Introduction
Colorectal cancer is the fourth most common cancer
diagnosed in the United States, accounting for 8% of all new cancer
cases (1). It is estimated that there
were 132,700 new cases of colorectal cancer, and an estimated
49,700 people died of this disease in the US in 2015. Among 4,877
patients who received first-line chemotherapy for metastatic
colorectal cancer (mCRC), identified in a nationwide and
commercially available chemotherapy order entry system from 2004 to
2011, 53% (n=2,575) received second-line treatment, 28% (n=1,373)
received third-line treatment, and only 13% (n=640) received
fourth-line treatment (2). Patients
with mCRC infrequently go on to receive third-line or later
treatment, and this might negatively impact on their overall
survival (OS).
Regorafenib (Stivarga®, Bayer AG,
Leverkusen, Germany) is an oral multikinase inhibitor that blocks
the activity of several protein kinases associated with
angiogenesis [vascular endothelial growth factor (VEGF) receptors
1–3 and TIE2], oncogenesis (KIT, RET, RAF1 and BRAF), and the tumor
microenvironment (PDGF receptor and FGF receptor) (3).
Trifluridine/tipiracil (TAS-102;
Lonsurf®; Taiho Pharmaceutical Co. Ltd, Tokyo) is an
orally administered combination of a thymidine-based nucleic acid
analogue, trifluridine, and a thymidine phosphorylase inhibitor,
tipiracil hydrochloride. Trifluridine is the active cytotoxic
component of TAS-102; its triphosphate form is incorporated into
DNA, and this appears to result in its antitumor effects (4). Tipiracil hydrochloride is a potent
inhibitor of thymidine phosphorylase, and serves to prevent the
rapid degradation of trifluridine in TAS-102, providing more
prolonged maintenance of adequate plasma levels of the active
drug.
Regorafenib and TAS-102 are new salvage-line
treatment options (5,6), which provided statistically significant
improvements of OS, progression-free survival (PFS), and disease
control in placebo-controlled randomized phase III trials (CORRECT
(7), CONCUR (8), RECOURSE (9) and TERRA) (10). Despite this evidence, these two drugs
are often considered not clinically meaningful for patients based
on the relatively small incremental benefits for OS and PFS.
A multicenter observational study (REGOTAS)
(11) has recently demonstrated the
clinical benefit and tolerability of these drugs in real-life
clinical practice, and criteria to choose between regorafenib or
TAS-102. However, the following issues remain to be established
(12): The appropriate way of
administration for patients with advanced disease, and which
subpopulations of patients might derive the greatest benefit from
salvage-line treatment with these drugs, compared to best
supportive care only. To address these questions, we conducted a
retrospective cohort study to evaluate the efficacy and safety of
regorafenib and TAS-102 in patients with refractory mCRC with the
aim of assessing their practical value as salvage-line therapy. A
post-hoc exploratory subgroup analysis was carried out to obtain
predictive scores for survival benefit in patients treated with
these regimens.
Patients and methods
Patients
Patients with unresectable mCRC were eligible for
the study if they had received at least two prior regimens of
standard chemotherapies. All patients had been treated at Tokai
University Hospital (Kanagawa, Japan) between June 2013 and
November 2015, after the approval of each drug for medical
reimbursement under the national insurance scheme in Japan
(regorafenib and TAS-102 were approved in May 2013 and May 2014,
respectively). The eligibility criteria were as follows: i)
histologically confirmed adenocarcinoma of the colon or rectum, and
presence of unresectable metastatic disease; ii) history of
treatments with fluoropyrimidine, irinotecan, oxaliplatin, and
anti-VEGF antibody (bevacizumab), or anti-epidermal growth factor
receptor (EGFR) antibody (cetuximab or panitumumab) for patients
who had KRAS exon 2 wild-type tumor; iii) Eastern
Cooperative Oncology Group performance status (ECOG PS) of 0 to 2;
and iv) adequate bone-marrow, liver, and renal function at the
start of the treatment. Patients were excluded if they had
previously received regorafenib or TAS-102, or had uncontrolled
medical disorders.
The Institutional Review Board for Clinical Research
approved all procedures for this retrospective observational study
(no. 16R-190), which was conducted in accordance with the
Declaration of Helsinki.
Treatment
Regorafenib (160 mg as a standard dose) was
administered once daily on days 1–21, with 7 days of rest. TAS-102
(35 mg/m2) was administered twice daily 5 days a week,
with 2 days of rest, for 2 weeks, followed by a 14-day rest period.
Both regimens were repeated every 4 weeks. The treatments were
continued until disease progression, death, unacceptable toxicity,
withdrawal of consent by the patient, or decision by the treating
physician that discontinuation would be in the patient's best
interest.
Patients whose initial dose had been reduced at the
discretion of the treating physician were included in this study.
