Introduction
As a typical malignant urogenital tract cancer,
bladder cancer (BC) is the sixth most prevalent cancer (1) and the second most common cause of death
for cancer in the urinary system for men (2). About 330,000 new BC cases occur around
the world resulting in 130,000 deaths annually (3). The main pathogenic factors of bladder
are environmental and genetic factors (4). Although surgical operation, radiotherapy
and chemotherapy have made great improvement in treatment, BC is
still a common disease with a high mortality rate (5). Moreover, there are many limitations for
the established BC biomarkers to diagnose the various cancers
(6). Thus efficient gene therapies
should be developed to use for early diagnosis of cancer.
MicroRNAs (miRNAs) act as tumor suppressors or
oncogenes by regulating gene expression at the post-transcriptional
levels to get involved in tumor formation (7). Especially, dysregulation of miRNAs in BC
was closely related with bladder tumorigenesis (8). Therefore, miRNAs could be used to
diagnose cancer. There are many studies on the role of miRNAs which
influenced cell physiological activity in BC, such as cell
migration, invasion, proliferation and survival. For example, tumor
suppressors including miR-106a, miR-223, and miR-613 accommodated
BC by regulating the MAPKs, NCOA1 and SphK1, respectively (9–11). In
addition, tumor oncogenes containing miR-130, miR-200c, and miR-556
regulate BC by regulating the PTEN, RECK and DAB2IP, respectively
(12–14). However, research on the function of
miR-145-5p is still rare in the pathogenesis of BC.
Transgelin-2 (TAGLN2) belonging to the ABP family
was firstly discovered in 1998 (15).
Recently, the dysregulated expression of TAGLN2 was identified in
various cancers. TAGLN2 function as an oncogene to promote cancer
cell proliferation, invasion and migration (16–18). In
addition, Yoshino et al indicated that TAGLN2 along with
miR-1/133a affected cell proliferation, apoptosis, invasion and
migration in BC (19). Nonetheless,
there are no reports on TAGLN2/miR-145-5p in BC.
This study proposed the hypothesis that miR-145-5p
suppressed tumor formation by regulating TAGLN2. In order to verify
the hypothesis, miR-145-5p and TAGLN2 expressions were detected in
BC. Besides, we also examined cell proliferation and migration to
further expound the roles of miR-145-5p and TAGLN2 in BC.
Materials and methods
Clinical tissues
Clinical tissues were collected from 22 patients
with BC who were undergoing transurethral resection or cystectomy
at Beijing Ditan Hospital, Capital Medical University (Beijing,
China). The study was approved by the Ethics Committee of Beijing
Ditan Hospital, Capital Medical University. Signed written informed
consents were obtained from the patients or guardians. BC adjacent
tissues were obtained from areas about 2 cm away from tumor
lesions.
Cell culture and transfection
The human BC cell lines T24 and 5637, and the
immortalized urothelial cell line SV-HUC-1 were applied in this
study. All the cell lines were obtained from the Shanghai Institute
of Cell Biology, Chinese Academy of Sciences (Shanghai, China). The
cells were grown in RPMI-1640 medium supplemented by 10% fetal
bovine serum (FBS). The cells were incubated at 37°C, with 5%
CO2 atmosphere.
The miR-145-5p mimic and inhibitor, TAGLN2 siRNA
were purchased from RiBoBio Co., Ltd. (Guangzhou, China) and then
they were transferred into T24 or 5637 cells with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) according to manufacturer's
instructions.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was applied for extracting total RNA containing
miRNA to quantitate the miR-145-5p expression in BC tissues and
cell lines. RNA was reverse transcribed using the RevertAid First
Strand cDNA kit (Thermo Fisher Scientifc, Inc.) according to the
manufacture's introduction. The reactions were incubated at 25°C
for 5 min, 42°C for 60 min and 70°C for 5 min. The miR-145-5p
reverse transcription primer is 5′-AGTCCAGTTTTCCCAGGAATCCCT-3′.
RT-qPCR was performed through the SYBR Premix Ex Taq (Takara Bio,
Inc., Otsu, Japan) on an ABI PRISM 7500 Real-time PCR system
(Applied Biosystems, Thermo Fisher Scientific, Inc.). The reactions
were incubated at 94°C for 10 min, followed by 45 cycles at 95°C
for 15 sec and 60°C for 30 sec. The primers were designed as
follows: miR-145-F 5′-CCTTGTCCTCACGGTCCAGT-3′, and R
5′-AACCATGACCTCAAGAACAGTATTT-3′; TAGLN2-F
5′-CTACCTGAAGCCGGTGTCC-3′, and R 5′-ATCCCCAGAGAAGAGCCCAT-3′; U6-F
5′- GCTTCGGCAGCACATATACTAAAAT −3′, and R 5′-
CGCTTCACGAATTTGCGTGTCAT −3′; GAPDH-F, 5′-GAGTCAACGGATTTGGTCGT-3′
and R, 5′-TTGATTTTGGAGGGATCTCG-3′. U6 and GAPDH were used as
control of miR-145 and TAGLN2. The miR-145-5p and TAGLN2 levels
were analyzed using the 2−ΔΔCq method (20).
