Introduction
TJ-100 (Daikenchuto, or TU-100), which comprises
three different medicinal herbs, is widely used in clinical
practice to treat ‘abdominal bloating and cold sensation in the
abdomen’ (1,2). Clinical studies support the use of
TJ-100 in the perioperative period to accelerate the recovery of
gastrointestinal function (3–6). The beneficial effect of TJ-100 is
mediated by enteric/sensory nerve stimulation via acetylcholine
receptors and/or serotonin receptors (7,8) and by the
release of adrenomedullin from epithelial cells (9–11).
Moreover, TJ-100 decrease the level of postoperative inflammatory
cytokines, ameliorating inflammation and postoperative ileus in
animal model (12).
JAPAN-PD study, a multicenter, randomized,
double-blinded, and placebo-control trial involving nearly 300
patients, was conducted to study the effect of TJ-100 on recovery
of gastrointestinal function after pancreaticoduodenectomy (PD)
(13). In contrast to the hypothesis,
perioperative administration of TJ-100 provided no positive effect
on time to first postoperative flatus and occurrence rate of
paralytic ileus. Concurrently, the cytokine levels in 180 of the
trial patient group were investigated, measuring the levels in
serum and drainage fluid on postoperative day (POD)1 and POD3.
Consistently, we found the lack of significant difference in 27
cytokine levels for both serum and drainage fluid between the
TJ-100- and placebo-treated patients ((n=180, P>0.05)) (13).
However, we noted that the cytokine levels differed
substantially among individuals. The range of factors potentially
influencing cytokine levels, including gene polymorphisms (14,15),
malignancy itself (16), and
different degree of pre/postoperative inflammation, might have
masked the effect of TJ-100. We also considered that cytokine
levels of drainage fluid are not suitable for evaluating the
effects of TJ-100, because they would be more influenced by the
local environment near the tip of the tube than by the whole
environment or systemic response. Thus, we determined to re-analyze
the effect of TJ-100 using the data from the previous study, but
with a special focus on the serum levels and efforts to minimize
the influence of other potentially confounding factors.
Materials and methods
Trial design and patient
recruitment
The details of the protocol and patient recruitment
were described previously (13).
Patients aged 20 years or older, who were undergoing PD for
periampullary tumor or pancreas head tumors, were recruited. The
patients were randomized according to four clinical variables: i)
type of primary tumor; ii) presence of neoadjuvant chemotherapy;
iii) preservation of gastric pylorus; and iv) presence of enteral
alimentation via a feeding tube. Administration of TJ-100 (15 g/day
divided into three doses; before every meal or every 8 h) or
placebo was initiated three days before surgery, and continued
until POD14. Patient serum was collected on POD1 and POD3, and
assayed for 27 different cytokines by LSI Medience Corporation
(Tokyo, Japan) using the Human Cytokine 27-plex assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and Bio-Plex 200 System
(Bio-Rad Laboratories, Inc.).
The study protocol was registered at UMIN Clinical
Trial Registry (no. 000007975) and at ClinicalTrials.gov (no. NCT01607307). The
institutional review board of each participating institute approved
the trial protocol, and the randomization and intervention was
started after obtaining the patients' written informed consent.
Additional written informed consent was obtained for the present
study.
Statistical analysis
The characteristic features were compared between
the patients receiving TJ-100 (TJ-100 Group) and placebo (Placebo
Group) using Student's t-test or Fisher's exact tests. First, we
compared the levels of serum cytokines on POD1 and POD3 between the
groups by using the Wilcoxon signed-rank test. Second, we compared
ratios of the cytokine levels on POD3 to those on POD1 for further
analysis using Wilcoxon signed-rank test. Note that the values
under the measurement threshold were excluded from the analysis,
because the assignment of lower limit may significantly distort the
ratios. P-values of <0.05 was considered significant. The
statistical analysis was performed using SAS 9.4 (SAS Institute,
Inc., Cary, NC, USA).
