Introduction
Epidermal growth factor receptor (EGFR) is a
receptor tyrosine kinase in the cell transmembrane that serves an
essential role in proliferation, metastasis, angiogenesis and
chemo-resistance of oral cancer cells (1,2). More than
80% patients with oral cancer and oral cancer cell lines exhibit
overexpression of EGFR (3–6). High expression of EGFR protein in oral
cancer is associated with poor prognosis, decreased survival time
and increased metastatic potential (7,8).
Inhibition of EGFR and regulation of downstream signaling
represents a novel approach for oral cancer therapy (9,10). Various
strategies have been developed to disrupt EGFR function and to
interfere with downstream signaling (11,12).
Anti-EGFR mAbs and EGFR inhibitors have been investigated the most
extensively (3,5,13).
Cetuximab is an EGFR-targeting mAb and the first
novel targeted agent for oral cancer treatment to obtain Food and
Drug Administration approval in the United States (11,13). In
combination with chemotherapeutic agents, cetuximab has been
demonstrated to increase the overall survival rate of patients with
oral cancer, and to have less toxicity (7,10,14). In oral cancer therapy, combination of
cetuximab with cisplatin, 5-fluorouracil (5-FU), docetaxel
(Taxotere) or paclitaxel (Taxol) has become the new standard
advanced treatment (8,9,15,16).
Curcumin is a traditional Chinese medicine isolated
from the rhizome of Curcuma longa (17–20).
Curcumin has been shown to exert anti-inflammatory, anti-oxidant,
and anticancer effects, it is pharmacologically safe and has
minimal toxicity (17,19,20). The
anticancer activities of curcumin are attributable to its
anti-proliferative, anti-angiogenic, anti-metastatic, pro-apoptotic
and autophagic characteristics (21–25). In
vitro studies reported that curcumin inhibited cell
proliferation in various oral cancer cell lines, including CAL 27,
1483, SCC-1, SCC-9, KB, SAS and SCC15 (26). Curcumin also suppressed EGFR
expression and its downstream signaling molecules (NF-κB, JNK, p38
and ERK) which are vital for oral cancer pathogenesis (27–29).
Furthermore, curcumin enhanced cisplatin cytotoxicity in PE/CA-PJ15
cells in vitro (30). The
combination of 5-FU, doxorubicin or cisplatin with curcumin
exhibited inhibited proliferation and induced apoptotic cell death
of NT8e oral squamous cell carcinoma cells (31). However, the molecular mechanism of the
suppression of cell proliferation and apoptotic induction of
drug-resistant oral cancer cells following co-incubation with
cetuximab and curcumin remains poorly understood. Herein, the
synergistic effects and underlying molecular mechanism of the
effect of combined treatment of cetuximab and curcumin in
cisplatin-resistant oral cancer CAR cells was explored.
Materials and methods
Chemicals and reagents
Erbitux (the active ingredient of cetuximab) was
provided by Hualien Tzu Chi Hospital (Taiwan) and originally
purchased from Merck KGaA (Darmstadt, Germany). Dulbecco's modified
Eagle's medium (DMEM), fetal bovine serum (FBS), L-glutamine and
penicillin-streptomycin solution were purchased from HyClone (GE
Healthcare, Logan, UT, USA). Caspase-3 and Caspase-9 colorimetric
assay kits were sourced from R&D Systems (Minneapolis, MN,
USA). All primary antibodies and anti-mouse/-rabbit immunoglobulin
G (IgG) horseradish peroxidase (HRP)-conjugated antibodies were
purchased from GeneTex (Hsinchu, Taiwan). Curcumin, Thiazolyl Blue
Tetrazolium Bromide (MTT) and other reagents were of analytical
grade from Sigma-Aldrich (Merck KGaA, Darmstadt Germany), unless
otherwise stated.
Cell culture
The human oral cancer cell line, CAL 27, was
obtained from American Type Culture Collection (ATCC; Manassas, VA,
USA). The cisplatin-resistant subline of CAL 27, CAR, was generated
in our laboratory, as previously described (32–34) and
exposed to increasing concentrations of cisplatin to generate a
stable subline with resistance to ≥80 µM cisplatin. CAR cells were
maintained in an environment of 5% CO2 at 37°C in DMEM
supplemented with 10% FBS, 2 mM L-glutamine, 100 µg/ml
streptomycin, 100 Units/ml penicillin and 80 µM cisplatin.
Cetuximab was diluted with cultured medium (DMEM with
supplementation as described above), and curcumin was dissolved in
dimethyl sulfoxide (DMSO).
Cytotoxicity assay
Cell viability was estimated by MTT assay. In brief,
CAR cells (1×104 cells/well) were plated in 96-well
tissue culture plates and treated with curcumin (10, 20, 40 or 50
µM), cetuximab (10, 20, 40 or 50 µg/ml) or 20 µg/ml cetuximab and
10, 20 or 40 µM curcumin for 24 h. Following exposure and removal
of the medium, the cells were cultured with 0.5 mg/ml MTT for an
additional 2 h. The blue formazan product was dissolved in 100 µl
DMSO and spectrophotometrically measured at a wavelength of 570 nm
using an ELISA plate reader (Anthos Labtec Instruments GmbH,
Salzburg, Austria), as previously described (35). The percentage of living cells was
calculated, and the ratio of optical density of the experimental
wells and control wells was calculated as % of control. Combination
index (CI) was determined using the Chou-Talalay method, as
previously described (36). A value
<1.0 indicated a synergistic effect.
Morphological determination
CAR cells (1×105 cells per well) were
seeded into a 24-well plate and treated with 20 µg/ml cetuximab and
10, 20 or 40 µM curcumin for 24 h. The cells were visualized using
a phase-contrast microscope to check for apoptotic characteristics
and photographed, as previously described (37).
Caspase-3 and −9 activity
measurement
CAR cells were seeded at a density of
5×106 cells per 75T flask and incubated with 20 µg/ml
cetuximab, 40 µM curcumin, or 20 µg/ml cetuximab and 40 µM curcumin
for 24 h. The cell lysate was collected, and the cell fraction was
analyzed for caspase-3/-9 activity using Caspase-3 and Caspase-9
Colorimetric Assay kits (R&D Systems, Inc., Minneapolis, MN,
USA), according to the manufacturer's protocol.