Cell proliferation and migration
assays
The cell proliferation and migration were performed
using MTT and Transwell assays to investigate the role of the
miR-145-5p and TAGLN2 using T24 or 5637 cells. The experimental
procedures were performed according to a previous study (18).
Dual luciferase report assays
The wt 3′-UTR of TAGLN2 or mut 3′-UTR of TAGLN2 were
inserted into the pGL3 promoter vector (GenScript, Nanjing, China)
for luciferase reporter experiments. Then, the vector and
miR-145-5p mimic were transfected into 5637 cells. Cells were
cultured in a 24-well plate. About 48 h after transfection, the
dual luciferase reporter assay system (Promega Corporation,
Madison, WI, USA) was applied to perform luciferase assays.
Western blot analysis
The protein samples were obtained using RIPA buffer.
Proteins were separated through SDS-PAGE and then incubated with 5%
non-fat milk blocked membranes at room temperature. Next we
incubated the membranes overnight at 4°C with rabbit polyclonal
TAGLN2 antibody (cat. no. ab233478; 1:1,000), rabbit polyclonal
GAPDH antibody (cat. no. ab9485; 1:1,000) and subsequently
incubated with matched goat polyclonal secondary antibody to rabbit
IgG - H&L (cat. no. ab150077; 1:1,000) all from Abcam
(Cambridge, MA, USA). The protein expression levels were measured
by a gel imaging system (JS-780; Pei Qing Technology Co., Ltd.,
Shanghai, China).
Statistical analysis
The experimental data are presented as the mean ±
SD. Enumeration data were analyzed using Student's t-test and
Chi-square test. Statistical analysis was analyzed with GraphPad
Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
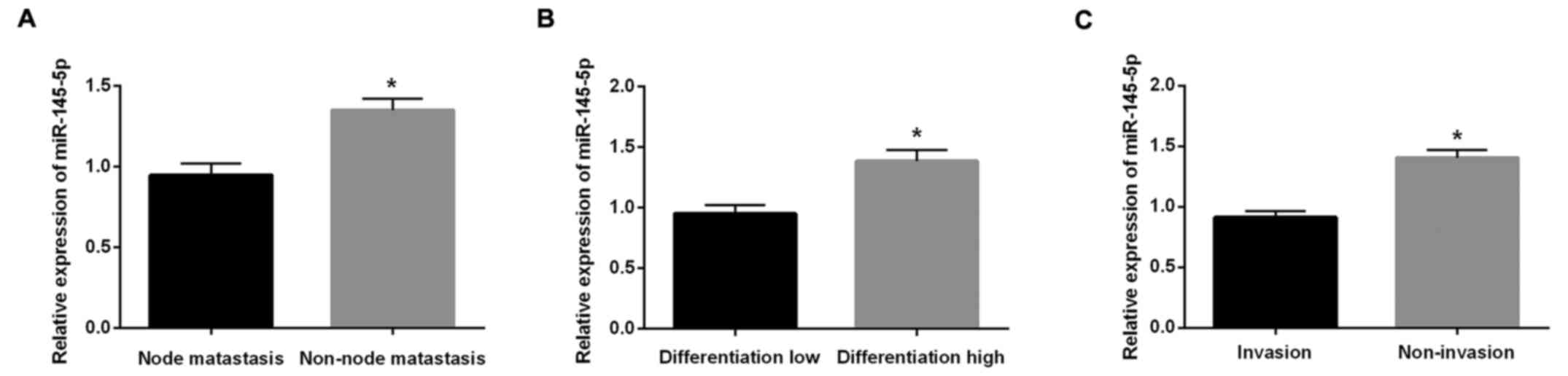
The relationship between miR-145-5p
expression and the clinicopathological features was analyzed in
BC
In Fig. 1, the
relationships between clinicopathological characteristics and the
expression of miR-145-5p are summarized. The miR-145-5p expression
was closely related with lymph node metastasis (Fig. 1A), differentiation (Fig. 1B) and vascular invasion (Fig. 1C; P<0.01).
