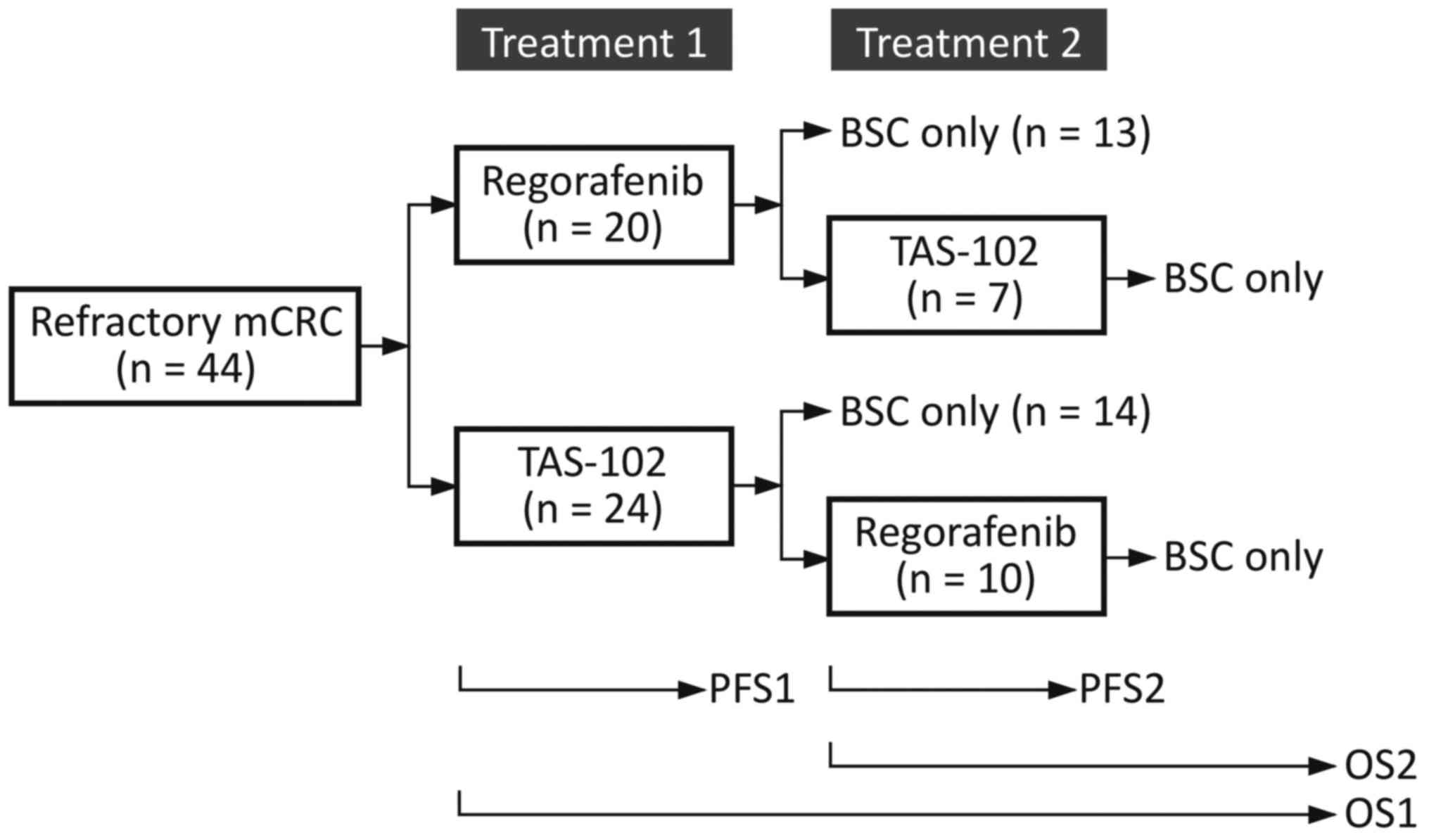
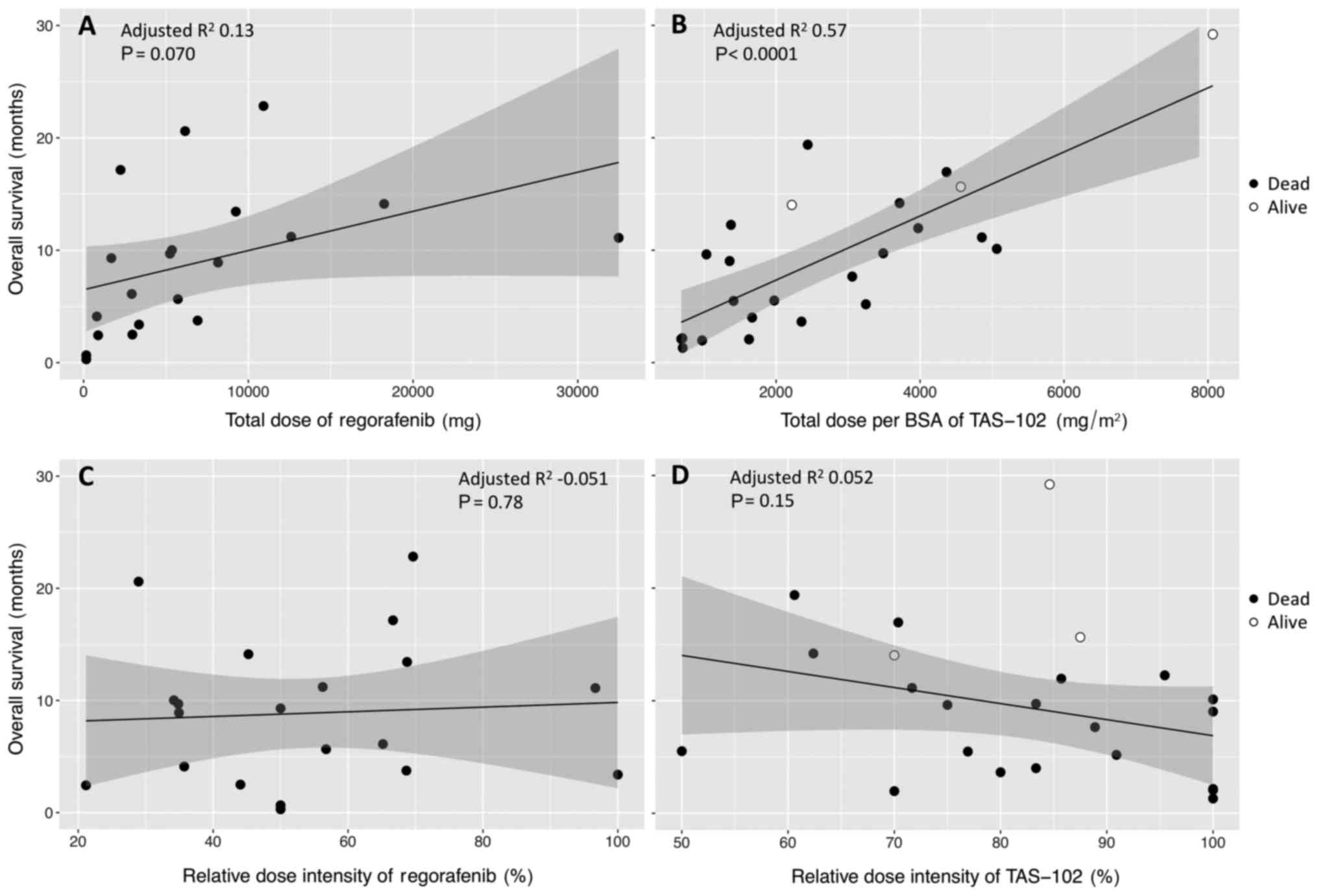
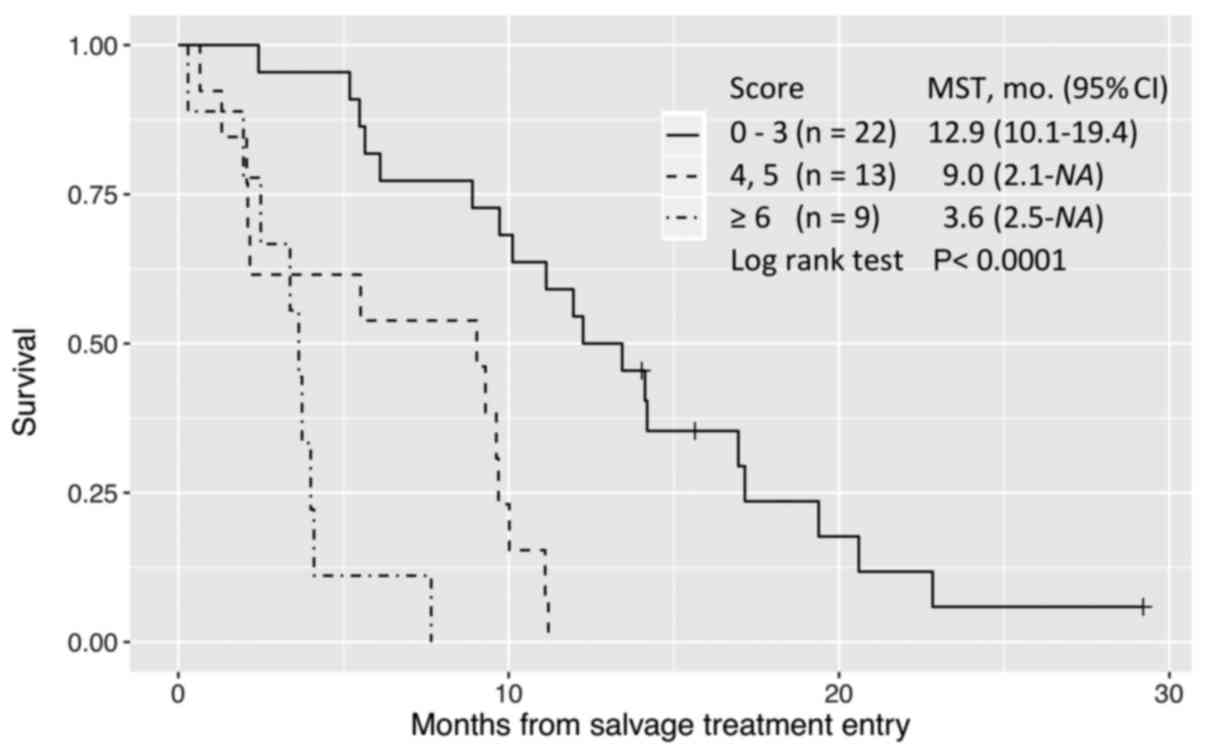
|
1
|
Howlader N, Noone A, Krapcho M, Garshell
J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
et al: SEER Cancer Statistics Review, 1975–2012. National Cancer
Institute; Bethesda, MD, USA: 2015
|
|
2
|
Abrams TA, Meyer G, Schrag D, Meyerhardt
JA, Moloney J and Fuchs CS: Chemotherapy usage patterns in a
US-wide cohort of patients with metastatic colorectal cancer. J
Natl Cancer Inst. 106:djt3712014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73–4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lenz HJ, Stintzing S and Loupakis F:
TAS-102, a novel antitumor agent: A review of the mechanism of
action. Cancer Treat Rev. 41:777–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crawford J, Becker PS, Armitage JO,
Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I,
Griffiths EA, et al: Myeloid growth factors, version 2.2017, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
15:1520–1541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aguilar Aranda E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu
J, Bai Y, Chi Y, Wang L, et al: Regorafenib plus best supportive
care versus placebo plus best supportive care in Asian patients
with previously treated metastatic colorectal cancer (CONCUR): A
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 16:619–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu J, Kim TW, Shen L, Sriuranpong V, Pan
H, Xu R, Guo W, Han SW, Liu T, Park YS, et al: Results of a
randomized, double-blind, placebo-controlled, phase III trial of
trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with
previously treated metastatic colorectal cancer: The TERRA study. J
Clin Oncol. 36:350–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moriwaki T, Fukuoka S, Taniguchi H,
Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama
C, Denda T, et al: Propensity score analysis of regorafenib versus
trifluridine/tipiracil in patients with metastatic colorectal
cancer refractory to standard chemotherapy (REGOTAS): A Japanese
society for cancer of the colon and rectum multicenter
observational study. Oncologist. 23:7–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
García-Alfonso P, Feliú J,
García-Carbonero R, Grávalos C, Guillén-Ponce C, Sastre J and
García-Foncillas J: Is regorafenib providing clinically meaningful
benefits to pretreated patients with metastatic colorectal cancer?