Patients who required dose reductions could re-escalate the dose up
to the recommended starting dose if the toxicity resolved to
baseline level. All patients received the best supportive care
available, but were not allowed to receive other antitumor agents,
hormonal therapy, or immunotherapy. The decisions regarding which
drug should be administered first, and whether to provide crossover
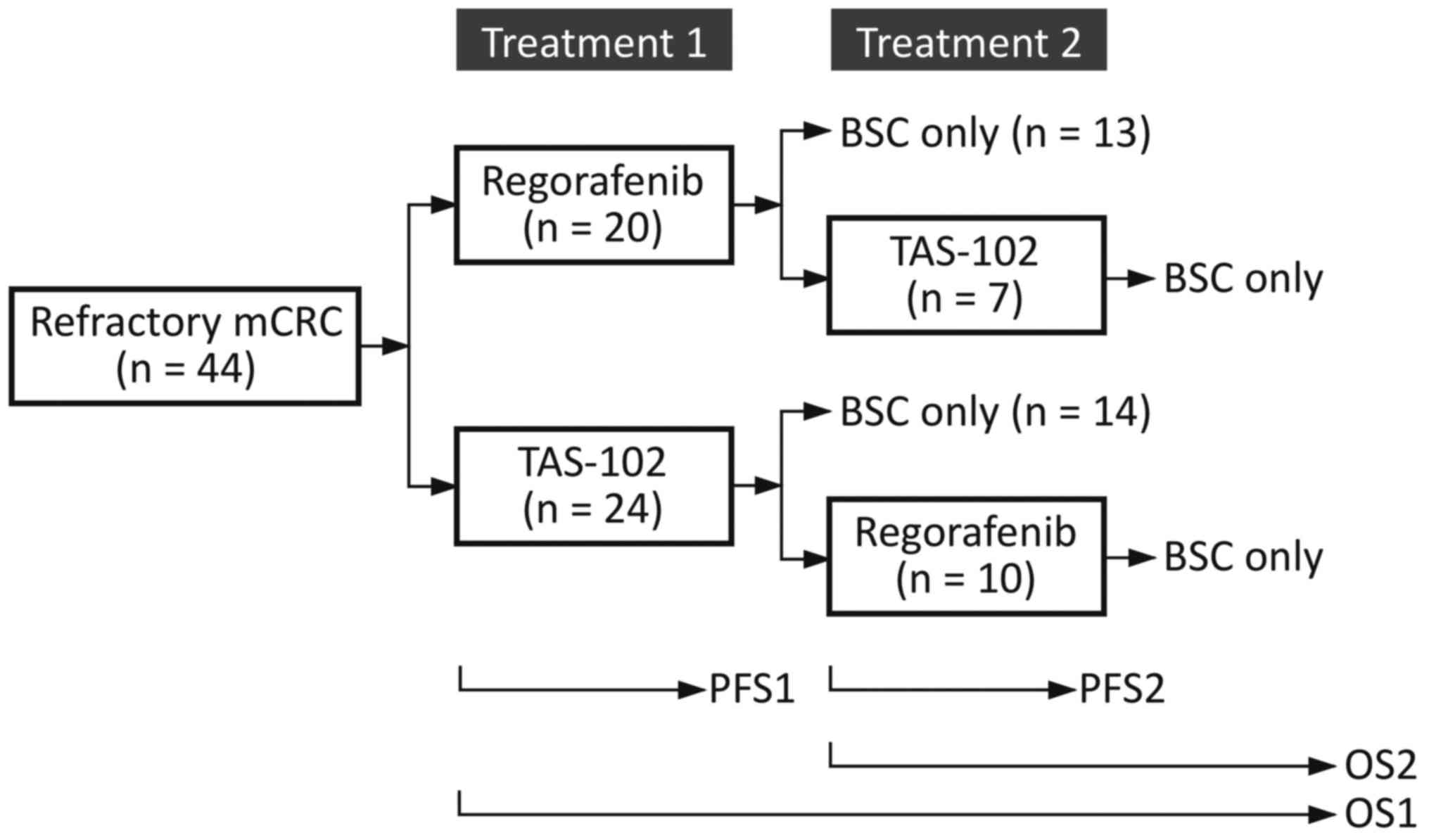
between treatments, were made by the treating physicians (Fig. 1).
Evaluation
All patients underwent computed tomography every 8
weeks to assess tumor responses to therapy in terms of change from
baseline during treatment according to the Response Evaluation
Criteria in Solid Tumors (RECIST) version 1.1 (13). We defined PFS1 as the interval from
the first administration of the primary treatment to the first
radiologic or clinical observation of disease progression or death
from any cause, whichever came first (Fig. 1).
We defined PFS2 as the interval from the initiation
of the secondary treatment to the second progression, for those who
had undertaken crossover between treatments after a first
progression. We defined OS1 as the time between the administration
date of the primary treatment and the date of death from any cause,
and OS2 as the time between the administration date of the
secondary treatment, if applicable, and the date of death. The
median PFS1, PFS2, OS1 and OS2 were estimated using the
Kaplan-Meier method.
The planned dose intensity (DI) for each drug was
defined as the total amount of drug in the entire treatment
intended based on the recommended dose and schedule. Then, the
relative dose intensity (RDI) for each drug was calculated as the
ratio between the delivered DI and the planned DI (14). Adverse events were classified and
graded according to the National Cancer Institute Common
Terminology Criteria for Adverse Events, version 4.03 (15).
Statistical methods and prognostic
score construction
Parametric data with P>0.05 for the
Kolmogorov-Smirnov test were analyzed using Welch's two sample
t-test, and non-parametric data using the Wilcoxon test.
Categorical data were analyzed using Fisher's exact test. The PFS1,
2 and OS1, 2 were compared using a log-rank test with 95%
confidence intervals (95% CIs).
The results of OS1 were plotted against the total
delivered dose or the RDI for each drug and fitted to a simple
linear regression model to calculate the regression coefficient
(16). A Cox proportional hazards
regression model was used to test each candidate variable predictor
associated with OS1 using stepwise model selection according to
Akaike's information criterion. To take account of the small number
of patients with PS 2, the values of ECOG PS were incorporated into
the model as a numerical variable. Hazard ratios were calculated by
taking the exponentials of the ß coefficients of Cox models.
Model discrimination was done by calculating the Harrell's C
(for concordance) index, which is the area under the
receiver operator curve (17,18). Hazard ratios of covariates were
rounded to the nearest integer to construct score weights. The
range of possible total score weights was divided into three groups
to stratify patients into poor-, intermediate- and long-survival
tertiles. P<0.05 was considered to indicate a statistically
significant difference. All analyses were performed using R version
3.3.2 (The R Foundation for Statistical Computing Platform)
(19).
Results
Patients
Patient demographics and characteristics are
outlined in Table I. Between June
2013 and November 2015, 44 patients with mCRC who were treated with
either regorafenib or TAS-102 for the first time were included in
the analysis. Of these patients, 7 went on to receive TAS-102 and
10 went on to receive regorafenib as secondary treatment (Fig. 1). Baseline demographic and disease
characteristics were well balanced between the two groups in terms
of the primary treatment. All the patients had received prior
chemotherapy regimens containing a fluoropyrimidine, oxaliplatin
and irinotecan; all but one patient (in the group with primary use
of TAS-102) had received bevacizumab.
 | Table I.Demographics. |
Table I.