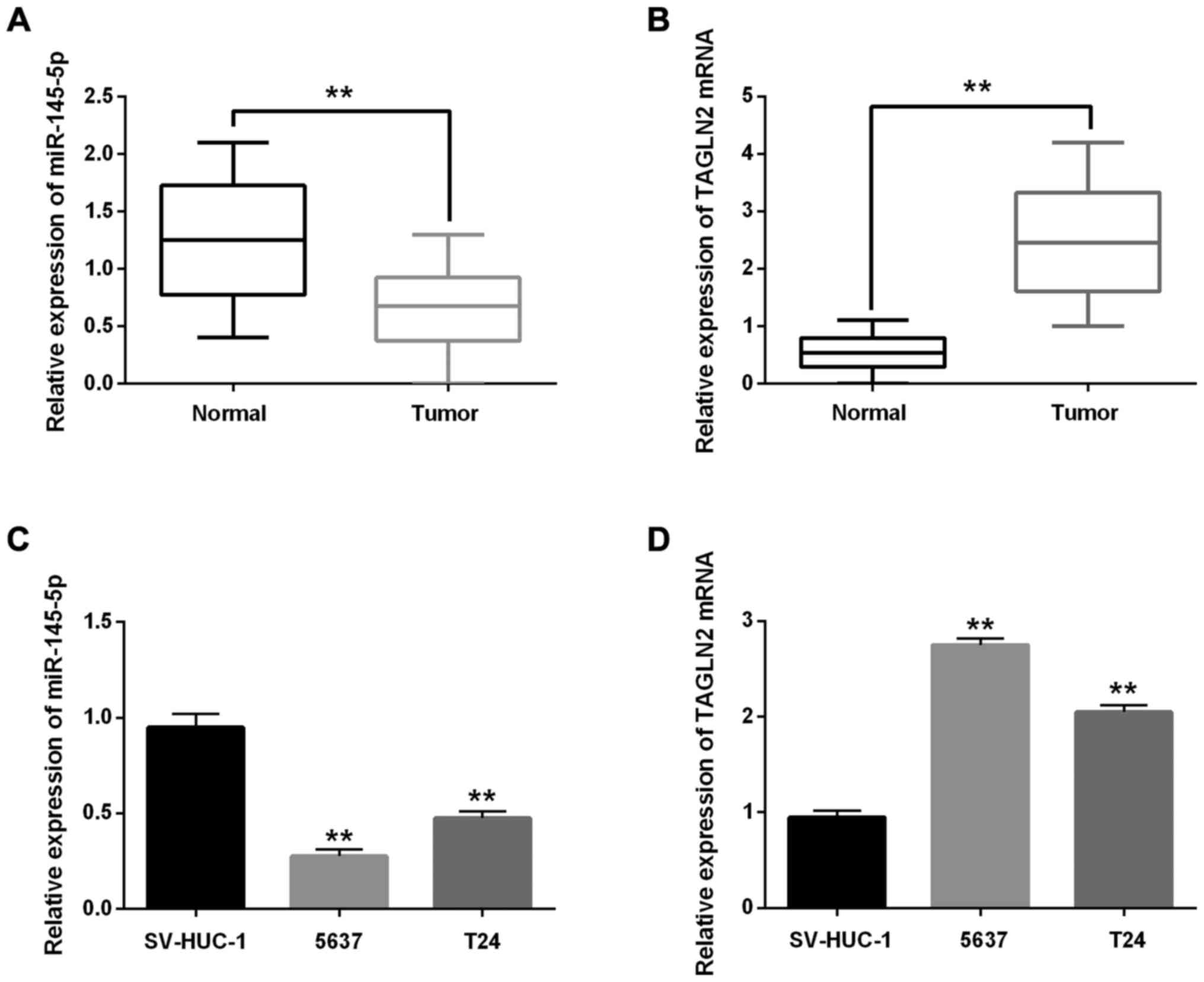
miR-145-5p and TAGLN2 expression in BC
tissues and cells
The miR-145-5p and TAGLN2 expression in BC tissues
was detected using RT-qPCR. The miR-145-5p expression was lower
than that of normal tissues in BC tissues but higher than
expression of TAGLN2 (Fig. 2A and B;
P<0.01). Furthermore, the miR-145-5p and TAGLN2 expression in BC
cell lines was detected simultaneously. The BC cell lines (5637 and
T24) had low expression for miR-145-5p but high expression for
TAGLN2 mRNA (Fig. 2C and D;
P<0.01). Because the expression levels of 5637 were considered
to have more difference than T24, 5637 cell line was selected in
the following experiments.