Western blot analysis
CAR cells (5×106 cells per 75T flask)
were treated with either 20 µg/ml cetuximab, 40 µM curcumin or both
for 24 h. Then, the cells were harvested and lysed with PRO-PREP
Protein Extraction Solution (iNtRON Biotechnology, Seongnam-si,
Gyeonggi-do, Korea). The protein concentration was determined using
the Pierce BCA protein assay kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and 40 µg protein was loaded per lane of 8–10%
SDS-PAGE gels. The protein was thereafter transferred into
Immobilon-P Transfer Membranes (Merck Millipore, Billerica, MA,
USA). Each membrane was blocked in 5% non-fat dry milk in
phosphate-buffered saline with Tween-20 (PBST; 8 mM
Na2HPO4, 0.15 M NaCl, 2 mM
KH2PO4, 3 mM KCl, 0.05% Tween-20, pH 7.4) for
1 h. The membraned were then incubated at 4°C overnight with
primary antibodies against p-EGFR (cat. no. GTX61353), EGFR (cat.
no. GTX100448), p-ERK (cat. no. GTX59568), ERK (cat. no. GTX59618),
p-JNK (cat. no. GTX52326), JNK (cat. no. GTX52360), p-p38 (cat. no.
GTX48614), p38 (cat. no. GTX110720) (all 1:1,000 dilution), and
β-actin (cat. no. GTX109639) (1:5,000 dilution) (GeneTex).
Following washing with PBST, the membrane was incubated with
appropriate anti-mouse (cat. No GTX213111-01)/-rabbit (cat. no.
GTX213110-01) HRP-conjugated secondary antibodies (1:10,000
dilution) for 1 h at room temperature. Proteins were visualized by
enhanced chemiluminescence (Immobilon Western Chemiluminescent HRP
Substrate; Merck Millipore) and using the LAS-4000 imaging system
(Fuji, Tokyo, Japan), as previously described (38–40). The
density of the immunoblots was analyzed using ImageJ (version 1.47;
National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
The values are presented as the mean ± standard
deviation of 3 independent experiments. Comparisons between the
drug-treated and -untreated groups were made using one-way analysis
of variance followed by Dunnett's test using SPSS software (version
16.0; SPSS, Inc., Chicago, IL, USA). P<0.001 was considered to
indicate a statistically significant difference.
Results
Effects of curcumin, cetuximab and
combination treatment on the viability of cisplatin-resistant oral
cancer CAR cells
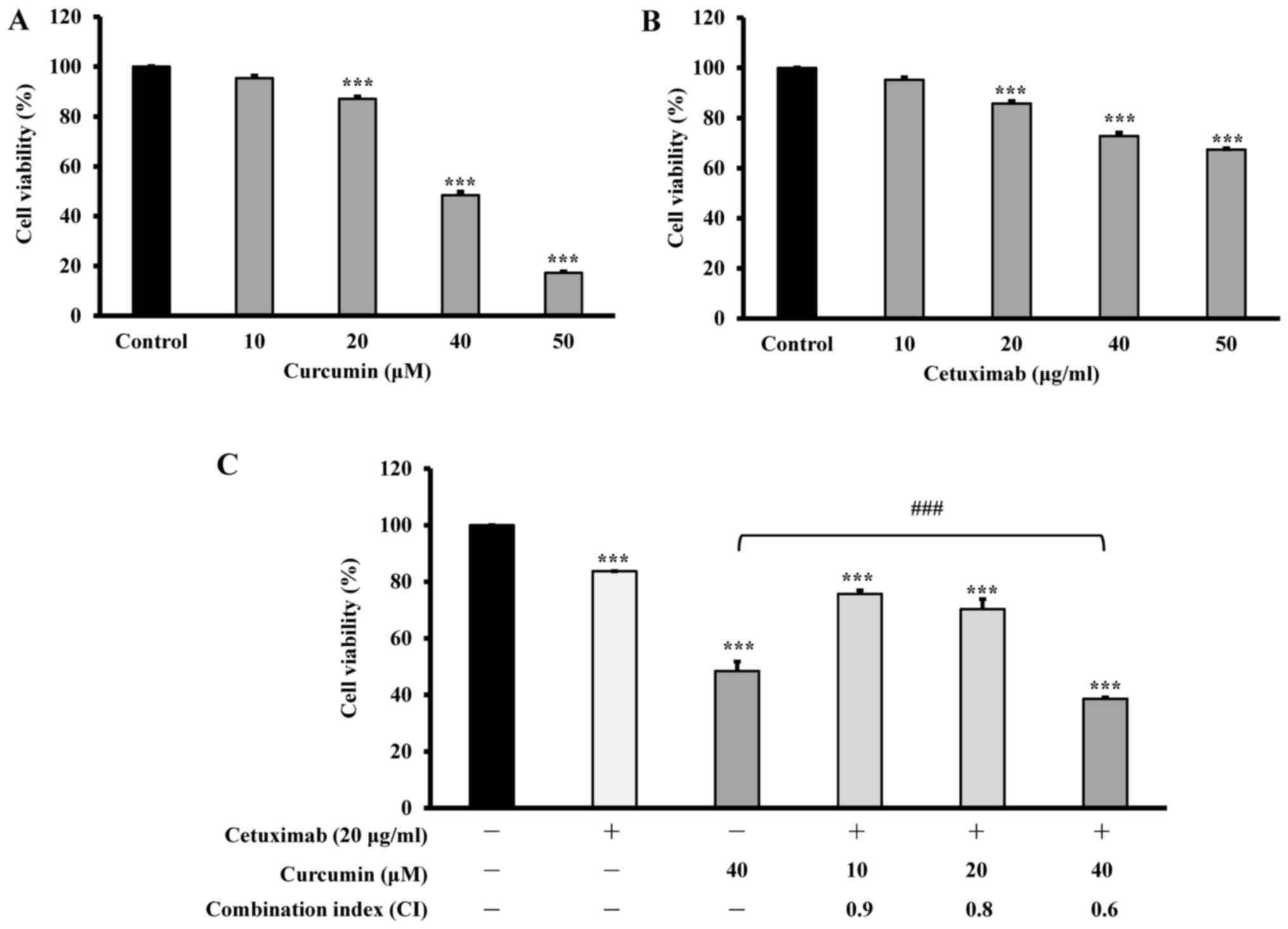
The cytotoxicity of curcumin and cetuximab on CAR
cells. The cells were cultured with various concentrations of
curcumin (10, 20, 40 or 50 µM), or cetuximab (10, 20, 40 or 50
µg/ml), or 20 µg/ml cetuximab combined with 10, 20 or 40 µM
curcumin, for 24 h. Cell viability was evaluated by MTT assay. The
results demonstrated that curcumin markedly decreased the viability
of CAR cells in a concentration-dependent manner, and the viability
at 20, 40 and 50 µM was 87.1, 48.5 and 12.3%, respectively
(Fig. 1A). It was also revealed that
20, 40 and 50 µg/ml cetuximab reduced CAR-cell viability to 85.8,
72.8 and 67.4%, respectively, another concentration-dependent
effect (Fig. 1B). However, 10 µg/ml
cetuximab demonstrated no significant inhibition. Thus, CAR cells
were more sensitive to curcumin than that to cetuximab. The cells
were treated with a combination of 20 µg/ml cetuximab and 0, 10, 20
and 40 µM curcumin for 24 h, and significant potentiation of
cytotoxicity and synergy of CAR cells was demonstrated by
viabilities of 83.7, 75.7, 70.3 and 38.6%, respectively (Fig. 1C). The combination index (CI) was 0.9,
0.8 and 0.6 at treatments of 20 µg/ml cetuximab and 10, 20 and 40
µM of curcumin, respectively, indicating the synergistic effects of
cetuximab and curcumin. The results imply that a significant
increase in cytotoxic effects was achieved with simultaneous
administration of cetuximab and curcumin to CAR cells.
