Introduction
Lymphoma, classified into Hodgkin lymphoma (HL) and
non-Hodgkin lymphoma (NHL) with various subtypes respectively,
originates from precursor cells in primary lymph organs or from
mature cells located in the peripheral lymphoid organs, arising
from a clone expansion of B-or-T lymphocytes transformed during the
pathways of lymphocyte differentiation (1,2). Chronic
myeloid leukemia (CML) and acute myeloid leukemia (AML) are clonal
expansion of hematopoietic progenitor cells characterized by
exaggerated proliferation of granulocytic lineage while CML
undergoes a chronic course relatively. Lymphoma and myeloid
leukemia are different malignancy originating from two lineages and
possess disparate cytogenetic, cell phenotype and biological
process. Generally, lymphoma combining with myeloid leukemia is
rarely seen except when CML in blast crisis with a bare possibility
occurs acute lymphocyte mutation. It is more rarely seen that
simultaneous bi-lineage malignancies without history treatment at
initial diagnosis. Shen et al reviewed 24 patients with CML
and T-lymphoblastic cell NHL (T-LBL) in the lymph node between 1980
and 2016, but most of those patients experienced chronic history of
CML followed by T-LBL afterwards (3).
Some scholars reported NHL or HL developing into leukemia during
remission or treatment (4–7). The cause about the bi-lineage
hematologic malignancies is unclear yet. Lam et al
analysedrisk factors ofsecondary acute myeloid
leukemia/myelodysplastic syndrome among survivors of NHL (6). Eichenauer et al reported
therapy-related acute myeloid leukemia and myelodysplastic
syndromes in patients with HL (4). To
our best knowledge, the therapy-related secondary tumor has been
frequently reported in patients who received various chemotherapy
regimens or radiotherapy or transplantation, however, there is no
systematic summary to individual cases about simultaneous
bi-lineage hematologic malignancies without previous therapy. So,
we summary simultaneous lymphoma and myeloid leukemia through
literature searching on PubMed (ncbi.nlm.nih.gov/pubmed) with the term ‘myeloid
leukemia’ or ‘myelogenous leukemia’ combined with ‘lymphoma’ and
‘simultaneous’ or ‘concurrent’ or ‘coinstantaneous’ or
‘co-existence’ to explore the features, prognosis and treatment. In
the meantime, we present our two cases diagnosed with concurrent
T-LBL and CML.
Patients and methods
Case report
Case 1
On April 27, 2009, a 43-year-old Chinese male was
admitted hospital because of finding a cervical mass for 10 days.
On physical examination, multiple enlarged lymph nodes no bigger
than 4×2 cm were found in bilateral cervical, submandibular and
submental region. Other physical findings were unremarkable. The
chest and abdomen CT scan was normal except splenomegaly. A
complete blood count revealed leucocyte count
43.81×109/l with 3.6% blasts, 7.2% promyelocytes,
erythrocyte count 4.45×1012/l, hemoglobin level 136.0
g/l, platelet count 123×109/l, aneutrophils count
25.06×109/l, β-microglobulin level 2.01 mg/l, lactate
dehydrogenase (LDH) level 382 U/l. A subsequent bone marrow
aspiration showed malignant proliferation of the myeloid Department
with myeloblasts >10% and that ratios of neutrophilic myelocyte,
metamyelocyte and segmented neutrophil all increased. The
chromosome indicated 46,XY,t(9,22). The FISH test for BCR/ABL was
positive with a rate of 7%. Biopsy of the right cervical lymph node
reveal T-LBL with lymphoma cells expressing CD3, CD4, CD45, TdT
(terminal deoxynucleotidyl transferase), but negative for CD20,
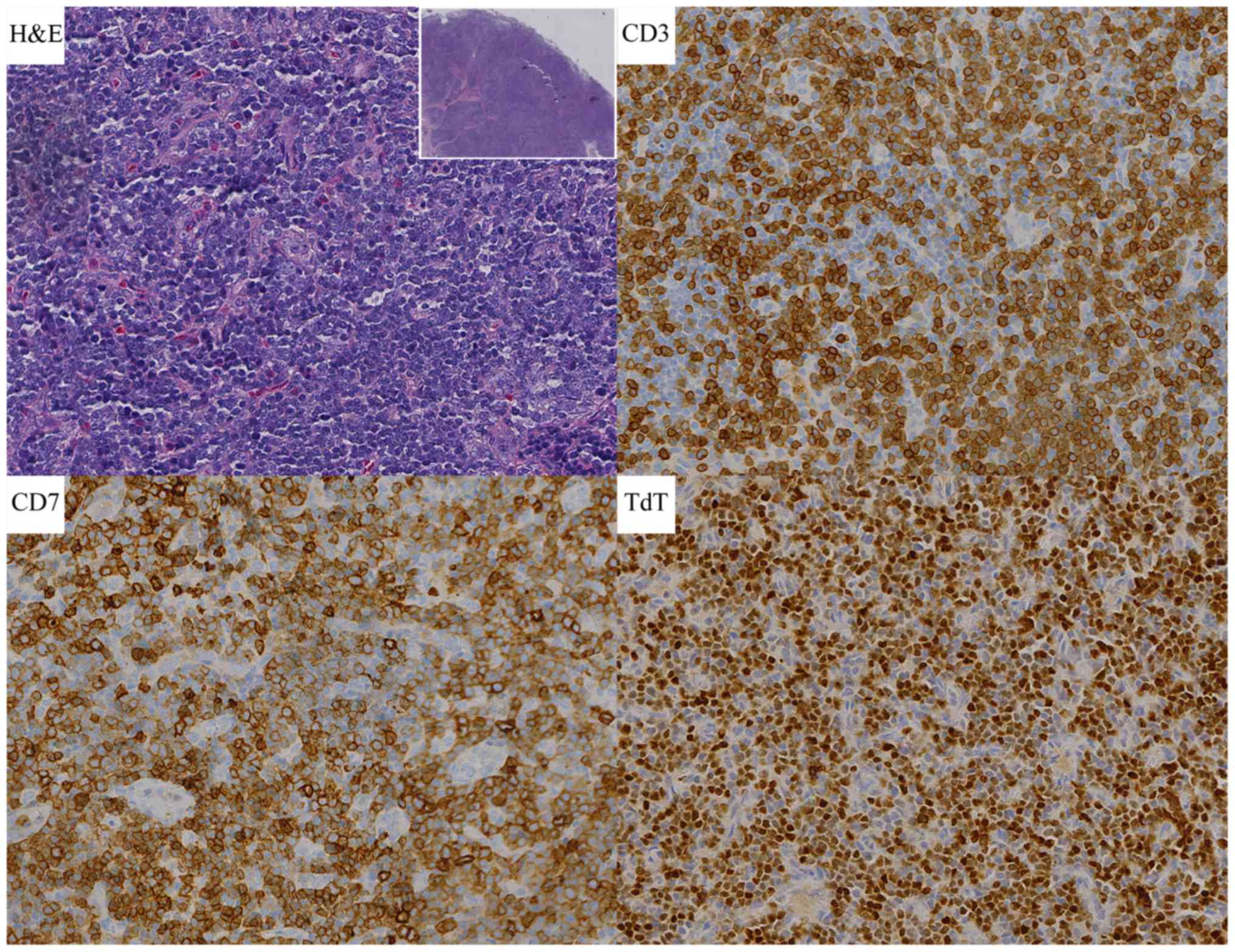
Pax-5, CD79a, ALK, MPO, Ki-67 level is 90% (Fig. 1). So ultimate diagnosis was T-LBL in
stage II according to the Ann Arbor classification, the IPI
(8) being 2, combining with CML in
blastic phase. The patient was treated with Hyper-CVAD A
(cyclophosphamide, vincristine, adriamycin and dexamethasone)
scheme one cycle and imatinib 600 mg qd. Then MOAP (mitoxantrone,
vincristine, arabinoside and prednisone) five cycles and
intrathecal injection four times. The patients obtained nearly
complete remission with bone marrow blasts and promyelocytes
reduced to 0.4%. Afterwards the patient accepted haploidentical
hematopoietic stem cell transplantation on December 15, 2009. Until
now (June 2017), the patient had obtained continuous complete
remission (CR) for over 8 years.