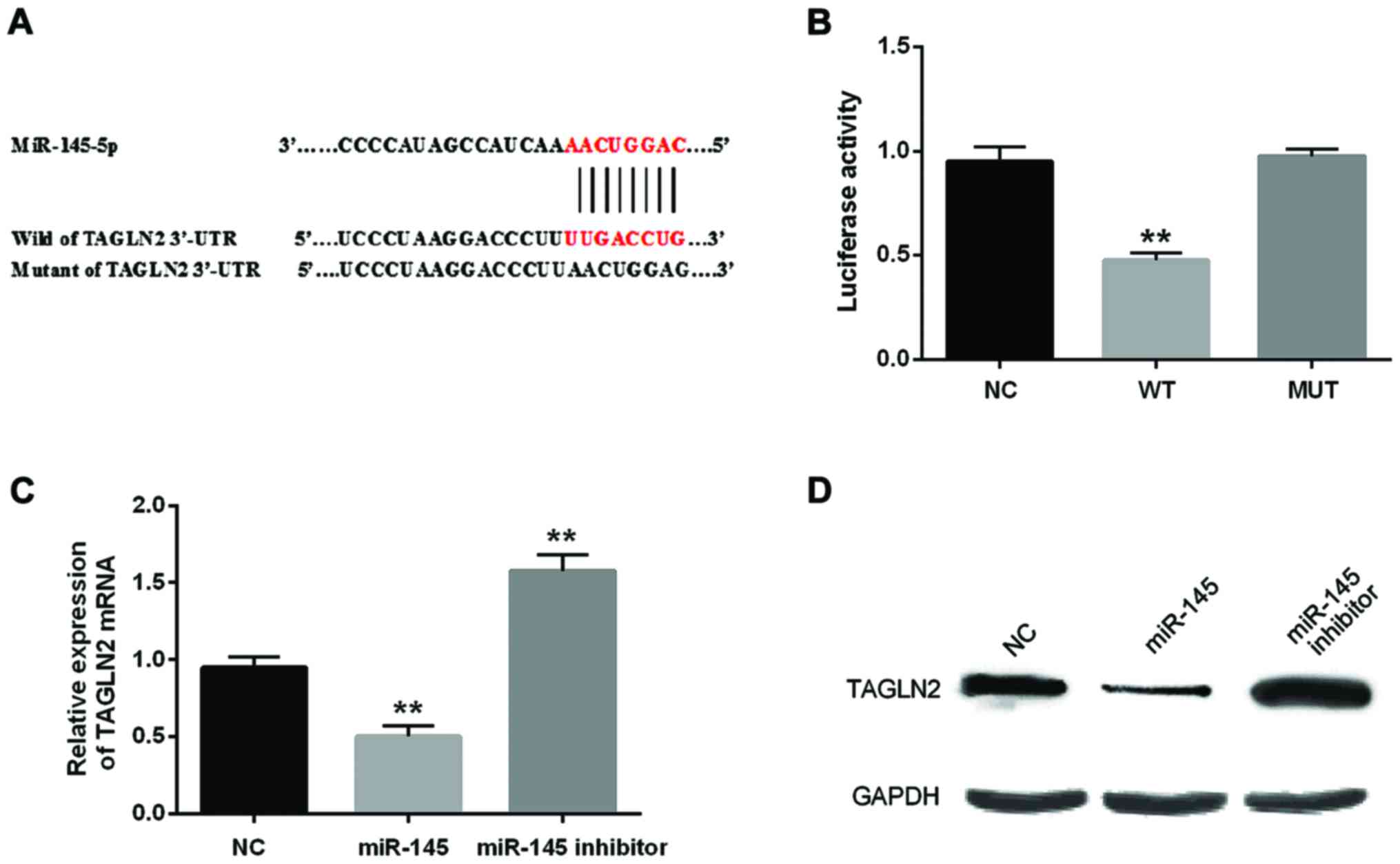
miR-145-5p directly targets TAGLN2 in
BC cells
TargetScan database predicted that TAGLN2 3′UTR has
a binding site for miR-145-5p (Fig.
3A). To verify the above result, we conducted luciferase
reporter assay in the 5637 cells. As predicted, luciferase activity
in the cells transfected with miR-145-5p mimic and wild-type TAGLN2
was distinctly deceased compared to the control group (P<0.01).
Additionally, little change was found in cells containing mutated
TAGLN2 and miR-145-5p mimic (Fig. 3B;
P>0.05). Furthermore, miR-145-5p overexpression downregulated
TAGLN2 mRNA and protein expression significantly (Fig. 3C and D).