Results
Clinical variables
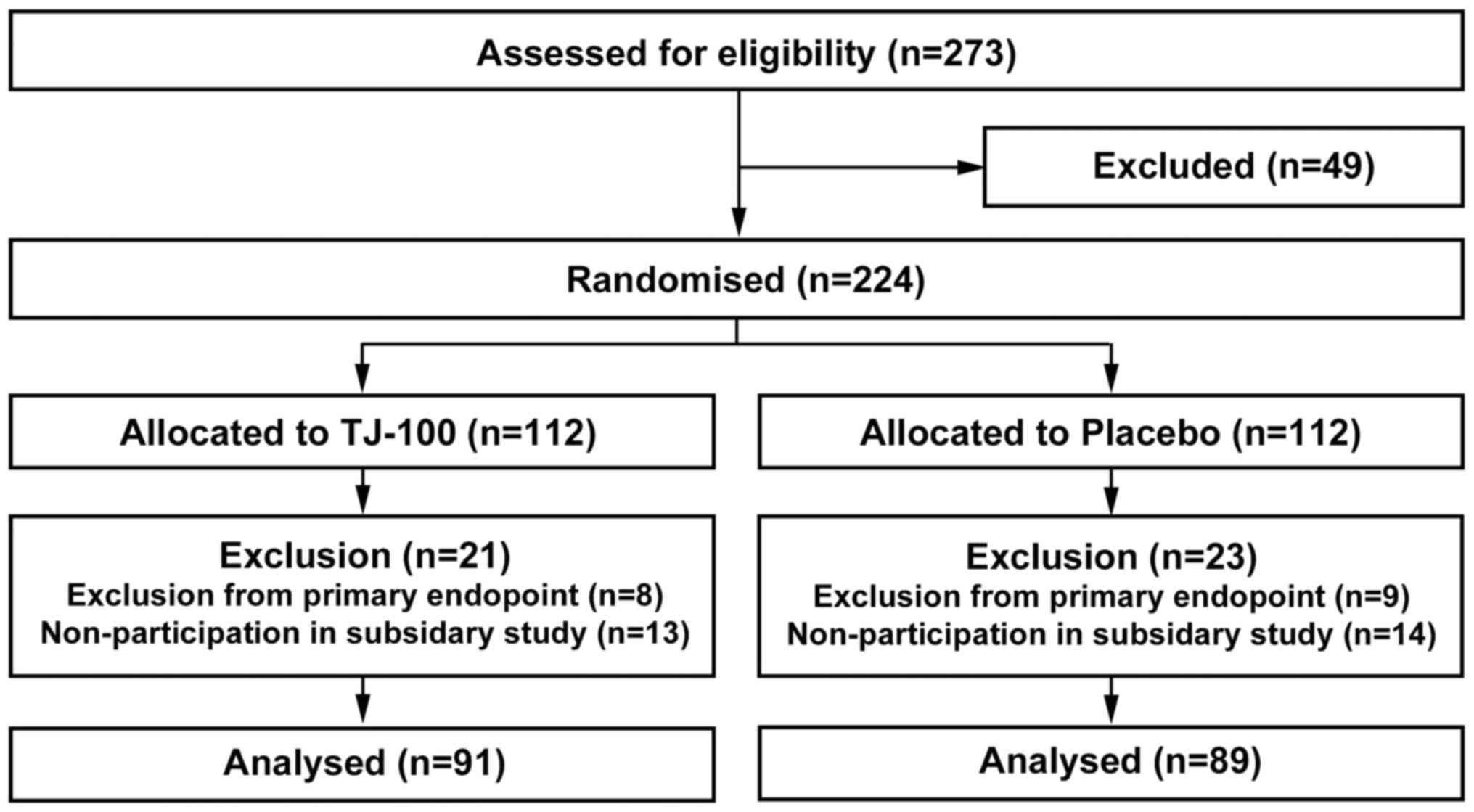
We analyzed clinical data of the patients who
participated in this additional explorative study after dividing
them into the TJ-100 Group and Placebo Group (Fig. 1 and Table
I). As in the original JAPAN-PD study (13), the TJ-100 Group included more patients
of an advanced age than did the Placebo Group (69.4±8.0 years vs.
65.1±11.4, P=0.039). We consider this difference was coincidentally
formed because the age was not used as a balancing variable of
randomization. Other clinical factors that could potentially alter
the cytokine levels did not differ between the groups.
 | Table I.Comparison between clinical variables
and the TJ-100 and Placebo Groups. |
Table I.
Comparison between clinical variables
and the TJ-100 and Placebo Groups.
| Patient
characteristic | TJ-100, n=91 | Placebo, n=89 | P-value |
|---|
| Age, mean ± SD
(years) | 69.4±8.0 | 65.1±11.4 | 0.004 |
| Sex, male/female
(%) | 50/41 (55/45) | 59/30 (66/34) | 0.130 |
| BMI, mean ± SD
(kg/m2) | 22.1±3.0 | 22.3±3.5 | 0.632 |
| Hx of abdominal
surgery (%) | 41 (45) | 30 (34) | 0.130 |
| Tumor
characteristics |
|
|
|
|
Pancreatic Ca, yes (%) | 52 (57) | 51 (57) | 1.000 |
|
Preoperative treatment, yes
(%) | 74 (81) | 71 (80) | 0.852 |
| Tumor
stage, 0/I/II/III/Iva/IVb/ND (%) |
3/12/7/25/27/3/13 | 3/9/6/25/24/4/18 | 0.303 |
|
|
(3/13/8/28/30/3/14) |
(3/10/7/28/27/20) |
|
| Surgical
variable |
|
|
|
| Surgical
time, mean ± SD (min) | 416.1±102.5 | 405.2±112.6 | 0.496 |
|
Bleeding, mean ± SD (ml) | 719.1±617.9 | 696.1±694.6 | 0.815 |
| Use of
epidural anesthesia (%) | 61 (67) | 65 (73) | 0.418 |
| Lymph
node dissection, D0/D1/D2/D3 (%) | 1/12/78/0 | 2/8/78/1 | 0.617 |
|
| (1/13/86/0) | (2/9/88/1) |
|
|
Dissection of nerve plexus
around the SMA (%) | 51 (56.0) | 59 (66.3) | 0.172 |
Absolute values of serum
cytokines
The values under the measurement threshold were
excluded in this analysis. As a result, IL-2 and IL-15 were
analyzed for only a small number of the patients, and thus
statistical comparison was invalid. As previously reported
(13), Wilcoxon signed-rank test
revealed no significant difference in cytokine levels between the
groups for POD1 (Table II) or
POD3.