Demographics.
|
| Primary
treatment |
|
|---|
|
|
|
|
|---|
| Characteristic | Regorafenib
(n=20) | TAS-102 (n=24) | P-value |
|---|
| Age, median
[range] | 68 [57–78] | 64 [44–86] | 0.087 |
| Sex |
| Male | 13 (65.0) | 15 (62.5) | 1.0 |
|
Female | 7 (35.0) | 9 (37.5) |
|
| ECOG PS |
| 0 | 6 (30.0) | 14 (58.3) | 0.077 |
| 1 | 12 (60.0) | 6 (25.0) |
|
| 2 | 2 (10.0) | 4 (16.7) |
|
| Primary site of
disease |
| Right
colon | 4 (20.0) | 10 (41.7) | 0.14 |
| Left
colon | 7 (35.0) | 3 (12.5) |
|
|
Rectum | 9 (37.5) | 11 (45.8) |
|
| KRAS exon 2
status |
|
Wild | 9 (45.0) | 14 (58.3) | 0.55 |
|
Mutation | 11 (55.0) | 10 (41.7) |
|
| Number of prior
regimens |
| 2 | 12 (60.0) | 12 (50.0) | 0.87 |
| 3 | 8 (40.0) | 11 (45.8) |
|
| ≥4 | 0 (0) | 1 (4.2) |
|
| Number of
metastatic sites |
| 1 | 6 (30.0) | 6 (25.0) | 0.46 |
| 2 | 12 (60.0) | 11 (45.8) |
|
| ≥3 | 2 (10.0) | 7 (29.2) |
|
| Metastatic
site |
|
Liver | 16 (80.0) | 19 (79.2) | 0.26 |
|
Lung | 10 (50.0) | 13 (54.2) |
|
|
Peritoneum | 6 (30.0) | 4 (16.7) |
|
| Lymph
node | 2 (10.0) | 8 (33.3) |
|
|
Others | 2 (10.0) | 8 (33.3) |
|
| Time from
initiation of first-line chemotherapy |
| ≤18
months | 5 (25.0) | 6 (25.0) | 1.0 |
| >18
months | 15 (75.0) | 18 (75.0) |
|
| History of systemic
anticancer agents |
|
Fluoropyrimidine | 20 (100) | 24 (100) | 0.99 |
|
Oxaliplatin | 20 (100) | 24 (100) |
|
|
Irinotecan | 20 (100) | 24 (100) |
|
|
Anti-VEGF antibody | 20 (100) | 23 (95.8) |
|
|
Anti-EGFR antibody (Wild
KRAS or all-RASa) | 9 (45.0) | 11 (45.8) |
|
| Post-treatment use
of regorafenib or TAS-102 | 7 (35.0) | 10 (41.7) | 0.76 |
Treatment exposure
Crossover between treatments was conducted for
patients with ECOG PS 0 or 1 at the time when the first treatment
was finished (Table II). The
durations of treatment were not significantly different for
regorafenib and TAS-102: median 2.6 months (range: 0.1–10.8) for
regorafenib and 3.8 months (0.9–20.3) for TAS-102 in Treatment 1,
and then 4.2 months (0.4–12.9) and 3.7 months (0.9–15.1),
respectively, in Treatment 2. The starting dose rate of regorafenib
was reduced to 0.78±0.26 mean ± standard deviation (SD) in
Treatment 1, and to 0.71±0.10 in Treatment 2. Although the
incidences of any dose modification were equivalent, the RDI over
the whole treatment period was greater for TAS-102: 0.83±0.14 for
TAS-102 vs. 0.54±0.21 for regorafenib in Treatment 1 (P<0.001),
and 0.90±0.11 vs. 0.63±0.16 in Treatment 2 (P<0.001).
 | Table II.Administration of study drugs,
response and survival. |
Table II.
Administration of study drugs,
response and survival.
| A, Treatment 1
(primary use). |
|---|
|
|---|
| Variable | Regorafenib
n=20 | TAS-102 n=24 | P-value |
|---|
| ECOG PS, n (%) |
| 0 | 6 (30.0) | 14 (58.3) | 0.077 |
| 1 | 12 (60.0) | 6 (25.0) |
|
| 2 | 2 (10.0) | 4 (16.7) |
|
| Median period of
medication, months | 2.6 [range:
0.1–10.8] | 3.8 [0.9–20.3] | 0.18 |
| Relative initial
dose, mean ± SD | 0.78±0.26 | 0.97±0.09 | 0.0031 |
| Any treatment
modification, n (%) | 19 (95.0) | 18 (75.0) | 0.11 |
| Mean RDI ± SD | 0.54 ± 0.21 | 0.83±0.14 | <0.001 |
| Median OS1,
months | 9.1 (95% CI:
4.1–13.4) | 9.3 (5.5–12.3) | 0.68 |
| Patients alive at
12 months, n (%) | 4 (20.0) | 6 (25.0) | 0.73 |
| Median PFS1,
months | 2.1 (95% CI:
1.3–3.6) | 3.1 (1.7–4.1) | 0.13 |
| Best overall
responsea, n (%) |
| CR | 0 (0) | 0 (0) | 1.0 |
| PR | 0 (0) | 0 (0) |
|
| SD | 15 (75.0) | 17 (70.8) |
|
| PD | 5 (25.0) | 7 (29.2) |
|
|
| B, Treatment 2
(secondary use). |
|
|
Variable | Regorafenib
n=10 | TAS-102
n=7 | P-value |
|
| ECOG PS, n (%) |
| 0 | 1 (10.0) | 3 (42.9) | 0.12 |
| 1 | 9 (90.0) | 4 (57.1) |
|
| 2 | 0 (0) | 0 (0) |
|
| Median period of
medication, months | 4.2 [range:
0.4–12.9] | 3.7 [0.9–15.1] | 0.80 |
| Relative initial
dose, mean ± SD | 0.71±0.10 | 0.94±0.15 | 0.0058 |
| Any treatment
modification, n (%) | 10 (100) | 4 (57.1) | 0.051 |
| Mean RDI ± SD | 0.63±0.16 | 0.90±0.11 | <0.001 |
| Median OS2,
months | 7.1 (95% CI:
5.0-NA) | 5.3
(3.0-NA) | 0.67 |
| Patients alive at
12 months, n (%) | 0 (0) | 0 (0) | 1.0 |
| Median PFS2,
months | 3.7 (95% CI:
3.1-NA) | 3.7
(0.8-NA) | 0.23 |
| Best overall
responsea, n (%) |
| CR | 0 (0) | 0 (0) | 1.0 |
| PR | 0 (0) | 0 (0) |
|
| SD | 6 (60.0) | 4 (57.1) |
|
| PD | 4 (40.0) | 3 (42.9) |
|
Efficacy
No patient had a complete response (CR) or partial
response (PR), as shown in Table II.