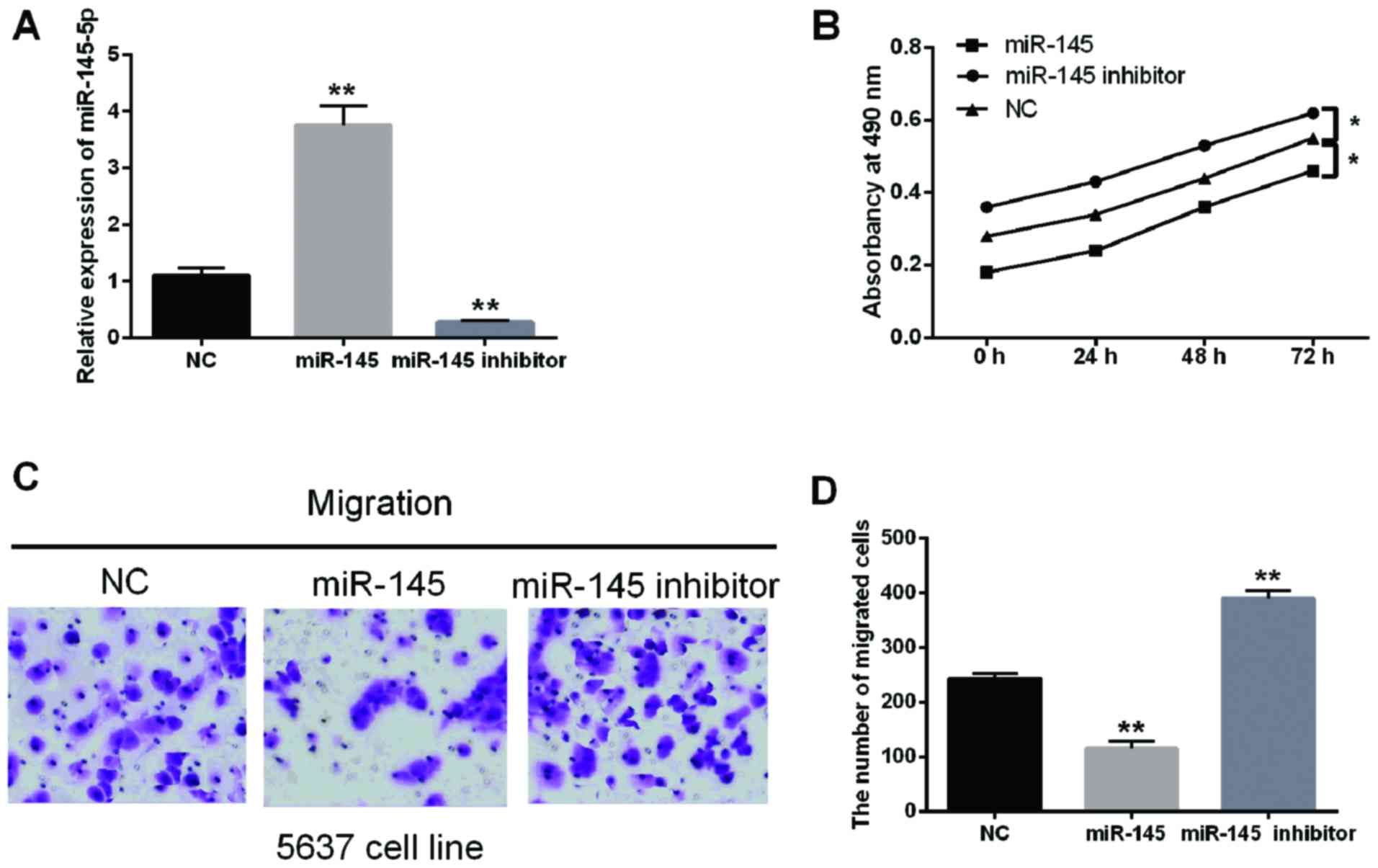
miR-145-5p suppresses the cell
proliferation and migration in BC
The function of miR-145-5p for regulating the cell
proliferation and migration in BC were investigated in the present
study. The high transfection efficiency was detected in cells
containing miR-145-5p mimics or inhibitor (Fig. 4A). Moreover, the MTT results
demonstrated that cell proliferation was suppressed by miR-145-5p
overexpression but promoted by the downregulation of miR-145-5p
(Fig. 4B). The Transwell analysis
suggested that the cell migration was also inhibited by miR-145-5p
overexpression while promoted by the miR-145-5p downregulation
(Fig. 4C and D; P<0.01).