Clin Transl Oncol. 18:1072–1081. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hryniuk W and Bush H: The importance of
dose intensity in chemotherapy of metastatic breast cancer. J Clin
Oncol. 2:1281–1288. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Health and Human Services, . National
Institutes of Health and National Cancer Institute: Common
terminology criteria for adverse events (CTCAE). v4.03.
2013.https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdfAugust
20–2013
|
|
16
|
Wilkinson GN and Rogers CE: Symbolic
description of factorial models for analysis of variance. Appl
Stat-J R Stat Soc. 22:392–399. 1973.
|
|
17
|
Harrell FE Jr, Lee KL and Mark DB:
Multivariable prognostic models: Issues in developing models,
evaluating assumptions and adequacy, and measuring and reducing
errors. Stat Med. 15:361–387. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pencina MJ and D'Agostino RB: Overall C as
a measure of discrimination in survival analysis: Model specific
population value and confidence interval estimation. Stat Med.
23:2109–2123. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
R Development Core Team, . R: A language
and environment for statistical computingR Foundation for
Statistical Computing. Vienna, Austria: 2016
|
|
20
|
Masuishi T, Taniguchi H, Hamauchi S,
Komori A, Kito Y, Narita Y, Tsushima T, Ishihara M, Todaka A,
Tanaka T, et al: Regorafenib versus trifluridine/tipiracil for
refractory metastatic colorectal cancer: A retrospective
comparison. Clin Colorectal Cancer. 16:e15–e22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sueda T, Sakai D, Kudo T, Sugiura T,
Takahashi H, Haraguchi N, Nishimura J, Hata T, Hayashi T, Mizushima
T, et al: Efficacy and safety of regorafenib or TAS-102 in patients
with metastatic colorectal cancer refractory to standard therapies.
Anticancer Res. 36:4299–4306. 2016.PubMed/NCBI
|
|
22
|
Adenis A, de la Fouchardiere C, Paule B,
Burtin P, Tougeron D, Wallet J, Dourthe LM, Etienne PL, Mineur L,
Clisant S, et al: Survival, safety, and prognostic factors for
outcome with Regorafenib in patients with metastatic colorectal
cancer refractory to standard therapies: Results from a multicenter
study (REBECCA) nested within a compassionate use program. BMC
Cancer. 16:4122016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bekaii-Saab TS, Ou FS, Anderson DM, Ahn
DH, Boland PM, Ciombor KK, Jacobs NL, Desnoyers RJ, Cleary JM,
Meyers JP, et al: Regorafenib dose optimization study (ReDOS):
Randomized phase II trial to evaluate dosing strategies for
regorafenib in refractory metastatic colorectal cancer (mCRC)- An
ACCRU network study. J Clin Oncol. 36 4 Suppl:S6112018.
|
|
24
|
Osawa H: Response to regorafenib at an
initial dose of 120 mg as salvage therapy for metastatic colorectal
cancer. Mol Clin Oncol. 6:365–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grothey A, Falcone A, Humblet Y, Bouche O,
Mineur L, Adenis A, Tabernero J, Yoshino T, Lenz HJ and Goldberg
RM: Characteristics of patients (pts) with metastatic colorectal
cancer (mCRC) treated with regorafenib (REG) who had
progression-free survival (PFS) >4 months (m): Subgroup analysis
of the phase 3 CORRECT trial. Ann Oncol. 27 Suppl 6:S516P2016.
View Article : Google Scholar
|
|
26
|
Puthiamadathil JM and Weinberg BA:
Emerging combination therapies for metastatic colorectal cancer -
impact of trifluridine/tipiracil. Cancer Manag Res. 9:461–469.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Austin PC: The performance of different
propensity score methods for estimating marginal hazard ratios.
Stat Med. 32:2837–2849. 2013. View
Article : Google Scholar : PubMed/NCBI
|