Effects of curcumin and cetuximab,
alone or combined, on the morphology of CAR cells
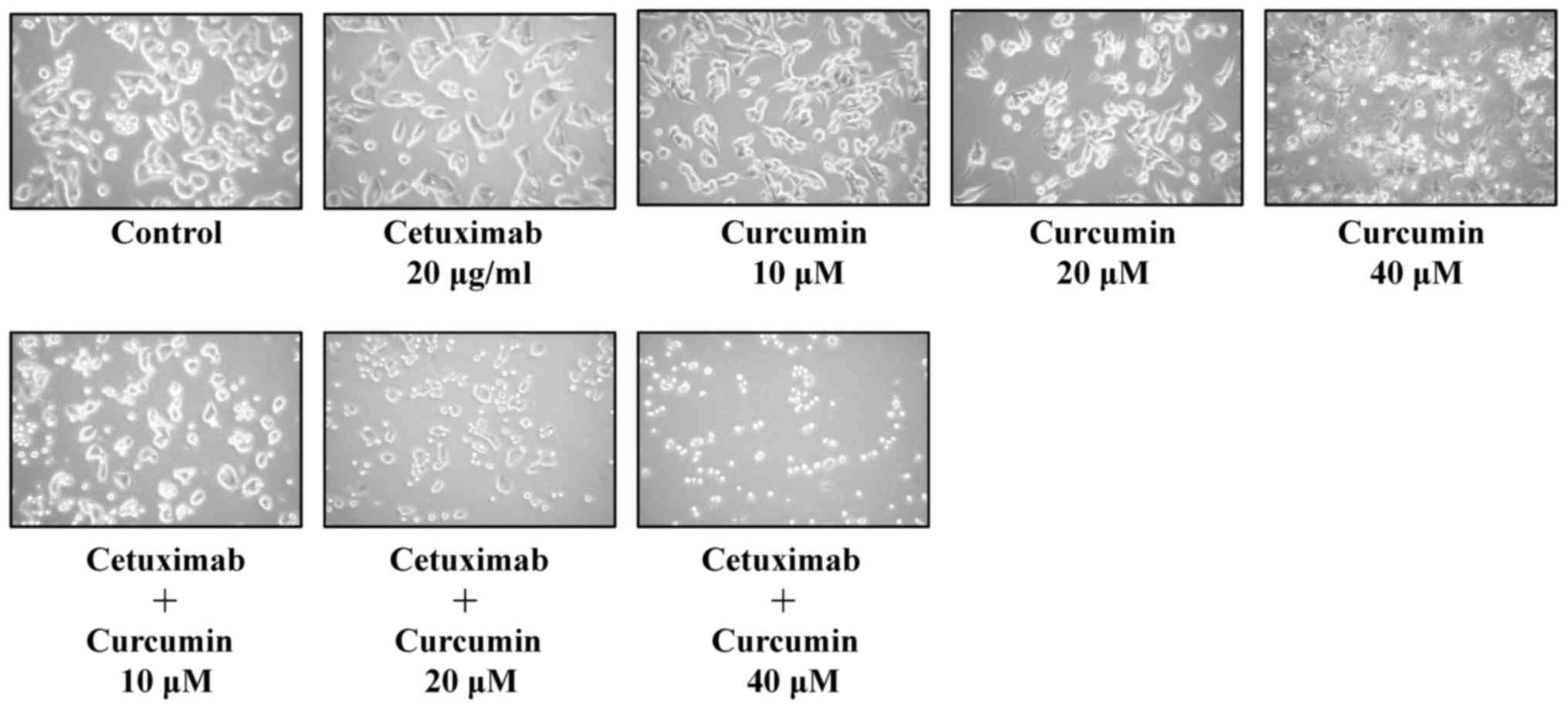
Photomicrographs demonstrated that combined
treatment resulted in cell shrinkage, cytoplasmic membrane blebbing
and cell death, compared with exposure to 20 µg/ml cetuximab alone
and untreated control (Fig. 2).
Furthermore, combined treatment resulted in increased inhibition in
viability of CAR cells compared with single-drug (cetuximab)
treatment and untreated control (Fig.
2). These data indicated that concurrent exposure to cetuximab
and curcumin synergistically induced apoptosis and reduced
proliferation of CAR cells.