Disease control was achieved in 15 out of 20 patients (75.0%) for
regorafenib and 17 out of 24 patients (70.8%) for TAS-102 in
Treatment 1, and in 6 out of 10 patients (60.0%) and 4 out of 7
patients (57.1%) in Treatment 2, respectively. There was no
difference in the best overall response for either treatment
line.
Median OS1 was 9.1 months and 20% of patients were
alive 12 months after starting regorafenib first; the corresponding
values were 9.3 months and 25% for patients treated with TAS-102
first (Table II). As for secondary
use, median OS2 values were 7.1 months and 5.3 months for
regorafenib and TAS-102, respectively, and no patient was alive in
either case at 12 months after crossover. There was no difference
in outcomes between regorafenib and TAS-102, regardless of the
order in which the two drugs were used.
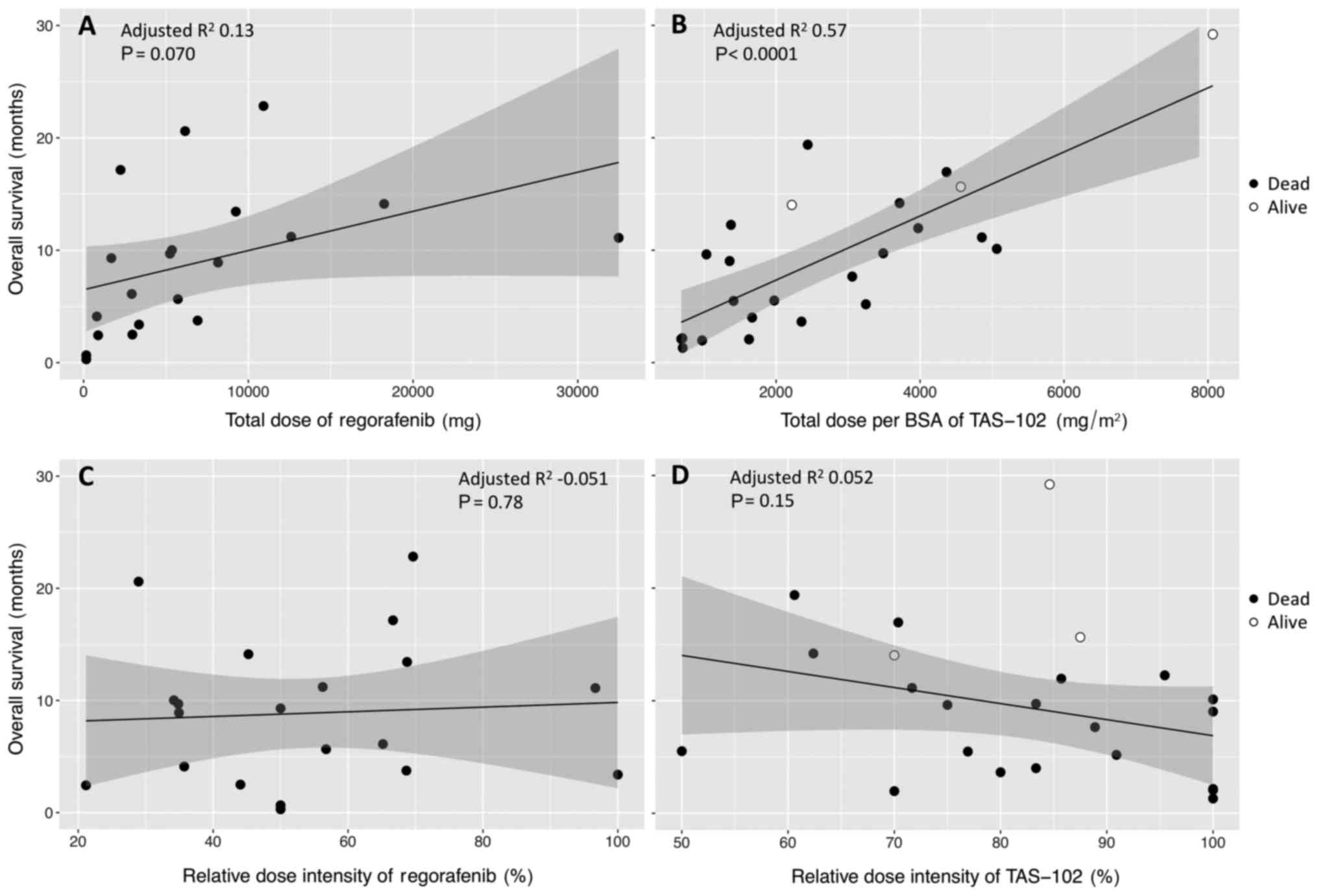
There was clearly a relationship between OS1 and a
total of delivered dose for each drug (Fig. 2A and B), even though OS1 was not
correlated to RDI (Fig. 2C and D).
The correlation between OS1 and the total of delivered dose was
higher for TAS-102, for which the data showed much less scatter, as
shown in Fig. 2B.
Safety
Table III summarizes
drug-related adverse events (AEs). Drug-related AEs occurred in 20
(100%) patients for regorafenib and in 22 (92%) patients for
TAS-102 in Treatment 1, and then in 8 (80%) and 7 (100%),
respectively, after crossover between the drugs in Treatment 2. The
frequencies of grade 3 or 4 hand-foot skin reaction (HFSR),
increased aspartate transaminase, increased alanine transaminase,
and increased bilirubin for the secondary use of regorafenib after
TAS-102 were not greater than the frequencies for the primary use
of regorafenib (10% vs. 45%, 0% vs. 5%, 0% vs. 5%, 0% vs. 10%,
respectively). Similarly, the frequencies of grade 3 or 4
leukopenia, neutropenia, anemia, and nausea for the secondary use
of TAS-102 posterior to regorafenib were similar to those for the
primary use of TAS-102 (57% vs. 29%, 29% vs. 34%, 57% vs. 38%, 0%
vs. 8%, respectively). One patient during the primary
administration of regorafenib suffered from severe
treatment-related liver dysfunction, and discontinued the treatment
after recovery.
 | Table III.Adverse events. |
Table III.