Case 2
On December 12, 2012, a 44-year-old Chinese male was
complained of finding a cervical mass with exacerbation for more
than 20 days. On physical examination, several enlarged lymph nodes
were observed in the bilateral neck, right collarbone and axillary.
In addition, the patient's left pharyngeal cavity was inflamed with
a random-shaped neoplasm. A complete blood test: leucocyte count
25.1×109/l, erythrocyte count 3.34×1012/l,
hemoglobin 103.0 g/l, platelet 123×109/l, neutrophils
19.6×109/l, β-microglobulin 2.0 mg/l, LDH 638 U/l. the
blasts, promyelocytes and metamyelocytes appeared in the peripheral
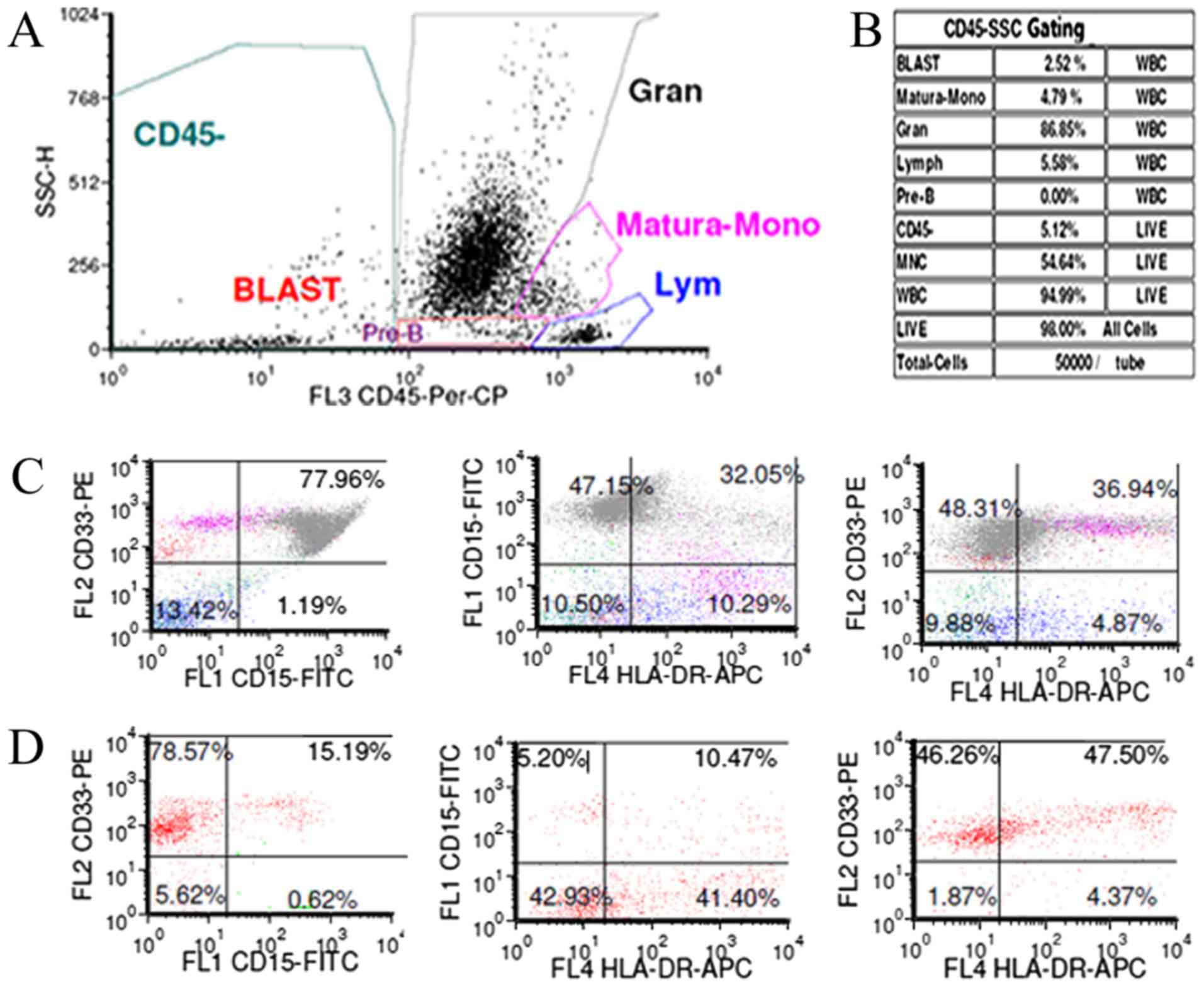
blood. Bone marrow analysis revealed granulocyte proliferation with
hyperactivity, blasts and promyelocytes accounted for 7.6%
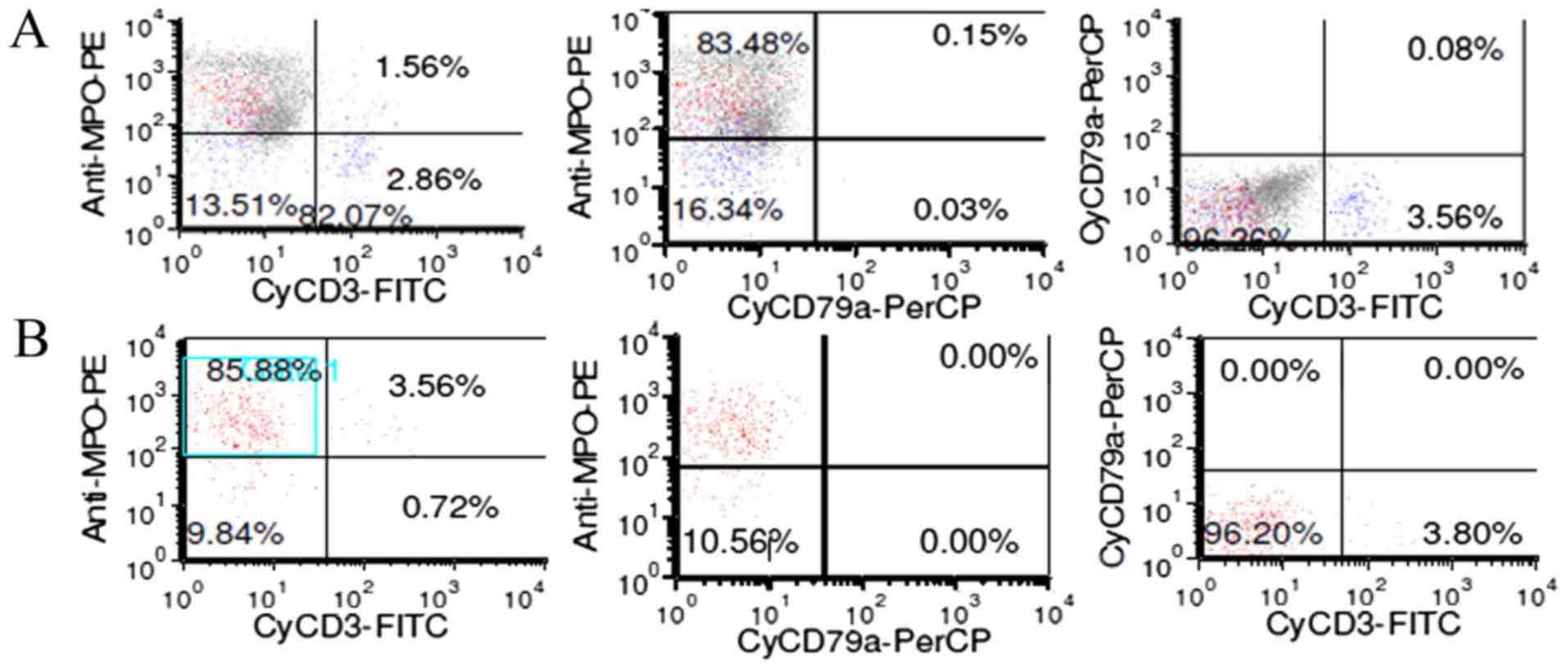
(Figs. 2 and 3). The fluorescence in situ
hybridization (FISH) test for BCR/ABL was positive with a rate of
70.2%. So, CML was diagnosed. The biopsy of left cervical lymph