Effects of curcumin and cetuximab,
alone or in combination, on caspase-3/-9-dependent apoptosis of CAR
cells
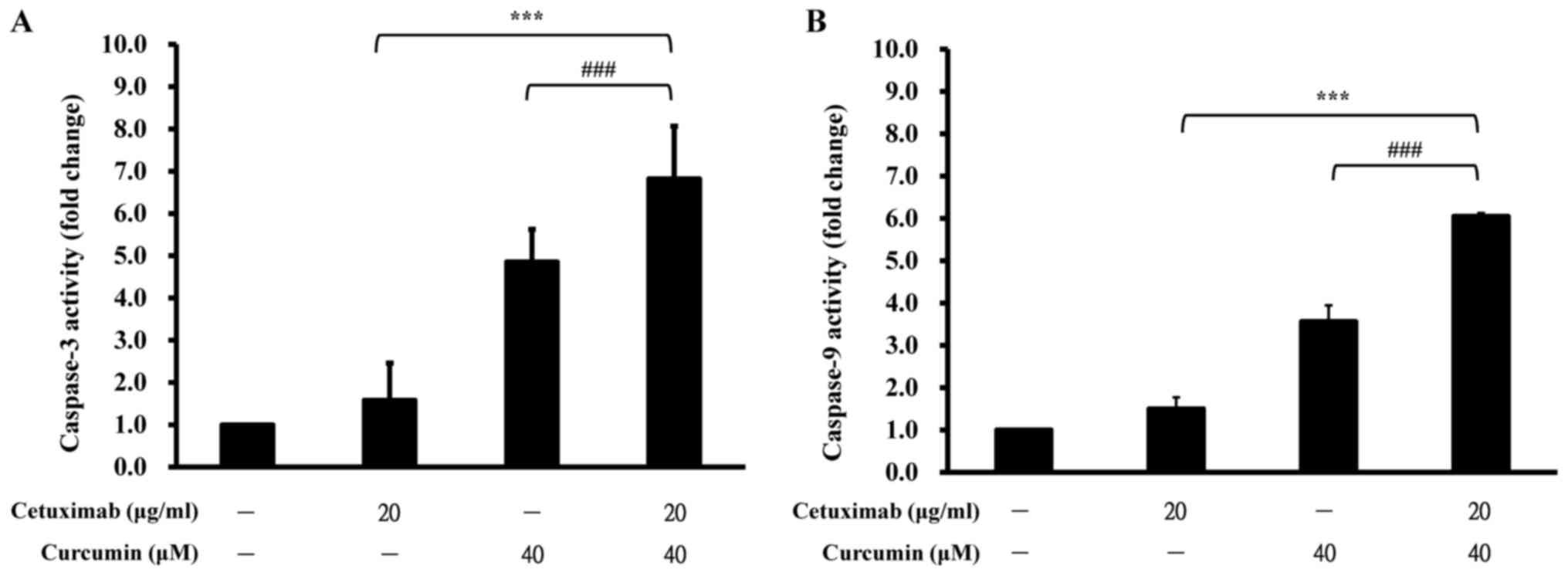
To further examine whether the observed suppression
of cell viability involved in apoptotic machinery, the cells were
treated with 20 µg/ml cetuximab, or 40 µM curcumin, or both, for 24
h prior to determination of caspase-3 and caspase-9 activities.
Individual treatment with cetuximab and curcumin induced 1.6- and
4.9-fold increases in caspase-3 activity compared with control,
whereas combination treatment stimulated a 6.8-fold increase in the
activity of caspase-3 of CAR cells (Fig.
3A). Similarly, caspase-9 activity was synergistically
increased in CAR cells when treated with cetuximab and curcumin
(6.0-fold increase; Fig. 3B).
However, either curcumin or cetuximab alone stimulated more minor
effects on caspase-9 activity, causing 1.5- and 3.6-fold increases
in CAR cells. These results indicate the synergistic cytotoxicity
of curcumin and cetuximab, and that the apoptotic mechanism was
caspase-3/-9-dependent in CAR cells.
Effect of curcumin and/or cetuximab
treatment on EGFR and MAPKs-regulated molecular signaling in CAR
cells
Cetuximab was reported to inhibit tumor growth,
invasion, angiogenesis and metastasis by binding to the
extracellular domain of EGFR by regulating the MAPKs pathway
(12,13). Furthermore, it has been documented
that colorectal cancer-cell resistance to cisplatin chemotherapy
may involve MAPK signaling (41). The
present study further analyzed the expression levels of EGFR and
the downstream MAPK pathway in CAR cells prior to cetuximab or
curcumin treatment, alone or in combination, using western blot
analysis. It was demonstrated that treatment combination treatment
effectively inhibited the phosphorylation of EGFR, but no effect on
total EGFR protein level was observed in CAR cells (Fig. 4). The levels of phosphorylated MAPKs
(ERK, JNK and p38) were also synergistically decreased compared
with cetuximab or curcumin treatment alone. However, there was no
effect on total ERK, JNK or p38 protein expression levels in any
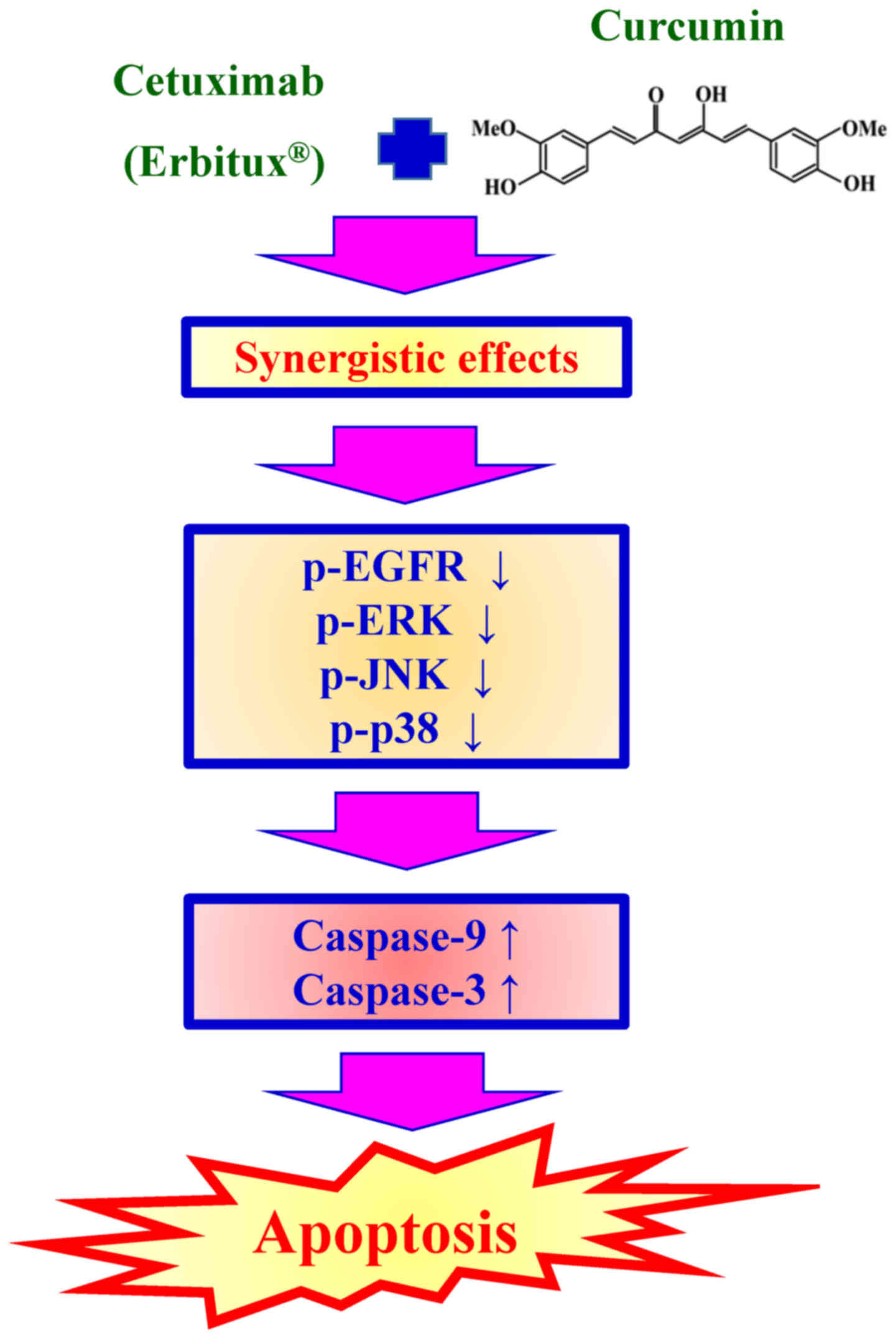
treatment group (Fig. 4). Taken
together, these results suggest that combined use of cetuximab and
curcumin triggered a dramatic increase in CAR-cell apoptosis by
suppressing EGFR and MAPKs signaling (Fig. 5), suggesting that the synergic effects
resulted in enhanced oral anticancer activity compared with
single-drug treatment in resistant oral cancer cells.