Adverse events.
|
| Regorafenib
(n=30) |
| TAS-102 (n=31) |
|
|---|
|
|
|
|
|
|
|---|
|
| REG-only or REG
prior to TAS (n=20) | REG posterior to
TAS (n=10) |
| TAS-only or TAS
prior to REG (n=24) | TAS posterior to
REG (n=7) |
|
|---|
|
|
|
|
|
|
|
|
|---|
| n (%) | Any grade | ≥Grade 3 | Any grade | ≥Grade 3 |
P-valuea | Any grade | ≥Grade 3 | grade | ≥Grade 3 |
P-valuea |
|---|
| Any event | 20 (100) | 13 (65) | 8 (80) | 5 (50) | 0.69 | 22 (92) | 15 (63) | 7 (100) | 6 (86) | 0.20 |
| Clinical AEs |
|
HFSR | 14 (70) | 9 (45) | 3 (30) | 1 (10) | 0.077 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
|
Nausea | 5 (25) | 0 (0) | 0 (0) | 0 (0) | 0.038 | 10 (42) | 2 (8) | 4 (57) | 0 (0) | 0.96 |
|
Anorexia | 9 (45) | 1 (5) | 1 (10) | 0 (0) | 0.33 | 8 (36) | 2 (9) | 4 (57) | 0 (0) | 1.0 |
|
Diarrhea | 2 (10) | 0 (0) | 1 (10) | 0 (0) | 0.090 | 3 (13) | 0 (0) | 1 (14) | 0 (0) | 0.021 |
|
Fatigue | 11 (55) | 2 (10) | 4 (40) | 0 (0) | 0.31 | 12 (50) | 1 (4) | 3 (43) | 0 (0) | 0.87 |
|
Mucositis oral | 4 (20) | 0 (0) | 0 (0) | 0 (0) | 0.30 | 6 (25) | 1 (4) | 1 (14) | 0 (0) | 0.37 |
|
Hypertension | 9 (45) | 2 (10) | 2 (20) | 2 (20) | 0.33 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Voice
alteration | 6 (30) | 0 (0) | 0 (0) | 0 (0) | 0.098 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
|
Alopecia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | 1 (4) | 0 (0) | 2 (29) | 0 (0) | 0.13 |
|
Others | 0 (0) | 0 (0) | 3 (29) | 3b (30) | 0.038 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Laboratory
abnormalities |
|
Leukopenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.0 | 12 (50) | 7 (29) | 4 (57) | 4 (57) | 0.70 |
|
Neutropenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.0 | 13 (54) | 9 (34) | 3 (43) | 2 (29) | 0.92 |
|
Anemia | 0 (0) | 0 (0) | 2 (20) | 0 (0) | 0.15 | 15 (63) | 9 (38) | 5 (71) | 4 (57) | 0.89 |
|
Thrombocytopenia | 6 (30) | 2 (10) | 2 (20) | 1 (10) | 0.96 | 7 (29) | 2 (8) | 2 (29) | 0 (0) | 0.96 |
| AST
increased | 15 (75) | 1 (5) | 4 (40) | 0 (0) | 0.26 | 6 (25) | 1 (4) | 4 (57) | 2 (29) | 0.44 |
| ALT
increased | 6 (30) | 1 (5) | 2 (29) | 0 (0) | 0.40 | 4 (17) | 0 (0) | 5 (71) | 1 (14) | 0.020 |
|
Hyperbilirubinemia | 7 (35) | 2 (10) | 1 (10) | 0 (0) | 0.61 | 1 (4) | 0 (0) | 3 (43) | 1 (14) | 0.030 |
|
Discontinuation due to
AEs | 5 (25) | 2 (20) | 0.75 | 3 (13) | 0 (0) | 0.78 |
Prognostic score
Table IV summarizes
the findings of univariate and multivariate analyses of baseline
characteristics as prognostic factors for OS and the score weights
assigned to each retained predictor variable. The total possible
score was 12 points; however, no patient had a score >10.
Patients who had a worse ECOG PS, time since diagnosis of
metastatic disease ≤18 months (rapid growth of tumor), and prior
chemotherapy continued ≥2 months beyond progressive disease (PD) on
the RECIST criteria (including so-called clinical PD) showed a
P-value <0.05 in the univariate analysis examining the
association between baseline characteristics and poor OS.
 | Table IV.Predictors for OS1 in patients
treated with regorafenib and/or TAS-102. |
Table IV.
Predictors for OS1 in patients
treated with regorafenib and/or TAS-102.
|
| Univariate
model | Multivariate
model |
|
|---|
|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value | Score weight |
|---|
| Male sex | 1.85
(0.912–3.75) | 0.089 |
|
|
|
| Age | 0.992
(0.960–1.03) | 0.62 |
|
|
|
| ECOG PS | 1.78
(1.02–3.10) | 0.044 | 2.00
(1.13–3.53) | 0.018 | 2 |
| Primary lesion |
| Right
colon | 1.53
(0.631–3.73) | 0.35 |
|
|
|
|
Rectum | 1.49
(0.642–3.47) | 0.35 |
|
|
|
| KRAS exon 2
status |
|
Mutant | 0.985
(0.526–1.84) | 0.96 |
|
|
|
| Metastatic
sites |
|
n≥3 | 1.21
(0.550–2.65) | 0.64 |
|
|
|
| Metastatic
sites |
|
Liver | 1.18
(0.538–2.58) | 0.68 |
|
|
|
|
Lung | 1.28
(0.688–2.40) | 0.43 |
|
|
|
|
Peritoneum | 1.01
(0.477–2.12) | 0.99 |
|
|
|
| Lymph
node | 0.596
(0.271–1.31) | 0.20 |
|
|
|
|
Other | 1.17
(0.570–2.41) | 0.67 |
|
|
|
| Number of prior
regimens | 0.876
(0.528–1.46) | 0.61 |
|
|
|
| History of
biologicals |
|
Anti-VEGF antibody | 1.13
(0.153–8.30) | 0.91 |
|
|
|
|
Anti-EGFR antibody | 0.827
(0.438–1.56) | 0.56 |
|
|
|
| Time since
diagnosis of metastatic disease |
| ≤18
months | 2.17
(1.04–4.55) | 0.039 | 2.51
(1.17–5.37) | 0.018 | 3 |
| Prior
chemotherapy |
|
Continued ≥2 months beyond
PD | 3.62
(1.72–7.63) | <0.001 | 4.95
(2.20–11.1) | <0.001 | 5 |
| Harrell's
C-index |
|
| 0.70 |
|
|
| Total possible
score |
|
|
|
| 12 |
Prior chemotherapy was repeated every 2 to 3 weeks
according to a regimen with an evaluation interval of <3 months,
so regorafenib or TAS-102 could be started within 6 weeks after
failure of the prior chemotherapy. Continuation of prior
chemotherapy ≥2 months beyond PD would represent prolonged
administration that clinicians intended to conduct for some
reason.
