire prostatectomy specimens from 125
patients. BMC Cancer. 16:6412016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minner S, Enodien M, Sirma H, Luebke AM,
Krohn A, Mayer PS, Simon R, Tennstedt P, Müller J, Scholz L, et al:
ERG status is unrelated to PSA recurrence in radically operated
prostate cancer in the absence of antihormonal therapy. Clin Cancer
Res. 17:5878–5888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fleischmann A, Saramäki OR, Zlobec I,
Rotzer D, Genitsch V, Seiler R, Visakorpi T and Thalmann GN:
Prevalence and prognostic significance of TMPRSS2-ERG gene fusion
in lymph node positive prostate cancers. Prostate. 74:1647–1654.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wise AM, Stamey TA, McNeal JE and Clayton
JL: Morphologic and clinical significance of multifocal prostate
cancers in radical prostatectomy specimens. Urology. 60:264–269.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clement T, Swars H, Boerner N, Klose KJ,
John H, Warnecke M and Weilemann LS: Venous occlusive disease of
the liver-a rare pregnancy complication. Internist (Berl).
31:297–300. 1990.(In German). PubMed/NCBI
|
|
23
|
Barry M, Perner S, Demichelis F and Rubin
MA: TMPRSS2-ERG fusion heterogeneity in multifocal prostate cancer:
Clinical and biologic implications. Urology. 70:630–633. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Svensson MA, LaFargue CJ, MacDonald TY,
Pflueger D, Kitabayashi N, Santa-Cruz AM, Garsha KE, Sathyanarayana
UG, Riley JP, Yun CS, et al: Testing mutual exclusivity of ETS
rearranged prostate cancer. Lab Invest. 91:404–412. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Pavlovitz B, Tull J, Wang Y, Deng
FM and Fuller C: Detection of TMPRSS2 gene deletions and
translocations in carcinoma, intraepithelial neoplasia, and normal
epithelium of the prostate by direct fluorescence in situ
hybridization. Diagn Mol Pathol. 19:151–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Font-Tello A, Juanpere N, de Muga S,
Lorenzo M, Lorente JA, Fumado L, Serrano L, Serrano S, Lloreta J
and Hernández S: Association of ERG and TMPRSS2-ERG with grade,
stage, and prognosis of prostate cancer is dependent on their
expression levels. Prostate. 75:1216–1226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hagen RM, Adamo P, Karamat S, Oxley J,
Aning JJ, Gillatt D, Persad R, Ladomery MR and Rhodes A:
Quantitative analysis of ERG expression and its splice isoforms in
formalin-fixed, paraffin-embedded prostate cancer samples:
Association with seminal vesicle invasion and biochemical
recurrence. Am J Clin Pathol. 142:533–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hägglöf C, Hammarsten P, Strömvall K,
Egevad L, Josefsson A, Stattin P, Granfors T and Bergh A:
TMPRSS2-ERG expression predicts prostate cancer survival and
associates with stromal biomarkers. PLoS One. 9:e868242014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klein EA, Falzarano SM, Maddala T,
Cherbavaz D, Novotny WF, Millward C and Magi-Galluzzi C: Use of
TMPRSS2-ERG gene rearrangement and quantitative ERG expression to
predict clinical recurrence after radical prostatectomy. J Clin
Oncol. 29 Suppl:S362011. View Article : Google Scholar
|
|
30
|
Terry S, Nicolaiew N, Basset V, Semprez F,
Soyeux P, Maillé P, Vacherot F, Ploussard G, Londoño-Vallejo A, de
la Taille A and Allory Y: Clinical value of ERG, TFF3, and SPINK1
for molecular subtyping of prostate cancer. Cancer. 121:1422–1430.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo CC, Wang Y, Xiao L, Troncoso P and
Czerniak BA: The relationship of TMPRSS2-ERG gene fusion between
primary and metastatic prostate cancers. Hum Pathol. 43:644–649.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perner S, Svensson MA, Hossain RR, Day JR,
Groskopf J, Slaughter RC, Jarleborn AR, Hofer MD, Kuefer R,
Demichelis F, et al: ERG rearrangement metastasis patterns in
locally advanced prostate cancer. Urology. 75:762–767. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tomlins SA, Laxman B, Dhanasekaran SM,
Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, et
al: Distinct classes of chromosomal rearrangements create oncogenic
ETS gene fusions in prostate cancer. Nature. 448:595–599. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carver BS, Tran J, Chen Z, Carracedo-Perez
A, Alimonti A, Nardella C, Gopalan A, Scardino PT, Cordon-Cardo C,
Gerald W and Pandolfi PP: ETS rearrangements and prostate cancer
initiation. Nature. 457(E1): discussion E2-E3. 2009.PubMed/NCBI
|