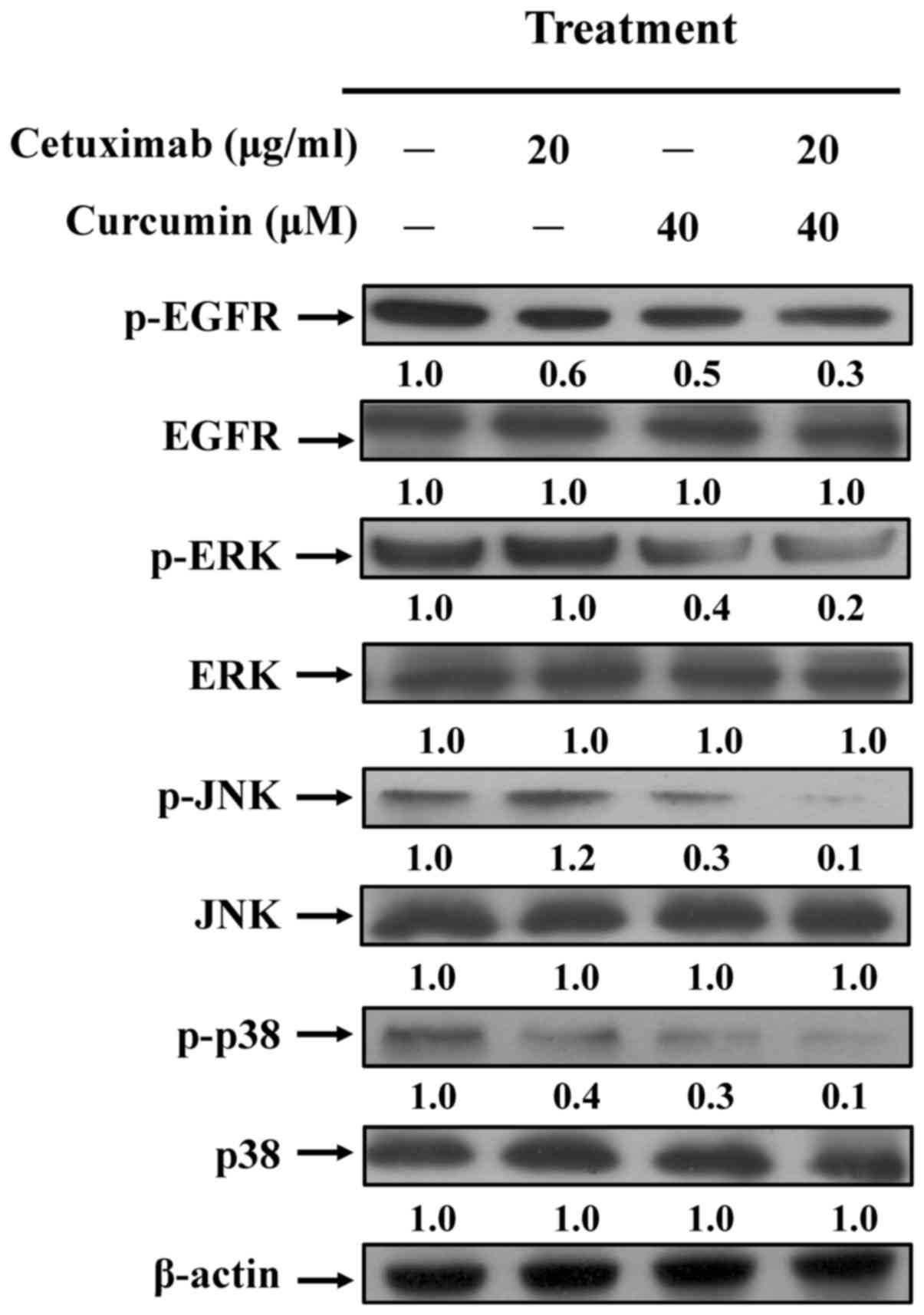 | Figure 4.Effects of curcumin and cetuximab on
EGFR and MAPK-regulated signaling in CAR cells. Protein expression
of p-EGFR, EFGR, p-ERK, ERK, p-JNK, JNK, p-p38 and p38 were
determined, and a β-actin control was applied to ensure equal
loading. Representative images were taken from 3 independent
experiments. EGFR, epidermal growth factor receptor; MAPK, mitogen
activated protein kinase; p-, phosphorylated; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase. |
Discussion
Surgery and brachytherapy are the major therapies
for oral cancer in the T1, T2 and artificial T3 groups
(Tumor-Node-Metastasis classification) in clinical practice
guidelines for head and neck cancer in Japan (42). Platinum-based chemotherapy (cisplatin
or carboplatin) is used for advanced stage cancer (42,43). In
1978, cisplatin was approved by the Food and Drug Administration
(FDA) for oral cancer treatment (44). Cisplatin is used for oral cancer
chemotherapy and functions via direct reaction with cellular
nucleophiles to achieve inter- and intra-stand DNA cross-links and
protein cross-links with DNA and RNA (45). However, oral cancer cells have gained
resistance to chemotherapeutic agents (46,47).