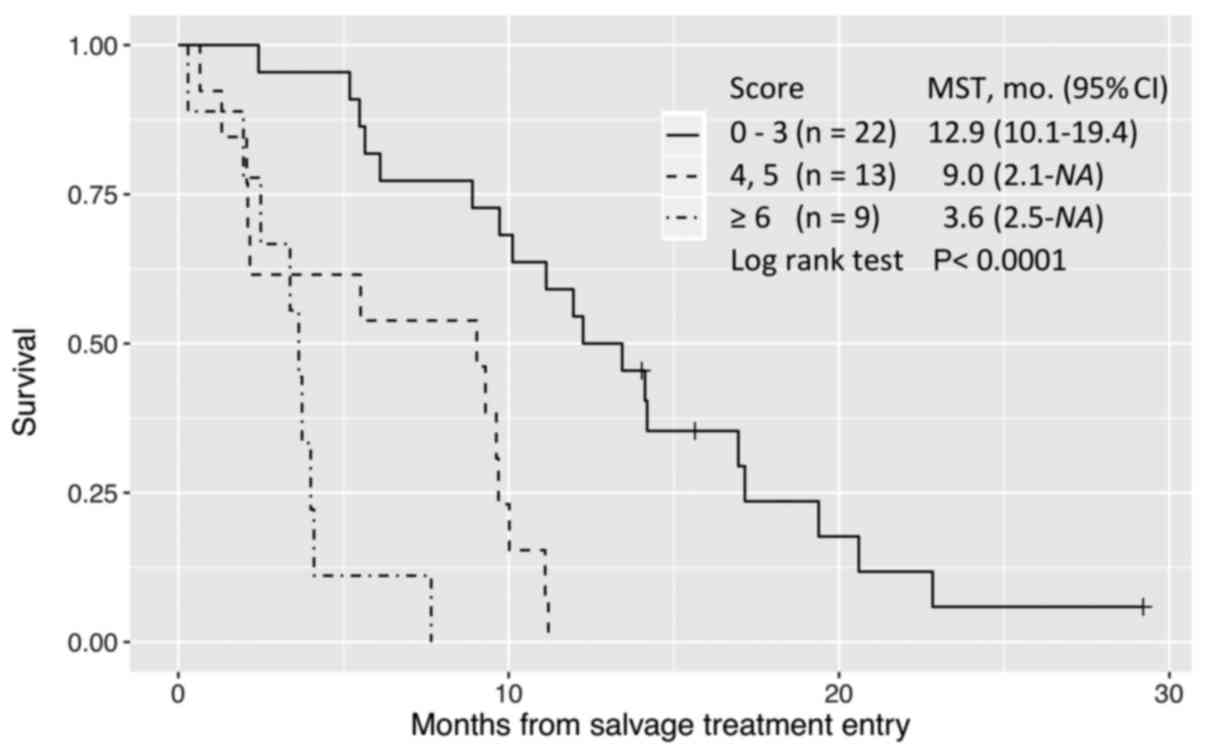
In multivariate analysis, these three factors
remained significant for the parsimonious model to predict OS while
retaining good discrimination (C-statistic=0.70). A score of
0–3 defined long survival; 4–5, intermediate survival; and ≥6, poor
survival (Fig. 3).
Discussion
Regorafenib and TAS-102 have been reported to show
similar efficacy but different toxicity profiles for regorafenib-
and TAS-102-naive patients in retrospective cohort studies
(11,20,21).
Analyses of efficacy and safety in patients treated with
regorafenib or TAS-102 in the real-life setting are important for
clinicians, because patient characteristics in real-life,
especially ECOG PS, may differ from those in phase III trials
(7–9).
No patient had a CR or PR for either drug in our
cohort. The two drugs were equivalent in terms of DCR: 75.0% for
regorafenib and 70.8% for TAS-102 in primary salvage treatment, and
60.0 and 57.1%, respectively, in secondary use. Crossover
administration was achieved in 7 out of 20 (35.0%) patients treated
with regorafenib first, and in 10 out of 24 (41.7%) patients
treated with TAS-102 first, but this does not imply inferiority: We
found that TAS-102 had provided a prolonged period of medication
for patients with poor performance status (ECOG PS=2), as shown in
Table II. Median OS1 of 4 patients
with ECOG PS=2 at the time of study entry was 3 months (range: 1.3
to 5.5 months) for TAS-102, providing better survival compared to
two weeks for 2 patients treated with regorafenib (P=0.020).
A cohort study of regorafenib in real-life clinical
practice for mCRC patients in France (REBECCA) (22) found that 50% of patients had a
treatment modification (dose reduction or interruption), and 31% of
patients discontinued regorafenib before progression mainly due to
toxicity or deterioration of general health status. According to
their data, survival was unfavorably affected by a low initial
daily dose of regorafenib.
Median OS1 in our study was consistent with those in
CORRECT (7) (median OS, 6.4 months;
12-month survival, 24%) and REBECCA (22) (5.6 months; 22%) for regorafenib, and
RECOURSE (9) (7.1 months; 27%) for
TAS-102, although 95 or 75% of our patients treated with
regorafenib or TAS-102 first, respectively, had a dose modification
(Table II). An initial dose was more
likely to be reduced for regorafenib compared to TAS-102 to avoid
early AE within the first 3 weeks of regorafenib treatment, but
there was no correlation between deterioration of OS1 and reduction
of initial daily dose (data not shown). This may be because we
commonly escalated the dosage thereafter, if possible, up to 120 mg
(4 out of 11 patients) or 160 mg (1 out of 10 patients), based on
each patient's response. The most common dosage was 120 mg daily
(19 out of 30 patients) in the first 2 cycles, as recently
recommended in the ReDOS study (23).