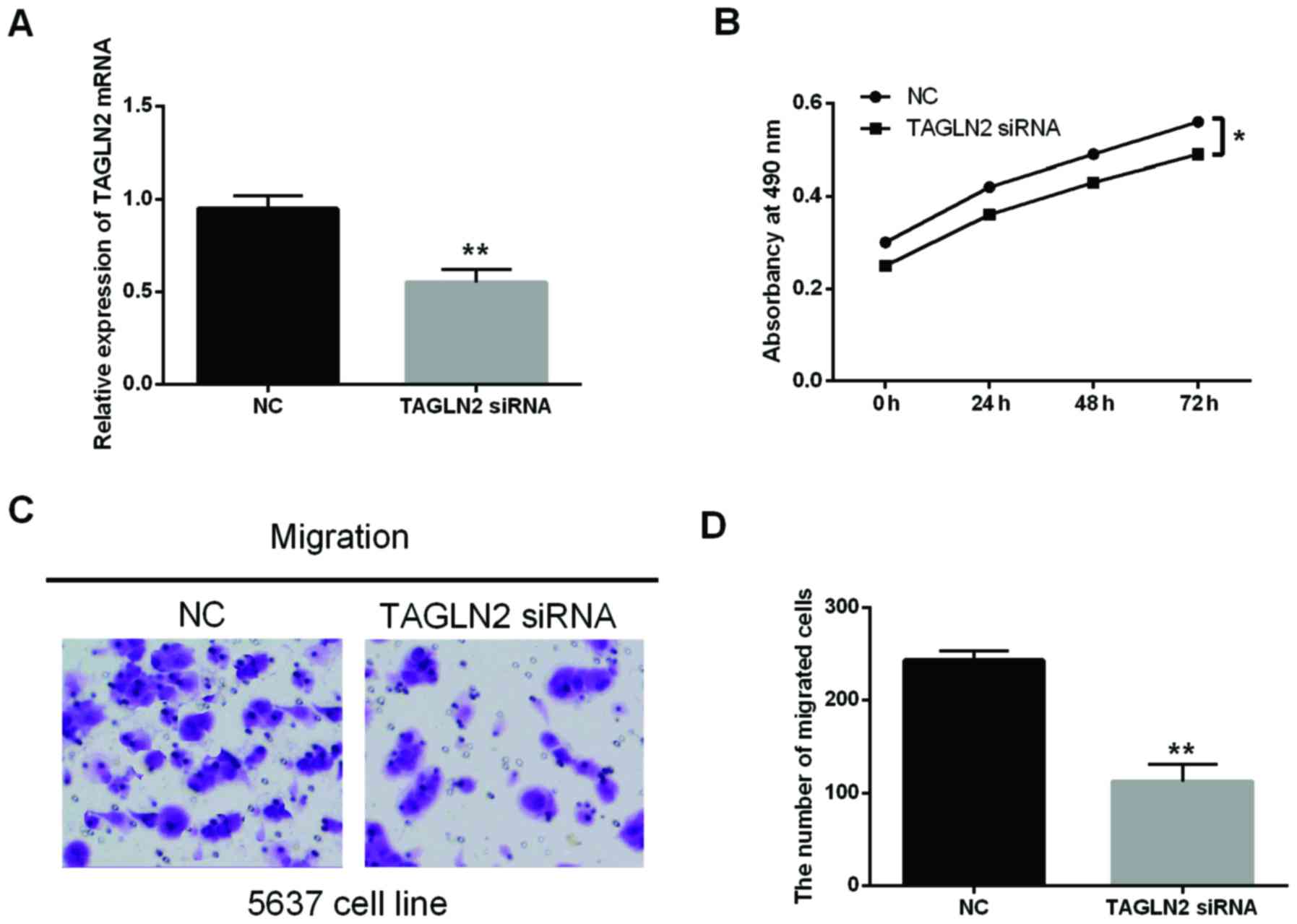
The regulated function of TAGLN2 in
BC
To analyze the regulated function of TAGLN2 in BC,
the TAGLN2 siRNA was transfected into the 5637 cells (Fig. 5A). Moreover, the MTT and Transwell
assay were conducted to identify the proliferation and migration.
The results suggested that the knockdown of TAGLN2 significantly
inhibited the proliferation and migration of 5367 cells (Fig. 5B-D; P<0.05). It indicated that
TAGLN2 might have the carcinogenic effect in BC to some extent.
Discussion
Many scholars have proposed that miRNAs act as
oncogenes or tumor suppressors through regulating the relative
target genes in various cancers (21), and that the change in miRNA expression
was related to the pathogenesis, metastasis and progression of
human cancer (22). Among them,
miR-145 plays a suppressive role in tumor progression and is
involved in tumorigenesis. Furthermore, miR-145 with anticancer
effect was identified in ovarian carcinoma (23), colorectal carcinoma (24) and non-small cell lung cancer (25). The same conclusion for BC was obtained
in this study. Furthermore, it has been reported that miR-145
participated in various physiological and pathological rhythms in
BCs, including differentiation (26),
apoptosis (27), and invasion
(28). Although miR-145 taking part
in pathogenesis of BC has been reported in many studies, its
regulated mechanisms on cell proliferation and migration in BC
continue to be confused.