Several molecular mechanisms are involved in cisplatin-resistance:
i) Increased activity of transporter protein function (MDR1 or
p-glycoprotein); ii) activated drug metabolism activity by enzymes;
iii) decreased drug binding to DNA; iv) promoted ROS production; v)
stimulated DNA repair; vi) increased tolerance to DNA damage; vii)
altered transcription of target genes; viii) changes in cell
cycle-associated events and ix) inhibition of cell death (46–48).
Recently, EGFR has been demonstrated as an important therapeutic
target in oral cancer, and it is expressed more highly in oral
cancer tissue than in normal tissues (7,10). In
addition, a correlation between high EGFR expression and
radio-resistance was demonstrated in patients with oral cancer
(49). Kuroda et al (50) demonstrated that cisplatin-resistance
is associated with EGFR-mediated signaling in lung cancer A549
cells. Chemo-sensitivity to cisplatin was restored by an
EGFR-selective tyrosine kinase inhibitor (AG1478) in A549 cells,
suggesting that the EGFR inhibitor may be a therapy for
cisplatin-resistance (50). To the
best of our knowledge, this is the first study to report the
synergistic inhibitory effect of cetuximab (an EGFR inhibitor) and
curcumin in cisplatin-resistant human oral cancer cells.
Evidence indicates that the molecular mechanisms of
cetuximab anticancer activity take 2 forms (51,52).
Firstly, EGF binding to the EGFR extracellular domain to inhibit
subsequent receptor dimerization/activation and to induce EGFR
degradation is inhibited. Secondly, antibody-dependent cellular
cytotoxicity or complement-dependent cell-mediated cytotoxicity
(51,52). Curcumin has been historically used in
traditional Chinese medicine, and its anticancer effects on various
types of solid cancers, such as colon cancer, multiple myeloma and
pancreatic cancer have reached phase II and III clinical trials
(53,54). Curcumin is also a potential
therapeutic agent for the treatment of diabetic patients (55,56). It
has been reported that curcumin is able to directly but partially
inhibit the enzymatic activity of the EGFR intracellular domain.
Inhibition of EGFR phosphorylation and induction of EGFR
ubiquitination then block the EGFR signaling pathway in cancer
cells (57). However, it has not been
reported whether combined cetuximab with curcumin synergistically
inhibit drug-resistant oral cancer-cell proliferation. The present
results demonstrated that a combined treatment of cetuximab and
curcumin synergistically inhibited cell viability (Figs. 1B and 2), induced cell death (Fig. 2), and stimulated caspase-3 and
caspase-9 activities (Fig. 3).
Furthermore, curcumin dramatically enhanced cetuximab-suppressed
phosphorylation of EGFR, ERK, JNK, and p38 levels in CAR cells
(Fig. 4). A previous study by Son
et al (58) demonstrated the
effect of cetuximab combined with cisplatin on colon cancer growth.
Cetuximab significantly decreased phosphorylation of EGFR and
phosphorylated p38/p38 ratio at 30 µg/ml, but there was no effect
on the phosphorylated ERK/ERK ratio in colon cancer HCT116 cells.
The present study also demonstrated that cetuximab treatment
decreased the protein level of phosphorylated EGFR and
phosphorylated p38. In addition, Li et al (59) demonstrated that the EGFR monoclonal
antibody, cetuximab, mildly evoked apoptosis of human vulvar
squamous carcinoma A431 cells. This is consistent with the present
finding that cetuximab triggered a non-significant increase in
caspase-3 and caspase-9 activities in CAR cells. However, the
activities of caspase-3 and caspase-9 were dramatically enhanced in
CAR cells prior to treatment with cetuximab in combination with
curcumin; or with exposure only to curcumin. The present results
are also consistent with previous studies (26,31)
showing that curcumin is effective against various types of cancer
via intrinsic apoptotic function. Although curcumin possesses
powerful biological activities, it does not reach the criteria of a
good drug candidate because it lacks adequate water solubility and
high bioavailability, and undergoes rapid in vivo metabolism
(60). To overcome these limitations,
novel forms of curcumin targeting, including nanoparticles,
liposomes, cyclodextrin encapsulation, micelles and phospholipid
complexes, have been synthesized and tested in recent years
(61,62).
In conclusion, combined cetuximab and curcumin
treatment is a novel therapeutic option for oral cancer treatment,
exhibiting synergistic anti-proliferative activity. The mechanism
results in a decreased activated EGFR level in cisplatin-resistant
oral cancer cells. With the results presented in the present study,
curcumin could be used as an adjuvant drug, and the combination of
cetuximab and curcumin may be a strategy to pursue in clinical
trials.
Acknowledgements
The authors would like to thank Dr Hong-Yi Chiu of
Department of Pharmacy, Hualien Tzu Chi Hospital (Taiwan) for
providing cetuximab (Erbitux) as a gift.
Funding
The present study was supported by China Medical
University Hospital (grant no. DMR-107-1230).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CFC, HJH and TDW conceived and designed the
experiments; CFC, CCL, JHC, HYC, JSY, and CYL performed the
experiments. CFC, CCL, JHC, and JSY analysed the data; CFC, HJH and
TDW wrote and modified the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Horn D, Hess J, Freier K, Hoffmann J and
Freudlsperger C: Targeting EGFR-PI3K-AKT-mTOR signaling enhances
radiosensitivity in head and neck squamous cell carcinoma. Expert
Opin Ther Targets. 19:795–805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prince A, Aguirre-Ghizo J, Genden E,
Posner M and Sikora A: Head and neck squamous cell carcinoma: New
translational therapies. Mt Sinai J Med. 77:684–699. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mendelsohn J and Baselga J: Epidermal
growth factor receptor targeting in cancer. Semin Oncol.
33:369–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Todd R and Wong DT: Epidermal growth
factor receptor (EGFR) biology and human oral cancer. Histol
Histopathol. 14:491–500. 1999.PubMed/NCBI
|
|
5
|
Pickhard A, Siegl M, Baumann A, Huhn M,
Wirth M, Reiter R, Rudelius M, Piontek G and Brockhoff G: The
response of head and neck squamous cell carcinoma to cetuximab
treatment depends on Aurora kinase A polymorphism. Oncotarget.
5:5428–5438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin MC, Huang MJ, Liu CH, Yang TL and
Huang MC: GALNT2 enhances migration and invasion of oral squamous
cell carcinoma by regulating EGFR glycosylation and activity. Oral
Oncol. 50:478–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cassell A and Grandis JR: Investigational
EGFR-targeted therapy in head and neck squamous cell carcinoma.