 | Table II.Comparison of serum cytokine levels
on POD1 between the TJ-100 and Placebo Groups. |
Table II.
Comparison of serum cytokine levels
on POD1 between the TJ-100 and Placebo Groups.
|
| TJ-100 (n=91) | Placebo (n=89) |
|
|---|
|
|
|
|
|
|---|
| Factor | n | Median | (Min-Max) | n | Median | (Min-Max) | P-value |
|---|
| Interleukin |
|
IL-2 | 5 | 45.5 | (35.7–739.2) | 13 | 37.2 | (17.9–1250.9) | 0.200 |
|
IL-7 | 90 | 14.7 | (2.6–129.7) | 89 | 18 | (3.1–248.3) | 0.316 |
|
IL-9 | 54 | 22.6 | (10.3–4061.4) | 52 | 25.4 | (11–2143.1) | 0.183 |
|
IL-13 | 91 | 7.5 | (1–124.4) | 88 | 7.6 | (1.6–152) | 0.466 |
|
IL-15 | 2 | 99.3 | (34.1–164.5) | 4 | 166.8 | (74.6–595.7) | 0.487 |
|
IL-5 | 91 | 5.9 | (0.5–108.1) | 89 | 6.6 | (0.5–230.1) | 0.331 |
|
GM-CSF | 52 | 34.1 | (13.8–146.8) | 45 | 51.5 | (14.4–173.9) | 0.065 |
|
IL-1β | 78 | 5.7 | (2.2–53.3) | 77 | 6.5 | (2.3–51.2) | 0.284 |
|
IL-6 | 91 | 216.1 | (66.4–2024.7) | 89 | 208.4 | (20.2–3084.5) | 0.773 |
|
IL-12 | 67 | 38.6 | (11.2–635.8) | 63 | 44.8 | (11.2–747.2) | 0.278 |
|
IL-17 | 56 | 102.8 | (34.3–1384.4) | 54 | 169.9 | (33–602.9) | 0.354 |
|
IL-1Ra | 87 | 339.2 | (36.4–2706.8) | 85 | 319.9 | (56.5–11374.9) | 0.547 |
|
IL-4 | 82 | 4.1 | (1.2–46.7) | 77 | 4.9 | (1.2–20) | 0.152 |
|
IL-10 | 80 | 34.5 | (8.7–271.4) | 74 | 41.2 | (8.7–1152.8) | 0.121 |
| Chemokine |
|
IL-8 | 91 | 55.2 | (22.4–422.7) | 89 | 56.5 | (20–188.1) | 0.925 |
|
IP-10 | 91 | 327.2 | (69.3–2574.7) | 89 | 306 | (54.3–2452.9) | 0.857 |
|
MCP-1 | 91 | 91.9 | (28.7–453.5) | 89 | 96 | (34.8–1877.4) | 0.854 |
|
MIP-1α | 90 | 8.6 | (2.5–34.9) | 89 | 8.7 | (2–31.3) | 0.640 |
|
MIP-1β | 91 | 114.8 | (36.7–302) | 89 | 100.5 | (27–287.4) | 0.381 |
|
RANTES | 91 | 9,121.7 |
(740.1–154119.1) | 89 | 8,984.5 |
(1263.2–212597.8) | 0.907 |
|
Eotaxin | 44 | 56.6 | (32.1–257.8) | 47 | 56.5 | (28.2–1500.3) | 0.821 |
| Growth factor |
| Basic
FGF | 72 | 61.4 | (13.6–379.4) | 70 | 64.4 | (14.4–289.7) | 0.202 |
|
PDGF-BB | 91 | 444.8 | (9.1–5535.9) | 88 | 435.6 | (31.3–11803.5) | 0.582 |
|
VEGF | 71 | 54.7 | (13.3–648.1) | 64 | 69.5 | (12.5–359.8) | 0.165 |
| Hematopoietic
factor |
|
G-CSF | 88 | 123.5 | (36.6–1240.3) | 87 | 122.9 | (33.7–911.1) | 0.946 |
|
Interferon-γ | 91 | 114.2 | (21.2–1451.8) | 88 | 137.5 | (21.2–854.5) | 0.324 |
| TNF
α | 90 | 91.2 | (22–1072.7) | 84 | 101.7 | (25.6–784.6) | 0.370 |
Of note, the cytokine levels ranged widely among
individuals. In TNF-α on POD1, for instance, 1,072 pg/ml was
observed as a maximum value, while the lower level was below the
lower measurement limit (20.64 pg/ml). Similarly, the ranges of
other cytokines levels were found quite large after PD.