It seemed important for successful escalation of regorafenib to
inform patients before administration about the likelihood of
weekly dose escalation. Furthermore, OS1 for regorafenib or
TAS-102, regardless of single use or crossover, was not correlated
to RDI, but was proportional to a total dose of each drug (Fig. 2). In third-line or later treatments,
clinicians may continue to prescribe the maximum recommended dose
to obtain the best outcome, but may withdraw treatment from
patients whose performance status deteriorates. In the former
scenario, patients could experience adverse effects without any
benefit, whereas possible responders could be missed in the latter
scenario. Our results indicate that lower dose-intensity provides a
longer duration of life under treatment compared to higher
dose-intensity in some cases. This can be interpreted as indicating
that there was a greater improvement in survival when these drugs
were administered at the appropriate dose for each individual
patient and continued for as long as possible until progression.
Regarding regorafenib, Osawa (24)
recommended an initial dose of 120 mg for salvage treatment of
mCRC, as this provided a significant effect with good
tolerability.
It has been considered that the toxic effects of
TAS-102 are generally mild and manageable compared with those of
regorafenib (21). The reported
incidence of clinical AEs for regorafenib, including grade ≥3 HFSR
(17% of patients in CORRECT) (7),
fatigue (10%), and hepatotoxicity (6% of Asian population in
CONCUR) (8), makes it difficult to
administer regorafenib to patients who have previously been treated
with TAS-102. In this study, the safety profiles of regorafenib and
TAS-102 were broadly consistent with those in previous pivotal
trials (7–9,25)
(Table III). In addition, the
incidences of HFSR, fatigue and hepatotoxicity in patients given
regorafenib were not significantly increased even if the drug was
used after TAS-102, while conversely, the frequencies of
myelosuppression including leukopenia and neutropenia, nausea and
anorexia in patients given TAS-102 were not greater in patients
with previous regorafenib treatment. Although treatment
discontinuation due to toxic effects was more frequently observed
for regorafenib treatment, the incidence of toxic effects was not
increased in patients with previous TAS-102 treatment, provided
that the initial dose of regorafenib was reduced to 120 mg in most
cases (Table II). These results
indicate that regorafenib can be safely administered to patients
with previous TAS-102 treatment.
Predictive biomarkers for OS have not yet been
identified for mCRC patients treated with regorafenib or TAS-102
(11,26). No association was identified between
KRAS, BRAF and PIK3CA mutation status and outcomes in
CORRECT (7) and RECOURSE (9). The post hoc analysis of CORRECT
indicated that patients treated with regorafenib who had long PFS
(>4 months) tended to have a better ECOG PS (score, 0), fewer
metastatic tumor sites (1 to 2 sites), and a longer time (≥18
months) since diagnosis of metastatic disease (25). In contrast, REBECCA (22) indicated that the following 6 baseline
variables were associated with poorer survival: poor ECOG PS, a
shorter time from diagnosis of metastases, a low initial dose of
regorafenib, >3 metastatic sites, liver metastases, and
KRAS mutations. A longer time since diagnosis of metastatic
disease is considered to reflect a better response to chemotherapy,
so PFS2 after success of crossover between regorafenib and TAS-102
tended to be longer than PFS1, which included patients with rapidly
growing tumors refractory to treatments (Table II).
In our study, a model with a good discrimination
(C-index 0.70), consisting of only 3 baseline predictors
(poor ECOG PS, ≤18 months from diagnosis of metastases, and prior
chemotherapy continued ≥2 months beyond PD) (Table IV), classified patients into similar
prognostic groups (Fig. 3). We
recommend that patients having a high probability of benefit should
be identified before starting treatment with regorafenib or TAS-102
among patients refractory to standard chemotherapy.
It remains an important clinical issue to decide
which drug should be administered first, but this has not been
established because of the lack of a head-to-head randomized trial.
A retrospective comparative analysis in 550 patients (REGOTAS)
(11) suggested that regorafenib
should be given first in patients aged <65 years, but TAS-102 in
patients aged ≥65 years, based on a favorable trend of OS. We tried
a propensity score method (inverse probability of treatment
weighting) (27) for choosing between
the two drugs in our study, but there was no clear result, except
for a favorable trend with age in the TAS-102-first group (hazard
ratio, 0.8; 95% confidence interval, 0.7–0.9). No difference in OS
was found between the two drugs.
There are some potential limitations of this study.
Our study is a retrospective single-center analysis. Although all
patients with refractory mCRC treated with regorafenib or TAS-102
in the period were included, the number of patients was relatively
small. All patients were treated by a team of six surgeons, all of
whom are colorectal cancer specialists. An all-RAS
(KRAS and NRAS) test was approved in Japan on April,
2015. Among our patients, 15 had wild-KRAS exon 2 tumor
identified before that date, and all of them received anti-EGFR
antibody regimens. Although 20% of them (3 patients) might have
other RAS mutation, the number is too small to permit any
conclusion; at worst, they would have had a short duration of
anti-EGFR treatment without benefit until tumor progression.
External validation is still needed to confirm the model used to
predict OS.