This study revealed downregulation of miR-145 in BC
tissues. Moreover, the inhibiting effect of miR-145-5p on cell
proliferation and migration were found in BC. In brief, all the
results indicated that miR-145-5p had inhibitory effects on the
pathogenesis of BC. Therefore, it is worth exploring BC
relationship between miR-145-5p and its target gene.
In this study, TAGLN2 was found to be a potential
target gene of miR-145-5p in BC through bioinformatics analysis. As
a member of the calponin family of actin-binding proteins, TAGLN2
is an oncogene. Many investigations have detected that TAGLN2 has
modulated cell proliferation, differentiation, migration and
apoptosis (29). Additionally,
Yoshino et al proved that downregulating TAGLN2 inhibited
cell proliferation, migration and invasion activity in BC (19). We also affirmed that miR-145-5p
directly targeted TAGLN2 in the present study. Moreover, we found
that miR-145-5p overexpression brought about downregulation of
TAGLN2 in BC. Furthermore, Transwell analysis revealed that TAGLN2
overexpression promoted BC cell proliferation and migration which
was in keeping with the previous studies.
Besides, we also identified the relationship between
clinicopathological characteristics and miR-145-5p expression in
BC. It was found that the expression of miR-145-5p was closely
related to lymph node metastasis, differentiation and vessel
invasion (P<0.01). Follow-up experiments will be conducted and
the prognostic analysis for these patients will continue to be
further analyzed.
In conclusion, the present study emphasized that
miR-145-5p suppressed TAGLN2 expression and contributed to cell
proliferation and migration in BC. This novel miR-145-5p/TAGLN2
axis may provide new therapeutic implications for BC. Future
research needs to make full use of the potential impact of
miR-145-5p on cancer treatment.
Acknowledgements
Not applicable.
Funding
This research was funded by the project of 2017
Capital Medical University Basic Clinical Research Cooperation
(17JL37) from Capital Medical University (Beijing, China).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ contributed significantly to data analysis and
manuscript preparation. MJ performed the data analyses and wrote
the manuscript. QL performed the data analyses. ZH helped perform
the analysis with constructive discussions. YZ contributed in the
organisation of the experimental data. SJ contributed to the
conception of the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Beijing Ditan Hospital, Capital Medical University (Beijing,
China). Signed informed consents were obtained from the patients or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harb-de la Rosa A, Acker M, Kumar RA and
Manoharan M: Epigenetics application in the diagnosis and treatment
of bladder cancer. Can J Urol. 22:7947–7951. 2015.PubMed/NCBI
|
|
2
|
Bid HK: Words of wisdom. Re: Markers
predicting response to Bacillus Calmette-Guérin immunotherapy in
high-risk bladder cancer patients: a systematic review. Eur Urol.
61:846–847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ploeg M, Aben KKH and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye F, Wang L, Castillo-Martin M, McBride
R, Galsky MD, Zhu J, Boffetta P, Zhang DY and Cordon-Cardo C:
Biomarkers for bladder cancer management: Present and future. Am J
Clin Exp Urol. 2:1–14. 2014.PubMed/NCBI
|
|
5
|
Jiang QQ, Liu B and Yuan T: MicroRNA-16
inhibits bladder cancer proliferation by targeting Cyclin D1. Asian
Pac J Cancer Prev. 14:4127–4130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu X, Li S, Lin Y, Chen H, Hu Z, Mao Y, Xu
X, Wu J, Zhu Y, Zheng X, et al: MicroRNA-124-3p inhibits cell
migration and invasion in bladder cancer cells by targeting ROCK1.
J Transl Med. 11:2762013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang M, Zhuang Q and Cui L: MiR-194
inhibits cell proliferation and invasion via repression of RAP2B in
bladder cancer. Biomed Pharmacother. 80:268–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ichimi T, Enokida H, Okuno Y, Kunimoto R,
Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama
K, et al: Identification of novel microRNA targets based on
microRNA signatures in bladder cancer. Int J Cancer. 125:345–352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin SS, Park SS, Hwang B, Kim WT, Choi
YH, Kim WJ and Moon SK: MicroRNA-106a suppresses proliferation,
migration, and invasion of bladder cancer cells by modulating MAPK
signaling, cell cycle regulators, and Ets-1-mediated MMP-2
expression. Oncol Rep. 36:2421–2429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo J, Cao R, Yu X, Xiao Z and Chen Z:
MicroRNA-223-3p inhibits human bladder cancer cell migration and
invasion. Tumour Biol. 39:10104283176916782017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Duan P, Zhu H and Rao D: miR-613
inhibits bladder cancer proliferation and migration through
targeting SphK1. Am J Transl Res. 9:1213–1221. 2017.PubMed/NCBI
|
|
12
|
Egawa H, Jingushi K, Hirono T, Ueda Y,
Kitae K, Nakata W, Fujita K, Uemura M, Nonomura N and Tsujikawa K:
The miR-130 family promotes cell migration and invasion in bladder
cancer through FAK and Akt phosphorylation by regulating PTEN. Sci
Rep. 6:205742016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng Y, Zhang X and Li P, Yang C, Tang J,
Deng X, Yang X, Tao J, Lu Q and Li P: MiR-200c promotes bladder
cancer cell migration and invasion by directly targeting RECK.