Changes in serum cytokine levels after
treatment
Whole groups
The change in serum cytokine level was defined by
the ratio of cytokine levels on POD3 divided by those on POD1 in
each individual, and then the ratios were compared between the
groups. The ratios over time of IL-4, IL-9, and IL-17 were higher
in the TJ-100 Group compared to the Placebo Group, suggesting that
these cytokines increased more from POD1 to POD3 following
treatment with TJ-100 than with placebo.
Patients without Grade B/C pancreatic
fistula or abdominal abscess
The same analysis was performed for the patients
without complications related to strong abdominal inflammation,
namely Grade B/C pancreatic fistula or abdominal abscess. In this
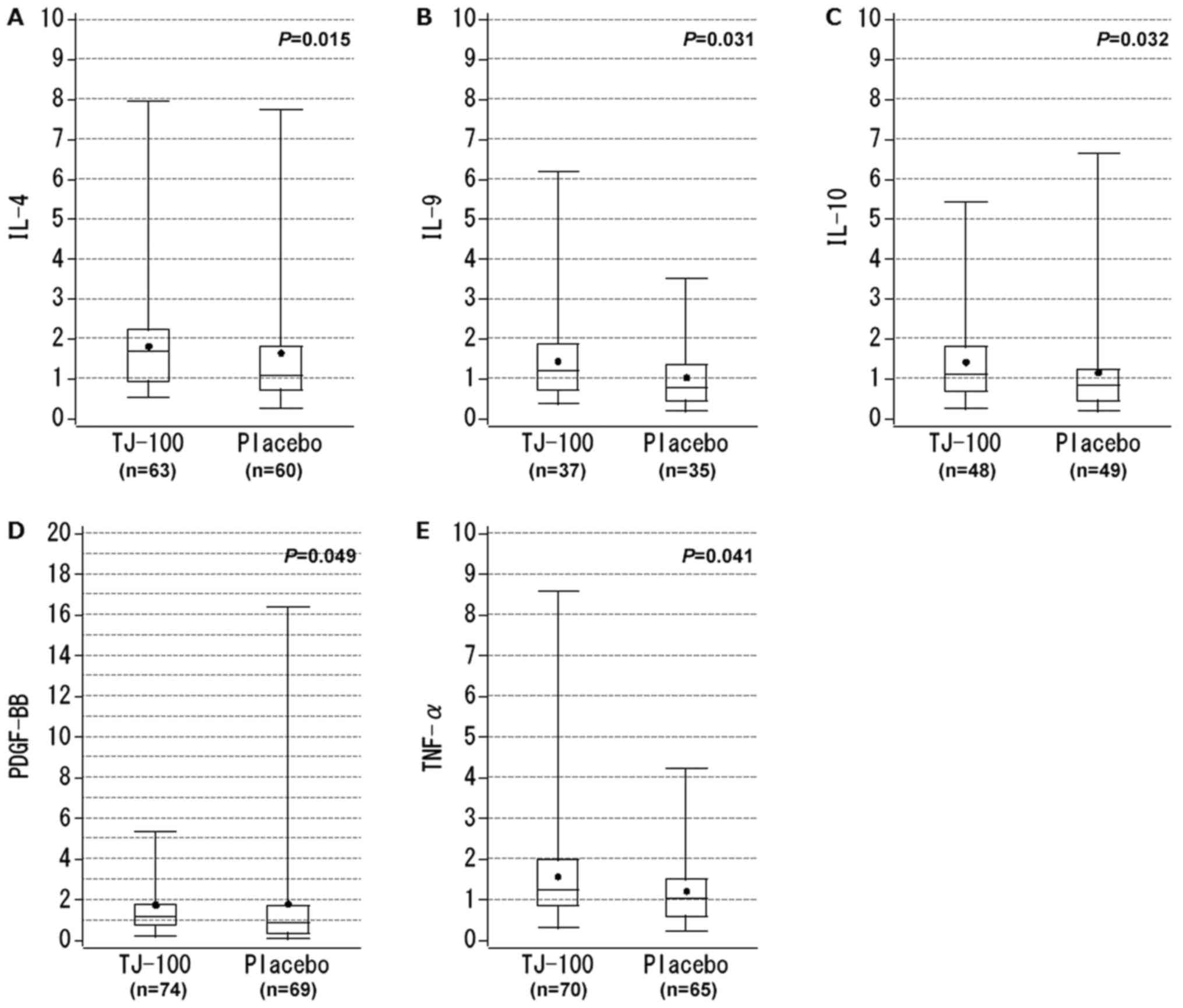
subgroup, the cytokine level ratios were significantly higher for
IL-4, IL-9, IL-10, PDGF-BB, and TNF-α in the TJ-100 Group than the
Placebo Group (Table III). For
IL-4, the median ratio was 1.7 in TJ-100 Group, compared to 1.1
with placebo, suggesting that serum level of IL-4 more increased
from POD1 to POD3 following TJ-100 treatment (Fig. 2A). For IL-9, IL-10, and PDGF-BB, the
median ratios slightly exceeded 1.0 in the TJ-100 Group, while the
median ratios of these cytokines were below 1.0 in the Placebo
Group (Fig. 2B-D). For TNF-α, a
statistical difference was demonstrated, although the difference in
median ratio was quite small between the groups (TJ-100 Group vs.