Expert Opin Investig Drugs. 19:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoch MA, Cousins K, Nartey R, Riley K and
Hartranft M: Two cases of combination therapy with cetuximab,
paclitaxel, and cisplatin for advanced head and neck cancer. J
Oncol Pharm Pract 1078155217722406. 2017.
|
|
9
|
Liebig H, Günther G, Kolb M, Mozet C,
Boehm A, Dietz A and Wichmann G: Reduced proliferation and colony
formation of head and neck squamous cell carcinoma (HNSCC) after
dual targeting of EGFR and hedgehog pathways. Cancer Chemother
Pharmacol. 79:411–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moon C, Chae YK and Lee J: Targeting
epidermal growth factor receptor in head and neck cancer: Lessons
learned from cetuximab. Exp Biol Med (Maywood). 235:907–920. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Astsaturov I, Cohen RB and Harari P:
EGFR-targeting monoclonal antibodies in head and neck cancer. Curr
Cancer Drug Targets. 6:691–710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai W, Li Y, Zhou Q, Xu Z, Sun C, Tan X
and Lu L: Cetuximab inhibits oral squamous cell carcinoma invasion
and metastasis via degradation of epidermal growth factor receptor.
J Oral Pathol Med. 43:250–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Psyrri A, Seiwert TY and Jimeno A:
Molecular pathways in head and neck cancer: EGFR, PI3K, and more.
Am Soc Clin Oncol Educ Book. 246–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Astsaturov I, Cohen RB and Harari P:
EGFR-targeting monoclonal antibodies in head and neck cancer. Curr
Cancer Drug Targets. 7:650–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burgy M, Barthélémy P, Lefevre F,
Dupret-Bories A, Truntzer P, Korenbaum C, Flesch H, Bronner G and
Borel C: Cetuximab-carboplatin-5-fluorouracil regimen in elderly
patients with recurrent or metastatic head and neck squamous-cell
carcinoma: A French retrospective survey. Oncology. 93:11–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guigay J, Even C, Mayache-Badis L, Debbah
M, Saada-Bouzid E, Tao Y, Deschamps F, Janot F, Lezghed N and
Michel C: Long-term response in patient with recurrent
oropharyngeal carcinoma treated with cetuximab, docetaxel and
cisplatin (TPEx) as first-line treatment followed by cetuximab
maintenance. Oral Oncol. 68:114–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Unlu A, Nayir E, Kalenderoglu Dogukan M,
Kirca O and Ozdogan M: Curcumin (Turmeric) and cancer. J BUON.
21:1050–1060. 2016.PubMed/NCBI
|
|
18
|
He Y, Yue Y, Zheng X, Zhang K, Chen S and
Du Z: Curcumin, inflammation, and chronic diseases: How are they
linked? Molecules. 20:9183–9213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J and Jiang YF: Natural compounds as
anticancer agents: Experimental evidence. World J Exp Med. 2:45–57.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen ZF and Liang H: Progresses in TCM
metal-based antitumour agents. Anticancer Agents Med Chem.
10:412–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Devi Pandima K, Rajavel T, Daglia M,
Nabavi SF, Bishayee A and Nabavi SM: Targeting miRNAs by
polyphenols: Novel therapeutic strategy for cancer. Semin Cancer
Biol. 46:146–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Owen HC, Appiah S, Hasan N, Ghali L,
Elayat G and Bell C: Phytochemical modulation of apoptosis and
autophagy: Strategies to overcome chemoresistance in leukemic stem
cells in the bone marrow microenvironment. Int Rev Neurobiol.
135:249–278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karimian MS, Pirro M, Johnston TP, Majeed
M and Sahebkar A: Curcumin and endothelial function: Evidence and
mechanisms of protective effects. Curr Pharm Des. 23:2462–2473.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jha A, Mohapatra PP, AlHarbi SA and Jahan
N: Curcumin: Not so spicy after all. Mini Rev Med Chem.
17:1425–1434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng SF, Lee CY, Hour MJ, Tsai SC, Kuo DH,
Chen FA, Shieh PC and Yang JS: Curcumin-loaded nanoparticles
enhance apoptotic cell death of U2OS human osteosarcoma cells
through the Akt-Bad signaling pathway. Int J Oncol. 44:238–246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Borges GÁ, Rêgo DF, Assad DX, Coletta RD,
De Luca Canto G and Guerra EN: In vivo and in vitro effects of
curcumin on head and neck carcinoma: A systematic review. J Oral
Pathol Med. 46:3–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vander Broek R, Snow GE, Chen Z and Van
Waes C: Chemoprevention of head and neck squamous cell carcinoma
through inhibition of NF-kB signaling. Oral Oncol. 50:930–941.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agrawal DK and Mishra PK: Curcumin and its
analogues: Potential anticancer agents. Med Res Rev. 30:818–860.
2010.PubMed/NCBI
|
|
29
|
Lin JK: Molecular targets of curcumin. Adv
Exp Med Biol. 595:227–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fetoni AR, Paciello F, Mezzogori D, Rolesi
R, Eramo SL, Paludetti G and Troiani D: Molecular targets for
anticancer redox chemotherapy and cisplatin-induced ototoxicity:
The role of curcumin on pSTAT3 and Nrf-2 signalling. Br J Cancer.
113:1434–1444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sivanantham B, Sethuraman S and Krishnan
UM: Combinatorial effects of curcumin with an anti-neoplastic agent
on head and neck squamous cell carcinoma through the regulation of
EGFR-ERK1/2 and apoptotic signaling pathways. ACS Comb Sci.
18:22–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gosepath EM, Eckstein N, Hamacher A,
Servan K, von Jonquieres G, Lage H, Györffy B, Royer HD and Kassack
MU: Acquired cisplatin resistance in the head-neck cancer cell line
Cal27 is associated with decreased DKK1 expression and can
partially be reversed by overexpression of DKK1. Int J Cancer.
123:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang PY, Peng SF, Lee CY, Lu CC, Tsai SC,
Shieh TM, Wu TS, Tu MG, Chen MY and Yang JS: Curcumin-loaded
nanoparticles induce apoptotic cell death through regulation of the
function of MDR1 and reactive oxygen species in cisplatin-resistant
CAR human oral cancer cells. Int J Oncol. 43:1141–1150. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee MR, Lin C, Lu CC, Kuo SC, Tsao JW,
Juan YN, Chiu HY, Lee FY, Yang JS and Tsai FJ: YC-1 induces
G0/G1 phase arrest and mitochondria-dependent
apoptosis in cisplatin-resistant human oral cancer CAR cells.