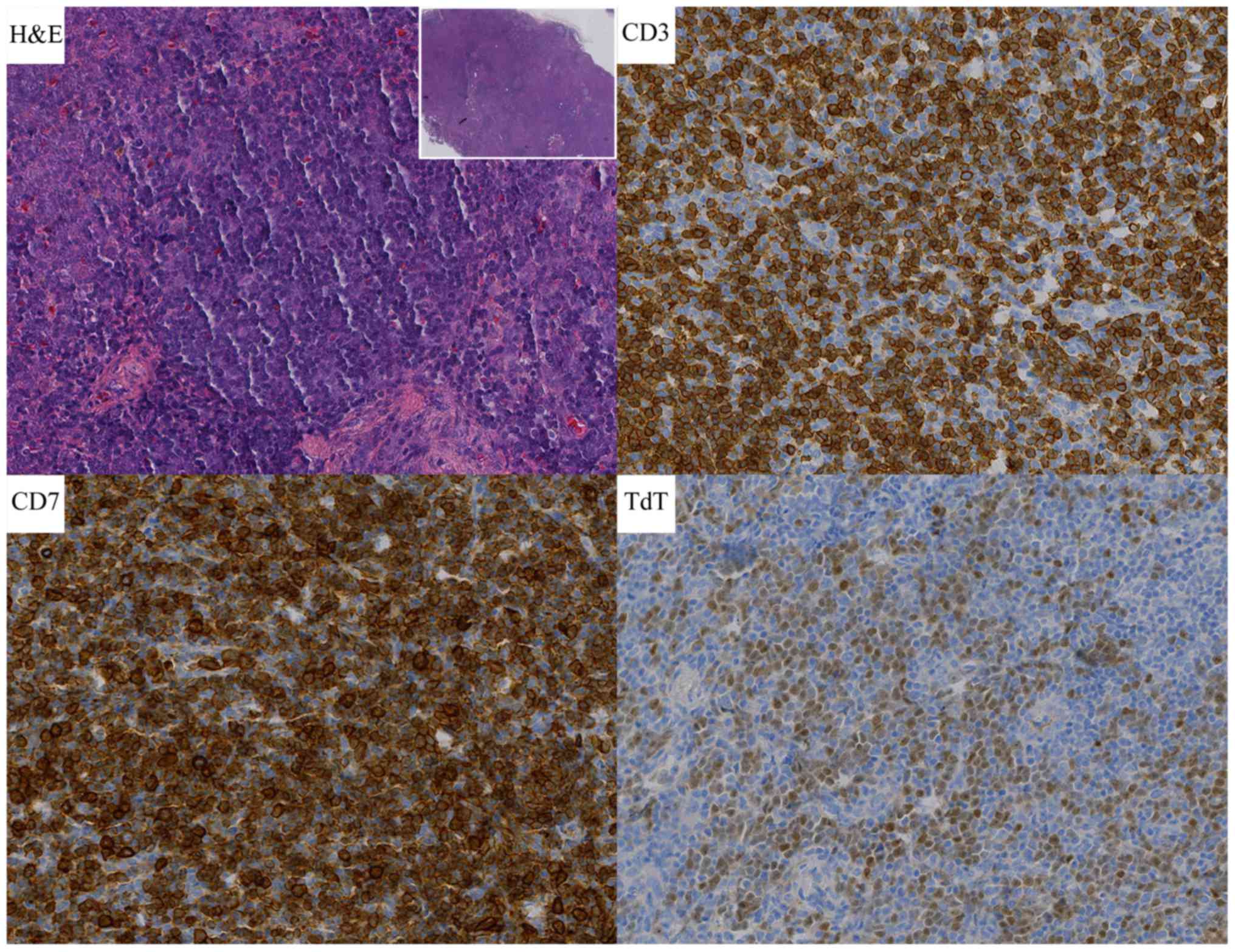
node conformed to T-LBL with lymphoma cells expressing CD20, CD3,
CD21 (part of the FDC were destroyed), CD10, Bcl-2, TdT, CD43, CD7,
CD2 and CD34, while MPO, CD30, ALK, EMA were negative (Fig. 4). The Ki-67 labeling index was 50 to
60%. According to these findings, a preliminary diagnosis was: CML
in chronic phase, mydoid sarcoma (MS) and T-LBL. Without treatment,
the patient left the hospital. On March 2, 2013, the patient
re-hospitalized. Repeated examination was the same as it was before
except lymph nodes bigger. From March 5, 2013 to July 8, 2013, the
patient was treated with Hyper-CVAD A and B alternately for six
cycles, and intrathecal injection for 11 times and reached partial
remission, but he didn't take imatinib for lack of money during
this period. Since August 2, 2013, two cycles of Hyper-CVAD B were
given again with taking imatinib 400 mg qd. However the disease
progressed. The patient did not continue treatment later, and
succumbed on March 12, 2014.