Placebo Group: 1.2 vs. 1.0, P=0.041; Fig.
2E).
 | Table III.Change in serum cytokine levels
between POD1 and POD3 in patients without severe abdominal
inflammation. |
Table III.
Change in serum cytokine levels
between POD1 and POD3 in patients without severe abdominal
inflammation.
|
| TJ-100 (n=74) | Placebo (n=70) |
|
|---|
|
|
|
|
|
|---|
|
| (Value on
POD1)/(Value on POD3) | (Value on
POD1)/(Value on POD3) |
|
|---|
|
|
|
|
|
|---|
| Factor | n | Median | (Min-Max) | n | Median | (Min-Max) | P-value |
|---|
| Interleukin |
|
IL-2 | 5 | 1.1 | (0.5–1.4) | 4 | 0.7 | (0.4–1) | 0.178 |
|
IL-7 | 73 | 1.3 | (0.3–16.5) | 70 | 1.2 | (0.3–7.2) | 0.164 |
|
IL-9 | 37 | 1.2 | (0.4–6.2) | 35 | 0.8 | (0.2–3.5) | 0.031 |
|
IL-13 | 74 | 1.9 | (0.4–18.1) | 69 | 1.5 | (0.2–14.3) | 0.177 |
|
IL-15 | 0 | – | (0.4–18.1) | 2 | 0.9 | (0.9–0.9) | – |
|
IL-5 | 74 | 1.5 | (0.2–7.3) | 70 | 1.1 | (0.2–5.4) | 0.095 |
|
GM-CSF | 26 | 0.8 | (0.1–4.5) | 26 | 0.6 | (0.2–2.8) | 0.185 |
|
IL-1β | 60 | 1.2 | (0.3–7.4) | 59 | 1 | (0.3–4.9) | 0.140 |
|
IL-6 | 73 | 0.3 | (0–50.5) | 70 | 0.3 | (0–1) | 0.785 |
|
IL-12 | 47 | 1.2 | (0.3–3.8) | 42 | 0.8 | (0.2–4.3) | 0.105 |
|
IL-17 | 39 | 1.2 | (0.2–5.2) | 34 | 0.8 | (0.1–7) | 0.094 |
|
IL-1Ra | 66 | 1.1 | (0.3–10.3) | 66 | 1 | (0.2–7.8) | 0.116 |
|
IL-4 | 63 | 1.7 | (0.5–7.9) | 60 | 1.1 | (0.2–7.7) | 0.015 |
|
IL-10 | 48 | 1.1 | (0.2–5.4) | 49 | 0.8 | (0.2–6.6) | 0.032 |
| Chemokine |
|
IL-8 | 74 | 0.8 | (0.1–14.1) | 70 | 0.8 | (0.3–4.7) | 0.444 |
|
IP-10 | 74 | 1.1 | (0.4–27) | 70 | 1.1 | (0.4–12.2) | 0.516 |
|
MCP-1 | 74 | 0.7 | (0.3–30.1) | 70 | 0.7 | (0.2–2.7) | 0.461 |
|
MIP-1α | 73 | 1.1 | (0.5–6.5) | 70 | 1.1 | (0.3–6.5) | 0.230 |
|
MIP-1β | 74 | 0.9 | (0.5–3.8) | 70 | 0.8 | (0.3–2.4) | 0.430 |
|
RANTES | 74 | 0.9 | (0.2–8.8) | 70 | 0.8 | (0–18.2) | 0.345 |
|
Eotaxin | 23 | 0.8 | (0.4–2.1) | 27 | 0.9 | (0.2–1.9) | 0.668 |
| Growth factors |
| Basic
FGF | 53 | 1.1 | (0.3–12) | 48 | 0.8 | (0.1–5.9) | 0.195 |
|
PDGF-BB | 74 | 1.2 | (0.1–22.8) | 69 | 0.8 | (0–16.3) | 0.049 |
|
VEGF | 50 | 1.2 | (0.3–10.6) | 42 | 1 | (0.1–5.1) | 0.195 |
| Hematopoietic
factor |
|
G-CSF | 69 | 1.1 | (0.1–93.5) | 66 | 1 | (0.2–7.5) | 0.370 |
|
Interferon-γ | 74 | 1.2 | (0.2–8) | 69 | 1.1 | (0.3–6.3) | 0.276 |
|
TNF-α | 70 | 1.2 | (0.3–8.6) | 65 | 1 | (0.2–4.2) | 0.041 |
Patients with Grade B/C pancreatic
fistula or abdominal abscess
There was no significant difference in transition of
the serum cytokine level when patients with Grade B/C pancreatic
fistula or abdominal abscess were analyzed.