OncoTargets Ther. 9:5091–5099. 2016. View Article : Google Scholar
|
|
14
|
Feng C, Sun P, Hu J, Feng H, Li M, Liu G,
Pan Y, Feng Y, Xu Y, Feng K, et al: miRNA-556-3p promotes human
bladder cancer proliferation, migration and invasion by negatively
regulating DAB2IP expression. Int J Oncol. 50:2101–2112. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stanier P, Abu-Hayyeh S, Murdoch JN,
Eddleston J and Copp AJ: Paralogous sm22alpha (Tagln) genes map to
mouse chromosomes 1 and 9: Further evidence for a paralogous
relationship. Genomics. 51:144–147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nohata N, Sone Y, Hanazawa T, Fuse M,
Kikkawa N, Yoshino H, Chiyomaru T, Kawakami K, Enokida H, Nakagawa
M, et al: miR-1 as a tumor suppressive microRNA targeting TAGLN2 in
head and neck squamous cell carcinoma. Oncotarget. 2:29–42. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao G, Xia C, Yang J, Liu J, Du H, Kang
X, Lin Y, Guan R, Yan P and Tang S: miR-133b regulates the
expression of the Actin protein TAGLN2 during oocyte growth and
maturation: A potential target for infertility therapy. PLoS One.
9:e1007512014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du YY, Zhao LM, Chen L, Sang MX, Li J, Ma
M and Liu JF: The tumor-suppressive function of miR-1 by targeting
LASP1 and TAGLN2 in esophageal squamous cell carcinoma. J
Gastroenterol Hepatol. 31:384–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshino H, Chiyomaru T, Enokida H,
Kawakami K, Tatarano S, Nishiyama K, Nohata N, Seki N and Nakagawa
M: The tumour-suppressive function of miR-1 and miR-133a targeting
TAGLN2 in bladder cancer. Br J Cancer. 104:808–818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshino H, Seki N, Itesako T, Chiyomaru T,
Nakagawa M and Enokida H: Aberrant expression of microRNAs in
bladder cancer. Nat Rev Urol. 10:396–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nair VS, Maeda LS and Ioannidis JPA:
Clinical outcome prediction by microRNAs in human cancer: A
systematic review. J Natl Cancer Inst. 104:528–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Wu X, Wang B, Wang Q and Han L:
Mechanisms of miR-145 regulating invasion and metastasis of ovarian
carcinoma. Am J Transl Res. 9:3443–3451. 2017.PubMed/NCBI
|
|
24
|
Salem SM, Hamed AR and Mosaad RM: MTDH and
MAP3K1 are direct targets of apoptosis-regulating miRNAs in
colorectal carcinoma. Biomed Pharmacother. 94:767–773. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen GM, Zheng AJ, Cai J, Han P, Ji HB and
Wang LL: microRNA-145-3p inhibits non-small cell lung cancer cell
migration and invasion by targeting PDK1 via the mTOR signaling
pathway. J Cell Biochem. 119:885–895. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujii T, Shimada K, Tatsumi Y, Hatakeyama
K, Obayashi C, Fujimoto K and Konishi N: microRNA-145 promotes
differentiation in human urothelial carcinoma through
downregulation of syndecan-1. BMC Cancer. 15:8182015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blick C, Ramachandran A, McCormick R,
Wigfield S, Cranston D, Catto J and Harris AL: Identification of a
hypoxia-regulated miRNA signature in bladder cancer and a role for
miR-145 in hypoxia-dependent apoptosis. Br J Cancer. 113:634–644.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kou B, Gao Y, Du C, Shi Q, Xu S, Wang CQ,
Wang X, He D and Guo P: miR-145 inhibits invasion of bladder cancer
cells by targeting PAK1. Urol Oncol. 32:846–854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dvorakova M, Nenutil R and Bouchal P:
Transgelins, cytoskeletal proteins implicated in different aspects
of cancer development. Expert Rev Proteomics. 11:149–165. 2014.
View Article : Google Scholar : PubMed/NCBI
|