Biomedicine (Taipei). 7:122017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: Dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840:2310–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu Y, Xin Y, Diao Y, Lu C, Fu J, Luo L and
Yin Z: Synergistic effects of apigenin and paclitaxel on apoptosis
of cancer cells. PLoS One. 6:e291692011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu CC, Huang BR, Liao PJ and Yen GC:
Ursolic acid triggers nonprogrammed death (necrosis) in human
glioblastoma multiforme DBTRG-05MG cells through MPT pore opening
and ATP decline. Mol Nutr Food Res. 58:2146–2156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH,
Yang JS, Lai KC, Lin JP, Tang NY, Lin JG and Chung JG: Antitumor
effects of emodin on LS1034 human colon cancer cells in vitro and
in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts
model. Food Chem Toxicol. 50:1271–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF,
Li KH, Chen PY, Chung JG and Yang JS: Kaempferol induced apoptosis
via endoplasmic reticulum stress and mitochondria-dependent pathway
in human osteosarcoma U-2 OS cells. Mol Nutr Food Res.
54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grossi V, Peserico A, Tezil T and Simone
C: p38alpha MAPK pathway: A key factor in colorectal cancer therapy
and chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nibu KI, Hayashi R, Asakage T, Ojiri H,
Kimata Y, Kodaira T, Nagao T, Nakashima T, Fujii T, Fujii H, et al:
Japanese clinical practice guideline for head and neck cancer.
Auris Nasus Larynx. 44:375–380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brockstein BE and Vokes EE: Oral
chemotherapy in head and neck cancer. Drugs. 58 Suppl 3:S91–S97.
1999. View Article : Google Scholar
|
|
44
|
Köberle B, Tomicic MT, Usanova S and Kaina
B: Cisplatin resistance: Preclinical findings and clinical
implications. Biochim Biophys Acta. 1806:172–182. 2010.PubMed/NCBI
|
|
45
|
Carlsson L, Bratman SV, Siu LL and
Spreafico A: The cisplatin total dose and concomitant radiation in
locoregionally advanced head and neck cancer: Any recent evidence
for dose efficacy? Curr Treat Options Oncol. 18:392017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang C, Liu XQ, Hou JS, Wang JN and Huang
HZ: Molecular mechanisms of chemoresistance in oral cancer. Chin J
Dent Res. 19:25–33. 2016.PubMed/NCBI
|
|
47
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brozovic A: The relationship between
platinum drug resistance and epithelial-mesenchymal transition.
Arch Toxicol. 91:605–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alorabi M, Shonka NA and Ganti AK: EGFR
monoclonal antibodies in locally advanced head and neck squamous
cell carcinoma: What is their current role? Crit Rev Oncol Hematol.
99:170–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kuroda H, Takeno M, Murakami S, Miyazawa
N, Kaneko T and Ishigatsubo Y: Inhibition of heme oxygenase-1 with
an epidermal growth factor receptor inhibitor and cisplatin
decreases proliferation of lung cancer A549 cells. Lung Cancer.
67:31–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Seo Y, Ishii Y, Ochiai H, Fukuda K,
Akimoto S, Hayashida T, Okabayashi K, Tsuruta M, Hasegawa H and
Kitagawa Y: Cetuximab-mediated ADCC activity is correlated with the
cell surface expression level of EGFR but not with the KRAS/BRAF
mutational status in colorectal cancer. Oncol Rep. 31:2115–2122.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kondo N, Tsukuda M, Sakakibara A,
Takahashi H, Hyakusoku H, Komatsu M, Niho T, Nakazaki K and Toth G:
Combined molecular targeted drug therapy for EGFR and HER-2 in head
and neck squamous cell carcinoma cell lines. Int J Oncol.
40:1805–1812. 2012.PubMed/NCBI
|
|
53
|
Ji JL, Huang XF and Zhu HL: Curcumin and
its formulations: Potential anti-cancer agents. Anticancer Agents
Med Chem. 12:210–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shehzad A, Wahid F and Lee YS: Curcumin in
cancer chemoprevention: Molecular targets, pharmacokinetics,
bioavailability, and clinical trials. Arch Pharm (Weinheim).
343:489–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qadir MI, Naqvi ST and Muhammad SA:
Curcumin: A polyphenol with molecular targets for cancer control.
Asian Pac J Cancer Prev. 17:2735–2739. 2016.PubMed/NCBI
|
|
56
|
Weisberg S, Leibel R and Tortoriello DV:
Proteasome inhibitors, including curcumin, improve pancreatic
β-cell function and insulin sensitivity in diabetic mice. Nutr
Diabetes. 6:e2052016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee JY, Lee YM, Chang GC, Yu SL, Hsieh WY,
Chen JJ, Chen HW and Yang PC: Curcumin induces EGFR degradation in
lung adenocarcinoma and modulates p38 activation in intestine: The
versatile adjuvant for gefitinib therapy. PLoS One. 6:e237562011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Son DJ, Hong JE, Ban JO, Park JH, Lee HL,
Gu SM, Hwang JY, Jung MH, Lee DW, Han SB and Hong JT: Synergistic
inhibitory effects of cetuximab and cisplatin on human colon cancer
cell growth via inhibition of the ERK-dependent EGF receptor
signaling pathway. Biomed Res Int. 2015:3975632015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li X, Lu Y, Pan T and Fan Z: Roles of
autophagy in cetuximab-mediated cancer therapy against EGFR.
Autophagy. 6:1066–1077. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Douglass BJ and Clouatre DL: Beyond yellow
curry: Assessing commercial curcumin absorption technologies. J Am
Coll Nutr. 34:347–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chang LC, Hsieh MT, Yang JS, Lu CC, Tsai
FJ, Tsao JW, Chiu YJ, Kuo SC and Lee KH: Effect of
bis(hydroxymethyl) alkanoate curcuminoid derivative MTH-3 on cell
cycle arrest, apoptotic and autophagic pathway in triple-negative
breast adenocarcinoma MDA-MB-231 cells: An in vitro study. Int J
Oncol. 52:67–76. 2018.PubMed/NCBI
|
|
62
|
Hsieh MT, Chang LC, Hung HY, Lin HY, Shih
MH, Tsai CH, Kuo SC and Lee KH: New bis(hydroxymethyl) alkanoate
curcuminoid derivatives exhibit activity against triple-negative
breast cancer in vitro and in vivo. Eur J Med Chem. 131:141–151.
2017. View Article : Google Scholar : PubMed/NCBI
|