The effect of patient age on ratios of
cytokine levels
We also assessed the impact of patient age on the
cytokine levels and the ratios. Patients were divided into two
groups, younger than 70 or 70 years and older. Comparison of the
ratios for IL-4, IL-9, IL-10, PDGF-BB, and TNF-α showed no
significant difference between younger and elder patients (Wilcoxon
signed-rank test, P=0.75, 0.465, 0.695, 0.297, and 0.688,
respectively).
Discussion
There are two intriguing findings in the initial
analysis of this study. First, comparing the serum cytokine levels
by Wilcoxon signed-rank testing revealed no significant difference
at POD1 or POD3 between the patients receiving TJ-100 treatment and
those receiving placebo. Second, the cytokine levels significantly
varied among the individuals even though the patients underwent
similar, invasive surgical procedures. The increase in cytokine
levels following surgery was quite small in some patients, even
with severe complications, while other patients had substantially
increased levels of the cytokines despite an uneventful
postoperative recovery (data not shown).
Preoperative tumor burden and inflammation might
explain these observations in part, because malignancies have been
shown to alter the levels of cytokines compared to benign diseases
needing surgical interventions (16).
Namely, the preoperative levels of IL-1β, IL-7, IL-8, G-CSF, IFN-γ,
and TNF-α were significantly increased in patients with colon
cancer, while these cytokines remained low in patients with other
diseases (16). The altered cytokine
levels before surgery were likely to have occurred in the present
study due to different states of cancer advancement and/or
different types of tumors, and such preoperative differences might
have consequently hampered detection of the effects of TJ-100.
Another possible explanation for the wide range of cytokine levels
observed in the present study is polymorphisms of genes encoding
cytokines. For instance, production of IL-1β, TNF-α, and IL-10 from
peripheral blood mononuclear cells (PBMCs) obtained from pancreatic
cancer patients or healthy volunteers was evidently affected by
particular gene polymorphisms (14,15).
Furthermore, the relationship between gene polymorphisms of
proinflammatory cytokines and their serum levels and short-term
operative results have been demonstrated in the field of
esophagectomy (17). Unfortunately,
these two potential differences in clinical background were not
addressed in the present study. At least, our findings clearly
suggested that application of serum cytokines as markers for the
strength of inflammation or severity of complication after PD might
not be as straightforward as previously considered.
By addressing the aforementioned potential
confounding factors by comparing ratios of cytokine levels on POD3
to those on POD1, we found that changes in IL-10, IL-4, IL-9,
TNF-α, and PDGF-BB were significantly reduced by TJ-100 in patients
without severe complications. In animal models, TJ-100 decreased
expression of TNF-α in target organs (12,18),
reducing postoperative paralytic ileus and bacterial translocation.
Furthermore, TJ-100 decreased levels of serum C-reactive protein in
patients following surgery for colon/rectal disease (19), suggesting that TJ-100 could ameliorate
inflammatory responses. In contrast, the present analysis
demonstrated a higher increase in TNF-α level from POD3 to POD1 in
patients who received TJ-100 compared to the Placebo Group,
indicating that TJ-100 might enhance the early inflammatory
response after PD. The contradiction between previous observations
and ours may be derived from the different extents of surgical
stress. While the uncontrolled expression of TNF-α is associated
with an overwhelming inflammatory response, multiple-organ
dysfunction, and impaired tissue recovery, extensive suppression of
TNF-α results in frequent occurrence of infectious complications
after surgery (20). The appropriate
inflammatory response elicited against surgical stress is clearly
essential for immunological defense and tissue repair, and the
biological influence of sustained serum levels of TNF-α in patients
receiving PD and TJ-100 should be investigated further.
IL-4, IL-10, and PDGF-BB are associated with wound
healing (21–24), while increased IL-9 might enhance
intestinal muscle contractility and protect against bacterial shock
by modulating inflammatory and anti-inflammatory mediators
(25,26). These observations are partially
consistent with our previous clinical study of 45 patients
undergoing PD (27); however, the
overall clinical significance of different changes in cytokine
levels due to TJ-100 administration was not determined by the
present study.
Several limitations related to this analysis should
be noted in addition to the lack of baseline (preoperative)
cytokine levels and information of gene polymorphisms. First, the
levels of cytokines were under the detection threshold in several
patients, precluding these samples from the final analysis.
Similarly, the number of patients with severe complications was not
large enough to detect statistical differences of cytokine
transitions in the present study. Finally, the level of the
cytokines at the later phase of recovery, such as 7 days after
operation, could have brought better understanding of the effect of
TJ-100.