Summary to the two cases
Our two cases were admitted because of a cervical
mass, and then found superficial lymphadenopathy with the
peripheric blood leucocyte soaring. Biopsy of the cervical lymph
node prove T-LBL depending on immunohistochemistry and typical
morphology. The FISH test for BCR/ABL of bone marrow was positive
with rates of 7 and 70.2%. So the two cases were diagnosed T-LBL
with CML finally. In terms of treatment, case 1 experienced durable
complete remission until present through chemotherapy combining
imatinib and then haploidentical hematopoietic stem cell
transplantation. However the second patient soon died after
chemotherapy and taking imatinib.
Methods
We here summary all concurrent myeloid leukemia and
lymphoma from 1976 to present (Table
I) to analyze the features, prognosis and treatment.
Statistical analyses were performed using IBM SPSS statistics
software, version 21.0 (IBM Corp., Armonk, NY, USA) and GraphPad
Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). OS
distributions were estimated using the Kaplan-Meier curve analysis,
time-to-event distributions were compared using the log-rank test
and two-tailed significance-level of 0.05 was considered
statistically significant.
 | Table I.Review of patients with myeloid
leukemia and lymphoma from 1976 to present. |
Table I.
Review of patients with myeloid
leukemia and lymphoma from 1976 to present.
| Case | First author,
year | Sex | Age (years) | Involvement
sites | Initial
diagnosis | Treatment | Follow-up
(months) | (Refs.) |
|---|
| 1 | Kapadia, 1976 | F | 64 | Lymph nodes and
liver and marrow | NHL-PDL and
AML | CTx | 11 | (27) |
| 2 | Youness, 1978 | M | 67 | Spleen and
marrow | NHL-PDL and
AML | CTx | 5 | (28) |
| 3 | Ramji, 1988 | NC | NC | NC | T-NHL and CML | NC | NC | (29) |
| 4 | Ohtsu, 1988 | M | 49 | NC | ATL and AML | NC | 6 | (30) |
| 5 | Tsukasaki,
1995 | F | 36 | NC | ATL and AML | NC | 30b | (11,16) |
| 6 | Abe, 1999 | F | 82 | Gallbladder and
marrow | MALT and AML | Untreated | 3 | (9) |
| 7 | Morales, 1999 | M | 63 | Lymph nodes and
marrow | T- NHL and CML | CTx | NC | (31) |
| 8 | Montefusco,
2001 | M | 64 | Spleen and
marrow | NHL-LG and AML | CTx and
hydroxyurea | 25 | (32) |
| 9
2002 | Zámecníková, | M | 34 | Lymph nodes and
liver and marrow | DLBCL and CML | CTx and RT | 4 | (2) |
| 10 | Au, 2003 | M | 67 | Mediastinal lymph
nodes and marrow | B-NHL and CML | CTx and RT and
hydroxyurea | 144b | (33) |
| 11 | Lamb, 2005 | M | 9 | Lymph nodes and
spleen and marrow | T-LBL and AML | CTx and
allo-HSCT | 48b | (34) |
| 12 | Metzgeroth,
2007 | M | 58 | Lymph nodes and
spleen and marrow | T-NHL and AEL | Imatinib | 18b | (12) |
| 13 | Capovilla,
2008 | M | 33 | Lymph nodes and
spleen and marrow | T-LBL and CEL | Imatinib | 12b | (35) |
| 14 | Li, 2011 | M | 12 | Lymph nodes and
marrow | T-LBL and AML | CTx | 4 | (36) |
| 15 | Chang, 2012 | M | 41 | Lymph nodes and
skin lesions and marrow | T-LBL and AML | CTx and
allo-HSCT | 14a | (13) |
| 16 | Sharkunov,
2012 | F | N | NC | HL and CML | CTx and
imatinib | NC | (37) |
| 17 | Wan, 2012 | M | 43 | Lymph nodes and
marrow | T-LBL and CML | CTx and
allo-HSCT | 19b | (38) |
| 18 | VanCrombrugge,
2012 | M | 47 | Sinonasal and
adjacent and marrow | NK-NHL IVB and
AML | CTx | Approximately 2
months | (10) |
| 19 | Kunitomi, 2014 | F | 62 | Lymph nodes and
marrow | EBV(+)DLBCL and
AML | CTx | 34 | (39) |
| 20 | Dong, 2016 | M | 25 | Lymph nodes and
marrow | T-LBL and AML | CTx and
allo-HSCT | 34b | (40) |
| 21 | Shen, 2016 | M | 28 | Lymph nodes and
spleen and marrow | T-LBL and CML | CTx | 3 months and lost
follow-up | (3) |
| 22 | Dai, 2017 | F | 37 | Lymph nodes and
skull and marrow | DLBCL and AML | CTx
andimatinib | Approximately 2
months | (41) |
| 23 | Our case | M | 43 | Lymph nodes and
marrow | T-LBL and CML | CTx and allo-HSCT