This analysis suggests that the administration of
regorafenib and TAS-102 can be recommended for patients with
refractory mCRC who have a better performance status, and a longer
time since diagnosis of metastatic disease. Prolongation of the
previous chemotherapy after diagnosis of disease progression
attenuated the survival benefit of regorafenib and TAS-102,
regardless of the order of their administration. We suggest that
the optimal survival benefit of regorafenib and TAS-102 is provided
by flexible and careful titration to the optimal dose for each
individual patient with initial dose reduction if necessary,
followed by prolonged administration until disease progression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conception, design, collection of patient
information, data interpretation, and drafting of the article was
undertaken by AT. SS, TS, KO, GS and HM participated in patient
treatment, and helped revise the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board for Clinical Research
approved all procedures for this retrospective observational study
(no. 16R-190), which was conducted in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TAS-102
|
trifluridine and tipiracil
|
|
mCRC
|
metastatic colorectal cancer
|
|
VEGF
|
vascular endothelial growth factor
|
|
EGFR
|
epidermal growth factor receptor
|
|
KRAS
|
Kirsten rat sarcoma
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
CTCAE
|
Common Terminology Criteria for
Adverse Events
|
References
|
1
|
Howlader N, Noone A, Krapcho M, Garshell
J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
et al: SEER Cancer Statistics Review, 1975–2012. National Cancer
Institute; Bethesda, MD, USA: 2015
|
|
2
|
Abrams TA, Meyer G, Schrag D, Meyerhardt
JA, Moloney J and Fuchs CS: Chemotherapy usage patterns in a
US-wide cohort of patients with metastatic colorectal cancer. J
Natl Cancer Inst. 106:djt3712014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73–4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lenz HJ, Stintzing S and Loupakis F:
TAS-102, a novel antitumor agent: A review of the mechanism of
action. Cancer Treat Rev. 41:777–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crawford J, Becker PS, Armitage JO,
Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I,
Griffiths EA, et al: Myeloid growth factors, version 2.2017, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
15:1520–1541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aguilar Aranda E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu
J, Bai Y, Chi Y, Wang L, et al: Regorafenib plus best supportive
care versus placebo plus best supportive care in Asian patients
with previously treated metastatic colorectal cancer (CONCUR): A
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 16:619–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu J, Kim TW, Shen L, Sriuranpong V, Pan
H, Xu R, Guo W, Han SW, Liu T, Park YS, et al: Results of a
randomized, double-blind, placebo-controlled, phase III trial of
trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with
previously treated metastatic colorectal cancer: The TERRA study. J
Clin Oncol. 36:350–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moriwaki T, Fukuoka S, Taniguchi H,
Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama
C, Denda T, et al: Propensity score analysis of regorafenib versus
trifluridine/tipiracil in patients with metastatic colorectal
cancer refractory to standard chemotherapy (REGOTAS): A Japanese
society for cancer of the colon and rectum multicenter
observational study. Oncologist. 23:7–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
García-Alfonso P, Feliú J,
García-Carbonero R, Grávalos C, Guillén-Ponce C, Sastre J and
García-Foncillas J: Is regorafenib providing clinically meaningful
benefits to pretreated patients with metastatic colorectal cancer?
Clin Transl Oncol. 18:1072–1081. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hryniuk W and Bush H: The importance of
dose intensity in chemotherapy of metastatic breast cancer. J Clin
Oncol. 2:1281–1288. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Health and Human Services, . National
Institutes of Health and National Cancer Institute: Common
terminology criteria for adverse events (CTCAE). v4.03.
2013.https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdfAugust
20–2013
|
|
16
|
Wilkinson GN and Rogers CE: Symbolic
description of factorial models for analysis of variance. Appl
Stat-J R Stat Soc. 22:392–399. 1973.
|
|
17
|
Harrell FE Jr, Lee KL and Mark DB:
Multivariable prognostic models: Issues in developing models,
evaluating assumptions and adequacy, and measuring and reducing
errors. Stat Med. 15:361–387. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pencina MJ and D'Agostino RB: Overall C as
a measure of discrimination in survival analysis: Model specific
population value and confidence interval estimation. Stat Med.
23:2109–2123. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
R Development Core Team, . R: A language
and environment for statistical computingR Foundation for
Statistical Computing. Vienna, Austria: 2016
|
|
20
|
Masuishi T, Taniguchi H, Hamauchi S,
Komori A, Kito Y, Narita Y, Tsushima T, Ishihara M, Todaka A,
Tanaka T, et al: Regorafenib versus trifluridine/tipiracil for
refractory metastatic colorectal cancer: A retrospective
comparison. Clin Colorectal Cancer. 16:e15–e22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sueda T, Sakai D, Kudo T, Sugiura T,
Takahashi H, Haraguchi N, Nishimura J, Hata T, Hayashi T, Mizushima
T, et al: Efficacy and safety of regorafenib or TAS-102 in patients
with metastatic colorectal cancer refractory to standard therapies.
Anticancer Res. 36:4299–4306. 2016.PubMed/NCBI
|
|
22
|
Adenis A, de la Fouchardiere C, Paule B,
Burtin P, Tougeron D, Wallet J, Dourthe LM, Etienne PL, Mineur L,
Clisant S, et al: Survival, safety, and prognostic factors for
outcome with Regorafenib in patients with metastatic colorectal
cancer refractory to standard therapies: Results from a multicenter
study (REBECCA) nested within a compassionate use program. BMC
Cancer. 16:4122016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bekaii-Saab TS, Ou FS, Anderson DM, Ahn
DH, Boland PM, Ciombor KK, Jacobs NL, Desnoyers RJ, Cleary JM,
Meyers JP, et al: Regorafenib dose optimization study (ReDOS):
Randomized phase II trial to evaluate dosing strategies for
regorafenib in refractory metastatic colorectal cancer (mCRC)- An
ACCRU network study. J Clin Oncol. 36 4 Suppl:S6112018.
|
|
24
|
Osawa H: Response to regorafenib at an
initial dose of 120 mg as salvage therapy for metastatic colorectal
cancer. Mol Clin Oncol. 6:365–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grothey A, Falcone A, Humblet Y, Bouche O,
Mineur L, Adenis A, Tabernero J, Yoshino T, Lenz HJ and Goldberg
RM: Characteristics of patients (pts) with metastatic colorectal
cancer (mCRC) treated with regorafenib (REG) who had
progression-free survival (PFS) >4 months (m): Subgroup analysis
of the phase 3 CORRECT trial. Ann Oncol. 27 Suppl 6:S516P2016.
View Article : Google Scholar
|
|
26
|
Puthiamadathil JM and Weinberg BA:
Emerging combination therapies for metastatic colorectal cancer -
impact of trifluridine/tipiracil. Cancer Manag Res. 9:461–469.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Austin PC: The performance of different
propensity score methods for estimating marginal hazard ratios.
Stat Med. 32:2837–2849. 2013. View
Article : Google Scholar : PubMed/NCBI
|