In conclusion, we examined the levels of 27
cytokines after PD and their transition from POD1 to POD3 in a
large number of the patients enrolled in a multi-institutional,
prospective trial. At initial assessment, a wide range of cytokine
levels among individuals after PD was found, suggesting the
difficulties of using the cytokines levels to assume the extent of
surgical stress. However, oral administration of TJ-100 altered the
postoperative changes in serum levels of IL-4, IL-9, IL-10, TNF-α,
and PDGF-BB. With consideration of gene polymorphism and
preoperative inflammatory status, studies using TJ-100 under
surgical stress should be performed to elucidate the role of
different transition pattern of cytokines.
Acknowledgements
Not applicable.
Funding
The present study was supported by the non-profit
organization Epidemiological and Clinical Research Information
Network (ECRIN).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
All authors read and approved the final manuscript.
HM performed data analysis, data interpretation, figure and table
creation, and wrote the manuscript. KO participated in study
design, study protocol creation, literature search, data
collection, data analysis and data interpretation. TF participated
in study design, data collection and manuscript revision. MSO
participated in study protocol creation, data analysis and figure
creation. MK, SH, YK, MS, TA, YS, YA, NK, YM, JO, HE and HN
participated in study design, study protocol creation, data
collection and manuscript revision. JS and HY participated in study
design, study protocol creation, literature search, data
collection, data analysis, data interpretation, figure and table
creation, and manuscript writing.
Ethics approval and consent to
participate
All patients provided written informed consent prior
to study inclusion. The institutional review board of each
participating institute approved the trial protocol.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kono T, Shimada M, Yamamoto M, Kaneko A,
Oomiya Y, Kubota K, Kase Y, Lee K and Uezono Y: Complementary and
synergistic therapeutic effects of compounds found in Kampo
medicine: Analysis of daikenchuto. Front Pharmacol. 6:1592015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manabe N, Camilleri M, Rao A, Wong BS,
Burton D, Busciglio I, Zinsmeister AR and Haruma K: Effect of
daikenchuto (TU-100) on gastrointestinal and colonic transit in
humans. Am J Physiol Gastrointest Liver Physiol. 298:G970–G975.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshikawa K, Shimada M, Wakabayashi G,
Ishida K, Kaiho T, Kitagawa Y, Sakamoto J, Shiraishi N, Koeda K,
Mochiki E, et al: Effect of daikenchuto, a traditional japanese
herbal medicine, after total gastrectomy for gastric cancer: A
multicenter, randomized, double-blind, placebo-controlled, phase II
trial. J Am Coll Surg. 221:571–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimada M, Morine Y, Nagano H, Hatano E,
Kaiho T, Miyazaki M, Kono T, Kamiyama T, Morita S, Sakamoto J, et
al: Effect of TU-100, a traditional Japanese medicine, administered
after hepatic resection in patients with liver cancer: A
multi-center, phase III trial (JFMC40-1001). Int J Clin Oncol.
20:95–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katsuno H, Maeda K, Kaiho T, Kunieda K,
Funahashi K, Sakamoto J, Kono T, Hasegawa H, Furukawa Y, Imazu Y,
et al: Clinical efficacy of Daikenchuto for gastrointestinal
dysfunction following colon surgery: A randomized, double-blind,
multicenter, placebo-controlled study (JFMC39-0902). Jpn J Clin
Oncol. 45:650–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katsuno H, Maeda K, Ohya M, Yoshioka K,
Tsunoda A, Koda K, Matsuoka H, Ohge H, Morita S, Saji S, et al:
Clinical pharmacology of daikenchuto assessed by transit analysis
using radiopaque markers in patients with colon cancer undergoing
open surgery: A multicenter double-blind randomized
placebo-controlled study (JFMC39-0902 additional study). J
Gastroenterol. 51:222–229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satoh K, Hayakawa T, Kase Y, Ishige A,
Sasaki H, Nishikawa S, Kurosawa S, Yakabi K and Nakamura T:
Mechanisms for contractile effect of Dai-kenchu-to in isolated
guinea pig ileum. Dig Dis Sci. 46:250–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukuda H, Chen C, Mantyh C, Ludwig K,
Pappas TN and Takahashi T: The herbal medicine, Dai-Kenchu-to,
accelerates delayed gastrointestinal transit after the operation in
rats. J Surg Res. 131:290–295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kono T, Kaneko A, Omiya Y, Ohbuchi K, Ohno
N and Yamamoto M: Epithelial transient receptor potential ankyrin 1
(TRPA1)-dependent adrenomedullin upregulates blood flow in rat
small intestine. Am J Physiol Gastrointest Liver Physiol.
304:G428–G436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuchiya K, Kubota K, Ohbuchi K, Kaneko A,
Ohno N, Mase A, Matsushima H, Yamamoto M, Miyano K, Uezono Y and
Kono T: Transient receptor potential ankyrin 1 agonists improve
intestinal transit in a murine model of postoperative ileus.