andimatinib | 98b |
|
| 24 | Our case | M | 44 | Lymph nodes and
marrow | T-LBL and CML | CTx and
imatinib | 15 |
|
Patient characteristics
From the statistics we conclude that the patients
ranged between 9 and 82 years old (median, 43 years). The
male/female ratio was 2.83:1 (17:6). 16 patients were involved
lymphadenectasis in bilateral cervical, submandibular, submental
and mediastinal region accompanying fatigue and fever, among of
which skull or liver or spleen or skin lesions was involved in 1
patient, 2 patients, 4 patients and 1 patient respectively. 2
patients was involved spleen without lymphadenectasis. Abe et
al (9) reported a patient was
admitted in the hospital because of progressive jaundice with MALT
lymphoma in gallbladder and AML in marrow. Van Crombrugge et
al (10) reported a patient
referred to hospital because of severe headache and progressive
facial pain and ultimately diagnosed NK cell lymphoma in sinonasal
and AML. The remaining patients were failed to get information. We
sum up total cases of different simultaneous lymphoma and myeloid
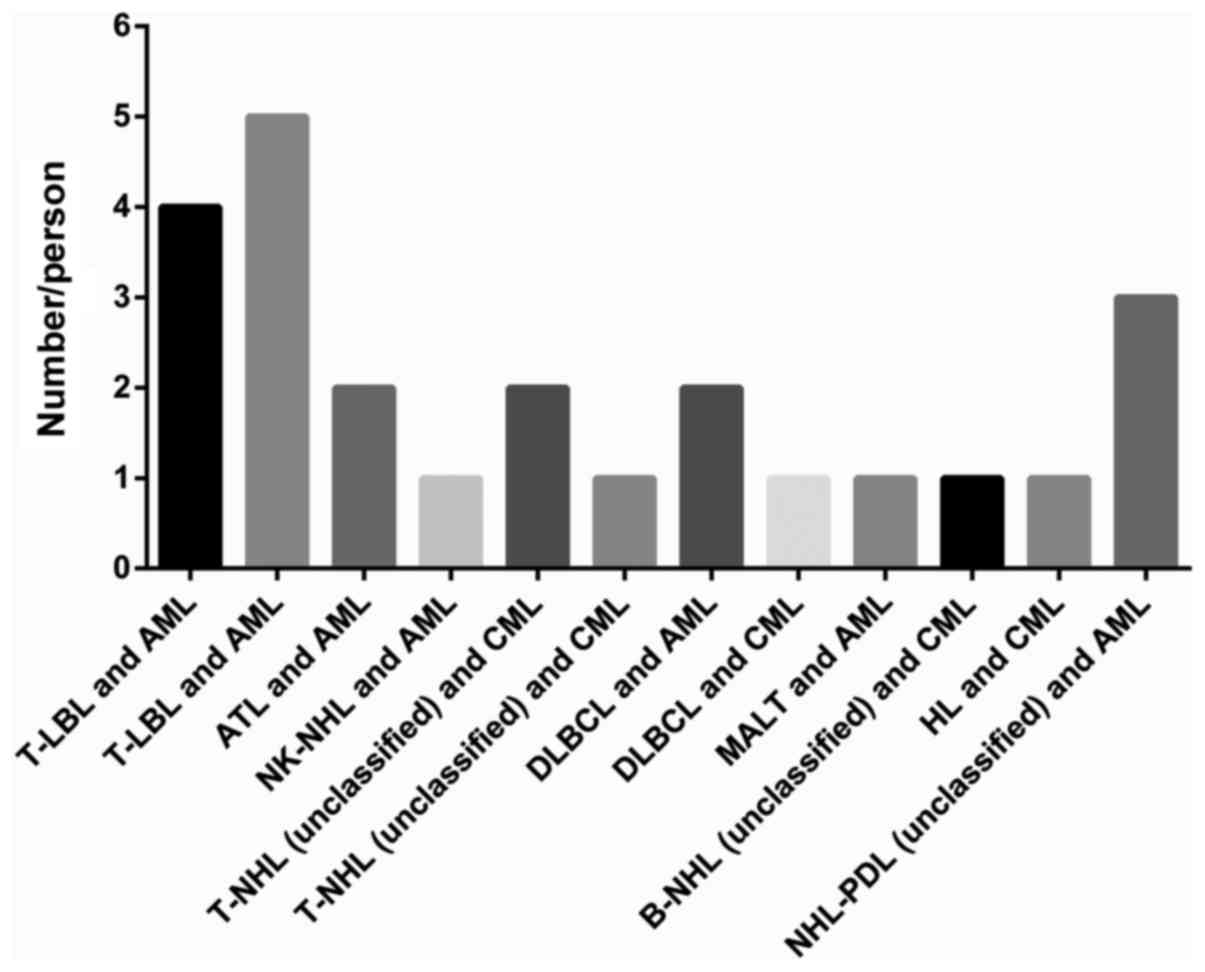
leukemia (Fig. 5). Simultaneous AML
and lymphoma is more than simultaneous CML and lymphoma being 14
and 10 respectively. The number of simultaneous T-cell
non-Hodgkin-lymphoma is 15 and the number of simultaneous B-cell
non-Hodgkin-lymphoma is 5. For the treatment, 10 patients were
treated with chemotherapy, 2 patients were treated with
chemotherapy andradiotherapy, 2 patients were treated with with
single imatinib, 5 patients were treated with with chemotherapy and
transplantation and 1 patient was untreated.
Survival and statistical analysis
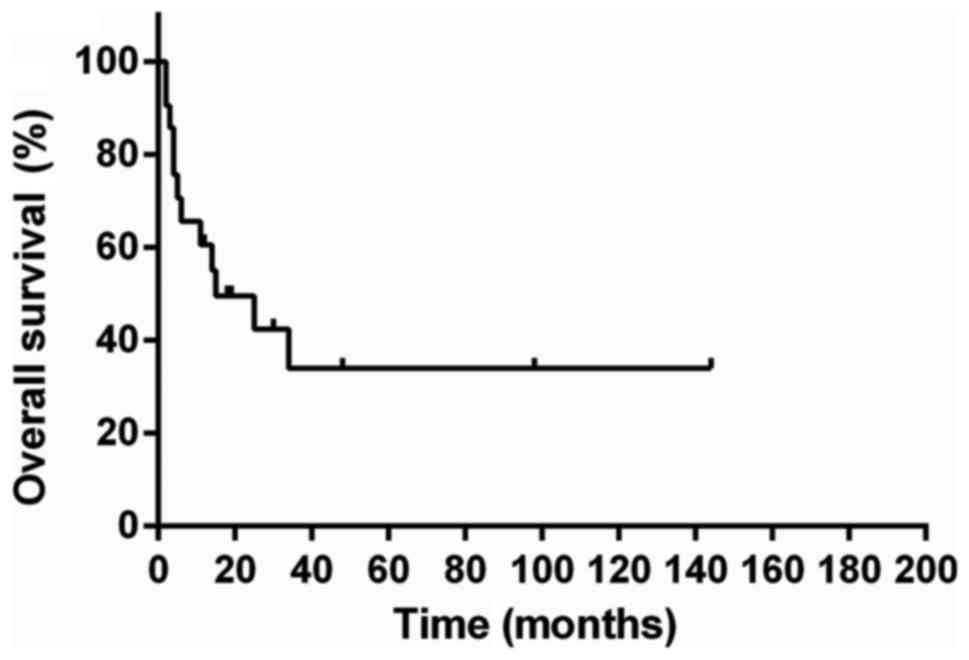
In the 24 patients, 21 patients were available to
analyze survival and the median survival was 15 months (Fig. 6). We performed univariate analysis to
evaluate the prognostic factors. There was no statistical
significance for sex (P=0.301) and for age (P=0.168) which was set
43.5 years as cut-off based on the ROC curve. There was no survival
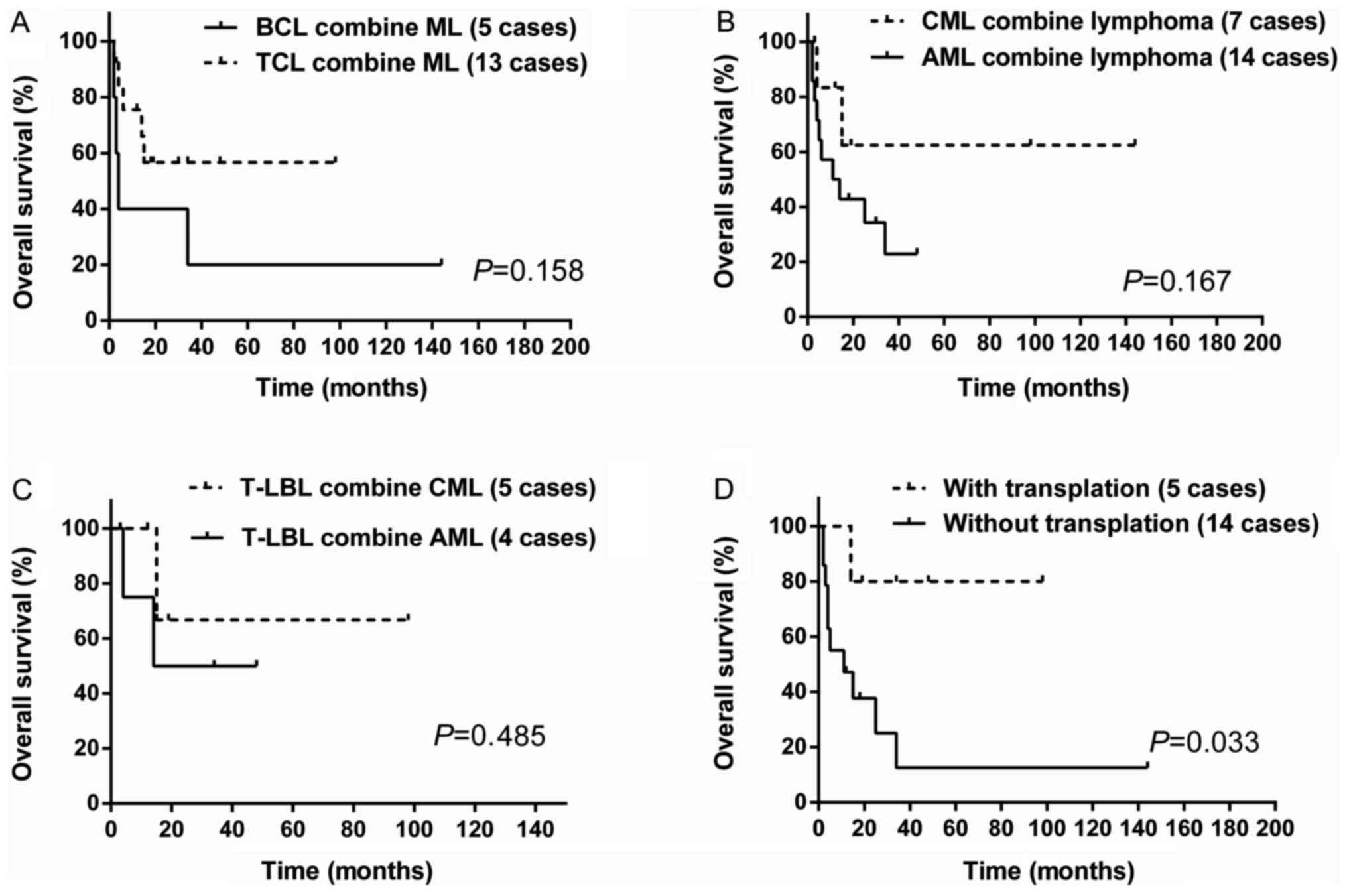
difference between B cell lymphoma companying myeloid leukemia and
T cell lymphoma companying myeloid leukemia (P=0.158; Fig. 7A). Similarly, no survival difference
was received between AML companying lymphoma and CML companying
lymphoma (P=0.167; Fig. 7B). Because
of concurrent T-LBL and myeloid leukemia being relatively common,
we performed statistical analysis between T-LBL combining CML and
T-LBL combining AML, but there was no statistical difference in
survival (P=0.485; (Fig. 7C). For the
treatment, chemotherapy together with transplantation are superior
to other treatment without transplantation (P=0.033; Fig. 7D). The median survival was unreached
for patients with transplantation and 11 months for those without
transplantation.
Discussion
Lymphoma and myeloid leukemia derive from different
tumour cells, and they mostly happen alone. It was frequently
reported that secondary or therapy-related hematologic
malignancies, but co-concurrent bi-lineage hematologic malignancies
are really rare. We present two cases simultaneous T-LBL and CML
and then reviewed all cases available to collect from Pubmed. The
characteristics are as follows: The simultaneous neoplasm tended to
occur in young to old with a good majority in male. Patients are
mostly admitted in the hospital because of enlarged lymph nodes
accompanying fatigue, fever and splenomegaly. Simultaneous HL and
myeloid leukemia is extremely rare and only one case was reported.
Simultaneous AML and lymphoma is more commonly seen than
simultaneous CML and lymphoma. Simultaneous T cell lymphoma and
myeloid leukemia is more thansimultaneous B cell lymphoma and
myeloid leukemia. The number of simultaneous T-LBL and myeloid
leukemia is maximum than any other subtypes. There is no