Neurogastroenterol Motil. 28:1792–1805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kono T, Kaneko A, Hira Y, Suzuki T,
Chisato N, Ohtake N, Miura N and Watanabe T: Anti-colitis and
-adhesion effects of daikenchuto via endogenous adrenomedullin
enhancement in Crohn's disease mouse model. J Crohns Colitis.
4:161–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Endo M, Hori M, Ozaki H, Oikawa T and
Hanawa T: Daikenchuto, a traditional Japanese herbal medicine,
ameliorates postoperative ileus by anti-inflammatory action through
nicotinic acetylcholine receptors. J Gastroenterol. 49:1026–1039.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okada K, Kawai M, Hirono S, Fujii T,
Kodera Y, Sho M, Nakajima Y, Satoi S, Kwon AH, Shimizu Y, et al:
Evaluation of the efficacy of daikenchuto (TJ-100) for the
prevention of paralytic ileus after pancreaticoduodenectomy: A
multicenter, double-blind, randomized, placebo-controlled trial.
Surgery. 159:1333–1341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barber MD, Powell JJ, Lynch SF, Fearon KC
and Ross JA: A polymorphism of the interleukin-1 beta gene
influences survival in pancreatic cancer. Br J Cancer.
83:1443–1447. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Warlé MC, Farhan A, Metselaar HJ, Hop WC,
Perrey C, Zondervan PE, Kap M, de Rave S, Kwekkeboom J, Ijzermans
JN, et al: Cytokine gene polymorphisms and acute human liver graft
rejection. Liver Transpl. 8:603–611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crucitti A, Corbi M, Tomaiuolo PM, Fanali
C, Mazzari A, Lucchetti D, Migaldi M and Sgambato A: Laparoscopic
surgery for colorectal cancer is not associated with an increase in
the circulating levels of several inflammation-related factors.
Cancer Biol Ther. 16:671–677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakamoto K, Oka M, Yoshino S, Hazama S,
Takeda S, Yoshimura K, Okayama N and Hinoda Y: Relationship between
cytokine gene polymorphisms and risk of postoperative pneumonia
with esophageal cancer. J Gastrointest Surg. 18:1247–1253. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshikawa K, Kurita N, Higashijima J,
Miyatani T, Miyamoto H, Nishioka M and Shimada M: Kampo medicine
‘Dai-kenchu-to’ prevents bacterial translocation in rats. Dig Dis
Sci. 53:1824–1831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Osawa G, Yoshimatsu K, Yokomizo H, Otani
T, Matsumoto A, Nakayama M and Ogawa K: The clinical effects of
dai-kenchu-to on postoperative intestinal movement and inflammatory
reaction in colorectal surgery. Hepatogastroenterology. 62:807–810.
2015.PubMed/NCBI
|
|
20
|
Yang ZP, Hong L, Wu Q, Wu KC and Fan DM:
Preoperative infliximab use and postoperative complications in
Crohn's disease: A systematic review and meta-analysis. Int J Surg.
12:224–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kieran I, Knock A, Bush J, So K, Metcalfe
A, Hobson R, Mason T, O'Kane S and Ferguson M: Interleukin-10
reduces scar formation in both animal and human cutaneous wounds:
Results of two preclinical and phase II randomized control studies.
Wound Repair Regen. 21:428–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salmon-Ehr V, Ramont L, Godeau G,
Birembaut P, Guenounou M, Bernard P and Maquart FX: Implication of
interleukin-4 in wound healing. Lab Invest. 80:1337–1343. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gupta M, Poonawala T, Farooqui M, Ericson
ME and Gupta K: Topical fentanyl stimulates healing of ischemic
wounds in diabetic rats. J Diabetes. 7:573–583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhansali A, Venkatesh S, Dutta P, Dhillon
MS, Das S and Agrawal A: Which is the better option: Recombinant
human PDGF-BB 0.01% gel or standard wound care, in diabetic
neuropathic large plantar ulcers off-loaded by a customized contact
cast? Diabetes Res Clin Pract. 83:e13–e16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khan WI, Richard M, Akiho H, Blennerhasset
PA, Humphreys NE, Grencis RK, Van Snick J and Collins SM:
Modulation of intestinal muscle contraction by interleukin-9 (IL-9)
or IL-9 neutralization: Correlation with worm expulsion in murine
nematode infections. Infect Immun. 71:2430–2438. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grohmann U, Van Snick J, Campanile F,
Silla S, Giampietri A, Vacca C, Renauld JC, Fioretti MC and
Puccetti P: IL-9 protects mice from Gram-negative bacterial shock:
Suppression of TNF-alpha, IL-12, and IFN-gamma, and induction of
IL-10. J Immunol. 164:4197–4203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okada K, Kawai M, Hirono S, Miyazawa M,
Shimizu A, Kitahata Y and Yamaue H: Perioperative administration of
Daikenchuto (TJ-100) reduces the postoperative paralytic ileus in
patients with pancreaticoduodenectomy. Hepatogastroenterology.
62:466–471. 2015.PubMed/NCBI
|