statistical difference in survival for different bi-lineage
malignancy.
However, due to the rarity of patients with
bi-lineage tumors, little is known concerning the pathogenesis.
Early in 1998, Tsukasaki et al reported the possible
association between adult T-cell leukemia/lymphoma and acute
myeloid leukemia. One of the possible mechanism is that immune
system is compromised severely in Adult T-Cell Leukemia/Lymphoma
(ATL) patients which results in the occurrence of AML. The other
possible explanation for the association of ATL and AML is that
growth factors such as M-CSF, G-CSF, and GM-CSF produced by the ATL
cells support the growth of the AML cells (11). Metzgeroth et al demonstrated
the association of the FIP1L1-PDGFRA fusion gene with lymphoblastic
T-NHL and eosinophilia-associated acute myeloid leukemia (12). Chang et al (13) and Holroyd et al (14) also recognized that FIP1L1-PDGFA is
associated with differentiation into both myeloid and lymphoid
lineages. Therefore FIP1L1-PDGFRA fusion gene, growth factors and
compromised immune system may lead to the co-concurrent bi-lineage
malignancies. Besides, the recently studies have confirmed that
retrovirus could cause leukemia and lymphoma in reptiles, primates
and mammals (15). Furthermore,
hematologic neoplasms have been reported to be complications of ATL
(16).
From the research, we can see that simultaneous
T-LBL and myeloid leukemia are more commonly seen than other
subtypes. The reason is still unclear. For T-LBL, combining
cytomorphology and flow cytometric immunophenotyping (FCI) enables
the accurate and rapid diagnosis (17). The diagnosis of T-LBL is based on the
identification of a neoplastic proliferation of small to
medium-sized blasts. Blasts express T-cell lineage markers (CD2,
CD3, CD4, CD5, CD7, and/or CD8) as well as markers of precursor T
lymphoblasts (CD1a, CD34, CD99, and/or TDT) (18). Concurrent T-LBL and CML is likely to
misdiagnosed with 8p11 myeloproliferative syndrome which is
characterized in its typical form by the simultaneously or
sequentially occurrence of a bcr/abl-negative myeloproliferative
disorder and a lymphoma, usually a precursor T lymphoblastic
lymphoma (19). The genetic testing
can identify.
The prognosis of the simultaneous bi-lineage
malignancies is poor with the median survival 15 months in this
study. For the treatment, there is not yet consensus with regard to
the optimal therapeutic modality due to the limited number of case
reports and absence of prospective studies of treatments and
outcomes. As we know, in terms of leukemia, hematopoietic stem cell
transplantation may be the best choice to reach complete remission.
Many studies highlighted the advantages of ASCT to AML (20–22).
Meanwhile, some researches show that Allo-geneic BMT treated for
young patients is feasible and can result in long-term disease-free
survival for advanced LGL or CLL (23). For highly invasive lymphoma, such as
T-LBL, allogeneic hematopoietic stem cell transplantation is
alternative after reaching complete remission from high dose
chemotherapy (24). For bi-lineage
hematologic malignancies, chemotherapy is necessary. Withregard to
bcr/abl-positive CML, imatinib may improve the survival time even
though some reports stated that the targeted drug might lead to the
secondary neoplasm. After complete remission, hematopoietic stem
cell transplantation is recommended. From our chart, we concluded
that those who were treated with transplantation survived longer
than those without transplantation (P=0.033). Unfortunately, one
patient died for graft-versus-host disease (GVHD) after
transplantation. It is obvious that the allogeneic hematopoietic
stem cell transplantation is good to the lymphoma with myeloid
leukemia, but GVHD should be taken high attention. In recent years,
immunotherapies paly crucial roles in hematologic neoplasms.
CD19-directed CAR-T cells can reach a complete remission rate of
94% in patients with refractory/relapsed ALL, much higher than that
of chemotherapy (25). Bispecific
antibodies (BsAbs) can bind simultaneously two different antigens
or epitopes, which leads to a wide range of applications including
redirecting T cells or NK cells to tumor cells, blocking two
different signaling pathways, dual targeting of different disease
mediators, and delivering payloads to targeted sites. Immunotherapy
has been demonstrating promising clinical results (26). For simultaneous bi-lineage
malignancies, immunotherapy may provide a possible remedy.
In conclusion, simultaneous bi-lineage malignancies
of myeloid leukemia and lymphomais rarely seen and there is no
statistical difference in survival for different types of
bi-lineage malignancy in this study. Simultaneous T-NHL and myeloid
leukemia is much more than simultaneous B-NHL andmyeloid leukemia,
so it is deserved vigilant to the occurence of myeloid leukemia
when diagnosed T-NHL. The pathogenesis in unclear and quickly
accurate diagnosis is important. For treatment, allogeneic
hematopoietic stem cell transplantation may improve survival. More
cases are needed to explore pathogenesis and validate our
conclusion.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81570203), the Health
Science and Technology Innovation Talents Project of Henan Province
(grant no. 2109901), the Key Science and Technology Research
Project of Henan province (grant no. 162102310194), the Medical Key
Science and Technology Research Project of Henan province (grant
no. 201503044).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MZZ has made substantial contributions to the
conception and design of the study and critically revised the
manuscript. YFS collated and analyzed the patient data and wrote
the manuscript. XRF was responsible for managing the patients,
provided the two cases and revised the manuscript. LZ, LL, XL, XHW
and ZCS analyzed and interpreted the data and critically revised
the manuscript for important intellectual content. All authors
approved the final version of the paper for publication.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee for
Scientific Research and Clinical Trials of Zhengzhou University and
informed consent was obtained from all patients.
Patient consent for publication
All patients provided written informed consent for
the publication of their data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Szumera-Ciećkiewicz A, Gałązka K, Szpor J,
Rymkiewicz G, Jesionek-Kupnicka D, Gruchała A,
Ziarkiewicz-Wróblewska B, Poniatowska-Broniek G, Demczuk S and
Prochorec-Sobieszek M: Distribution of lymphomas in Poland
according to World Health Organization classification: Analysis of
11718 cases from National Histopathological Lymphoma Register
project-the Polish Lymphoma Research Group study. Int J Clin Exp
Pathol. 7:3280–3286. 2014.PubMed/NCBI
|
|
2
|
Zámecníková A, Vranovský A and Hlavcák P:
Coexistence of Philadelphia-positive chronic granulocytic leukemia
and diffuse large B-cell lymphoma at initial diagnosis. Leuk
Lymphoma. 43:429–431. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen ZL, Yin LF, Mao WW, Liang J and Yang
L: Philadelphia chromosome-negative non-Hodgkin's lymphoma
occurring in Philadelphia chromosome-positive chronic myeloid
leukemia: A case report and literature review. Oncol Lett.
11:2909–2912. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eichenauer DA, Thielen I, Haverkamp H,
Franklin J, Behringer K, Halbsguth T, Klimm B, Diehl V, Sasse S,
Rothe A, et al: Therapy-related acute myeloid leukemia and
myelodysplastic syndromes in patients with Hodgkin lymphoma: A
report from the German Hodgkin Study Group. Blood. 123:1658–1664.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roberts E III, Oncale M, Safah H and
Schmieg J: Therapy-related T/myeloid mixed phenotype acute leukemia
in a patient treated with chemotherapy for cutaneous diffuse large
B cell lymphoma. J La State Med Soc. 168:16–20. 2016.PubMed/NCBI
|
|
6
|
Lam CJ, Curtis RE, Dores GM, Engels EA,
Caporaso NE, Polliack A, Warren JL, Young HA, Levine PH, Elmi AF,
et al: Risk factors for second acute myeloid
leukemia/myelodysplastic syndrome among survivors of non-Hodgkin
lymphoma. Leukemia. 30:1187–1190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhatt VR, Giri S, Verma V, Dahal S, Shah
BK, Pathak R, Bociek RG, Vose JM and Armitage JO: Secondary acute
myeloid leukemia in survivors of Hodgkin lymphoma. Future Oncol.
12:1565–1575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
International Non-Hodgkin's Lymphoma
Prognostic Factors Project, . A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abe Y, Takatsuki H, Okada Y, Saito A,
Kimura T and Nishimura J: Mucosa-associated lymphoid tissue type
lymphoma of the gallbladder associated with acute myeloid leukemia.
Intern Med. 38:442–444. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Crombrugge L, De Vos G, Vanclooster C,
Lemmerling M and Kerre T: The simultaneous appearance of a nasal
natural killer-cell lymphoma and acute myelogenous leukemia. B-ENT.
8:49–52. 2012.PubMed/NCBI
|
|
11
|
Tsukasaki K, Koba T, Iwanaga M, Murata K,
Maeda T, Atogami S, Nakamura H, Yamada Y, Kamihira S and Tomonaga
M: Possible association between adult T-cell leukemia/lymphoma and
acute myeloid leukemia. Cancer. 82:488–494. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Metzgeroth G, Walz C, Score J, Siebert R,
Schnittger S, Haferlach C, Popp H, Haferlach T, Erben P, Mix J, et
al: Recurrent finding of the FIP1L1-PDGFRA fusion gene in
eosinophilia-associated acute myeloid leukemia and lymphoblastic
T-cell lymphoma. Leukemia. 21:1183–1188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang H, Chuang WY, Sun CF and Barnard MR:
Concurrent acute myeloid leukemia and T lymphoblastic lymphoma in a
patient with rearranged PDGFRB genes. Diagn Pathol. 7:192012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holroyd A, Cross NC and Macdonald DH: The
two faces of myeloproliferative neoplasms: Molecular events
underlying lymphoid transformation. Leuk Res. 35:1279–1285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Melo JV and Deininger MW: Biology of
chronic myelogenous leukemia-signaling pathways of initiation and
transformation. Hematol Oncol Clin North Am. 18(545–568): vii–viii.
2004.
|
|
16
|
Tsukasaki K, Fujimoto T, Hata T, Yamada Y,
Kamihira S and Tomonaga M: Concomitant complete remission of APL
and smoldering ATL following ATRA therapy in a patient with the two
diseases simultaneously. Leukemia. 9:1797–1798. 1995.PubMed/NCBI
|
|
17
|
Bhaker P, Das A, Rajwanshi A, Gautam U,
Trehan A, Bansal D, Varma N and Srinivasan R: Precursor
T-lymphoblastic lymphoma: Speedy diagnosis in FNA and effusion
cytology by morphology, immunochemistry, and flow cytometry. Cancer
Cytopathol. 123:557–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jain N, Lamb AV, O'Brien S, Ravandi F,
Konopleva M, Jabbour E, Zuo Z, Jorgensen J, Lin P, Pierce S, et al:
Early T-cell precursor acute lymphoblastic leukemia/lymphoma
(ETP-ALL/LBL) in adolescents and adults: A high-risk subtype.
Blood. 127:1863–1869. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goradia A, Bayerl M and Cornfield D: The
8p11 myeloproliferative syndrome: Review of literature and an
illustrative case report. Int J Clin Exp Pathol. 1:448–456.
2008.PubMed/NCBI
|
|
20
|
Vellenga E, van Putten W, Ossenkoppele GJ,
Verdonck LF, Theobald M, Cornelissen JJ, Huijgens PC, Maertens J,
Gratwohl A, Schaafsma R, et al: Autologous peripheral blood stem
cell transplantation for acute myeloid leukemia. Blood.
118:6037–6042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gorin NC, Labopin M, Reiffers J, Milpied
N, Blaise D, Witz F, de Witte T, Meloni G, Attal M, Bernal T, et
al: Higher incidence of relapse in patients with acute myelocytic
leukemia infused with higher doses of CD34+ cells from
leukapheresis products autografted during the first remission.
Blood. 116:3157–3162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gorin NC, Labopin M, Blaise D, Reiffers J,
Meloni G, Michallet M, de Witte T, Attal M, Rio B, Witz F, et al:
Higher incidence of relapse with peripheral blood rather than
marrow as a source of stem cells in adults with acute myelocytic
leukemia autografted during the first remission. J Clin Oncol.
27:3987–3993. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toze CL, Shepherd JD, Connors JM, Voss NJ,
Gascoyne RD, Hogge DE, Klingemann HG, Nantel SH, Nevill TJ,
Phillips GL, et al: Allogeneic bone marrow transplantation for
low-grade lymphoma and chronic lymphocytic leukemia. Bone Marrow
Transplant. 25:605–612. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haioun C, Mounier N, Emile JF, Ranta D,
Coiffier B, Tilly H, Récher C, Fermé C, Gabarre J, Herbrecht R, et
al: Rituximab versus observation after high-dose consolidative
first-line chemotherapy with autologous stem-cell transplantation
in patients with poor-risk diffuse large B-cell lymphoma. Ann
Oncol. 20:1985–1992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei G, Ding L, Wang J, Hu Y and Huang H:
Advances of CD19-directed chimeric antigen receptor-modified T
cells in refractory/relapsed acute lymphoblastic leukemia. Exp
Hematol Oncol. 6:102017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei G, Wang J, Huang H and Zhao Y: Novel
immunotherapies for adult patients with B-lineage acute
lymphoblastic leukemia. J Hematol Oncol. 10:1502017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kapadia SB and Kaplan SS: Simultaneous
occurrence of non-Hodgkin's lymphoma and acute myelomonocytic
leukemia. Cancer. 38:2557–2560. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Youness E, Ahearn MJ and Drewinko B:
Simultaneous occurrence of non-Hodgkin's lymphoma and spontaneous
acute granulocytic leukemia. Am J Clin Pathol. 70:415–420. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramji S, Rusia U and Basu TK: Simultaneous
occurrence of a chronic myeloid leukemia and a malignant T-cell
lymphoma. Indian Pediatr. 25:566–568. 1988.PubMed/NCBI
|
|
30
|
Ohtsu T, Tobinai K, Minato K, Mukai K,
Kagami Y, Miwa M, Arai C and Shimoyama M: Concurrent adult T-cell
leukemia and acute myeloblastic leukemia. Jpn J Clin Oncol.
18:33–41. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morales E, Bancalari G, Fahrenkrog AM and
Rossle A: Chronic myeloid leukemia and non Hodgkin lymphoma in the
same patient. Clinical case. Rev Med Chil. 127:1105–1107. 1999.(In
Spanish). PubMed/NCBI
|
|
32
|
Montefusco E, Fazi F, Cordone I, Ariola C,
Nanni M, Spadea A, Spiriti MA, Fenu S, Mandelli F and Petti MC:
Molecular remission following high-dose hydroxyurea and fludarabine
plus cytarabine in a patient with simultaneous acute myeloid
leukemia and low-grade lymphoma. Leuk Lymphoma. 40:671–674. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Au WY, Ma SK, Wan TS, Wang EP, Lau TC and
Kwong YL: Concurrent mediastinal B cell lymphoma and chronic
myeloid leukemia with an unusually favorable response to
chemotherapy. Leuk Lymphoma. 44:535–538. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lamb LS Jr, Neuberg R, Welsh J, Best R,
Stetler-Stevenson M and Sorrell A: T-cell lymphoblastic
leukemia/lymphoma syndrome with eosinophilia and acute myeloid
leukemia. Cytometry B Clin Cytom. 65:37–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Capovilla M, Cayuela JM, Bilhou-Nabera C,
Gardin C, Letestu R, Baran-Marzak F, Fenaux P and Martin A:
Synchronous FIP1L1-PDGFRA-positive chronic eosinophilic leukemia
and T-cell lymphoblastic lymphoma: A bilineal clonal malignancy.
Eur J Haematol. 80:81–86. 2008.PubMed/NCBI
|
|
36
|
Li YH, Xiao Y and Jiang ZJ: Childhood
lymphoblastic lymphoma with acute myeloid leukemia: A case report
and literature review. Zhonghua Xue Ye Xue Za Zhi. 32:127–128.
2011.(In Chinese). PubMed/NCBI
|
|
37
|
Sharkunov NN, Moiseyeva TN, Zybunova EE,
Vinogradova OY and Kravchenko SK: Successful treatment for
Hodgkin's lymphoma in a female patient with Ph+ chronic myeloid
leukemia. Ter Arkh. 84:71–74. 2012.(In Russian). PubMed/NCBI
|
|
38
|
Wan DM, Zhang SP and Zhang C:
Haploidentical hematopoietic stem cell transplantation for
treatment of T-lymphoblastic lymphoma with chronic myeloid
leukemia: A case report and literature review. Zhonghua Xue Ye Xue
Za Zhi. 33:227–228. 2012.(In Chinese). PubMed/NCBI
|
|
39
|
Kunitomi A, Kotani S, Ukyo N, Ono K,
Nakamine H and Nohgawa M: Epstein-Barr virus-positive diffuse large
B-cell lymphoma of the elderly complicated by the onset of acute
myeloid leukemia. Intern Med. 53:51–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dong HJ, Wu W, Wang JH, Zhu HF, Gao S, Hou
LP and Bai QX: Acute myeloid leukemia complicated with complex
karyotypes and T-lymphoblastic lymphoma: A case report. Zhonghua
Xue Ye Xue Za Zhi. 37:2372016.(In Chinese). PubMed/NCBI
|
|
41
|
Dai Y, Shuai X, Kuang P, Wang L, Liu T and
Niu T: Philadelphia chromosome with acute myeloid leukemia and
concurrent large B cell lymphoma of different origins: A case
report. Oncol Lett. 13:1189–1193. 2017. View Article : Google Scholar : PubMed/NCBI
|